Terroir Effect on the Phenolic Composition and Chromatic Characteristics of Mencía/Jaen Monovarietal Wines: Bierzo D.O. (Spain) and Dão D.O. (Portugal)
Abstract
1. Introduction
2. Results and Discussion
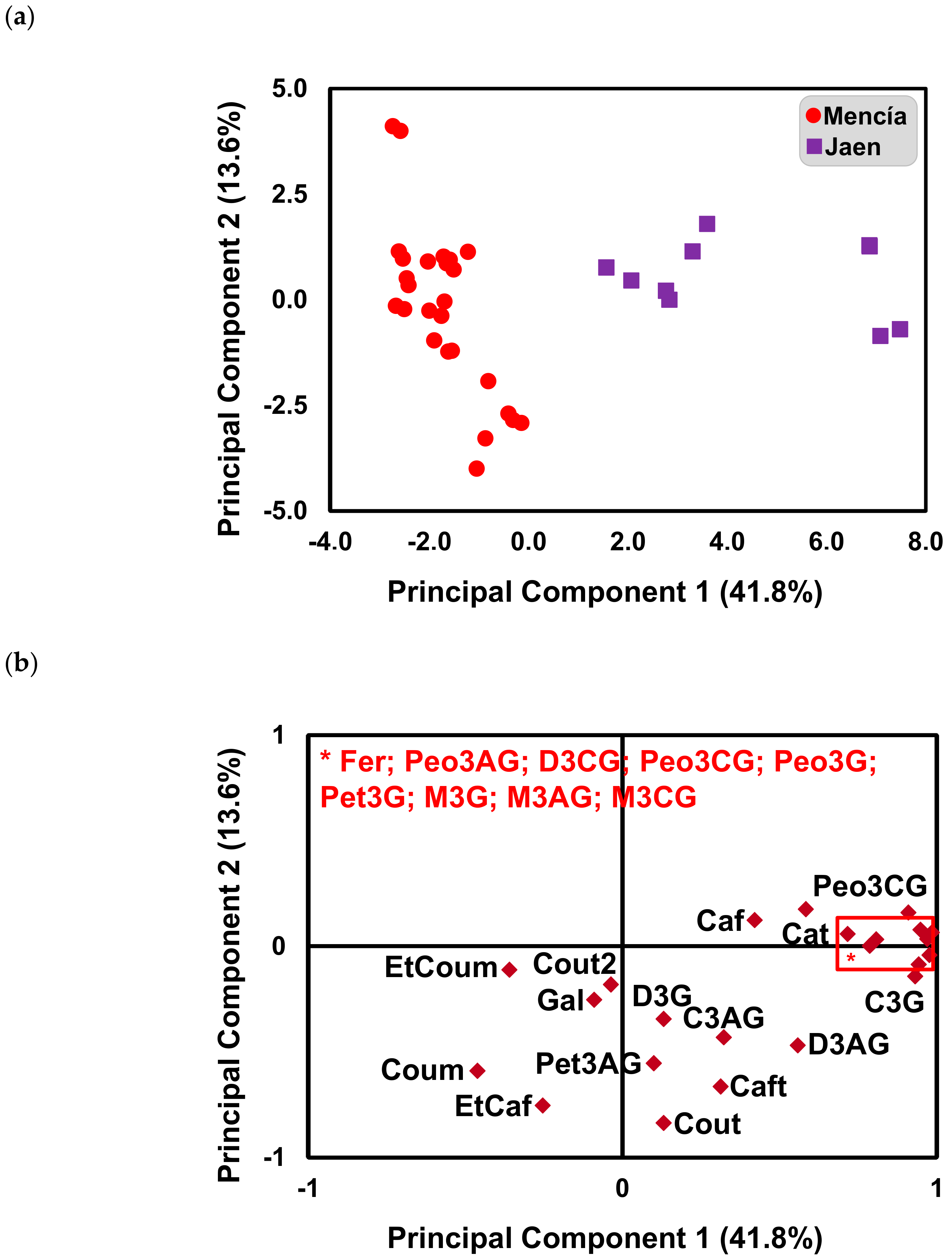
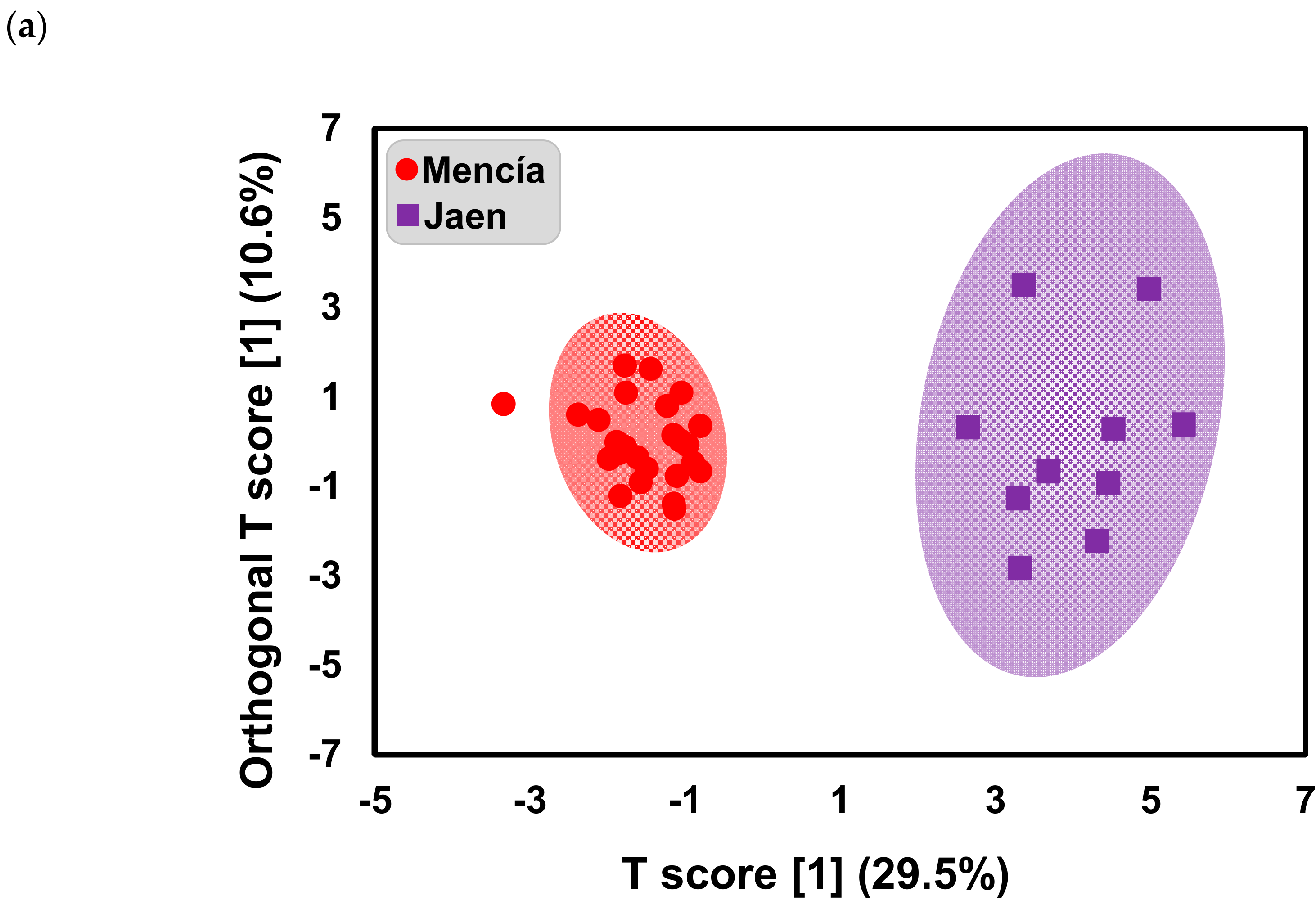
2.1. Effect of Bierzo D.O. and Dão D.O. “Terroirs” on the Anthocyanins and Phenolic Acid Composition of ‘Mencía’/‘Jaen’ Wines—Unsupervised Analysis
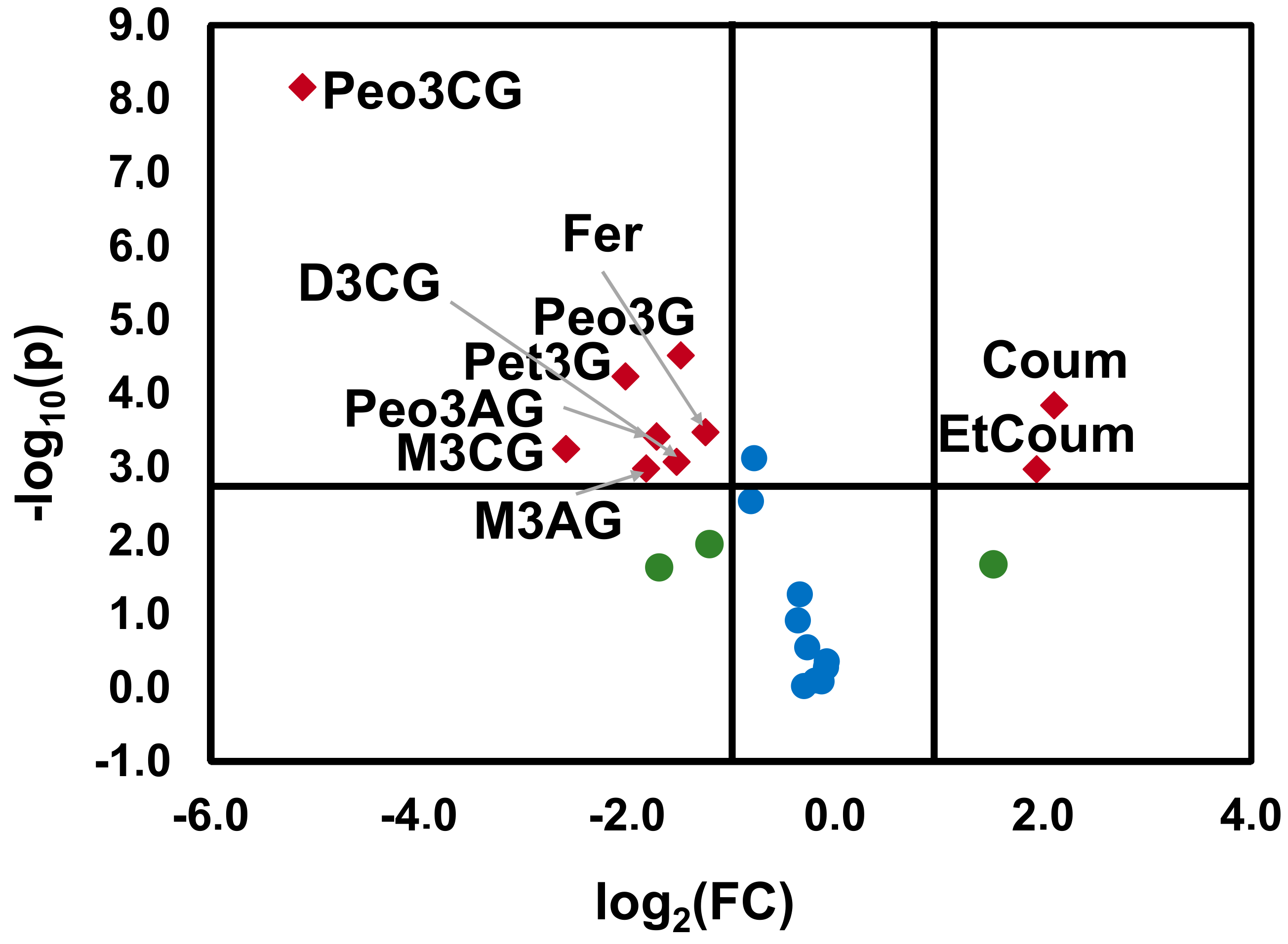
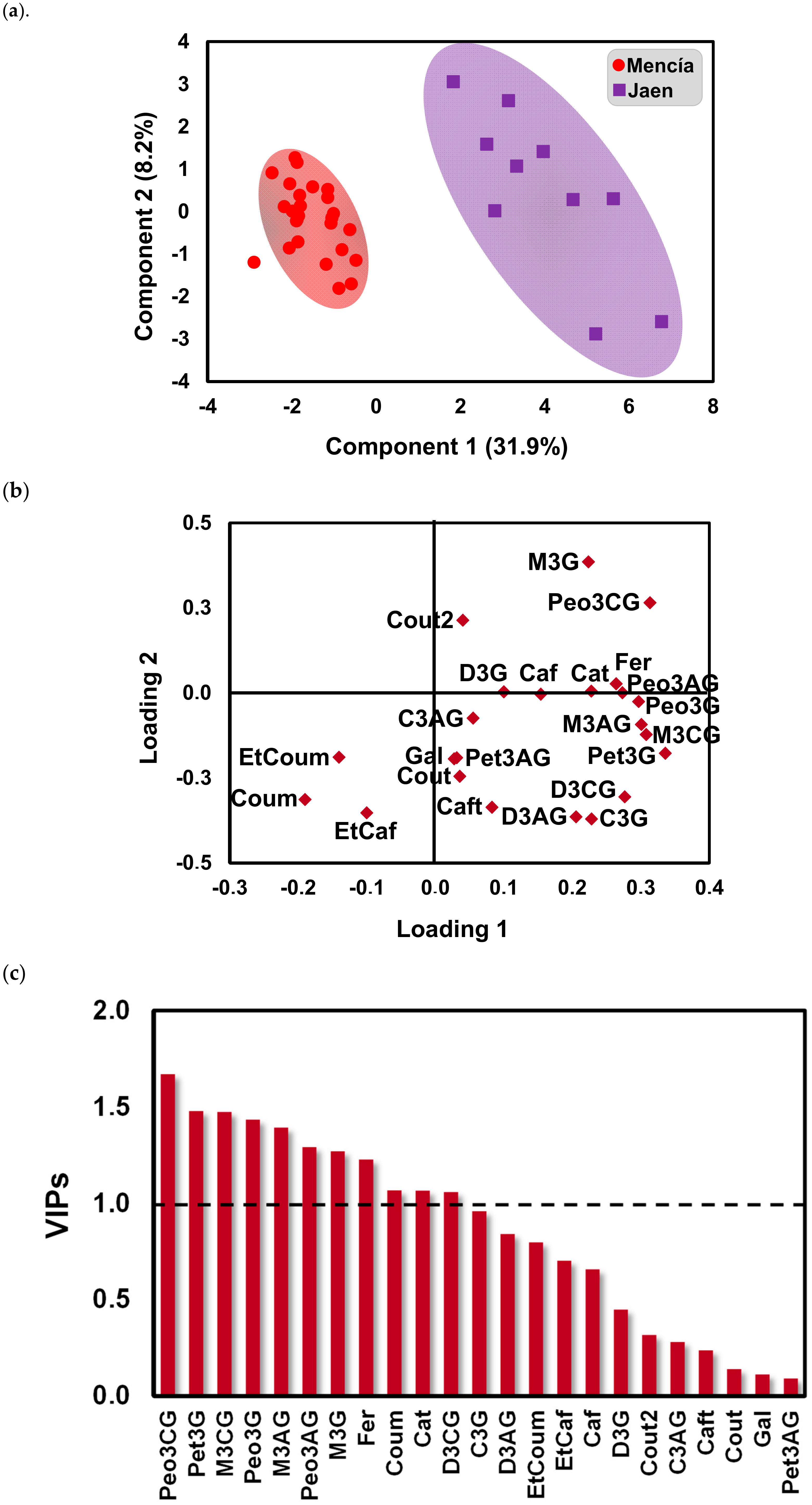
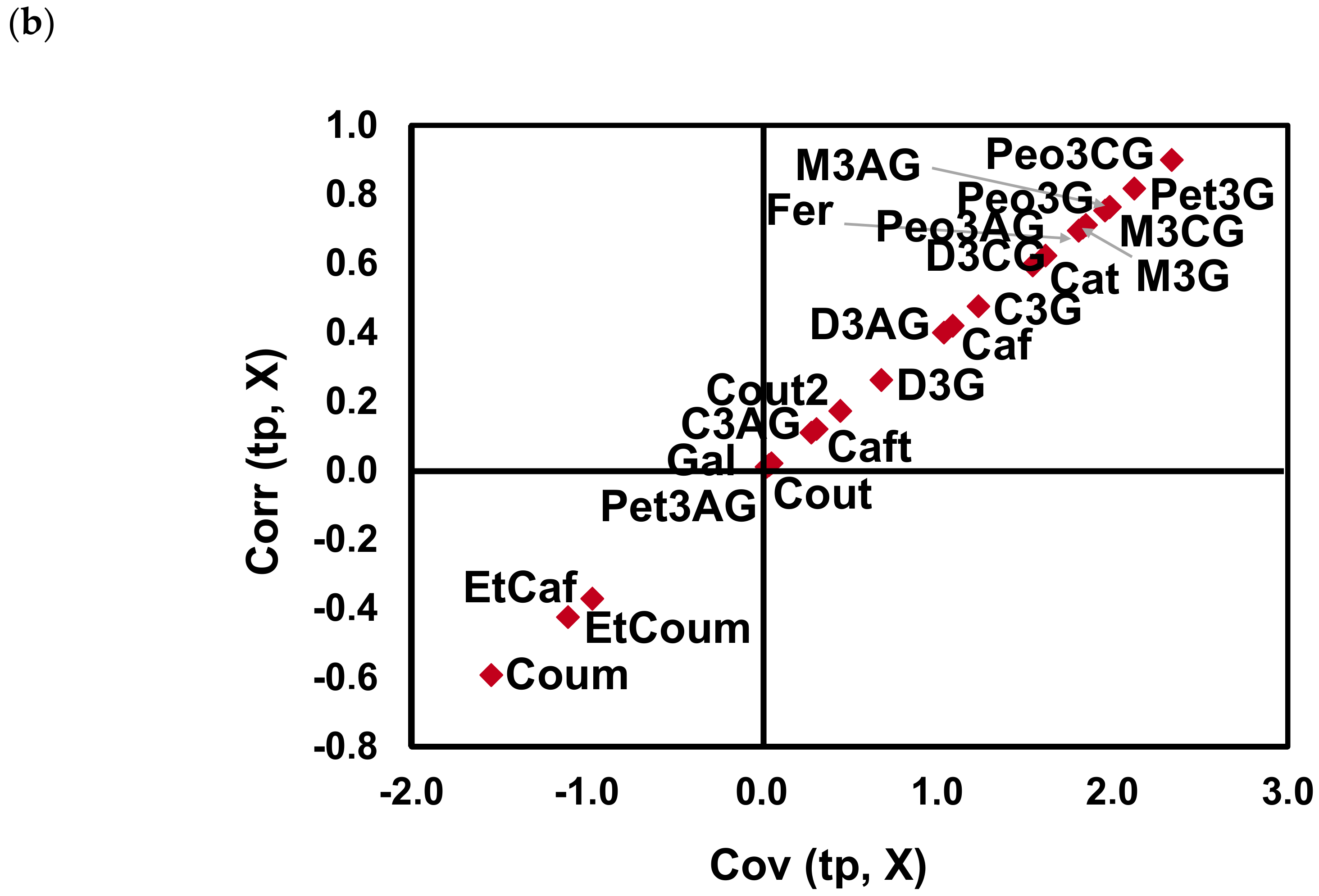
2.2. Effect of Bierzo D.O. and Dão D.O. Terroirs on the Anthocyanins and Phenolic Acid Composition of ‘Mencía’/‘Jaen’ Wines—Discrimination and Variable Importance
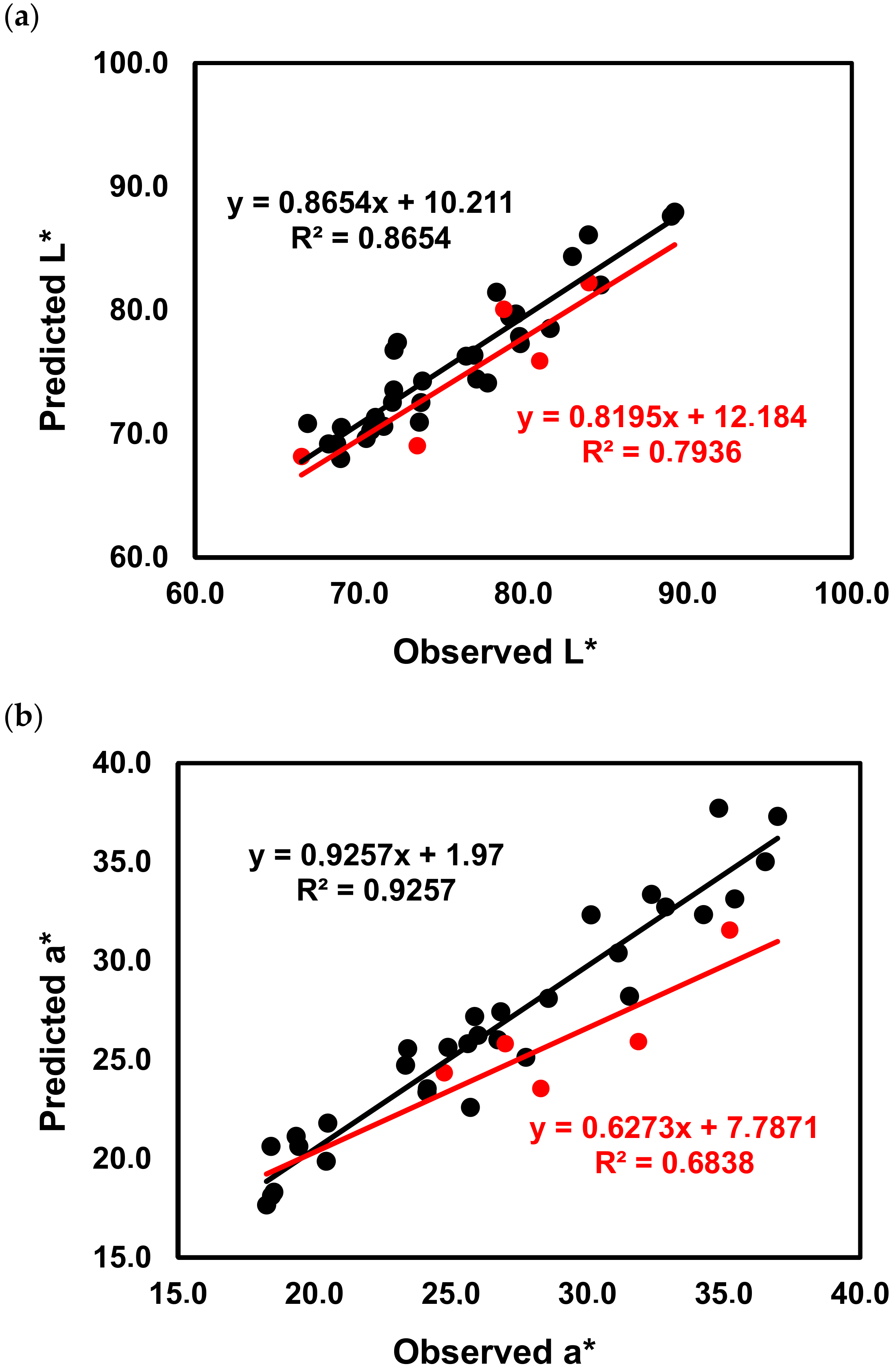
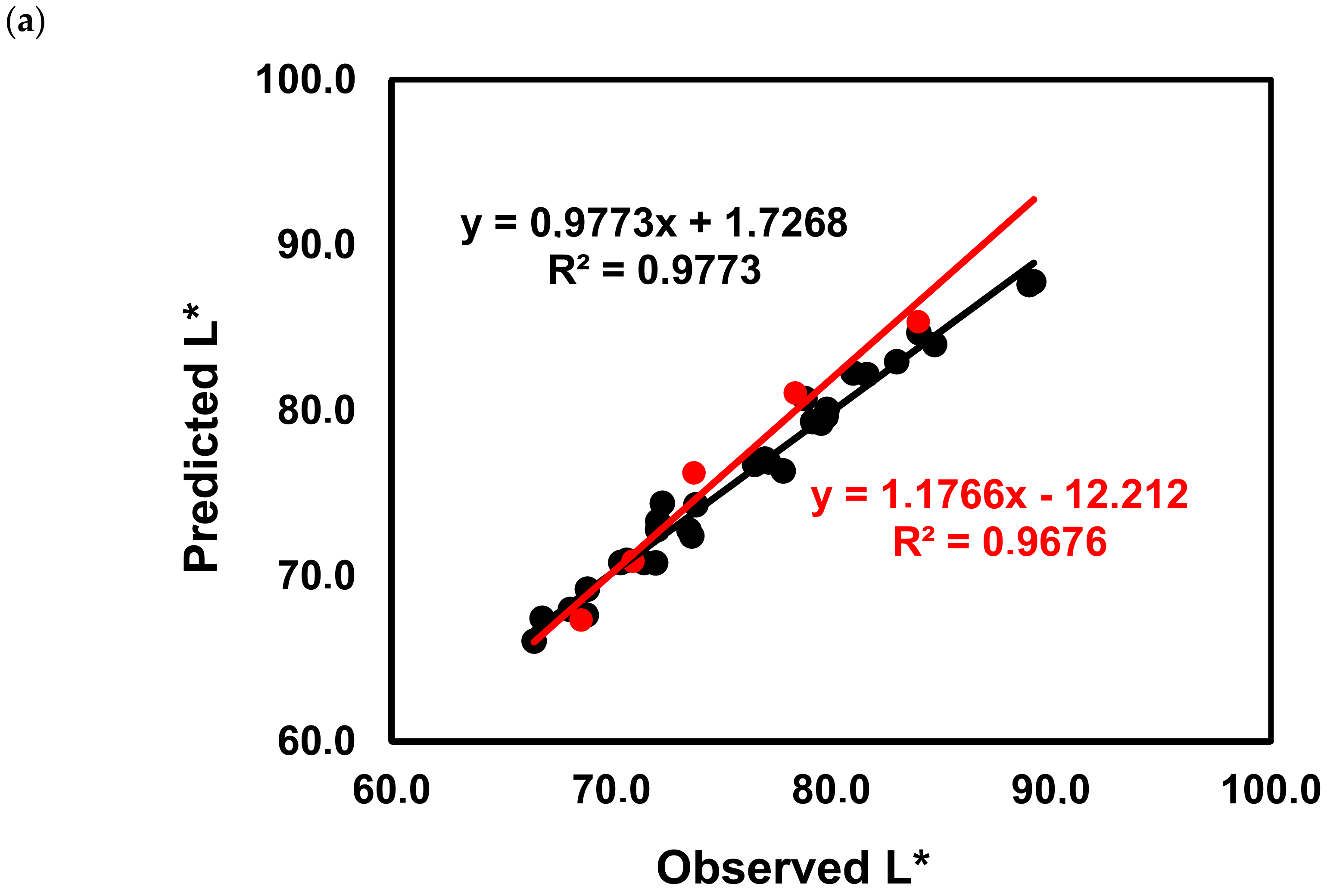
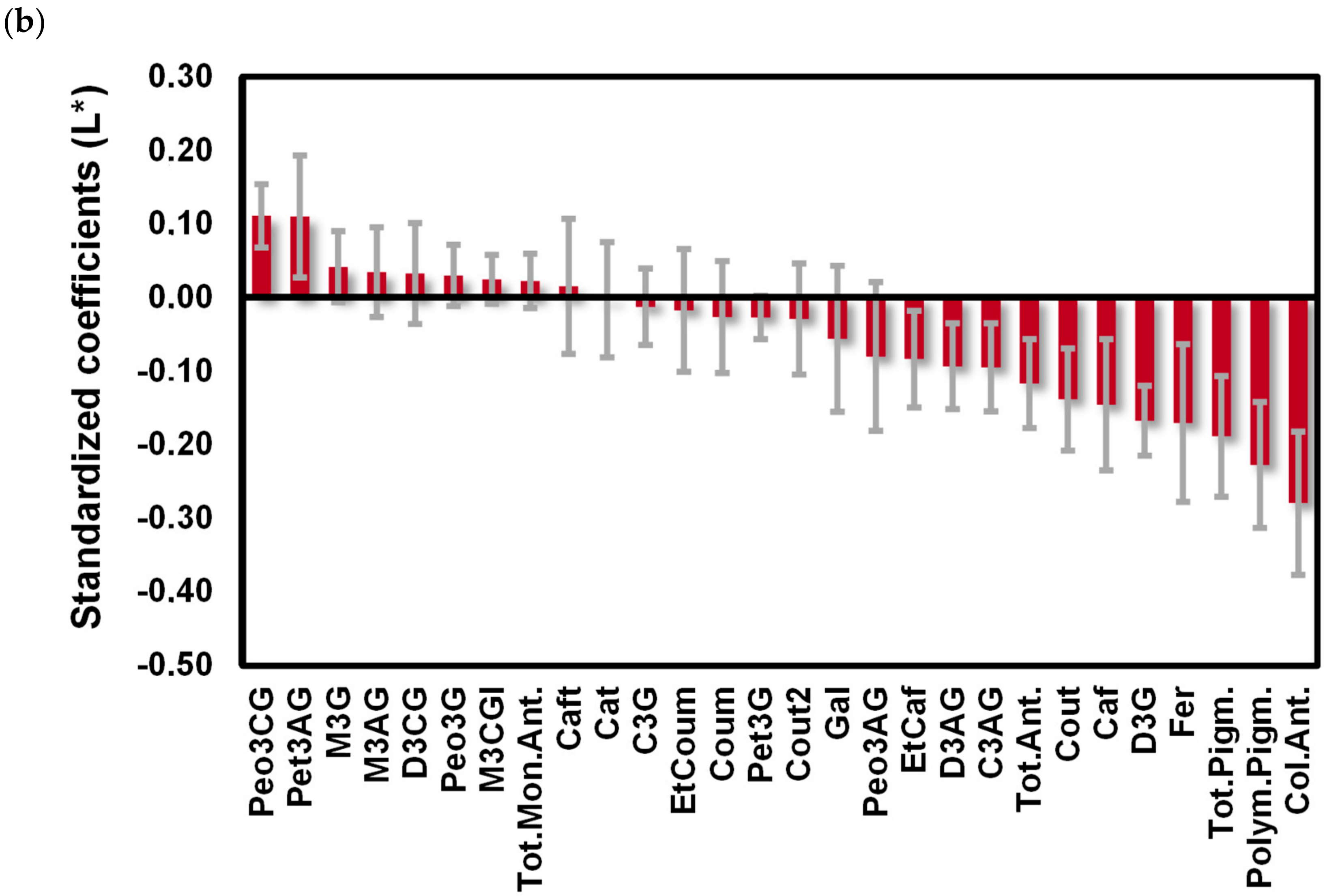
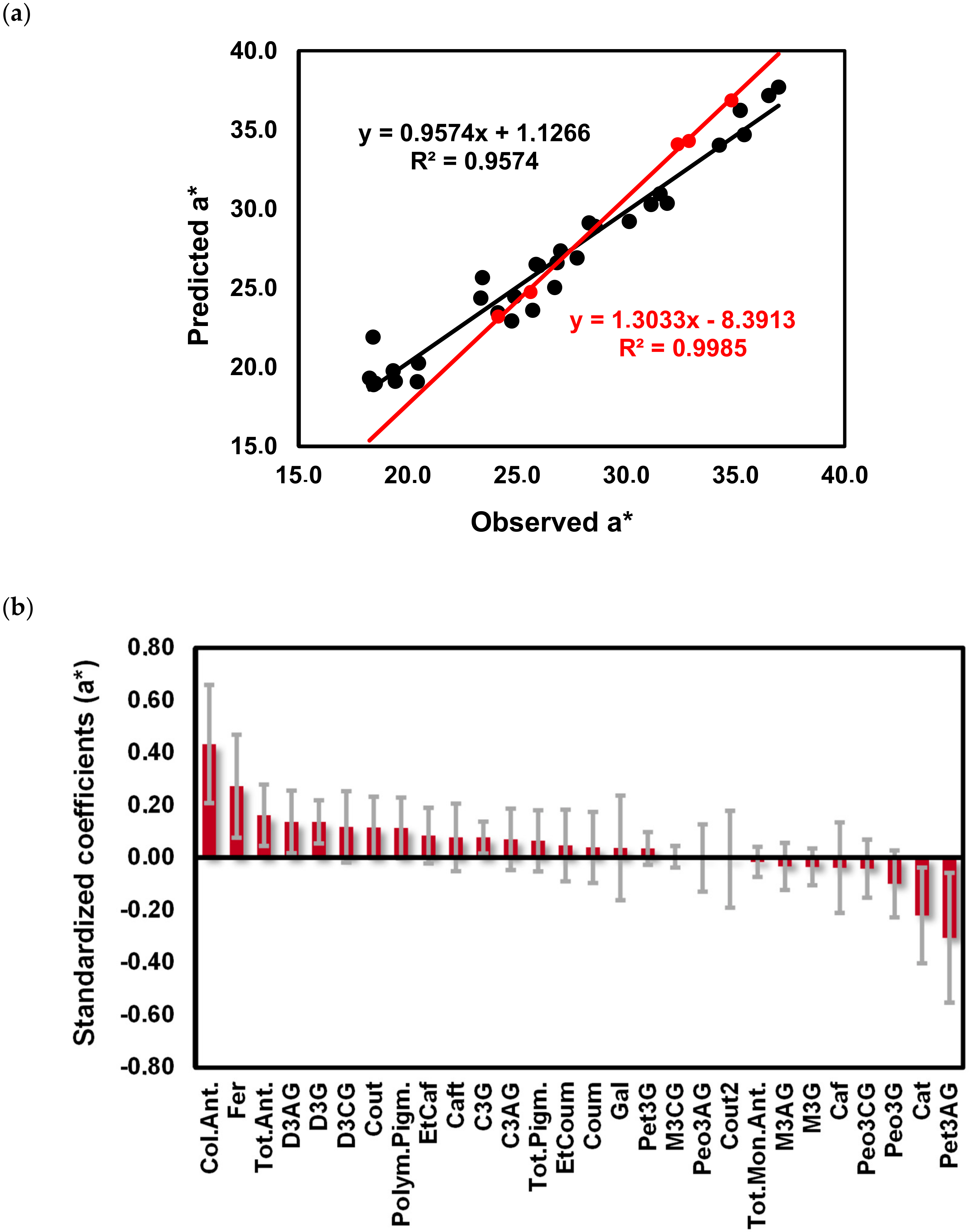
