Ferrocenyl Phosphorhydrazone Dendrimers Synthesis, and Electrochemical and Catalytic Properties
Abstract
1. Introduction
2. Synthesis of Ferrocenyl Phosphorhydrazone Dendrimers
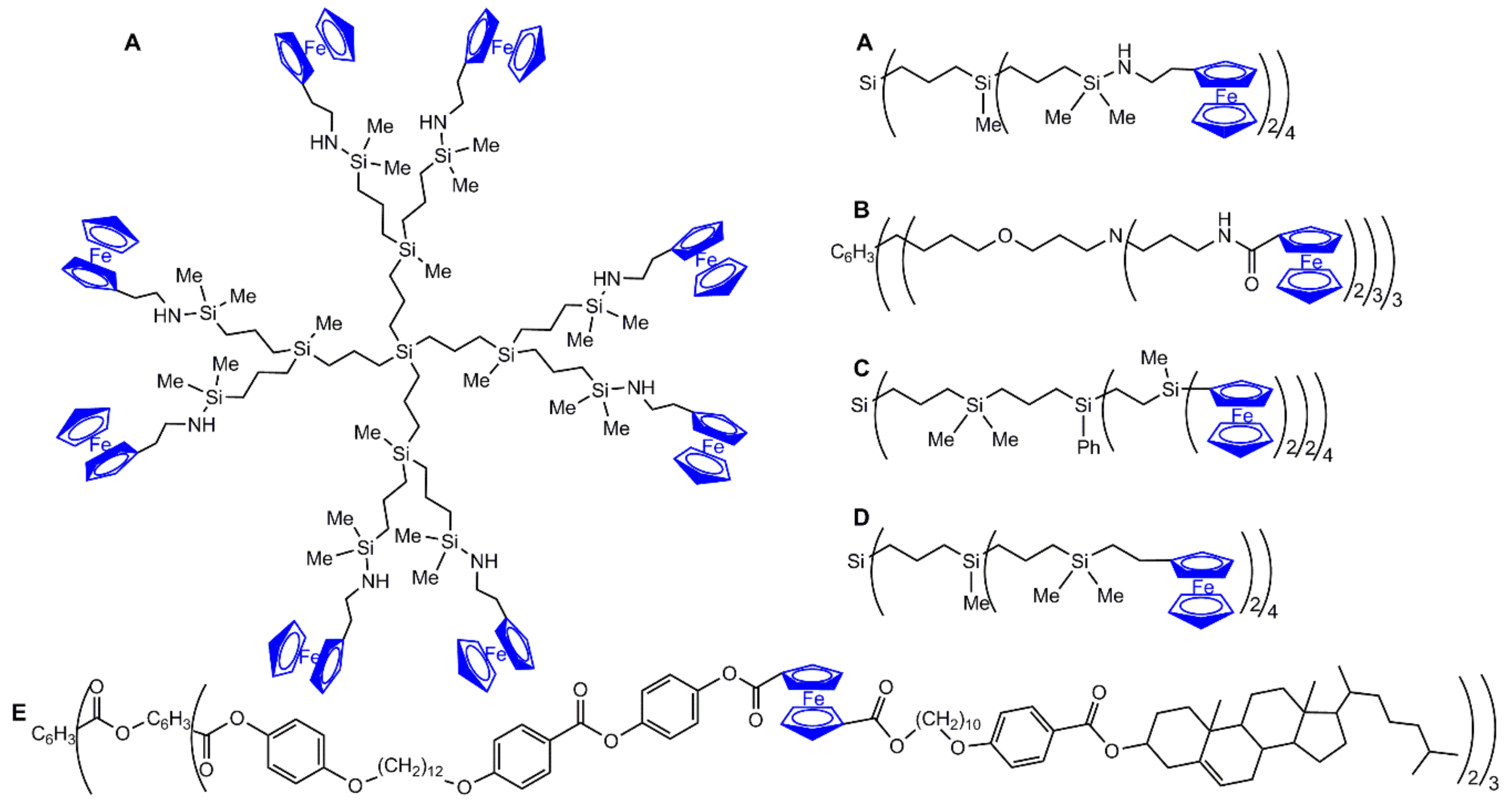
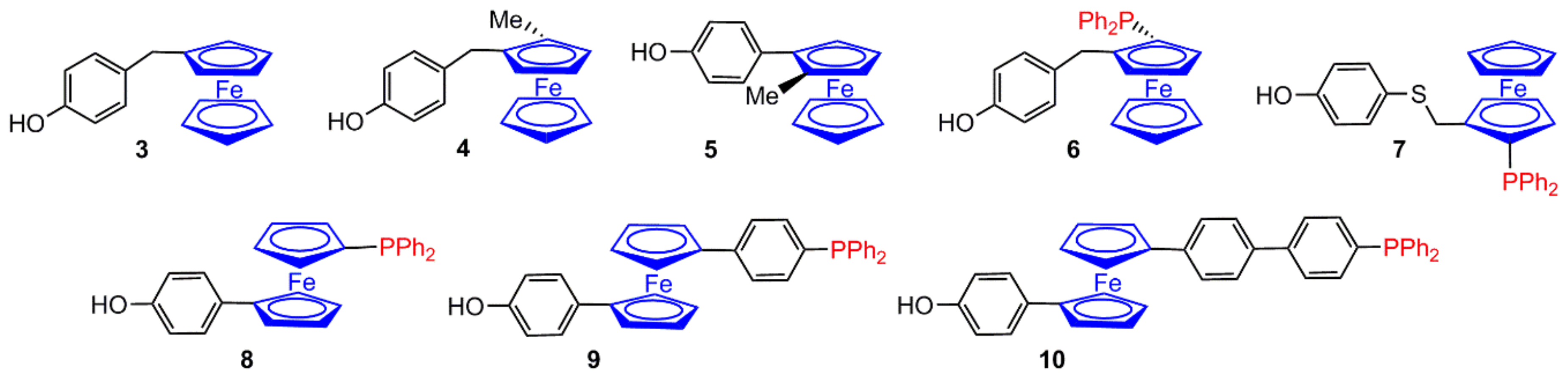
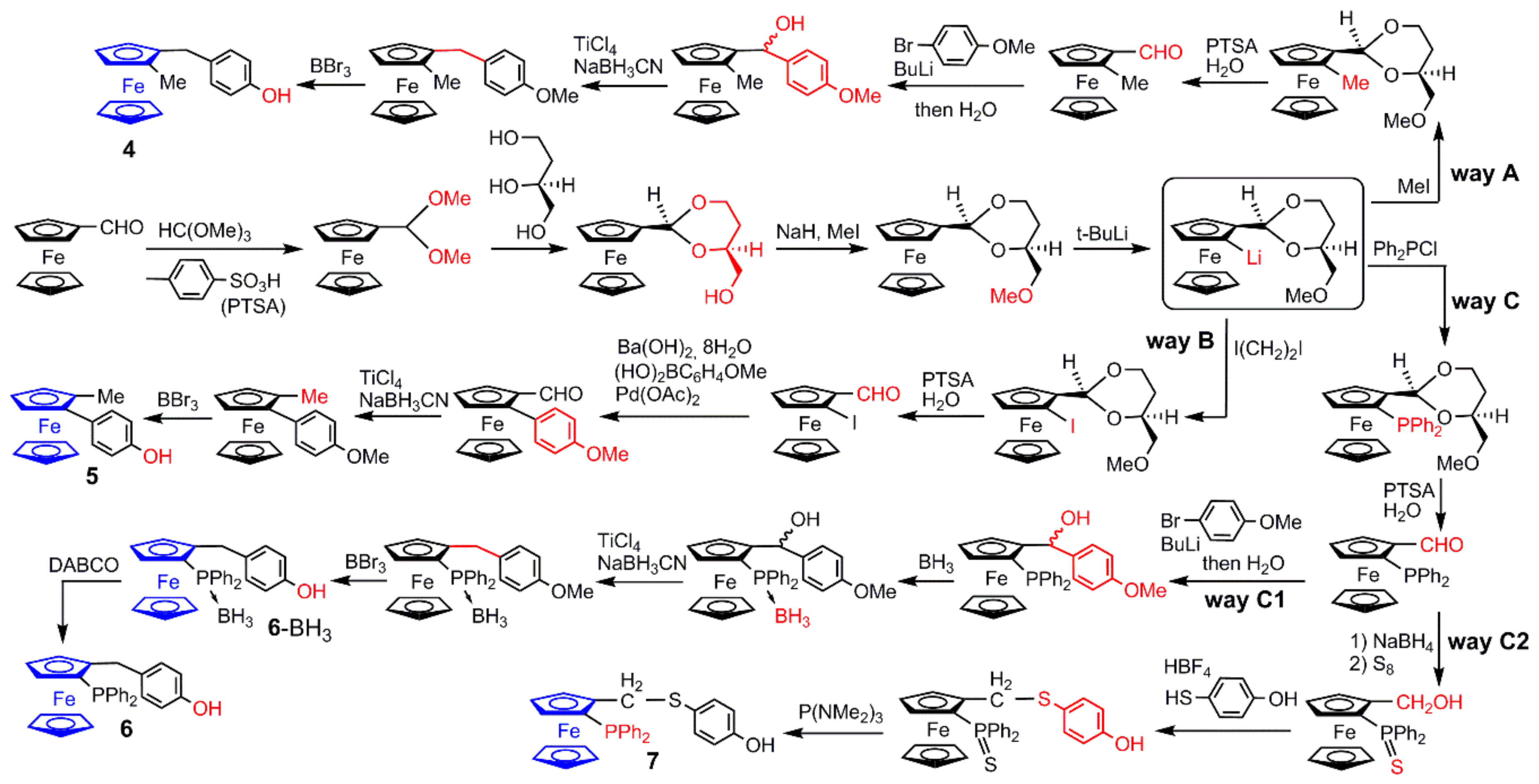
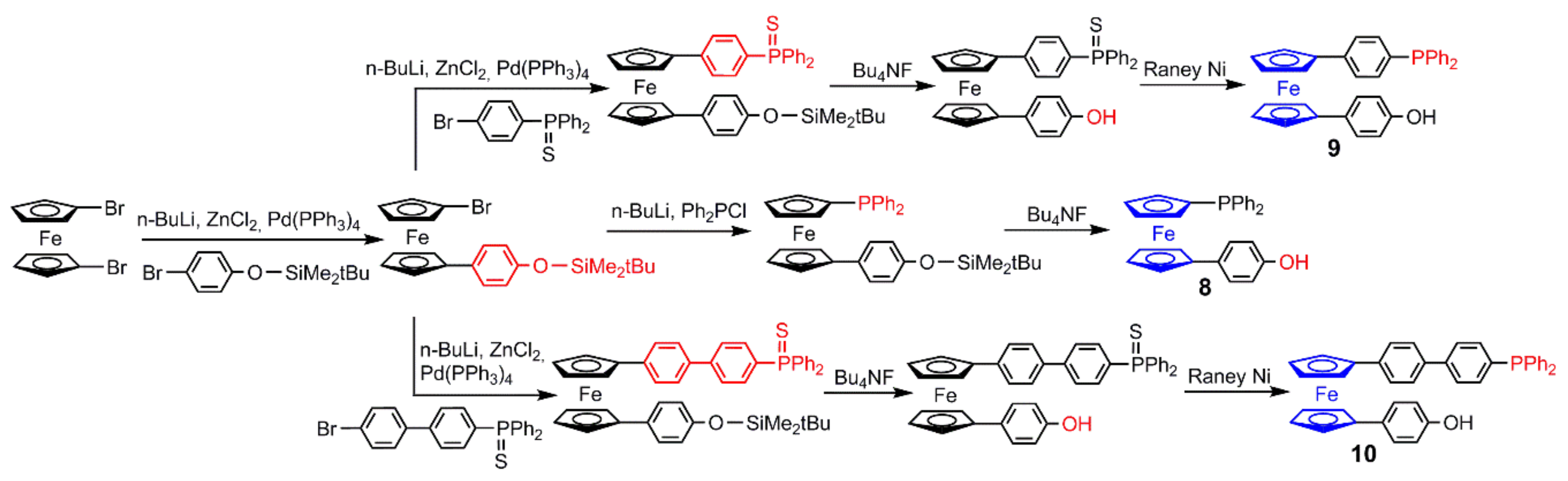
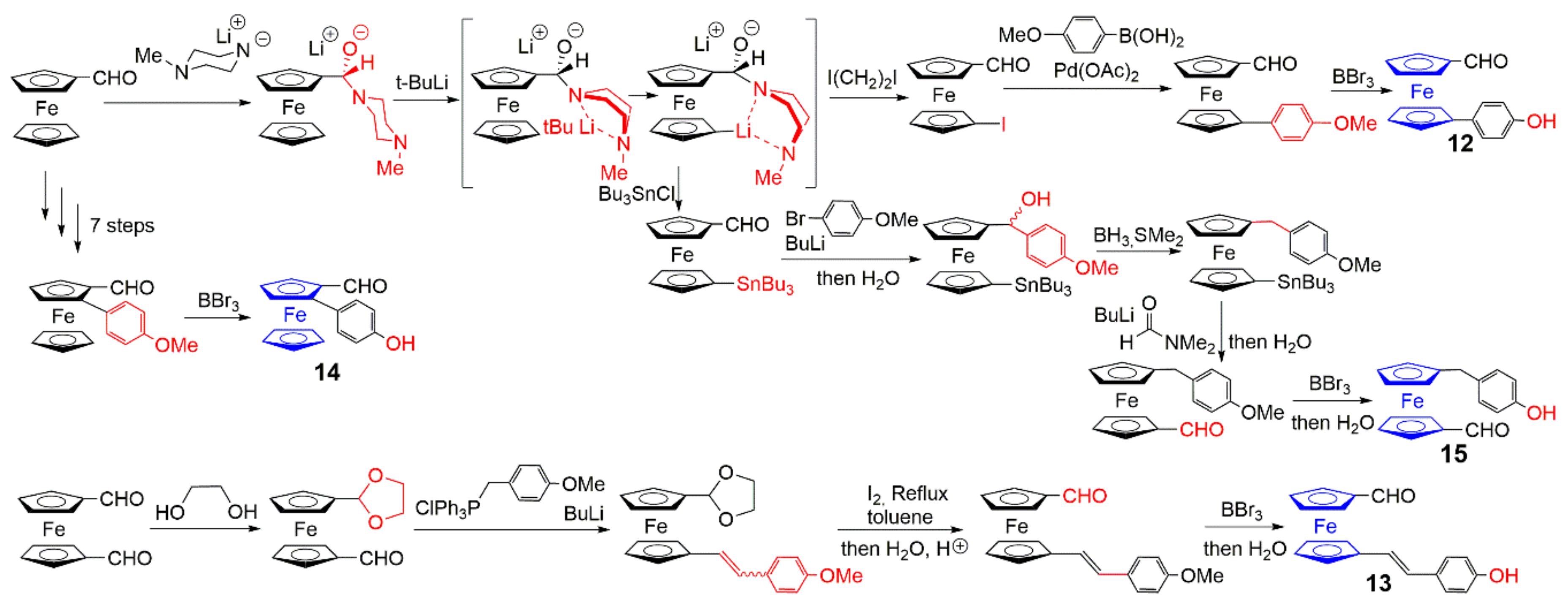
2.1. Ferrocenes on the Periphery of Dendrimers
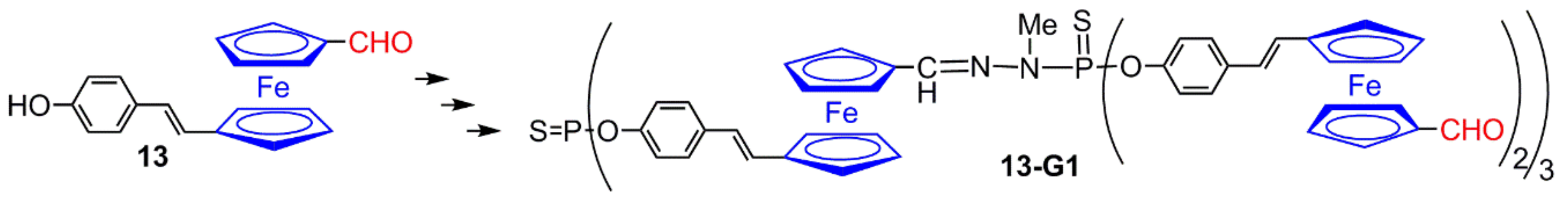
2.2. Ferrocene at the Core of Dendrimers
2.3. Ferrocenes as Branches of Dendrimers at one or Several Layers
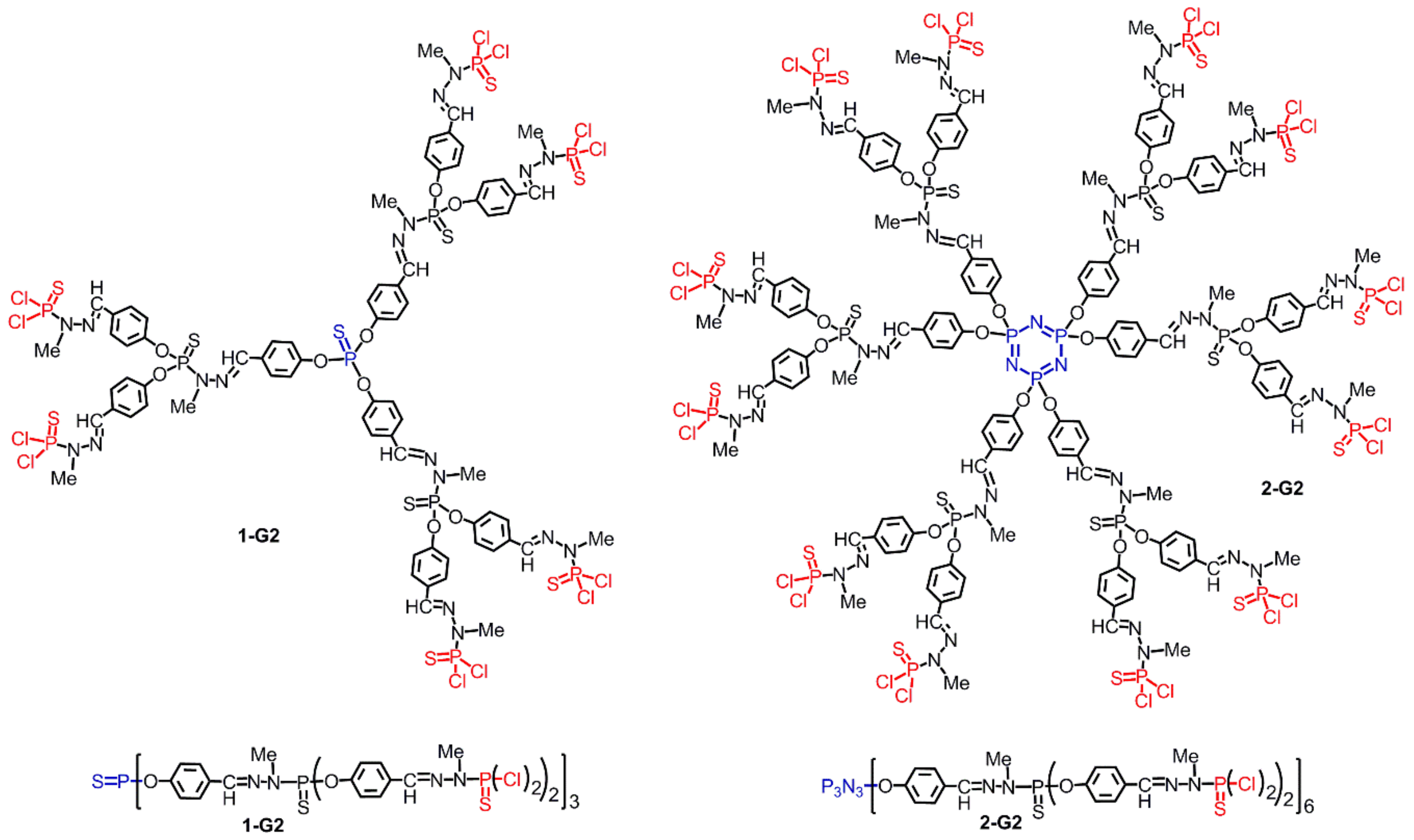
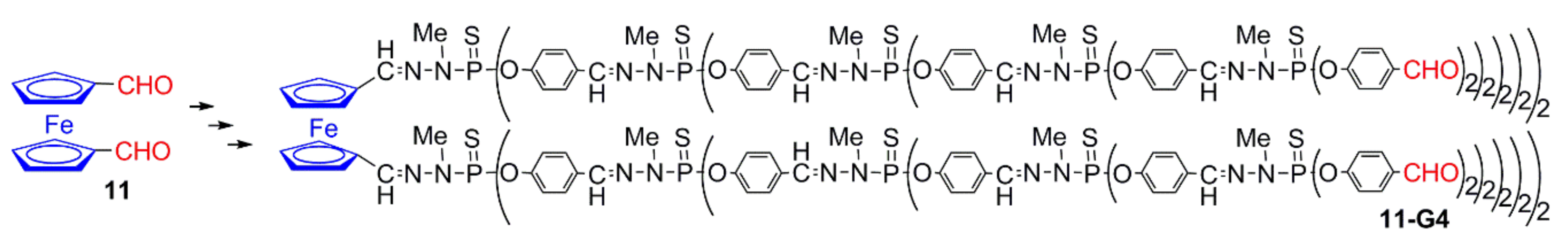
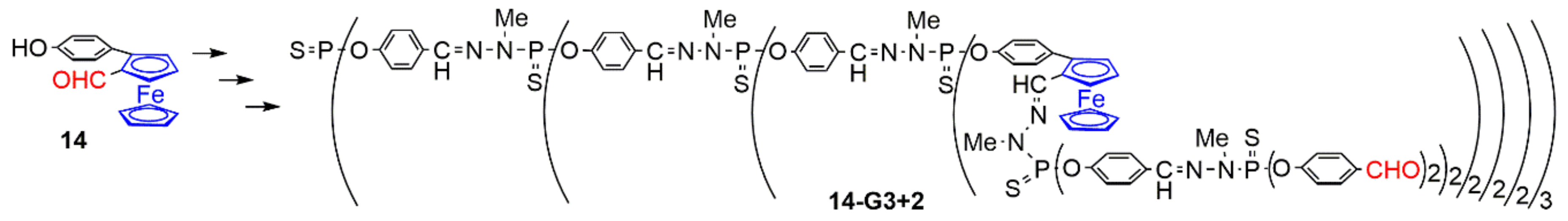
2.3.1. Ferrocenes at all Layers of Dendrimers
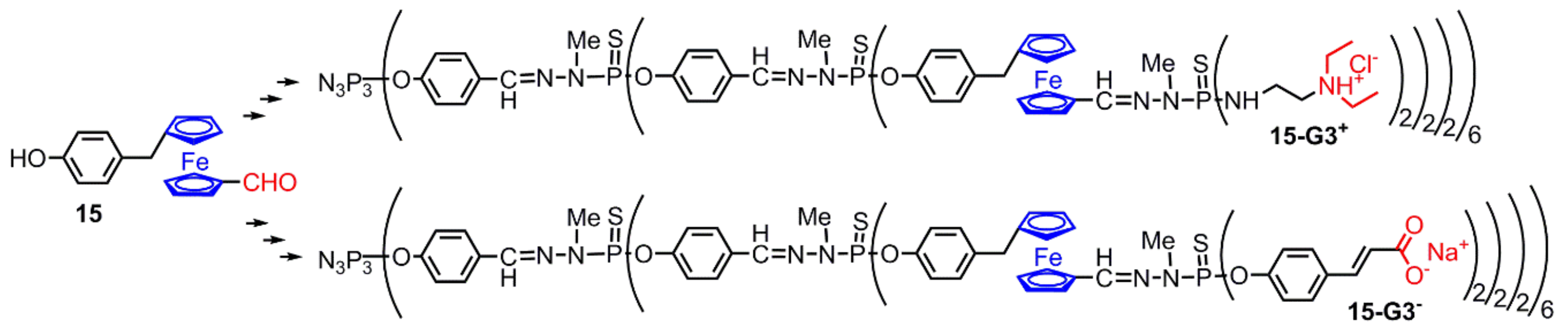
2.3.2. Ferrocenes at a Single Layer Inside Dendrimers
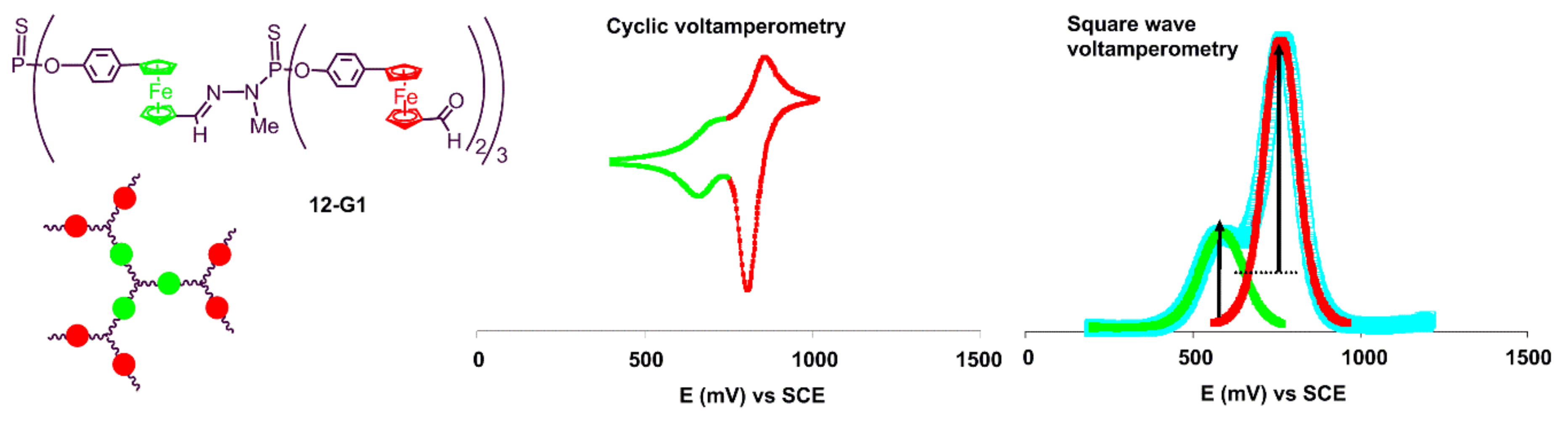
3. Electrochemical Properties
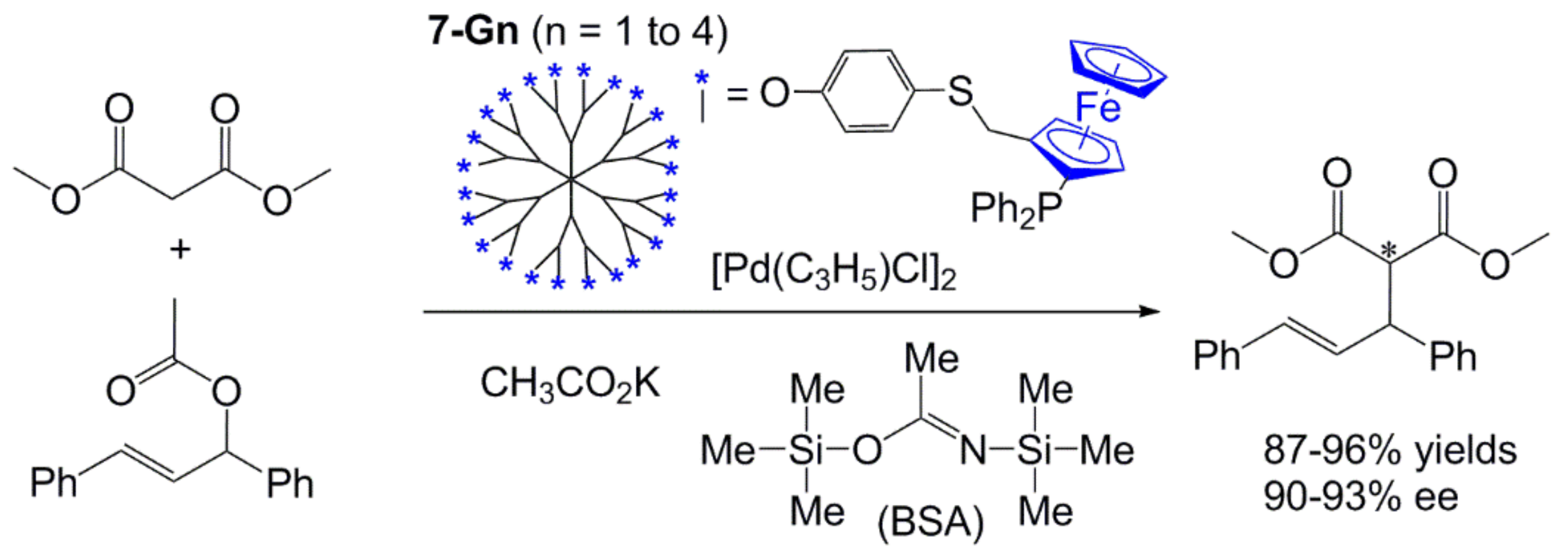
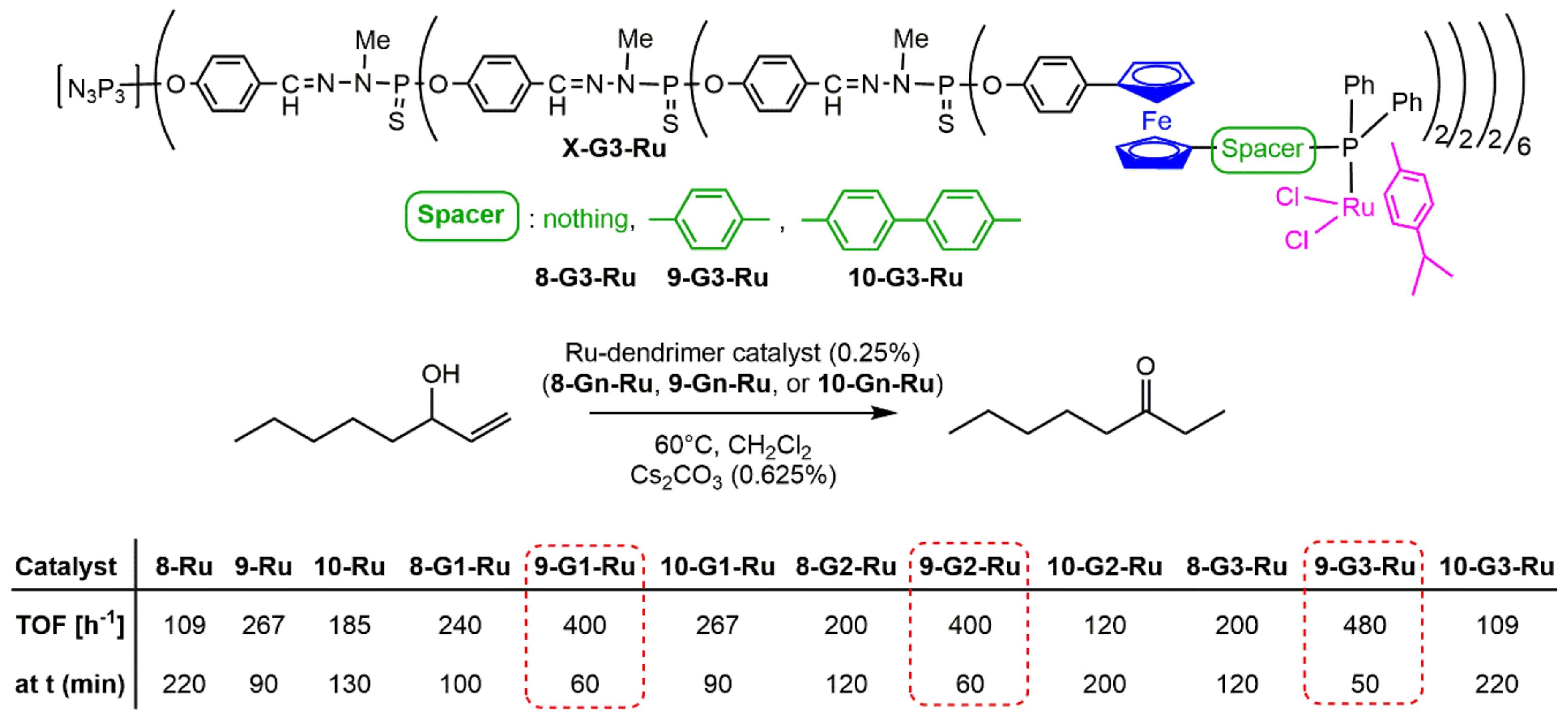
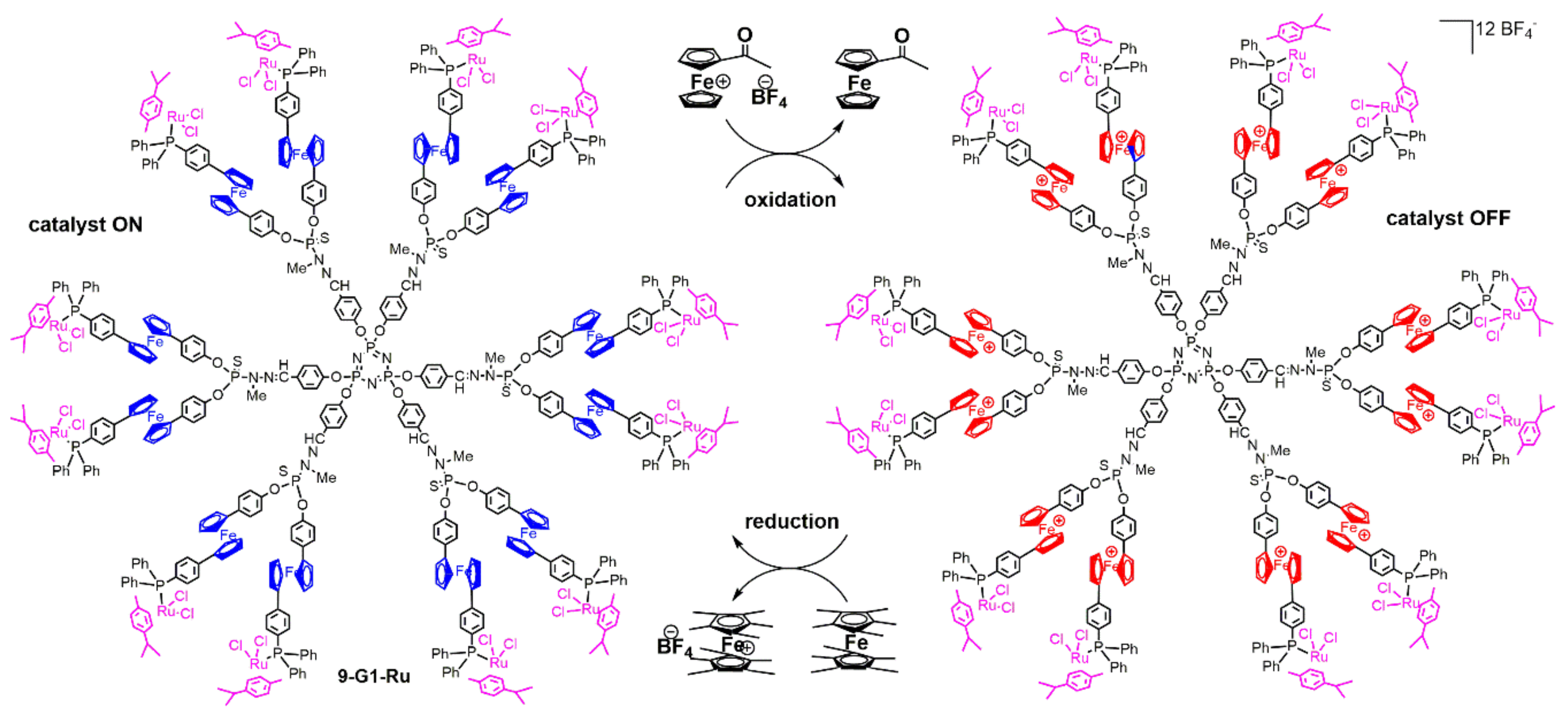
4. Catalysis Experiments with Ferrocenyl Phosphine Complexes on the Periphery of Dendrimers
