4.4.2. Synthesis of Compounds
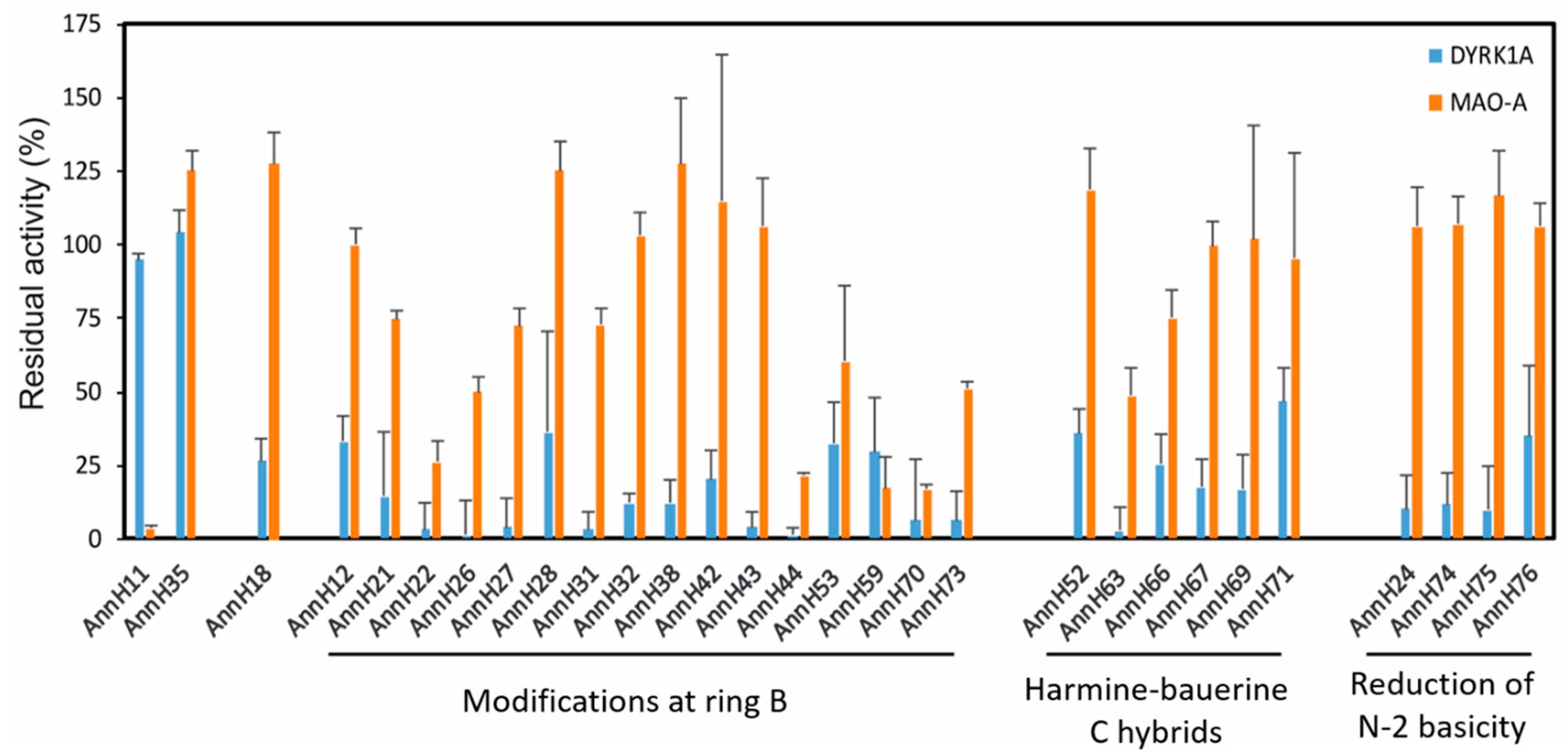
General procedure A for the N-alkylation of varios β-carbolines with using of sodium hydride as base in DMF. To the appropiate β-carboline in dry DMF sodium hydride (60% in mineral oil) was added. The mixture was stirred at 40 °C for 20 min and the appropriate alkyl halide was added. The mixture was stirred at 40 °C for the specified time period and then over night at room temperature. The following detailed procedure for isolation of AnnH44 is representative for the preparation of AnnH44, AnnH31, AnnH70, AnnH73, AnnH35, AnnH36, AnnH39, AnnH40, AnnH38, AnnH12, AnnH26, AnnH32, AnnH30, AnnH28, AnnH34, AnnH43, AnnH74, AnnH75, AnnH76, AnnH80, AnnH79, AnnH67, AnnH69 and AnnH66.
General procedure B for the N-alkylation of β-carbolines using potassium tert-butoxide as base in DMSO. The appropriate β-carboline and potassium tert-butoxide were dissolved in anhydrous DMSO and stirred for 30 min at 80 °C. The reaction mixture was cooled to room temperature and then the appropriate alkyl halide was added and the mixture stirred at 80 °C for the specified time period and then cooled to room temperature. After quenching with cold aqueous ammonia solution (10%, 10 mL), the mixture was treated with water until a solid was precipitated. The aqueos layer was extracted with ethyl acetate (3 × 50 mL). The combined organics were dried over sodium sulfate and evaporated to dryness. The residue was purified by column chromatography (silica gel, iso-hexane/ethyl acetate/ethanol 2:2:1) to afford the corresponding alkylated β-carbolines AnnH9, AnnH11, AnnH14, AnnH3, AnnH4, AnnH22 and AnnH27.
General procedure C for the N-alkylation of β-carbolines using sodium hydride as base in THF. A suspension of the appropriate β-carboline and sodium hydride (60% in mineral oil) in THF was stirred for 20 min, then the appropriate alkyl halide was added and the mixture was stirred under reflux for 65 h. The solution was allowed to cool to room temperature and water (10 mL) was added. The organic layer was separated and the aqueos layer was extracted with DCM (3 × 25 mL). The combined organic layers were dried over sodium sulfate and evaporated to dryness. The residue was purified by column chromatography (silica gel, iso-hexane/ethyl acetate/ethanol/triethylamine 2:2:1 +1%) to afford the corresponding alkylated β-carbolines AnnH16, AnnH21, AnnH53, AnnH55, AnnH57, AnnH71 and AnnH77.