2.3. Impact of the Phenolic Composition on the Color of Red Wines from ‘Mencía’/‘Jaen’
3. Materials and Methods
3.1. Wine Samples
3.2. High-Performance Liquid Chromatography (HPLC) Analysis of Anthocyanins, Catechin, and Phenolic Acids
3.3. Color Intensity, Total Anthocyanins, Colored Anthocyanins, Pigments, and Chromatic Characteristics
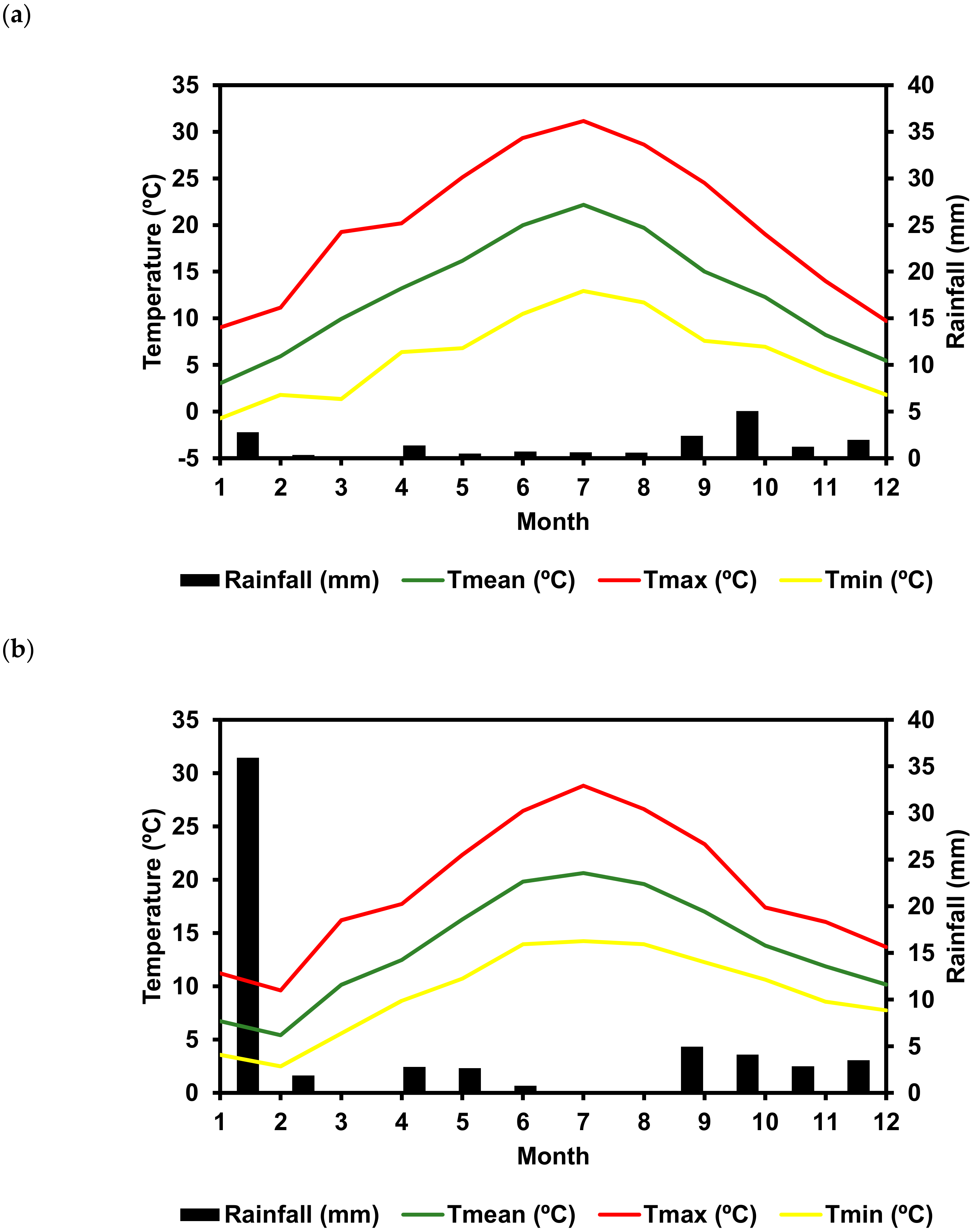
3.4. Climacteric Data, Bioclimatic Indexes, and Soil Characteristics
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Martín, J.P.; Santiago, J.L.; Pinto-Carnide, O.; Leal-Martínez, F.M.C.; Ortiz, J.M. Determination of relationships among autochthonous grapevine varieties (Vitis vinifera L.) in the Northwest of the Iberian Peninsula by using microsatellite markers. Genet. Resour. Crop Evol. 2006, 53, 1255–1261. [Google Scholar] [CrossRef]