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Caminade, A.-M.; Turrin, C.-O.; Laurent, R.; Ouali, A.; Delavaux-Nicot, B. Dendrimers: Towards Catalytic, Material and Biomedical Uses; John Wiley & Sons Ltd.: Chichester, UK, 2011; pp. 1–538. [Google Scholar]
- Alonso, B.; Cuadrado, I.; Moran, M.; Losada, J. Organometallic Silicon Dendrimers. J. Chem. Soc.-Chem. Commun. 1994, 2575–2576. [Google Scholar] [CrossRef]
- Valerio, C.; Fillaut, J.L.; Ruiz, J.; Guittard, J.; Blais, J.C.; Astruc, D. The dendritic effect in molecular recognition: Ferrocene dendrimers and their use as supramolecular redox sensors for the recognition of small inorganic anions. J. Am. Chem. Soc. 1997, 119, 2588–2589. [Google Scholar] [CrossRef]
- Castro, R.; Cuadrado, I.; Alonso, B.; Casado, C.M.; Moran, M.; Kaifer, A.E. Multisite inclusion complexation of redox active dendrimer guests. J. Am. Chem. Soc. 1997, 119, 5760–5761. [Google Scholar] [CrossRef]
- Losada, J.; Cuadrado, I.; Moran, M.; Casado, C.M.; Alonso, B.; Barranco, M. Ferrocenyl silicon-based dendrimers as mediators in amperometric biosensors. Anal. Chim. Acta 1997, 338, 191–198. [Google Scholar] [CrossRef]
- Deschenaux, R.; Serrano, E.; Levelut, A.M. Ferrocene-containing liquid-crystalline dendrimers: A novel family of mesomorphic macromolecules. Chem. Commun. 1997, 1577–1578. [Google Scholar] [CrossRef]