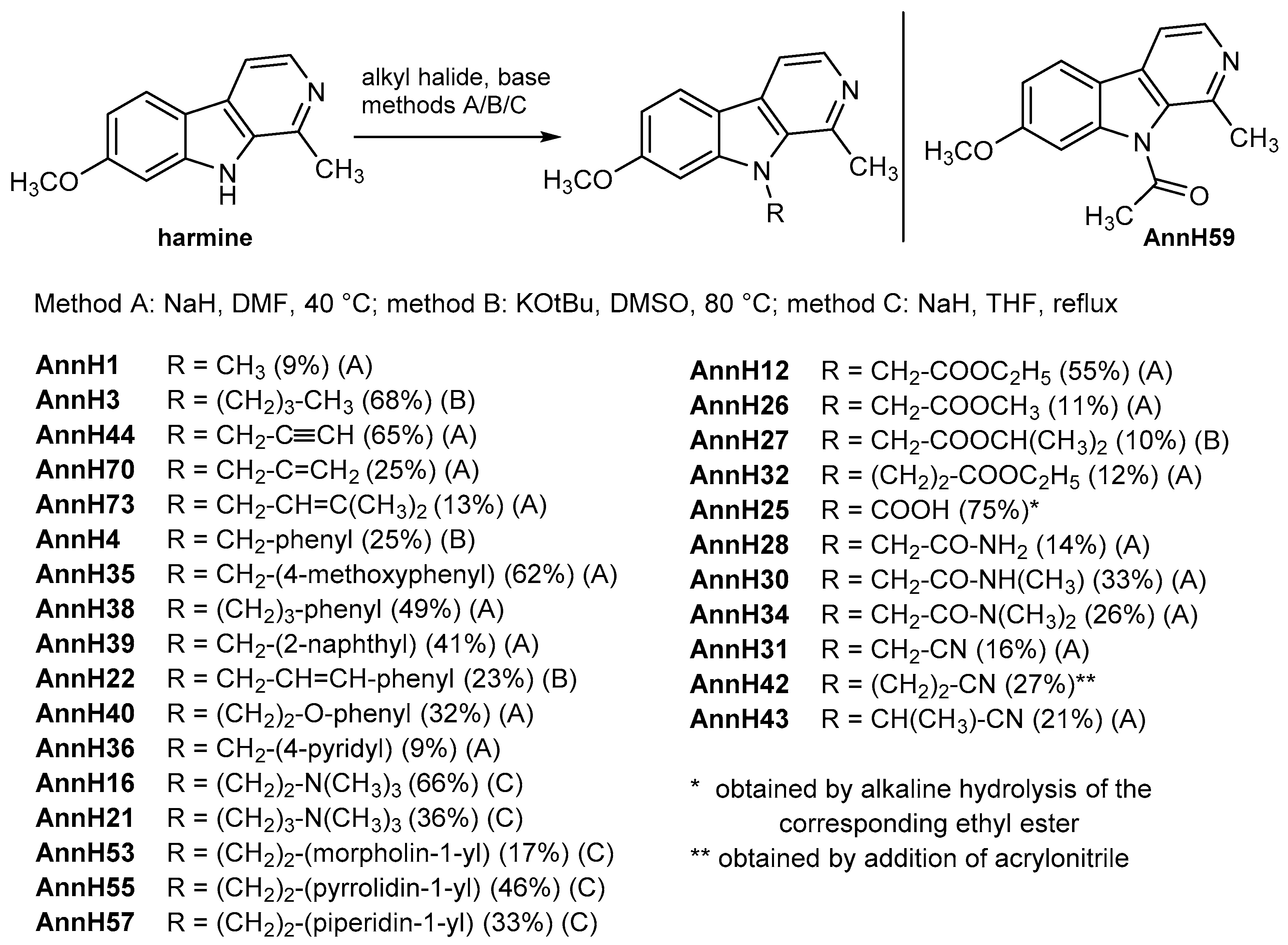
7-Methoxy-1-methyl-9-(prop-2-yn-1-yl)-9H-pyrido[3,4-b]indole (AnnH44). Prepared from harmine (153 mg, 0.720 mmol), sodium hydride (41 mg, 1.0 mmol), dry DMF (5.0 mL) and propargyl bromide (80% wt in toluene, 0.080 mL, 0.72 mmol). The mixture was stirred at 40 °C for 2 h. After treatment with a saturated aqueous solution of sodium carbonate (10 mL) and water (5 mL) the mixture was extracted with ethyl acetate (5 × 20 mL). The combined organic layers were dried over magnesium sulfate and evaporated to dryness. The residue was purified by column chromatography (silica gel, iso-hexane/ethyl acetate/ethanol 2:2:1) to afford AnnH44 as a light brown solid (118 mg, 65%). m.p. 146–147 °C; 1H-NMR (400 MHz, CDCl3) δ = 8.31 (d, J = 5.2 Hz, 1 H, 3-H), 7.96 (d, J = 9.0 Hz, 1 H, 5-H), 7.71 (d, J = 5.2 Hz, 1 H, 4-H), 6.94–6.88 (m, 2 H, 6-H, 8-H), 5.18 (d, J = 2.5 Hz, 2 H, 1’-H), 3.94 (s, 3 H, OCH3), 3.10 (s, 3 H, 1-CH3), 2.35 (t, J = 2.5 Hz, 1 H, 3′-H); 13C-NMR (100 MHz, CDCl3) δ = 161.1, 142.7, 141.0, 139.1, 135.0, 129.7, 122.5, 115.4, 112.3, 109.5, 93.2, 78.5, 73.5, 55.7, 34.7, 23.0; IR (KBr) (cm−1) = 3431, 3064, 2119, 1615, 1171; MS (EI) m/z (relative intensity, %) = 250 [M+•] (100), 211 (60), 196 (8), 168 (21); HRMS (EI) calcd. for C16H14N2O: 250.1106; found 250.1107; purity: 100% (λ = 210 nm), 100% (λ = 254 nm) (by HPLC).