- Cunha, J.; Zinelabidine, L.H.; Teixeira-Santos, M.; Brazão, J.; Fevereiro, P.; Martínez-Zapater, J.M.; Ibáñez, J.; Eiras-Dias, J.E. Grapevine cultivar ‘Alfrocheiro’ or ‘Bruñal’ plays a primary role in the relationship among Iberian grapevines. Vitis 2015, 54, 59–65. [Google Scholar]
- Instituto da Vinha e do Vinho (IVV). Available online: https://www.ivv.gov.pt/np4/35/ (accessed on 30 September 2020).
- Jiang, B.A.O.; Zhang, Z.W.E.N.; Zhang, X.Z. Influence of terrain on phenolic compounds and antioxidant activities of Cabernet Sauvignon wines in loess plateau region of China. J. Chem. Soc. Pak. 2011, 33, 900. [Google Scholar]
- Cortell, J.M.; Halbleib, M.; Gallagher, A.V.; Righetti, T.L.; Kennedy, J.A. Influence of Vine Vigor on Grape (Vitis vinifera L. Cv. Pinot Noir) and Wine Proanthocyanidins. J. Agric. Food Chem. 2005, 53, 5798–5808. [Google Scholar] [CrossRef]
- Russell, K.; Zivanovic, S.; Morris, W.C.; Penfield, M.; Weiss, J. The Effect of Glass Shape on the Concentration of Polyphenolic Compounds and Perception of Merlot Wine. J. Food Qual. 2005, 28, 377–385. [Google Scholar] [CrossRef]
- Kumsta, M.; Pavlousek, P.; Kupsa, J. Influence of terroir on the concentration of selected stilbenes in wines of the cv. Riesling in the Czech Republic. Hortic. Sci. 2012, 39, 38–46. [Google Scholar] [CrossRef]
- Roullier-Gall, C.; Boutegrabet, L.; Gougeon, R.D.; Schmitt-Kopplin, P. A grape and wine chemodiversity comparison of different appellations in Chemical Signatures of Two ‘‘Climats de Bourgogne’’ Burgundy: Vintage vs terroir effects. Food Chem. 2014, 152, 100–107. [Google Scholar] [CrossRef]
- Definition of Vitivinicultural “Terroir”; Resolution OIV/Viti 333/2010; OIV: Paris, France, 2010; Available online: http://www.oiv.int/public/medias/379/viti-2010-1-en.pdf (accessed on 30 September 2020).