- Launay, N.; Caminade, A.M.; Lahana, R.; Majoral, J.P. A general synthetic strategy for neutral phosphorus-containing dendrimers. Angew. Chem.-Int. Ed. Engl. 1994, 33, 1589–1592. [Google Scholar] [CrossRef]
- Caminade, A.M. Inorganic dendrimers: Recent advances for catalysis, nanomaterials, and nanomedicine. Chem. Soc. Rev. 2016, 45, 5174–5186. [Google Scholar] [CrossRef]
- Launay, N.; Slany, M.; Caminade, A.M.; Majoral, J.P. Phosphorus-containing dendrimers. Easy access to new multi-difunctionalized macromolecules. J. Org. Chem. 1996, 61, 3799–3805. [Google Scholar] [CrossRef]
- Ouali, A.; Laurent, R.; Caminade, A.M.; Majoral, J.P.; Taillefer, M. Enhanced catalytic properties of copper in O- and N-arylation and vinylation reactions, using phosphorus dendrimers as ligands. J. Am. Chem. Soc. 2006, 128, 15990–15991. [Google Scholar] [CrossRef]
- Keller, M.; Colliere, V.; Reiser, O.; Caminade, A.M.; Majoral, J.P.; Ouali, A. Pyrene-Tagged Dendritic Catalysts Noncovalently Grafted onto Magnetic Co/C Nanoparticles: An Efficient and Recyclable System for Drug Synthesis. Angew. Chem. Int. Ed. 2013, 52, 3626–3629. [Google Scholar] [CrossRef]
- Caminade, A.-M.; Laurent, R. Homogeneous catalysis with phosphorus dendrimer complexes. Coord. Chem. Rev. 2019, 389, 59–72. [Google Scholar] [CrossRef]