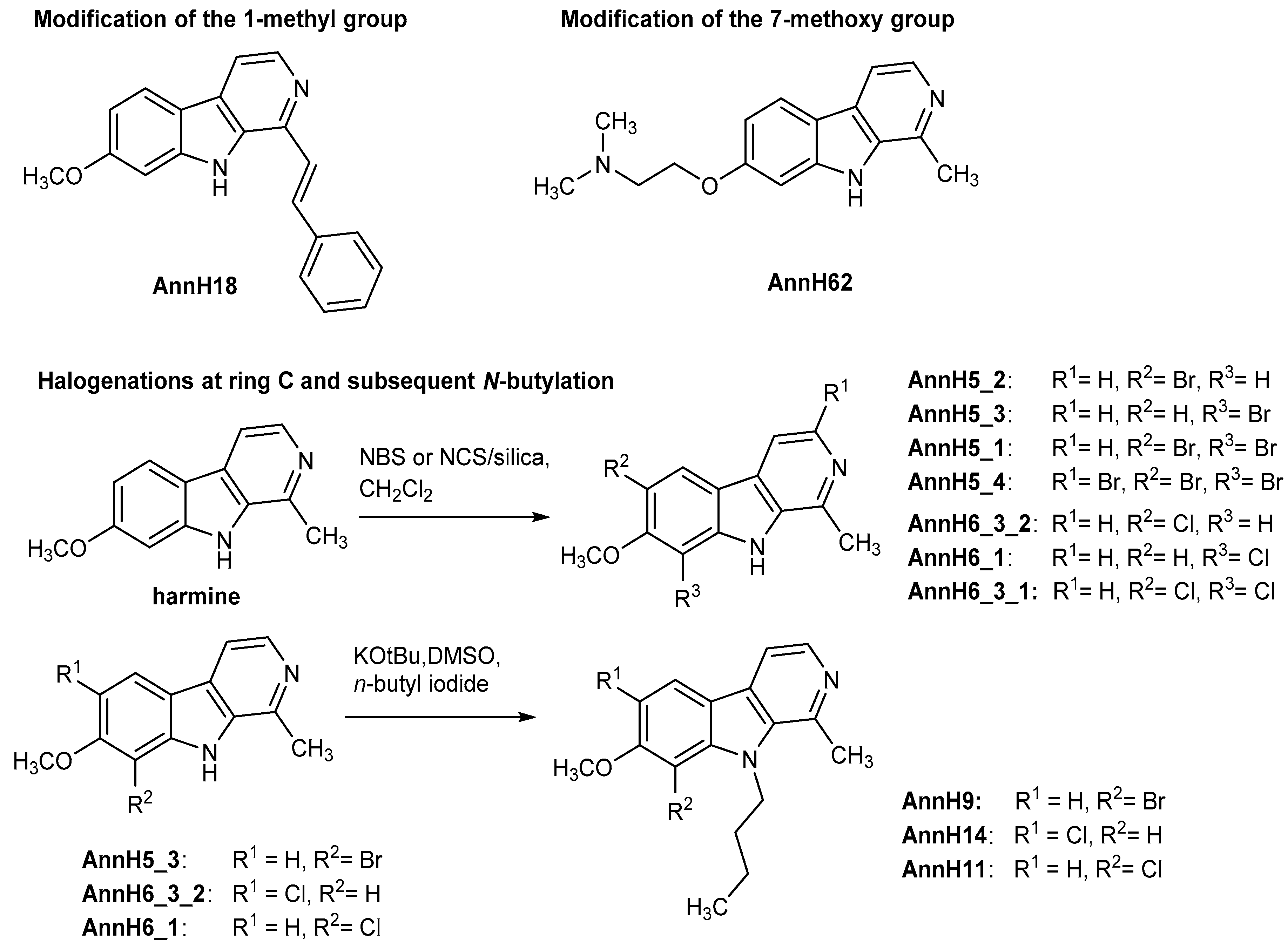
8-Bromo-9-butyl-7-methoxy-1-methyl-9H-pyrido[3,4-b]indole (AnnH9). Prepared from 8-bromo-7-methoxy-1-methyl-9H-pyrido[3,4-b]indole (AnnH5_3) (48 mg, 0.17 mmol), potassium tert-butoxide (22 mg, 0.20 mmol), dry DMSO (5 mL) and n-butyl iodide (0.10 mL, 0.88 mmol). The mixture was stirred at 80 °C for 3 h. Beige solid (34 mg, 60%). m.p. 90–96 °C; 1H-NMR (500 MHz, CDCl3) δ = 8.32 (d, J = 5.2 Hz, 1 H, 3-H), 7.98 (d, J = 8.5 Hz, 1 H, 5-H), 7.71 (d, J = 5.2 Hz, 1 H, 4-H), 6.93 (d, J = 8.5 Hz, 1 H, 6-H), 5.10–4.90 (m, 2 H, 1′-H), 4.02 (s, 3 H, OCH3), 3.03 (s, 3 H, 1-CH3), 1.72–1.63 (m, 2 H, 2′-H), 1.38–1.24 (m, 2 H, 3′-H), 0.93 (t, J = 7.4 Hz, 3 H, 4′-H); 13C-NMR (100 MHz, CDCl3) δ = 156.8, 141.6, 140.0, 138.9, 137.2, 129.5, 120.8, 119.2, 111.9, 105.7, 93.9, 57.2, 45.3, 33.7, 24.6, 19.5, 13.9; IR (KBr) (cm−1) = 3422, 2926, 1653, 1026; MS (EI) m/z (relative intensity, %) = 348 (54), 346 [M+•] (56), 307 (98), 305 (100), 225 (10), 57 (26); HRMS (EI) calcd. for C17H19N2OBr: 346.0681; found 346.0691; purity: 100% (λ = 210 nm), 100% (λ = 254 nm) (by HPLC).
9-Butyl-8-chloro-7-methoxy-1-methyl-9H-pyrido[3,4-b]indole (AnnH11). Prepared from 8-chloro-7-methoxy-1-methyl-9H-pyrido[3,4-b]indole (AnnH6_1) (54 mg, 0.22 mmol), potassium tert-butoxide (27 mg, 0.24 mmol), dry DMSO (5 mL) and n-butyl iodide (0.13 mL, 1.1 mmol). The mixture was stirred at 80 °C for 4 h. Beige solid (35 mg, 53%). m.p. 88–90 °C; 1H-NMR (500 MHz, DMSO-d6) δ = 8.24 (d, J = 5.1 Hz, 1 H, 3-H), 8.20 (d, J = 8.6 Hz, 1 H, 5-H), 7.95 (d, J = 5.1 Hz, 1 H, 4-H), 7.17 (d, J = 8.6 Hz, 1 H, 6-H), 4.88 (t, J = 7.8 Hz, 2 H, 1′-H), 3.98 (s, 3 H, OCH3), 2.95 (s, 3 H, 1-CH3), 1.65 (p, J = 7.7 Hz, 2 H, 2′-H), 1.31–1.24 (m, 2 H, 3′-H), 0.88 (t, J = 7.4 Hz, 3 H, 4′-H); 13C-NMR (100 MHz, DMSO-d6) δ = 150.2 135.8, 133.2, 132.2, 130.7, 123.2, 115.3, 112.6, 106.9, 100.8, 98.3, 51.4, 40.3, 33.5, 18.2, 13.5, 8.1; IR (KBr) (cm−1) = 3426, 2954, 1623, 1078; MS (EI) m/z (relative intensity, %) = 304 (17), 302 [M+•] (48), 261 (32), 259 (100), 246 (6), 244 (10), 218 (4), 216 (13); HRMS (EI) calcd. for C17H19N2OCl: 302.1186; found 302.1190; purity: 100% (λ = 210 nm), 100% (λ = 254 nm) (by HPLC).