- Rebolo, S.; Peña, R.M.; Latorre, M.J.; García, S.; Botana, A.M.; Herrero, C. Characterization of Galician (NW Spain) Ribeira Sacra wines using pattern recognition analysis. Anal. Chim. Acta 2000, 417, 211–220. [Google Scholar] [CrossRef]
- Calleja, A.; Falque, E. Volatile composition of Mencia wines. Food Chem. 2005, 90, 357–363. [Google Scholar] [CrossRef]
- Noguerol-Pato, R.; Gonzalez-Barreiro, C.; Cancho-Grande, B.; Simal-Gandara, J. Quantitative determination and characterisation of the main odorants of Mencia monovarietal red wines. Food Chem. 2009, 117, 473–484. [Google Scholar]
- Noguerol-Pato, R.; González-Rodríguez, R.M.; González-Barreiro, C.; Cancho-Grande, B.; Simal-Gándara, J. Influence of tebuconazole residues on the aroma composition of Mencía red wines. Food Chem. 2011, 124, 1525–1532. [Google Scholar] [CrossRef]
- González-Rodríguez, R.M.; Cancho-Grande, B.; Torrado-Agrasar, A.; Simal-Gándara, J.; Mazaira-Pérez, J. Evolution of tebuconazole residues through the winemaking process of Mencía grapes. Food Chem. 2009, 117, 529–537. [Google Scholar] [CrossRef]
- Canosa, P.; Oliveira, J.M.; Masa, A.; Vilanova1, M. Study of the Volatile and Glycosidically Bound Compounds of Minority Vitis vinifera Red Cultivars from NW Spain. J. Inst. Brew. 2011, 117, 462–471. [Google Scholar] [CrossRef]
- Bouzas-Cid, Y.; Trigo-Córdoba, E.; Orriols, I.; Falqué, E.; Mirás-Avalos, J.M. Influence of Soil Management on the Red Grapevine (Vitis vinifera L.) Mencía Must Amino Acid Composition and Wine Volatile and Sensory Profiles in a Humid Region. Beverages 2018, 4, 76. [Google Scholar] [CrossRef]
- Vilanova, M.; Campo, E.; Escudero, A.; Graña, M.; Masa, A.; Cacho, J. Volatile composition and sensory properties of Vitis vinifera red cultivars from North West Spain: Correlation between sensory and instrumental analysis. Anal. Chim. Acta 2012, 720, 104–111. [Google Scholar] [CrossRef]
- Vilanova, M.; Rodríguez, I.; Canosa, P.; Otero, I.; Gamero, E.; Moreno, D.; Talaverano, I.; Valdés, E. Variability in chemical composition of Vitis vinifera cv Mencía from different geographic areas and vintages in Ribeira Sacra (NW Spain). Food Chem. 2015, 169, 187–196. [Google Scholar] [CrossRef]
- Añón, A.; López, J.F.; Hernando, D.; Orriols, I.; Revilla, E.; Losada, M.M. Effect of five enological practices and of the general phenolic composition on fermentation-related aroma compounds in Mencia young red wines. Food Chem. 2014, 148, 268–275. [Google Scholar] [CrossRef]
- Mihnea, M.; González-SanJosé, M.L.; Ortega-Heras, M.; Pérez-Magariño, S. A comparative study of the volatile content of Mencía wines obtained using different pre-fermentative maceration techniques. LWT-Food Sci. Technol. 2015, 64, 32–41. [Google Scholar] [CrossRef]
- Blanco, P.; Mirás-Avalos, J.M.; Pereira, E.; Fornos, D.; Orriols, I. Modulation of chemical and sensory characteristics of red wine from Mencía by using indigenous Saccharomyces cerevisiae yeast strains. J. Int. Sci. Vigne. Vin. 2014, 48, 63–74. [Google Scholar] [CrossRef]
- Nuñez, M.; Peña, R.M.; Herrero, C.; García-Martín, S. Analysis of some metals in wine by means of capillary electrophoresis. Application to the differentiation of Ribeira Sacra Spanish red wines. Analusis 2000, 28, 432–437. [Google Scholar]
- Peña, R.M.; Latorre, M.J.; García, S.; Botana, A.M.; Herrero, C. Pattern recognition analysis applied to classification of Galician (NW Spain) wines with certified brand of origin Ribeira Sacra. J. Food Sci. 1999, 79, 2052. [Google Scholar] [CrossRef]
- Vilanova, M.; Soto, B. The impact of geographic origin on sensory properties of Vitis vinifera cv. Mencía. J. Sens. Stud. 2005, 20, 503–511. [Google Scholar] [CrossRef]
- Harbertson, J.F.; Spayd, S. Measuring phenolics in the winery. Am. J. Enol. Vitic. 2006, 57, 280–288. [Google Scholar]
- Downey, M.O.; Dokoozlian, N.K.; Krstic, M.P. Cultural practice and environmental impacts on the flavonoid composition of grapes and wine: A review of recent research. Am. J. Enol. Vitic. 2006, 57, 257–268. [Google Scholar]
- Garcia-Falcón, M.S.; Perez-Lamela, C.; Martinez-Carballo, E.; Simal-Gandara, J. Determination of phenolic compounds in wines: Influence of bottle storage of young red wines on their evolution. Food Chem. 2007, 105, 248–259. [Google Scholar] [CrossRef]
- Alén-Ruiz, F.; García-Falcón, M.S.; Pérez-Lamela, M.C.; Martínez-Carballo, E.; Simal-Gándara, J. Influence of major polyphenols on antioxidant activity in Mencía and Brancellao red wines. Food Chem. 2009, 113, 53–60. [Google Scholar] [CrossRef]
- Soto Vázquez, E.; Río Segade, S.; Orriols Fernández, I. Effect of the winemaking technique on phenolic composition and chromatic characteristics in young red wines. Eur. Food Res. Technol. 2010, 231, 789–802. [Google Scholar] [CrossRef]
- Ortega-Heras, M.; Perez-Magariño, S.; Gonzalez-Sanjose, M.L. Comparative study of the use of maceration enzymes and cold prefermentative maceration on phenolic and anthocyanic composition and color of a Mencía red wine. LWT-Food Sci. Technol. 2012, 48, 1–8. [Google Scholar] [CrossRef]
- Moreno, A.; Castro, M.; Falqué, E. Evolution of trans- and cis-resveratrol content in red grapes (Vitis vinifera L. cv Mencía, Albarello and Merenzao) during ripening. Eur. Food Res. Technol. 2008, 227, 667–674. [Google Scholar]
- Rivas-Gonzalo, J.C.; Gutierrez, Y.; Hebrero, E.; Santos-Buelga, C. Comparisons of methods for the determination of anthocyanins in red wines. Am. J. Enol. Vitic. 1992, 43, 210–214. [Google Scholar]
- Perez-Enciso, M.; Tenenhaus, M. Prediction of clinical outcome with microarray data: A partial least squares discriminant analysis (PLS-DA) approach. Hum. Genet. 2003, 112, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, P.; Bruce, S.J.; Moritz, T.; Trygg, J.; Sjoström, M.; Plumb, R.; Granger, J.; Maibaum, E.; Nicholson, J.K.; Holmes, E.; et al. Extraction, interpretation and validation of information for comparing samples in metabolic LC/MS data sets. Analyst 2005, 130, 701–707. [Google Scholar] [CrossRef] [PubMed]
- Wold, S.; Antti, H.; Lindgren, F.; Ohman, J. Orthogonal signal correction of near-infrared spectra. Chemom. Intell. Lab. Syst. 1998, 44, 175–185. [Google Scholar] [CrossRef]
- Wiklund, S.; Johansson, E.; Sjoestroem, L.; Mellerowicz, E.J.; Edlund, U.; Shockcor, J.P.; Gottfries, J.; Moritz, T.; Trygg, J. Visualization of GC/TOF-MS-based metabolomics data for identification of biochemically interesting compounds using OPLS class models. Anal. Chem. 2008, 80, 115–122. [Google Scholar] [CrossRef]
- Van Leeuwen, C.; Roby, J.-P.; de Rességuier, L. Soil-related terroir factors: A review. OENO One 2018, 52, 2. [Google Scholar] [CrossRef]
- Costa, R.; Fraga, H.; Fonseca, A.; Cortázar-Atauri, I.G.; Val, M.C.; Carlos, C.; Reis, S.; Santos, J.A. Grapevine Phenology of cv. Touriga Franca and Touriga Nacional in the Douro Wine Region: Modelling and Climate Change Projections. Agronomy 2019, 9, 210. [Google Scholar] [CrossRef]
- Lorenzo, M.N.; Taboada, J.J.; Lorenzo, J.F.; Ramos, A.M. Influence of climate on grape production and wine quality in the Rías Baixas, north-western Spain. Reg. Environ. Chang. 2013, 13, 887–896. [Google Scholar] [CrossRef]
- Amerine, M.A.; Winkler, A.J. Composition and quality of musts and wines of California grapes. Hilgardia 1944, 15, 493–675. [Google Scholar] [CrossRef]
- Riou, C.; Becker, N.; Sotes Ruiz, V.; Gomez-Miguel, V.; Carbonneau, A.; Panagiotou, M.; Calo, A.; Costacurta, A.; De Castro, R.; Pinto, A.; et al. Le déterminisme Climatique de la Maturation du Raisin: Application au Zonage de la Teneur en Sucre Dans la Communauté Européenne; Office des Publications Officielles des Communautés Européennes: Luxembourg, 1994; 322p. [Google Scholar]
- Tonietto, J.; Carbonneau, A. A multicriteria climatic classification system for grape-growing regions worldwide. Agric. For. Meteorol. 2004, 124, 81–97. [Google Scholar] [CrossRef]
- Jackson, D.I.; Lombard, P.B. Environmental and management practices affecting grape composition and wine quality-a review. Am. J. Enol. Vitic. 1993, 44, 409–430. Available online: http://www.ajevonline.org/content/44/4/409 (accessed on 30 September 2020).