- Caminade, A.M.; Ouali, A.; Laurent, R.; Turrin, C.O.; Majoral, J.P. Coordination chemistry with phosphorus dendrimers. Applications as catalysts, for materials, and in biology. Coord. Chem. Rev. 2016, 308, 478–497. [Google Scholar] [CrossRef]
- Blanzat, M.; Turrin, C.O.; Aubertin, A.M.; Couturier-Vidal, C.; Caminade, A.M.; Majoral, J.P.; Rico-Lattes, I.; Lattes, A. Dendritic catanionic assemblies: In vitro anti-HIV activity of phosphorus-containing dendrimers bearing Gal beta(1)cer analogues. ChemBioChem 2005, 6, 2207–2213. [Google Scholar] [CrossRef] [PubMed]
- Blattes, E.; Vercellone, A.; Eutamene, H.; Turrin, C.O.; Theodorou, V.; Majoral, J.P.; Caminade, A.M.; Prandi, J.; Nigou, J.; Puzo, G. Mannodendrimers prevent acute lung inflammation by inhibiting neutrophil recruitment. Proc. Natl. Acad. Sci. USA 2013, 110, 8795–8800. [Google Scholar] [CrossRef] [PubMed]
- Caminade, A.M.; Fruchon, S.; Turrin, C.O.; Poupot, M.; Ouali, A.; Maraval, A.; Garzoni, M.; Maly, M.; Furer, V.; Kovalenko, V.; et al. The key role of the scaffold on the efficiency of dendrimer nanodrugs. Nat. Commun. 2015, 6, 7722. [Google Scholar] [CrossRef] [PubMed]