9-Butyl-6-chloro-7-methoxy-1-methyl-9H-pyrido[3,4-b]indole (AnnH14). Prepared from 6-chloro-7-methoxy-1-methyl-9H-pyrido[3,4-b]indole (AnnH6_3_2) (53 mg, 0.21 mmol), potassium tert-butoxide (31 mg, 0.27 mmol), dry DMSO (5 mL) and n-butyl iodide (0.13 mL, 1.1 mmol). The mixture was stirred at 80 °C for 2 h. Beige solid (35 mg, 54%). m.p. 133–137 °C; 1H-NMR (400 MHz, DMSO-d6) δ = 8.34 (s, 1 H, 5-H), 8.19 (d, J = 5.2 Hz, 1 H, 3-H), 7.94 (d, J = 5.2 Hz, 1 H, 4-H), 7.37 (s, 1 H, 8-H), 4.58 (t, J = 7.5 Hz, 2 H, 1′-H), 4.02 (s, 3 H, OCH3), 2.95 (s, 3 H, 1-CH3), 1.72–1.68 (m, 2 H, 2′-H), 1.37–1.31 (m, 2 H, 3′-H), 0.92 (t, J = 7.4 Hz, 3 H, 4′-H); 13C-NMR (100 MHz, DMSO-d6) δ = 150.7, 136.7, 136.5, 133.5, 130.1, 123.2, 117.9, 109.9, 109.6, 108.1, 89.6, 52.1, 35.5, 28.0, 18.6, 15.0, 9.3; IR (KBr) (cm−1) = 3443, 3048, 1622, 1040; MS (EI) m/z (relative intensity, %) = 304 (14), 302 [M+•] (30), 261 (34), 259 (100), 246 (4), 244 (8), 218 (2), 216 (14); HRMS (EI) calcd. for C17H19N2OCl: 302.1186; found 302.1193; purity: 100% (λ = 210 nm), 93% (λ = 254 nm) (by HPLC).
1-(7-Methoxy-1-methyl-9H-pyrido[3,4-b]indol-9-yl)ethan-1-one (N9-acetylharmine; AnnH59). Harmine (103 mg, 0.484 mmol) and piperonal (160 mg, 1.07 mmol) in 3.0 mL of acetic anhydride were stirred at reflux for 3 h and at room temperature for 2 h. The reaction was cooled to room temperature and then treated with 60 mL of ice water. The pH was adjusted to 7–8 with a saturated aqueous solution of sodium hydrogen carbonate. The mixture was extracted with ethyl acetate (3 × 150 mL). The combined organic layers were dried over sodium sulfate and evaporated to dryness. The residue was purified by column chromatography (silica gel, iso-hexane/ethyl acetate/ethanol 2:2:1) to afford AnnH59 as a beige solid (38 mg, 31%). m.p. 107–108 °C; 1H-NMR (400 MHz, CDCl3) δ = 8.50 (d, J = 5.1 Hz, 1 H, 3-H), 7.89 (d, J = 8.6 Hz, 1 H, 5-H), 7.63 (d, J = 5.1 Hz, 1 H, 4-H), 7.59 (d, J = 2.2 Hz, 1 H, 8-H), 7.00 (dd, J = 8.6, 2.2 Hz, 1 H, 6-H), 3.94 (s, 3 H, OCH3), 2.73 (s, 3 H, CO-CH3), 2.69 (s, 3 H, 1-CH3); 13C-NMR (100 MHz, CDCl3) δ = 170.6, 161.6, 145.8, 143.6, 141.8, 134.8, 134.2, 122.1, 117.7, 111.7, 111.1, 99.8, 55.8, 27.1, 24.7; IR (KBr) (cm−1) = 3443, 2979, 1702, 1626, 1170; MS (ESI) m/z = 255 [M++ H], 213; HRMS (ESI) calcd. for C15H14N2O2 +H+: 255.1133; found 255.1133; purity: 99% (λ = 210 nm), 99% (λ = 254 nm) (by HPLC).
9-Butyl-7-methoxy-1-methyl-9H-pyrido[3,4-b]indole (
AnnH3). Prepared from harmine (102 mg, 0.481 mmol), potassium
tert-butoxide (58 mg, 0.52 mmol), dry DMSO (9 mL) and
n-butyl iodide (0.27 mL, 2.4 mmol). The mixture was stirred at 80 °C for 2 h. Beige solid (85 mg, 68%). The analytical data were in accordance with those published in ref. [
34].
7-Methoxy-1-methyl-9-(prop-2-yn-1-yl)-9H-pyrido[3,4-b]indole (AnnH70). Prepared from harmine (104 mg, 0.491 mmol), sodium hydride (24 mg, 0.60 mmol), dry DMF (2.5 mL) and allyl bromide (0.040 mL, 0.50 mmol). The mixture was stirred at 40 °C for 7 h. Beige solid (30 mg, 25%). m.p. 95–96 °C; 1H-NMR (500 MHz, CDCl3) δ = 8.29 (d, J = 5.2 Hz, 1H, 3-H), 7.99 (d, J = 8.7 Hz, 1 H, 5-H), 7.74 (d, J = 5.2 Hz, 1 H, 4-H), 6.90 (dd, J = 8.6, 2.2 Hz, 1 H, 6-H), 6.79 (d, J = 2.1 Hz, 1 H, 8-H), 6.09 (ddt, J = 17.2, 10.4, 4.0 Hz, 1 H, 2′-H), 5.20–5.16 (m, 1 H, 3′-H), 5.10–5.08 (m, 2 H, 1′-H), 4.83–4.79 (m, 1 H, 3′-H), 3.93 (s, 3 H, OCH3), 2.98 (s, 3 H, 1-CH3); 13C-NMR (100 MHz, CDCl3) δ = 170.0, 143.2, 140.9, 138.5, 135.6, 133.2, 129.3, 122.3, 116.5, 115.1, 112.2, 109.0, 93.3, 55.6, 46.9, 22.9; IR (KBr) (cm−1) = 3443, 2957, 1618, 1173; MS (APCI) m/z = 253 [M++ H], 212; HRMS (ESI) calcd. for C16H16N2O + H+: 253.1338; found 253.1341; purity: 97% (λ = 210 nm), 100% (λ = 254 nm) (by HPLC).
7-Methoxy-1-methyl-9-(3-methylbut-2-en-1-yl)-9H-pyrido[3,4-b]indole (AnnH73). Prepared from harmine (107 mg, 0.505 mmol), sodium hydride (23 mg, 0.56 mmol), dry DMF (3 mL) and 3,3-dimethylallyl bromide (0.055 mL, 0.47 mmol). The mixture was stirred at 40 °C for 4 h. Beige solid (18 mg, 14%). m.p. 102–104 °C; 1H-NMR (500 MHz, CDCl3) δ = 8.24 (d, J = 5.2 Hz, 1 H, 3-H), 7.94 (d, J = 8.6, 1 H, 5-H), 7.69 (d, J = 5.2 Hz, 1 H, 4-H), 6.85 (dd, J = 8.6, 2.2 Hz, 1 H, 6-H), 6.78 (d, J = 2.1 Hz, 1 H, 8-H), 5.22–5.18 (m, 1 H, 2′-H), 5.08–5.06 (m, 2 H, 1′-H), 3.90 (s, 3 H, OCH3), 2.96 (s, 3 H, 1-CH3), 1.86 (d, J = 1.3 Hz, 3 H, C(CH3)2), 1.69 (d, J = 1.3 Hz, 3 H, C(CH3)2); 13C-NMR (125 MHz, CDCl3) δ = 160.8, 143.1, 140.9, 138.3, 135.5, 134.5, 129.3, 122.3, 121.8, 115.3, 112.2, 108.9, 93.4, 55.6, 43.4, 25.5, 23.3, 18.4; IR (KBr) (cm−1) = 3433, 2928, 1620, 1255; MS (APCI) m/z = 281 [M++ H], 213; HRMS (ESI) calcd. for C18H20N2O + H+: 281.1653; found 281.1650; purity: 100% (λ = 210 nm), 100% (λ = 254 nm) (by HPLC).