- Branas, J.; Bernon, G.; Levadoux, L. Élements de Viticulture Générale; Imp. De Dehan: Montpellier/Bordeaux, France, 1974. [Google Scholar]
- Carbonneau, A. Ecophysiologie de la vigne et terroir. In Terroir, Zonazione, Viticoltura; Schuster, D., Paoletti, A., Eds.; Trattato Internazionale, Phytoline: Piacenza, Italy, 2003; pp. 61–102. [Google Scholar]
- Fraga, H.; Malheiro, A.C.; Moutinho-Pereira, J.; Jones, G.V.; Alves, F.; Pinto, J.G.; Santos, J.A. Very high resolution bioclimatic zoning of Portuguese wine regions: Present and future scenarios. Reg. Environ. Chang. 2014, 14, 295–306. [Google Scholar] [CrossRef]
- Blanco-Ward, D.; García Queijeiro, J.M.; Jones, G.V. Spatial climate variability and viticulture in the Miño River Valley of Spain. Vitis 2007, 46, 63–70. [Google Scholar]
- Yamane, T.; Jeong, S.T.; Goto-Yamamoto, N.; Koshita, Y.; Kobayashi, S. Effects of Temperature on Anthocyanin Biosynthesis in Grape Berry Skins. Am. J. Enol. Vitic. 2006, 57, 54–59. [Google Scholar]
- Mori, K.; Goto-Yamamoto, N.; Kitayama, M.; Hashizume, K. Loss of anthocyanins in red-wine grape under high temperature. J. Exp. Bot. 2007, 58, 1935–1945. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Sugaya, S.; Gemma, H. Decreased anthocyanin biosynthesis in grape berries grown under elevated night temperature condition. Sci. Hort. 2005, 105, 319–330. [Google Scholar] [CrossRef]
- Kliewer, W.M.; Torres, R.E. Effect of Controlled Day and Night Temperatures on Grape Coloration. Am. J. Enol. Vitic. 1972, 23, 71–77. [Google Scholar]
- Revilla, E.; Losada, M.M.; Gutiérrez, E. Phenolic Composition and Color of Single Cultivar Young Red Wines Made with Mencia and Alicante-Bouschet Grapes in AOC Valdeorras (Galicia, NW Spain). Beverages 2016, 2, 18. [Google Scholar] [CrossRef]
- Cheminat, A.; Brouillard, R. PMR investigation of 3-O-(β-Dglucosyl) malvidin structural transformations in aqueous solutions. Tetrahedron Lett. 1986, 27, 4457–4460. [Google Scholar] [CrossRef]
- Boulton, R. The copigmentation of anthocyanins and its role in the color of red wine: A critical review. Am. J. Enol. Vitic. 2001, 52, 67–87. [Google Scholar]
- Berke, B.; Chèze, C.; Vercauteren, J.; Deffieux, G. Bisulfite addition to anthocyanins: Revisited structures of colourless adducts. Tetrahedron Lett. 1998, 39, 5771–5774. [Google Scholar] [CrossRef]
- Somers, T. The polymeric nature of wine pigments. Phytochemistry 1971, 10, 2175–2186. [Google Scholar] [CrossRef]
- Darias-Martín, J.; Carrillo, M.; Díaz, E.; Boulton, R.B. Enhancement of red wine color by pre-fermentation addition of copigments. Food Chem. 2001, 73, 217–220. [Google Scholar] [CrossRef]
- Darias-Martín, J.; Martín-Luis, B.; Carrillo-López, M.; LamuelaRaventós, R.; Díaz-Romero, C.; Boulton, R. Effect of caffeic acid on the color of red wine. J. Agric. Food Chem. 2002, 50, 2062–2067. [Google Scholar] [CrossRef] [PubMed]
- Guise, R.; Filipe-Ribeiro, L.; Nascimento, D.; Bessa, O.; Nunes, F.M.; Cosme, F. Comparison between different types of carboxylmethylcellulose and other oenological additives used for white wine tartaric stabilization. Food Chem. 2014, 156, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Filipe-Ribeiro, L.; Milheiro, J.; Matos, C.C.; Cosme, F.; Nunes, F.M. Reduction of 4-ethylphenol and 4-ethylguaiacol in red wine by activated carbons with different physicochemical characteristics: Impact on wine quality. Food Chem. 2017, 229, 242–251. [Google Scholar] [CrossRef]
- Filipe-Ribeiro, L.; Milheiro, J.; Matos, C.C.; Cosme, F.; Nunes, F.M. Data on changes in red wine phenolic compounds, headspace aroma compounds and sensory profile after treatment of red wines with activated carbons with different physicochemical characteristics. Data Brief 2017, 12, 188–202. [Google Scholar] [CrossRef]
- OIV Organisation International de la Vigne et du Vin Recueil de Méthodes Internationales d’Analyse des Vins et des Moûts. Edition Officielle. Paris. 2015. Available online: http://www.oiv.int/fr/normes-et-documents-techniques (accessed on 30 September 2020).
- Ribéreau-Gayon, P.; Stonestreet, E. Le dosage des anthocyanes dans le vin rouge. Bull. Soc. Chim. Fr. 1965, 9, 2649–2652. [Google Scholar]
- Somers, T.C.; Evans, M.E. Spectral evaluation of young red wines: Anthocyanin equilibria, total phenolics, free and molecular O2, “Chemical age”. J. Sci. Food Agric. 1977, 28, 279–287. [Google Scholar] [CrossRef]
- Available online: http://www.inforiego.org/opencms/opencms/info_meteo/index.html (accessed on 1 December 2020).
- Available online: https://en.tutiempo.net/climate/2015/ws-85600.html (accessed on 1 December 2020).
- Huglin, P. New Method of Evaluating the Solar Thermal Possibilities of a Wine-Growing Environment; Reports from the French Academy of Agriculture; Académie d’Agriculture de France: Paris, France, 1978; Volume 64, pp. 1117–1126. [Google Scholar]
- Hargreaves, G.H.; Asce, F.; Allen, R.G. History and evaluation of Hargreaves evapotranspiration equation. J. Irrig. Drain. Eng. ASCE 2003, 129, 53–63. [Google Scholar] [CrossRef]
- Allen, R.G.; Pruitt, W.O.; Wright, J.L.; Howell, T.A.; Ventura, F.; Snyder, R.; Itenfisu, D.; Steduto, P.; Berengena, J.; Baselga, J.; et al. A recommendation on standardized surface resistance for hourly calculation of reference ETo by the FAO56 Penman–Monteith method. Agric. Water Manag. 2006, 81, 1–22. [Google Scholar] [CrossRef]
- Santos, J.A.; Malheiro, A.C.; Pinto, J.G.; Jones, G.V. Macroclimate and viticultural zoning in Europe: Observed trends and atmospheric forcing. Clim. Res. 2012, 51, 89–103. [Google Scholar] [CrossRef]
- Specifications of the PDO <<Bierzo>> PDO-ES-A0615 Reglamento_1_1601369319.pdf. Available online: http://www.crdobierzo.es/en/regulator-board/legislation/ (accessed on 1 December 2020).
- Instituto da Vinha e do Vinho (IVV). Available online: https://www.ivv.gov.pt/np4/%7BclientServletPath%7D/?newsId=8617&fileName=DOP_D_O___Caderno_de_especifica__es_fina.pdf (accessed on 1 December 2020).
- Gouveia, J.; Lopes, C.M.; Pedroso, V.; Martins, S.; Rodrigues, P.; Alves, I. Effect of irrigation on soil water depletion, vegetative growth, yield and berry composition of the grapevine variety Touriga Nacional. Ciência Téc. Vitiv. 2012, 27, 115–122. [Google Scholar]
- Rubert, J.; Lacina, O.; Fauhl-Hassek, C.; Hajslova, J. Metabolic fingerprinting based on high-resolution tandem mass spectrometry: A reliable tool for wine authentication? Anal. Bioanal. Chem. 2014, 406, 6791–6803. [Google Scholar] [CrossRef] [PubMed]