- Caminade, A.-M. Phosphorus dendrimers for nanomedicine. Chem. Commun. 2017, 53, 9830–9838. [Google Scholar] [CrossRef]
- Caminade, A.-M.; Hameau, A.; Majoral, J.-P. The specific functionalization of cyclotriphosphazene for the synthesis of smart dendrimers. Dalton Trans. 2016, 45, 1810–1822. [Google Scholar] [CrossRef]
- Iftime, G.; Moreau-Bossuet, C.; Manoury, E.; Balavoine, G.G.A. Selective functionalization of the 1′-position of ferrocenecarbaldehyde. Chem. Commun. 1996, 527–528. [Google Scholar] [CrossRef]
- Chiffre, J.; Averseng, F.; Balavoine, G.G.A.; Daran, J.C.; Iftime, G.; Lacroix, P.G.; Manoury, E.; Nakatani, K. A Novel and Perfectly Aligned Crystal of A Ferrocenyl Chromophore displaying High Quadratic Non Linear Optical Bulk Efficiency. Eur. J. Inorg. Chem. 2001, 2001, 2221–2226. [Google Scholar] [CrossRef]
- Routaboul, L.; Chiffre, J.; Balavoine, G.G.A.; Daran, J.C.; Manoury, E. Highly efficient reduction of ferrocenyl derivatives by borane. J. Organomet. Chem. 2001, 637–639, 364–371. [Google Scholar] [CrossRef]
- Routaboul, L.; Vincendeau, S.; Daran, J.C.; Manoury, E. New ferrocenyl P,S and S,S ligands for asymmetric catalysis. Tetrahedron Asymmetry 2005, 16, 2685–2690. [Google Scholar] [CrossRef]