9-Benzyl-7-methoxy-1-methyl-9H-pyrido[3,4-b]indole (
AnnH4). Prepared from harmine (101 mg, 0.476 mmol), potassium
tert-butoxide (58 mg, 0.52 mmol), dry DMSO (10 mL) and benzyl bromide (0.30 mL, 2.4 mmol). The mixture was stirred at 80 °C for 4 h. Beige solid (36 mg, 25%). The analytical data were in accordance with those published in ref. [
34].
7-Methoxy-9-(4-methoxybenzyl)-1-methyl-9H-pyrido[3,4-b]indole (AnnH35). Prepared from harmine (150 mg, 0.706 mmol), sodium hydride (32 mg, 0.82 mmol), dry DMF (5.0 mL) and 4-methoxybenzyl chloride (0.10 mL, 0.70 mmol). The mixture was stirred at 40 °C for 3 h. Beige solid (145 mg, 62%). m.p. 164–166 °C; 1H-NMR (500 MHz, CDCl3) δ = 8.30 (d, J = 5.2 Hz, 1 H, 3-H), 8.01 (d, J = 8.6 Hz, 1 H, 5-H), 7.77 (d, J = 5.2 Hz, 1 H, 4-H), 6.95–6.87 (m, 3 H, 6-H, 3″/5″-H), 6.82–6.78 (m, 2 H, 2″/6″-H), 6.77 (d, J = 2.1 Hz, 1 H, 8-H), 5.66 (s, 2 H, 1′-H), 3.85 (s, 3 H, OCH3), 3.75 (s, 3 H, 4″-OCH3), 2.86 (s, 3 H, 1-CH3); 13C-NMR (125 MHz, CDCl3) δ = 161.0, 158.9, 143.5, 141.0, 138.6, 135.7, 129.8, 129.3, 126.6 (2 C), 122.4, 115.1, 114.4 (2 C), 112.3, 109.2, 93.3, 55.6, 55.3, 47.7, 23.1; IR (KBr) (cm−1) = 3045, 1619, 1027; MS (EI) m/z (relative intensity, %) = 332 [M+•] (8), 121 (100); HRMS (EI) calcd. for C21H20N2O2: 332.1525; found 332.1524; purity: 100% (λ = 210 nm), 100% (λ = 254 nm) (by HPLC).
7-Methoxy-1-methyl-9-(3-phenylpropyl)-9H-pyrido[3,4-b]indole (
AnnH38). Prepared from harmine (151 mg, 0.712 mmol), sodium hydride (29 mg, 0.72 mmol), dry DMF (5.0 mL) and 1-bromo-3-phenylpropane (0.11 mL, 0.73 mmol). The mixture was stirred at 40 °C for 4 h. Beige solid (114 mg, 49%). The analytical data were in accordance with those published in ref. [
34].
7-Methoxy-1-methyl-9-(2-naphthylmethyl)-9H-pyrido[3,4-b]indole (AnnH39). Prepared from harmine (150 mg, 0.709 mmol), sodium hydride (28 mg, 0.71 mmol), dry DMF (5.0 mL) and 2-(bromomethyl)naphthalene (0.159 mg, 0.716 mmol). The mixture was stirred at 40 °C for 4 h. Beige solid (102 mg, 41%). m.p. 143 °C; 1H-NMR (500 MHz, CD2Cl2) δ = 8.27 (d, J = 5.2 Hz, 1 H, 3-H), 8.05 (d, J = 8.6 Hz, 1 H, 5-H), 7.82–7.80 (m, 3 H, 4-H, 4′′-H, 5′′-H), 7.60 (d, J = 7.8 Hz, 1 H, 8′′-H), 7.48–7.37 (m, 2 H, 6′′-H, 7′′-H), 7.33 (s, 1 H, 1′′-H), 7.26 (dd, J = 8.5, 1.8 Hz, 1 H, 3′′-H), 6.91 (dd, J = 8.6, 2.2 Hz, 1 H, 6-H), 6.81 (d, J = 2.1 Hz, 1 H, 8-H), 5.88 (s, 2 H, 1′-H), 3.80 (s, 3 H, OCH3), 2.81 (s, 3 H, 1-CH3); 13C-NMR (124 MHz, CD2Cl2) δ = 161.3, 143.7, 140.7, 138.7, 135.9, 133.6, 132.9 (2 C), 130.9, 128.9, 127.8 (2 C). 126.5, 126.0, 124.2, 123.8, 122.5, 115.3, 112.3, 109.4, 93.4, 55.7, 53.2, 23.0; IR (KBr) (cm−1) = 3427, 3091, 1627, 1147; MS (EI) m/z (relative intensity, %) = 352 [M+•] (20), 141 (100); HRMS (EI) calcd. for C24H20N2O: 352.1576; found 352.1574; purity: 100% (λ = 210 nm), 100% (λ = 254 nm) (by HPLC).
7-Methoxy-1-methyl-9-((E)-3-phenylallyl)-9H-pyrido[3,4-b]indole (AnnH22). Prepared from harmine (100 mg, 0.472 mmol), potassium tert-butoxide (65 mg, 0.58 mmol), dry DMSO (10 mL) and (E)-3-phenylallyl bromide (509 mg, 2.58 mmol). The mixture was stirred at 80 °C for 3 h Beige solid (36 mg, 23%). m.p. 104–108 °C; 1H-NMR (400 MHz, CDCl3) δ = 8.30 (d, J = 5.3 Hz, 1 H, 3-H), 8.01 (d, J = 8.6 Hz, 1 H, 5-H), 7.78 (d, J = 5.2 Hz, 1 H, 4-H), 7.32–7.14 (m, 5 H, aromatic protons), 6.92 (dd, J = 8.6, 2.2 Hz, 1 H, 6-H), 6.85 (d, J = 2.0 Hz, 1 H, 8-H), 6.42 (dt, J = 16.0, 4.5 Hz, 1 H, 2‘-H), 6.21 (d, J = 16.0 Hz, 1 H, 3‘-H), 5.26 (dd, J = 4.4, 1.8 Hz, 2 H, 1‘-H), 3.92 (s, 3 H, OCH3), 3.03 (s, 3 H, 1-CH3); 13C-NMR (100 MHz, CDCl3) δ = 161.2, 143.4, 140.6, 138.1, 136.0, 135.5, 131.4, 129.7, 128.6 (2 C), 127.9 (2 C), 126.4, 124.7, 122.5, 115.1, 112.4, 109.3, 93.4, 55.7, 46.6, 22.7; IR (KBr) (cm−1) = 3362, 2926, 1624, 1169; MS (EI) m/z (relative intensity, %) = 328 [M+•] (36), 117 (100); HRMS (EI) calcd. for C22H20N2O: 328.1576; found 328.1574; purity: 92% (λ = 210 nm), 100% (λ = 254 nm) (by HPLC).