| Wine Samples | Gallic Acid | Catechins | trans-Caftaric Acid | Coutaric Acid Isomer | Coutaric Acid | Caffeic Acid | p-Coumaric Acid | Ferulic Acid | Caffeic Acid Ethyl Ester | Coumaric Acid Ethyl Ester |
|---|---|---|---|---|---|---|---|---|---|---|
| Mencía | 21.42 ± 0.02 d | 8.14 ± 0.27 ab | 33.77 ± 0.43 d | 0.83 ± 0.01 b | 21.00 ± 0.21 g | 2.95 ± 0.94 ab | 5.63 ± 0.16 g | 0.81 ± 0.03 ab | 2.22 ± 0.10 e | 0.41 ± 0.00 a |
| Mencía | 20.76 ± 0.01 d | 7.28 ± 0.47 a | 5.10 ± 0.02 a | 1.25 ± 0.01 c | 5.63 ± 0.00 b | 3.96 ± 0.01 b | 2.58 ± 0.02 d | 2.40 ± 0.00 d | 1.40 ± 0.14 d | 2.06 ± 0.06 c |
| Mencía | 7.93 ± 0.10 ab | 7.10 ± 0.07 a | 35.42 ± 0.58 d | 0.60 ± 0.03 a | 19.76 ± 0.35 fg | 2.19 ± 0.30 a | 3.38 ± 0.04 e | 3.22 ± 0.02 e | 2.93 ± 0.07 f | 0.51 ± 0.33 ab |
| Mencía | 19.54 ± 0.34 cd | 8.79 ± 2.38 b | 17.28 ± 3.29 b | 0.56 ± 0.07 a | 9.83 ± 0.29 c | 2.38 ± 0.05 a | 5.78 ± 0.10 f | 0.92 ± 0.01 ab | 1.27 ± 0.02 d | 2.72 ± 0.09 d |
| Mencía | 17.46 ± 0.44 c | 2.37 ± 0.00 a | 34.37 ± 0.39 c | 0.72 ± 0.00 b | 13.29 ± 0.19 d | 3.78 ± 0.05 b | 0.86 ± 0.01 b | 0.66 ± 0.01 a | 0.40 ± 0.01 ab | 0.51 ± 0.02 a |
| Mencía | 21.88 ± 0.33 d | 6.11 ± 0.18 a | 36.50 ± 2.39 e | 1.30 ± 0.13 c | 19.91 ± 1.26 fg | 2.91 ± 0.018 ab | 4.01 ± 0.26 f | 1.62 ± 0.13 c | 1.43 ± 0.19 d | 2.38 ± 0.14 cd |
| Mencía | 21.61 ± 0.03 d | 7.26 ± 80.71 a | 29.78 ± 1.16 d | 0.73 ± 0.01 b | 15.77 ± 0.63 e | 2.80 ± 0.15 a | 1.31 ± 0.04 c | 2.70 ± 0.10 d | 1.02 ± 0.15 c | 0.65 ± 0.03 b |
| Mencía | 6.16 ± 0.35 a | 11.40 ± 0.85 bc | 4.90 ± 0.24 a | 0.33 ± 0.04 a | 1.68 ± 0.42 a | 2.13 ± 0.52 a | 1.59 ± 0.01 c | 0.79 ± 0.00 ab | 0.11 ± 0.07 a | 0.47 ± 0.02 a |
| Mencía | 22.55 ± 2.25 d | 10.35 ± 1.27 b | 30.24 ± 0.12 d | 0.81 ± 0.00 b | 16.34 ± 0.08 e | 2.82 ± 0.01 a | 4.28 ± 0.14 f | 0.61 ± 0.05 a | 0.94 ± 0.09 c | 0.58 ± 0.04 b |
| Mencía | 29.04 ± 2.75 e | 10.28 ± 3.52 b | 18.14 ± 0.38 b | 0.69 ± 0.17 ab | 6.91 ± 0.23 b | 3.46 ± 0.02 b | 3.01 ± 0.04 de | 2.44 ± 0.14 d | 0.22 ± 0.02 a | 0.85 ± 0.10 b |
| Mencía | 18.98 ± 0.18 cd | 10.45 ± 4.14 b | 23.61 ± 0.21 c | 1.11 ± 0.02 bc | 10.15 ± 0.05 c | 2.80 ± 0.13 a | 1.59 ± 0.01 c | 0.84 ± 0.08 ab | 0.49 ± 0.01 b | 0.92 ± 0.00 b |
| Mencía | 20.55 ± 0.06 d | 8.52 ± 0.64 b | 33.47 ± 2.62 d | 1.00 ± 0.25 bc | 18.81 ± 1.18 f | 2.68 ± 0.19 a | 3.17 ± 0.18 e | 0.84 ± 0.02 ab | 0.70 ± 0.05 b | 0.85 ± 0.17 b |
| Mencía | 22.01 ± 0.10 d | 6.22 ± 0.01 a | 39.24 ± 0.77 ef | 0.23 ± 0.09 a | 14.93 ± 0.29 de | 1.96 ± 0.03 a | 0.93 ± 0.02 b | 0.99.1 ± 0.00 b | 0.71 ± 0.02 bc | 0.62 ± 0.01 b |
| Jaen | 17.52 ± 0.37 c | 13.91 ± 0.54 bc | 29.64 ± 0.24 d | 0.64 ± 0.11 a | 9.60 ± 0.24 c | 3.76 ± 0.05 b | 1.01 ± 0.15 b | 4.28 ± 0.17 f | 0.64 ± 0.02 b | 0.67 ± 0.08 b |
| Jaen | 19.52 ± 0.46 cd | 14.11 ± 0.28 bc | 42.18 ± 1.21 f | 0.46 ± 0.09 a | 16.61 ± 0.29 e | 3.44 ± 0.08 b | 0.59 ± 0.09 ab | 2.51 ± 0.04 d | 0.16 ± 0.02 a | 0.17 ± 0.05 a |
| Jaen | 20.87 ± 1.08 d | 17.03 ± 0.80 c | 28.18 ± 0.69 c | 0.77 ± 0.02 b | 15.81 ± 0.21 e | 3.56 ± 0.09 b | 0.36 ± 0.00 a | 2.67 ± 0.03 d | 0.24 ± 0.08 a | 0.26 ± 0.01 a |
| Jaen | 16.32 ± 0.50 c | 7.36 ± 0.22 a | 17.14 ± 0.33 b | 0.55 ± 0.26 a | 15.81 ± 0.21 e | 3.69 ± 0.12 b | 0.43 ± 0.33 a | 3.74 ± 0.16 c | 0.14 ± 0.01 a | 0.35 ± 0.01 a |
| Jaen | 11.18 ± 0.51 b | 10.26 ± 1.37 b | 26.06 ± 0.36 c | 1.93 ± 0.16 d | 10.15 ± 0.12 c | 2.18 ± 0.17 a | 0.96 ± 0.08 b | 1.92 ± 0.07 c | 0.56 ± 0.03 b | 0.51 ± 0.03 a |
| Wine Samples | D3G | C3G | Pet3G | Peo3G | M3G | D3AG | C3AG | Pet3AG | Peo3AG | M3AG | D3CG | Peo3CG | M3CG |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mencía | 1.98 ± 0.01 c | 6.31 ± 0.19 c | 9.84 ± 0.19 c | 10.02 ± 0.10 b | 43.44 ± 0.69 b | 12.82 ± 0.48 f | 1.96 ± 0.15 f | 1.00 ± 0.19 b | 3.57 ± 0.04 e | 5.08 ± 0.21 ab | nd | nd | 3.01 ± 0.08 b |
| Mencía | 2.76 ± 0.02 d | 5.52 ± 0.07 b | 8.19 ± 0.03 bc | 13.19 ± 0.06 bc | 44.31 ± 0.10 b | 3.34 ± 0.01 a | 0.52 ± 0.00 b | 1.05 ± 0.04 b | 2.23 ± 0.01 c | 5.84 ± 0.05 b | nd | nd | 3.45 ± 0.03 bc |
| Mencía | 4.52 ± 0.14 e | 11.04 ± 1.30 e | 16.10 ± 0.26 d | 16.80 ± 0.18 c | 83.43 ± 1.30 c | 3.06 ± 0.03 a | nd | 1.95 ± 0.01 c | 1.99 ± 0.01 c | 11.96 ± 0.16 c | nd | nd | 5.75 ± 0.06 d |
| Mencía | 1.69 ± 0.02 b | 4.58 ± 0.05 b | 8.29 ± 0.04 bc | 9.28 ± 0.04 b | 48.57 ± 0.38 b | 2.96 ± 0.02 a | 0.49 ± 0.02 b | 1.09 ± 0.00 b | 1.58 ± 0.05 b | 7.31 ± 0.03 b | nd | nd | 4.17 ± 0.03 c |
| Mencía | 0.81 ± 0.02 a | 1.72 ± 0.01 a | 4.12 ± 0.10 a | 4.07 ± 0.10 a | 36.05 ± 0.54 ab | 3.67 ± 0.83 b | 0.84 ± 0.01 c | 0.89 ± 0.01 b | nd | 3.83 ± 0.03 a | nd | nd | 1.72 ± 0.02 a |
| Mencía | 2.99 ± 0.29 d | 8.00 ± 0.62 c | 14.89 ± 1.15 d | 14.30 ± 0.14 c | 79.00 ± 5.97 c | 7.37 ± 0.49 d | 1.27 ± 0.06 d | 0.56 ± 0.02 a | 2.80 ± 0.16 d | 10.15 ± 0.75 c | nd | nd | 7.52 ± 0.57 e |
| Mencía | 2.09 ± 0.15 c | 4.00 ± 0.36 b | 6.30 ± 0.56 ab | 7.06 ± 0.66 a | 28.64 ± 2.40 a | 5.01 ± 0.24 bc | 0.91 ± 0.10 c | 0.79 ± 0.0 b | 0.84 ± 0.09 a | 2.88 ± 0.30 a | nd | nd | 1.89 ± 0.18 a |
| Mencía | 2.04 ± 0.09 c | 3.51 ± 0.14 ab | 4.98 ± 0.17 a | 4.36 ± 0.08 a | 30.27 ± 0.37 a | 1.97 ± 0.64 a | 0.28 ± 0.05 a | 0.16 ± 0.08 a | 0.76 ± 0.01 a | 3.18 ± 0.01 a | nd | nd | 2.30 ± 0.07 a |
| Mencía | 1.36 ± 0.00 b | 4.63 ± 0.01 b | 7.24 ± 0.04 bc | 9.86 ± 0.03 b | 36.78 ± 0.17 ab | 9.75 ± 0.00 e | 1.46 ± 0.00 de | 0.70 ± 0.01 b | 1.48 ± 0.02 b | 3.77 ± 0.00 a | nd | nd | 2.34 ± 0.02 a |
| Mencía | 1.78 ± 0.09 b | 4.71 ± 0.22 b | 7.09 ± 0.18 bc | 9.24 ± 0.40 b | 31.43 ± 1.35 a | 8.30 ± 0.46 de | 1.14 ± 0.01 c | 0.61 ± 0.03 a | 1.18 ± 0.09 ab | 3.51 ± 0.19 a | nd | nd | 2.87 ± 0.14 b |
| Mencía | 2.22 ± 0.01 c | 4.31 ± 0.07 b | 7.29 ± 0.08 bc | 13.14 ± 0.07 bc | 35.46 ± 0.42 ab | 2.58 ± 0.05 a | 0.51 ± 0.02 b | 0.89 ± 0.02 b | 1.06 ± 0.01 a | 2.83 ± 0.03 a | nd | nd | 2.94 ± 0.0 3 b |
| Mencía | 1.77 ± 0.26 bc | 5.51 ± 0.63 b | 7.84 ± 1.94 bc | 9.56 ± 3.12 b | 34.73 ± 5.59 ab | 4.08 ± 0.66 b | 0.75 ± 0.15 b | 0.70 ± 0.19 b | 1.10 ± 0.17 a | 3.34 ± 0.45 a | nd | nd | 2.63 ± 0.42 ab |
| Mencía | 0.65 ± 0.01 a | 8.79 ± 0.03 d | 14.70 ± 0.26 d | 27.01 ± 0.10 e | 133.27 ± 0.59 de | 1.83 ± 0.04 c | 0.45 ± 0.01 b | 0.95 ± 0.02 b | 2.64 ± 0.03 d | 6.64 ± 0.23 b | nd | nd | 2.07 ± 0.06 a |
| Jaen | 1.55 ± 0.01 b | 19.70 ± 0.19 f | 40.10 ± 0.41 g | 29.89 ± 0.06 f | 202.51 ± 0.24 g | 7.34 ± 0.01 d | 0.69 ± 0.01 bc | 0.82 ± 0.01 b | 8.10 ± 0.03 g | 35.56 ± 0.12 f | 0.45 ± 0.01 a | 3.59 ± 0.17 c | 24.72 ± 0.15 h |
| Jaen | 2.02 ± 0.08 c | 27.54 ± 0.05 g | 41.78 ± 0.03 g | 32.22 ± 0.11 f | 198.12 ± 0.00 g | 13.33 ± 0.13 f | 1.68 ± 0.20 ef | 0.99 ± 0.03 b | 3.07 ± 0.15 d | 26.88 ± 0.05 e | 0.54 ± 0.15 a | 3.91 ± 0.16 d | 28.02 ± 0.01 i |