- Turrin, C.O.; Chiffre, J.; de Montauzon, D.; Daran, J.C.; Caminade, A.M.; Manoury, E.; Balavoine, G.; Majoral, J.P. Phosphorus-containing dendrimers with ferrocenyl units at the core, within the branches, and on the periphery. Macromolecules 2000, 33, 7328–7336. [Google Scholar] [CrossRef]
- Turrin, C.O.; Chiffre, J.; Daran, J.C.; de Montauzon, D.; Caminade, A.M.; Manoury, E.; Balavoine, G.; Majoral, J.P. New chiral phosphorus-containing dendrimers with ferrocenes on the periphery. Tetrahedron 2001, 57, 2521–2536. [Google Scholar] [CrossRef]
- Routaboul, L.; Vincendeau, S.; Turrin, C.O.; Caminade, A.M.; Majoral, J.P.; Daran, J.C.; Manoury, E. New phosphorus dendrimers with chiral ferrocenyl phosphine-thioether ligands on the periphery for asymmetric catalysis. J. Organomet. Chem. 2007, 692, 1064–1073. [Google Scholar] [CrossRef]
- Neumann, P.; Dib, H.; Sournia-Saquet, A.; Grell, T.; Handke, M.; Caminade, A.M.; Hey-Hawkins, E. Ruthenium Complexes with Dendritic Ferrocenyl Phosphanes: Synthesis, Characterization, and Application in the Catalytic Redox Isomerization of Allylic Alcohols. Chem.-Eur. J. 2015, 21, 6590–6604. [Google Scholar] [CrossRef] [PubMed]
- Riant, O.; Samuel, O.; Flessner, T.; Taudien, S.; Kagan, H.B. An efficient asymmetric synthesis of 2-substituted ferrocenecarboxaldehydes. J. Org. Chem. 1997, 62, 6733–6745. [Google Scholar] [CrossRef]