7-Methoxy-1-methyl-9-(2-phenoxyethyl)-9H-pyrido[3,4-b]indole (AnnH40). Prepared from harmine (150 mg, 0.709 mmol), sodium hydride (28 mg, 0.71 mmol), dry DMF (5.0 mL) and 2-bromoethyl phenyl ether (0.144 mg, 0.716 mmol). The mixture was stirred at 40 °C for 6 h. Beige solid (72 mg, 31%). m.p. 143–144 °C; 1H-NMR (500 MHz, CD2Cl2) δ = 8.24 (d, J = 5.2 Hz, 1 H, 3-H), 7.96 (d, J = 8.6 Hz, 1 H, 5-H), 7.72 (d, J = 5.2 Hz, 1 H, 4-H), 7.27–7.14 (m, 2 H, aromatic protons), 7.00 (d, J = 2.1 Hz, 1 H, 8-H), 6.92–6.87 (m, 2 H, 6-H, 4′-H), 6.78–6.76 (m, 2 H, aromatic protons), 4.91 (t, J = 5.7 Hz, 2 H, 1′-H), 4.35 (t, J = 5.7 Hz, 2 H, 2′-H), 3.92 (s, 3 H, OCH3), 3.05 (s, 3 H, 1-CH3); 13C-NMR (125 MHz, CD2Cl2) δ = 161.1, 158.4, 143.4, 141.1, 138.6, 135.8, 129.6 (2 C), 129.4, 122.3, 121.3, 115.3, 114.4 (2 C), 112.2, 109.4, 93.7, 67.0, 54.0, 44.3, 23.8; IR (KBr) (cm−1) = 3021, 2869, 1631, 1197; MS (EI) m/z (relative intensity, %) = 332 [M+•] (34), 225 (100), 182 (16); HRMS (EI) calcd. for C21H20N2O2: 332.1525; found 332.1528; purity: 100% (λ = 210 nm), 100% (λ = 254 nm) (by HPLC).
7-Methoxy-1-methyl-9-(pyridin-4-yl-methyl)-9H-pyrido[3,4-b]indole (AnnH36). Prepared from harmine (157 mg, 0.708 mmol), sodium hydride (41 mg, 0.71 mmol), dry DMF (5.0 mL) and 4-(chloromethyl)pyridine hydrochloride (0.125 mg, 0.708 mmol). The mixture was stirred at 40 °C for 7 h. Brown solid (20 mg, 9%). m.p. 143–146 °C; 1H-NMR (400 MHz, CD2Cl2) δ = 8.49 (dd, J = 4.5, 1.6 Hz, 2 H, 3′′-H, 5′′-H), 8.28 (d, J = 5.6 Hz, 1 H, 3- H), 8.11 (d, J = 8.7 Hz, 1 H, 5- H), 7.96 (d, J = 5.6 Hz, 1 H, 4-H), 7.00 (dd, J = 8.7, 2.1 Hz, 1 H, 6-H), 6.94 (d, J = 6.0 Hz, 2 H, 2′′-H, 6′′-H), 6.77 (d, J = 2.1 Hz, 1 H, 8-H), 5.78 (s, 2 H, 1′-H), 3.87 (s, 3 H, OCH3), 2.87 (s, 3 H, 1-CH3); 13C-NMR (100 MHz, CD2Cl2) δ = 162.8, 150.7 (2 C), 147.2, 145.0, 140.0, 136.2, 135.5, 131.7, 123.6, 121.1 (2 C), 114.9, 113.4, 111.2, 93.4, 56.1, 49.4, 21.1; IR (KBr) (cm−1) = 3425, 2922, 1620, 1166; MS (EI) m/z (relative intensity, %) = 303 [M+•] (100), 225 (26), 211 (95); HRMS (EI) calcd. for C19H17N3O: 303.1372; found 303.1371; purity: 93 (λ = 210 nm), 100% (λ = 254 nm) (by HPLC).
[2-(7-Methoxy-1-methyl-9H-pyrido[3,4-b]indol-9-yl)-ethyl]-dimethylamine (AnnH16). Prepared from harmine (103 mg, 0.490 mmol), sodium hydride (70 mg, 1.8 mmol), dry THF (5 mL) and N,N-dimethyl-2-chloroethylamine hydrochloride (107 mg, 0.750 mmol). Orange solid (91 mg, 66%). m.p. 88–92 °C; 1H-NMR (500 MHz, CDCl3) δ = 8.29 (d, J = 5.2 Hz, 1 H, 3-H), 7.97 (d, J = 7.1 Hz, 1 H, 5-H), 7.74 (d, J = 5.2 Hz, 1 H, 4-H), 6.91–6.89 (m, 2 H, 6-H, 8-H), 4.68–4.55 (m, 2 H, 1′-H), 3.95 (s, 3 H, OCH3), 3.06 (s, 3 H, 1-CH3), 2.76–2.63 (m, 2 H, 2′-H), 2.39 (s, 6 H, N(CH3)2); 13C-NMR (125 MHz, CDCl3) δ = 161.0, 143.1, 140.4, 138.2, 135.3, 129.5, 122.5, 115.2, 112.3, 109.1, 93.1, 58.6, 55.7, 46.0 (2 C), 43.5, 23.3; IR (KBr) (cm−1) = 3424, 2950, 1621, 1148; MS (EI) m/z (relative intensity, %) = 283 [M+•] (2), 58 (100); HRMS (EI) calcd. for C17H21N3O: 283.1685; found 283.1682; purity: 100% (λ = 210 nm), 100% (λ = 254 nm) (by HPLC).
[3-(7-Methoxy-1-methyl-9H-pyrido[3,4-b]indol-9-yl)-N,N-dimethylpropan-1-amine (
AnnH21). Prepared from harmine (100 mg, 0.470 mmol), sodium hydride (65 mg, 1.6 mmol), dry THF (5 mL) and
N,
N-dimethyl-3-chloropropylamine hydrochloride (129 mg, 0.820 mmol). Orange solid (50 mg, 36%). m.p. 46–48 °C. The analytical data were in accordance with those published in ref. [
33].