| Jaen | 2.36 ± 0.03 c | 10.84 ± 0.02 e | 21.52 ± 0.20 e | 21.48 ± 0.28 d | 123.52 ± 1.17 d | 8.34 ± 0.10 de | 1.62 ± 0.03 e | 0.96 ± 0.01 b | 3.65 ± 0.05 e | 16.85 ± 0.13 d | nd | 1.53 ± 0.14 a | 13.68 ± 0.01 f |
| Jaen | 2.07 ± 0.04 c | 10.27 ± 1.08 de | 27.41 ± 1.63 f | 19.98 ± 1.97 d | 162.69 ± 4.04 f | 6.16 ± 0.10 c | 0.74 ± 0.03 b | 0.76 ± 0.39 b | 7.11 ± 0.21 f | 27.40 ± 2.26 e | n.d. | 2.12 ± 0.12 b | 18.21 ± 0.05 g |
| Jaen | 3.11 ± 0.13 a | 8.13 ± 0.23 c | 13.66 ± 0.98 d | 24.57 ± 2.62 e | 141.99 ± 12.8 e | 5.96 ± 0.50 c | 1.00 ± 0.07 c | 0.79 ± 0.12 b | 2.67 ± 0.28 d | 19.39 ± 0.95 d | n.d. | 3.74 ± 0.03 cd | 13.64 ± 0.76 f |
| Bioclimatic Index | Bierzo DO | Dão DO | ||
|---|---|---|---|---|
| Wrinkler index | 1549 | Moderately cold | 1638 | Moderately cold |
| Huglin Heliothermic index | 2473 | Warm | 2217 | Temperate warm |
| Drying Index | −16 | Moderately dry | 130 | Sub-humid |
| Cool nigh index | 7.6 | Very cool nights | 12.3 | Cool nights |
| Branas Hydrothermic Index | 1818 | Low risk | 2279 | Low risk |
| N. of hot days (>30 °C) a | 14 | 7 | ||
| N. of cold nights (<10 °C) a | 32 | 12 |
| Wine Samples | Color Intensity | Hue | Chromatic Characteristics | ||||
|---|---|---|---|---|---|---|---|
| (a.u.) | L* | a* | b* | C* | °h | ||
| Mencía | 15.36 ± 0.36 h | 0.712 ± 0.041 b | 68.9 ± 0.0 b | 30.63 ± 0.71 f | 7.51 ± 0.08 fg | 31.54 ± 0.71 g | 13.79 ± 0.17 f |
| Mencía | 13.91 ± 0.05 g | 0.700 ± 0.002 ab | 73.6 ± 0.1 e | 27.37 ± 0.54 e | 7.82 ± 0.24 g | 28.46 ± 0.58 f | 15.96 ± 0.17 h |
| Mencía | 13.22 ± 0.01 f | 0.750 ± 0.004 bc | 73.8 ± 0.1 e | 18.43 ± 0.19 a | 6.43 ± 0.16 e | 19.52 ± 0.24 ab | 19.25 ± 0.26 i |
| Mencía | 8.18 ± 0.06 b | 0.791 ± 0.003 c | 81.3 ± 0.5 i | 20.45 ± 0.04 b | 7.12 ± 0.00 f | 21.66 ± 0.04 c | 19.20 ± 0.04 i |
| Mencía | 6.76 ± 0.06 a | 0.795 ± 0.011 c | 84.3 ± 0.5 j | 19.37 ± 0.07 ab | 4.95 ± 0.06 d | 19.99 ± 0.08 b | 14.33 ± 0.11 fg |
| Mencía | 16.23 ± 0.56 i | 0.685 ± 0.012 ª | 66.7 ± 0.3 a | 36.74 ± 0.32 i | 6.19 ± 0.10 e | 37.26 ± 0.33 i | 9.57 ± 0.07 d |
| Mencía | 13.06 ± 0.04 f | 0.782 ± 0.004 c | 72.2 ± 0.2 d | 31.70 ± 0.23 fg | 6.22 ± 0.15 e | 32.30 ± 0.26 g | 11.10 ± 0.19 e |
| Mencía | 10.43 ± 0.04 cd | 0.720 ± 0.005 b | 83.5 ± 0.7 j | 24.12 ± 0.01 c | 10.70 ± 0.12 h | 26.39 ± 0.04 d | 23.94 ± 0.25 k |
| Mencía | 11.95 ± 0.01 e | 0.784 ± 0.003 c | 76.8 ± 0.3 f | 18.37 ± 0.05 a | 3.47 ± 0.15 b | 18.69 ± 0.08 a | 10.71 ± 0.43 e |
| Mencía | 10.57 ± 0.03 d | 0.758 ± 0.006 bc | 77.5 ± 0.5 fg | 26.77 ± 0.08 e | 7.94 ± 0.07 g | 27.92 ± 0.05 ef | 16.53 ± 0.18 h |
| Mencía | 11.05 ± 0.09 d | 0.775 ± 0.001 c | 78.6 ± 0.3 gh | 18.32 ± 0.12 a | 4.79 ± 0.08 d | 18.93 ± 0.09 ab | 14.67 ± 0.33 g |
| Mencía | 9.67 ± 0.02 c | 0.731 ± 0.009 b | 79.3 ± 0.3 h | 25.66 ± 0.07 d | 4.46 ± 0.08 cd | 26.05 ± 0.06 d | 9.86 ± 0.20 d |
| Mencía | 6.12 ± 0.02 ª | 0.688 ± 0.017 a | 89.1 ± 0.1 k | 18.46 ± 0.07 a | 7.29 ± 0.01 f | 18.95 ± 0.06 b | 21.57 ± 0.08 j |
| Jaen | 14.06 ± 0.05 g | 0.710 ± 0.002 b | 70.6 ± 0.2 c | 32.60 ± 0.37 g | 1.82 ± 0.02 a | 32.65 ± 0.37 g | 3.20 ± 0.01 a |
| Jaen | 13.88 ± 0.45 g | 0.643 ± 0.032 a | 72.1 ± 0.1 d | 35.30 ± 0.12 h | 3.10 ± 0.11 b | 35.45 ± 0.12 h | 5.01 ± 0.17 b |
| Jaen | 14.89 ± 0.16 h | 0.799 ± 0.037 c | 71.2 ± 0.4 cd | 25.92 ± 0.09 d | 18.14 ± 0.16 j | 31.57 ± 0.07 g | 35.00 ± 0.15 l |
| Jaen | 15.26 ± 0.07 h | 0.714 ± 0.043 b | 68.4 ± 0.3 b | 34.53 ± 0.40 h | 4.27 ± 0.20 c | 34.81 ± 0.39 h | 7.05 ± 0.25 c |
| Jaen | 12.53 ± 0.14 ef | 0.684 ± 0.011 a | 79.8 ± 0.0 h | 24.82 ± 0.09 c | 11.19 ± 0.04 i | 27.22 ± 0.06 e | 24.29 ± 0.16 k |
| Wine Samples | Total Anthocyanins (mg/L) | Colored Anthocyanins (a.u.) | Total Pigments (a.u.) | Polymeric Pigments (a.u.) |
|---|---|---|---|---|
| Mencía | 277 ± 2 e | 2.83 ± 0.05 a | 20.00 ± 2.29 cd | 4.78 ± 0.11 g |
| Mencía | 257 ± 8 d | 4.23 ± 0.08 b | 17.88 ± 0.43 cd | 2.83 ± 0.07 fg |
| Mencía | 243 ± 4 cd | 4.80 ± 0.05 c | 19.04 ± 0.93 cd | 1.75 ± 0.06 c |
| Mencía | 208 ± 2 b | 2.28 ± 0.01 a | 18.53 ± 0.21 cd | 1.72 ± 0.03 c |
| Mencía | 190 ± 6 b | 2.15 ± 0.14 a | 9.80 ± 0.71 ab | 1.19 ± 0.04 b |
| Mencía | 333 ± 1 f | 5.20 ± 0.17 cd | 20.65 ± 0.007 cd | 3.07 ± 0.02 g |
| Mencía | 293 ± 6 e | 3.87 ± 0.02 b | 16.77 ± 2.57 c | 2.42 ± 0.04 e |
| Mencía | 203 ± 1 b | 4.34 ± 0.00 bc | 12.42 ± 0.00 b | 0.90 ± 0.01 a |
| Mencía | 254 ± 9 cd | 2.98 ± 0.14 a | 11.62 ± 0.14 b | 2.52 ± 0.014 e |
| Mencía | 232 ± 9 c | 3.03 ± 0.03 a | 10.61 ± 0.86 ab | 2.20 ± 0.04 d |
| Mencía | 231 ± 3 c | 3.05 ± 0.11 a | 10.66 ± 0.07 ab | 2.24 ± 0.09 d |
| Mencía | 236 ± 2 cd | 2.88 ± 0.01 a | 10.40 ± 0.14 ab | 2.05 ± 0.001 d |
| Mencía | 147 ± 8 a | 2.36 ± 0.00 a | 7.37 ± 0.00 a | 0.93 ± 0.025 a |
| Jaen | 515 ± 9 i | 5.04 ± 0.15 cd | 31.41 ± 0.71 e | 2.02 ± 0.06 d |
| Jaen | 525 ± 8 i | 5.32 ± 0.86 cd | 21.56 ± 1.07 d | 2.38 ± 0.01 e |
| Jaen | 382 ± 5 g | 3.69 ± 0.22 b | 27.52 ± 0.21 e | 2.74 ± 0.05 f |
| Jaen | 434 ± 1 h | 5.89 ± 0.18 d | 38.08 ± 1.00 f | 1.71 ± 0.10 c |
| Jaen | 381 ± 7 g | 2.96 ± 0.01 a | 16.97 ± 0.14 c | 1.77 ± 0.01 c |
Sample Availability: Samples of the compounds used are not available from the authors. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cosme, F.; Vilela, A.; Moreira, L.; Moura, C.; Enríquez, J.A.P.; Filipe-Ribeiro, L.; Nunes, F.M. Terroir Effect on the Phenolic Composition and Chromatic Characteristics of Mencía/Jaen Monovarietal Wines: Bierzo D.O. (Spain) and Dão D.O. (Portugal). Molecules 2020, 25, 6008. https://doi.org/10.3390/molecules25246008
Cosme F, Vilela A, Moreira L, Moura C, Enríquez JAP, Filipe-Ribeiro L, Nunes FM. Terroir Effect on the Phenolic Composition and Chromatic Characteristics of Mencía/Jaen Monovarietal Wines: Bierzo D.O. (Spain) and Dão D.O. (Portugal). Molecules. 2020; 25(24):6008. https://doi.org/10.3390/molecules25246008
Chicago/Turabian StyleCosme, Fernanda, Alice Vilela, Luís Moreira, Carla Moura, José A. P. Enríquez, Luís Filipe-Ribeiro, and Fernando M. Nunes. 2020. "Terroir Effect on the Phenolic Composition and Chromatic Characteristics of Mencía/Jaen Monovarietal Wines: Bierzo D.O. (Spain) and Dão D.O. (Portugal)" Molecules 25, no. 24: 6008. https://doi.org/10.3390/molecules25246008
APA StyleCosme, F., Vilela, A., Moreira, L., Moura, C., Enríquez, J. A. P., Filipe-Ribeiro, L., & Nunes, F. M. (2020). Terroir Effect on the Phenolic Composition and Chromatic Characteristics of Mencía/Jaen Monovarietal Wines: Bierzo D.O. (Spain) and Dão D.O. (Portugal). Molecules, 25(24), 6008. https://doi.org/10.3390/molecules25246008