- Turrin, C.O.; Chiffre, J.; Daran, J.C.; de Montauzon, D.; Balavoine, G.; Manoury, E.; Caminade, A.M.; Majoral, J.P. New phosphorus-containing dendrimers with ferrocenyl units in each layer. C. R. Chim. 2002, 5, 309–318. [Google Scholar] [CrossRef]
- Turrin, C.O.; Chiffre, J.; de Montauzon, D.; Balavoine, G.; Manoury, E.; Caminade, A.M.; Majoral, J.P. Behavior of an optically active ferrocene chiral shell located within phosphorus-containing dendrimers. Organometallics 2002, 21, 1891–1897. [Google Scholar] [CrossRef]
- De Jong, E.R.; Manoury, E.; Daran, J.C.; Turrin, C.O.; Chiffre, J.; Sournia-Saquet, A.; Knoll, W.; Majoral, J.P.; Caminade, A.M. Synthesis and characterization of water-soluble ferrocene-dendrimers. J. Organomet. Chem. 2012, 718, 22–30. [Google Scholar] [CrossRef]
- Caminade, A.M.; Servin, P.; Laurent, R.; Majoral, J.P. Dendrimeric phosphines in asymmetric catalysis. Chem. Soc. Rev. 2008, 37, 56–67. [Google Scholar] [CrossRef]
- Caminade, A.M.; Ouali, A.; Laurent, R.; Turrin, C.O.; Majoral, J.P. The dendritic effect illustrated with phosphorus dendrimers. Chem. Soc. Rev. 2015, 44, 3890–3899. [Google Scholar] [CrossRef] [PubMed]