4-[2-(7-Methoxy-1-methyl-9H-pyrido[3,4-b]indol-9-yl)-ethyl]morpholine (AnnH53). Prepared from harmine (104 mg, 0.491 mmol), sodium hydride (68 mg, 1.7 mmol), dry THF (5 mL) and 4-(2-chloroethyl)morpholine hydrochloride (150 mg, 0.834 mmol). Beige solid (26 mg, 17%). m.p. 183 °C; 1H-NMR (400 MHz, CDCl3) δ = 8.29 (d, J = 5.4 Hz, 1 H, 3-H), 8.00 (d, J = 9.3 Hz, 1 H, 5-H), 7.79 (d, J = 5.4 Hz, 1 H, 4-H), 6.94–6.92 (m, 2 H, 6-H, 8-H), 4.71–4.58 (m, 2 H, 1′-H), 3.97 (s, 3 H, OCH3), 3.73–3.68 (m, 4 H, 5′′-H, 3′′-H), 3.12 (s, 3 H, 1-CH3), 2.83–2.69 (m, 2 H, 2′-H), 2.55–2.53 (m, 4 H, 2′′-H, 6′′-H); 13C-NMR (125 MHz, CDCl3) δ = 161.4, 143.6, 139.4, 136.9, 135.1, 130.2, 122.7, 115.0, 112.6, 109.5, 93.4, 66.8 (2 C), 58.0, 55.8, 54.2 (2 C), 43.0, 22.5; IR (KBr) (cm−1) = 3442, 2927, 1623, 1115; MS (ESI) m/z = 326 [M+ H], 235, 118; HRMS (EI) calcd. for C19H23N3O2: 325.1790; found 325.1789; purity: 90% (λ = 210 nm), 100% (λ = 254 nm) (by HPLC).
7-Methoxy-1-methyl-9-[2-(pyrrolidin-1-yl)ethyl]-9H-pyrido[3,4-b]indole (AnnH55). Harmine (150 mg, 0.706 mmol) and sodium hydride (137 mg, 3.43 mmol) were stirred in dry THF (7.5 mL) for 1 h. After addition of 1-(2-chloroethyl)pyrrolidine hydrochloride (204 mg, 1.20 mmol) the mixture was stirred for 23 h. Beige solid (100 mg, 46%). m.p. 99–101 °C; 1H-NMR (400 MHz, CDCl3) δ = 8.29 (d, J = 5.2 Hz, 1 H, 3-H), 7.97 (d, J = 8.6 Hz, 1 H, 5-H), 7.74 (d, J = 5.2 Hz, 1 H, 4-H), 6.96 (d, J = 1.9 Hz, 1 H, 8-H), 6.90 (dd, J = 8.6, 2.2 Hz, 1 H, 6-H), 4.76–4.61 (m, 2 H, 1′-H), 3.96 (s, 3 H, OCH3), 3.07 (s, 3 H, 1-CH3), 2.98–2.83 (m, 2 H, 2′-H), 2.70–2.66 (m, 4 H, pyrrolidin), 1.94–1.79 (m, 4 H, pyrrolidin); 13C-NMR (100 MHz, CDCl3) δ = 161.1, 143.1, 140.4, 138.3, 135.3, 129.5, 122.4, 115.2, 112.3, 109.3, 93.1, 55.8, 55.4, 54.6 (4 C), 44.1, 23.5; IR (KBr) (cm−1) = 3450, 2929, 1622, 1139; MS (ESI) m/z = 310 [M++ H], 98; HRMS (EI) calcd. for C19H23N3O: 309.1841; found 309.1843; purity: 91% (λ = 210 nm), 100% (λ = 254 nm) (by HPLC).
7-Methoxy-1-methyl-9-[2-(piperidin-1-yl)ethyl]-9H-pyrido[3,4-b]indole (AnnH57). Harmine (106 mg, 0.501 mmol) and sodium hydride (93 mg, 0.23 mmol) were stirred in dry THF (5 mL) for 1 h. After addition of 1-(2-chloroethyl)piperidine hydrochloride (148 mg, 0.806 mmol) the mixture was stirred for 48 h. Light brown solid (75 mg, 33%). m.p. 108–110 °C; 1H-NMR (400 MHz, CDCl3) δ = 8.28 (d, J = 5.2 Hz, 1 H, 3-H), 7.96 (d, J = 8.6 Hz, 1 H, 5-H), 7.72 (d, J = 5.2 Hz, 1 H, 4-H), 6.93 (d, J = 2.0 Hz, 1 H, 8-H), 6.89 (dd, J = 8.6, 2.2 Hz, 1 H, 6-H), 4.71–4.56 (m, 2 H, 1′-H), 3.94 (s, 3 H, OCH3), 3.05 (s, 3 H, 1-CH3), 2.77–2.63 (m, 2 H, 2′-H), 2.55–2.50 (m, 4 H, 1′′-H, 5′′-H), 1.62–1.59 (m, 4 H, 2′′-H, 4′′-H), 1.48–1.42 (m, 2 H, 3′′-H); 13C-NMR (100 MHz, CDCl3) δ = 160.9, 143.1, 140.6, 138.3, 135.4, 129.4, 122.4, 115.2, 112.3, 109.0, 93.2, 58.3, 55.7, 55.3 (2 C), 42.9, 25.9 (2 C), 24.1, 23.5; IR (KBr) (cm−1) = 3444, 2932, 1621, 1112; MS (ESI) m/z = 324 [M++ H], 163, 112, 102; HRMS (EI) calcd. for C20H25N3O: 323.1998; found 323.1999; purity: 100% (λ = 210 nm), 100% (λ = 254 nm) (by HPLC).
Ethyl 2-(7-methoxy-1-methyl-9H-pyrido[3,4-b]indol-9-yl)acetate (
AnnH12). The preparation of
AnnH12 has been reported in a patent [
35] without presenting spectroscopic data.
1H-NMR (400 MHz, CDCl
3)
δ = 8.30 (d,
J = 5.2 Hz, 1 H, 3-H), 7.98 (d,
J = 8.6 Hz, 1 H, 5-H), 7.74 (d,
J = 5.3 Hz, 1 H, 4-H), 6.91 (dd,
J = 2.2, 8.6 Hz, 1 H, 6-H), 6.76 (d,
J = 2.2, Hz, 1 H, 8-H), 5.20 (s, 2 H, 1′-H), 4.27–4.20 (m, 2 H, O-CH
2), 3.93 (s, 3 H, OCH
3), 2.94 (s, 3 H, 1-CH
3), 1.25 (m, 3 H, CH
2-C
H3);
13C-NMR (100 MHz, CDCl
3)
δ = 168.6, 161.1, 143.4, 140.5, 139.0, 135.7, 129.8, 122.6, 115.4, 112.5, 109.4, 93.0, 62.0, 55.7, 46.8, 23.0, 14.1; IR (KBr)
(cm
−1) = 3461, 2872, 1736, 1622, 1252; MS (EI)
m/z (relative intensity, %)
= 298 [M
+•] (50), 225 (100), 182 (24); HRMS (EI) calcd. for C
17H
18N
2O
3: 298.1318; found 298.1311.