- Neumann, P.; Dib, H.; Caminade, A.M.; Hey-Hawkins, E. Redox Control of a Dendritic Ferrocenyl-Based Homogeneous Catalyst. Angew. Chem. Int. Ed. 2015, 54, 311–314. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: No sample of compounds is available from the authors. |

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turrin, C.-O.; Manoury, E.; Caminade, A.-M. Ferrocenyl Phosphorhydrazone Dendrimers Synthesis, and Electrochemical and Catalytic Properties. Molecules 2020, 25, 447. https://doi.org/10.3390/molecules25030447
Turrin C-O, Manoury E, Caminade A-M. Ferrocenyl Phosphorhydrazone Dendrimers Synthesis, and Electrochemical and Catalytic Properties. Molecules. 2020; 25(3):447. https://doi.org/10.3390/molecules25030447
Chicago/Turabian StyleTurrin, Cédric-Olivier, Eric Manoury, and Anne-Marie Caminade. 2020. "Ferrocenyl Phosphorhydrazone Dendrimers Synthesis, and Electrochemical and Catalytic Properties" Molecules 25, no. 3: 447. https://doi.org/10.3390/molecules25030447
APA StyleTurrin, C.-O., Manoury, E., & Caminade, A.-M. (2020). Ferrocenyl Phosphorhydrazone Dendrimers Synthesis, and Electrochemical and Catalytic Properties. Molecules, 25(3), 447. https://doi.org/10.3390/molecules25030447