Methyl 2-(7-methoxy-1-methyl-9H-pyrido[3,4-b]indol-9-yl)acetate (AnnH26). Prepared from harmine (105 mg, 0.490 mmol), sodium hydride (20 mg, 0.49 mmol), dry DMF (4.0 mL) and methyl bromoacetate (0.050 mL, 0.53 mmol). The mixture was stirred at 40 °C for 4 h. White solid (16 mg, 11%). m.p. 121–125 °C. 1H-NMR (500 MHz, CDCl3) δ = 8.31 (d, J = 5.2 Hz, 1 H, 3-H), 7.98 (d, J = 8.6 Hz, 1 H, 5-H), 7.74 (d, J = 5.2 Hz, 1 H, 4-H), 6.91 (dd, J = 8.6, 2.0 Hz, 1 H, 6-H), 6.75 (d, J = 2.1 Hz, 1 H, 8-H), 5.21 (s, 2 H, 1′-H), 3.93 (s, 3 H, OCH3), 3.77 (s, 3 H, COOCH3), 2.93 (s, 3 H, 1-CH3); 13C-NMR (100 MHz, CDCl3) δ = 169.4, 161.2, 143.3, 140.5, 139.0, 135.6, 129.8, 122.6, 115.4, 112.5, 109.4, 93.0, 55.7, 52.9, 46.7, 23.0; IR (KBr) (cm−1) = 3446, 2998, 1735, 1624, 1174; MS (EI) m/z (relative intensity, %) = 284 [M+•] (37), 225 (100), 182 (26); HRMS (EI) calcd. for C16H16N2O3: 284.1161; found 284.1162; purity: 100% (λ = 210 nm), 100% (λ = 254 nm) (by HPLC).
Isopropyl 2-(7-methoxy-1-methyl-9H-pyrido[3,4-b]indol-9-yl)acetate(AnnH27). Prepared from harmine (150 mg, 0.706 mmol), potassium tert-butoxide (144 mg, 1.28 mmol), dry DMSO (13 mL) and isopropyl bromoacetate (1.0 mL, 7.7 mmol). The mixture was stirred at 80 °C for 3 h. Beige solid (18 mg, 10%). m.p. 78–82 °C; 1H-NMR (400 MHz, CDCl3) δ = 8.30 (d, J = 5.3 Hz, 1 H, 3-H), 7.97 (d, J = 8.6 Hz, 1 H, 5-H), 7.74 (d, J = 5.2 Hz, 1 H, 4-H), 6.91 (dd, J = 8.6, 2.1 Hz, 1 H, 6-H), 6.76 (d, J = 2.0 Hz, 1 H, 8-H), 5.15 (s, 2 H, 1′-H), 5.12–5.10 (m, 1 H, OCH), 3.96 (s, 3 H, OCH3), 2.93 (s, 3 H, 1-CH3), 1.24 (s, 6 H, CH(CH3)2); 13C-NMR (100 MHz, CDCl3) δ = 168.3, 161.7, 143.5, 140.5, 138.8, 135.7, 129.8, 122.5, 115.3, 112.4, 109.3, 93.0, 69.8, 55.6, 47.0, 22.9, 21.7 (2 C); IR (KBr) (cm−1) = 3426, 2980, 1729, 1632, 1175; MS (EI) m/z (relative intensity, %) = 312 [M+•] (28), 270 (8), 225 (100), 182 (16); HRMS (EI) calcd. for C18H20N2O3: 312.1474; found 312.1474; purity: 100% (λ = 210 nm), 100% (λ = 254 nm) (by HPLC).
Ethyl 3-(7-Methoxy-1-methyl-9H-pyrido[3,4-b]indol-9-yl)propanoate (AnnH32). To harmine (105 mg, 0.494 mmol) in dry DMF (3.0 mL) was added sodium hydride (60% in mineral oil, 49 mg, 1.2 mmol). The mixture was stirred at room temperature for 1 h. Ethyl 3-bromopropionate (0.070 mL, 0.54 mmol) was added and the mixture was stirred for 18 h at room temperature. Then, additional sodium hydride (60% in mineral oil, 26 mg, 0.65 mmol) and ethyl 3-bromopropionate (0.070 mL, 0.54 mmol) were added and the mixture was stirred for 1 h. The reaction mixture was treated with DCM (20 mL) and washed with water (20 mL) and brine (20 mL). The organic layer was dried over magnesium sulfate and evaporated to dryness. The residue was purified by coulmn chromatography (silica gel, iso-hexane/ethyl acetate/ethanol 2:2:1) to afford AnnH32 as a white solid (18 mg, 12%). m.p. 95–96 °C; 1H-NMR (500 MHz, CDCl3) δ = 8.30 (d, J = 5.2 Hz, 1 H, 3-H), 7.97 (d, J = 8.6 Hz, 1 H, 5- H), 7.74 (d, J = 5.2 Hz, 1 H, 4-H), 6.93 (d, J = 2.0 Hz, 1 H, 8-H), 6.90 (dd, J = 8.6, 2.1 Hz, 1 H, 6-H), 4.90–4.77 (m, 2 H, 1′-H), 4.12 (q, J = 7.2 Hz, 2 H, O-CH2), 3.96 (s, 3 H, OCH3), 3.04 (s, 3 H, 1-CH3), 2.86–2.73 (m, 2 H, 2′-H), 1.24–1.13 (m, 3 H, CH2-CH3); 13C-NMR (100 MHz, CDCl3) δ = 170.9, 161.0, 142.7, 140.5, 138.6, 135.0, 129.8, 122.5, 115.3, 112.3, 109.3, 93.2, 61.1, 55.7, 40.5, 35.0, 23.3, 14.0; IR (KBr) (cm−1) = 3445, 2968, 1728, 1622, 1196; MS (EI) m/z (relative intensity, %) = 312 [M+•] (48), 225 (100), 182 (15); HRMS (EI) calcd. for C18H20N2O3: 312.1474; found 312.1474; purity: 100% (λ = 210 nm), 100% (λ = 210 nm) (by HPLC).
2-(7-Methoxy-1-methyl-9H-pyrido[3,4-b]indol-9-yl)acetic acid (AnnH25). Ethyl-2-(7-methoxy-1-methyl-9H-pyrido[3,4-b]indol-9-yl)acetate (AnnH12) (107 mg, 0.360 mmol) was dissolved in methanol (3.45 mL). After addition of water (3.45 mL) and aqueos sodium hydroxide (1 M, 0.38 mL, 0.38 mmol) the mixture was stirred at room temperature for 4 h. The solvent was removed under reduced pressure and water (5 mL) was added. The pH was adjusted to 5–6 with 2 M HCl. The formed precipitate was collected by filtration, washed with water and dried in vacuo to afford AnnH25 as a white solid (73 mg, 75%). m.p. 276–279 °C; 1H-NMR (400 MHz, DMSO-d6) δ = 9.90 (s, 1 H, OH), 8.18 (d, J = 5.2 Hz, 1 H, 3-H), 8.10 (d, J = 8.6 Hz, 1 H, 5-H), 7.91 (d, J = 5.3 Hz, 1 H, 4-H), 7.29 (d, J = 2.0 Hz, 1 H, 8-H), 6.88 (dd, J = 8.6, 2.0 Hz, 1 H, 6-H), 5.41 (s, 2 H, 1′-H), 3.90 (s, 3 H, OCH3), 2.85 (s, 3 H, 1-CH3); 13C-NMR (100 MHz, DMSO-d6) δ = 171.1, 160.6, 143.4, 140.4, 137.6, 135.0, 128.6, 122.3, 113.9, 112.3, 109.5, 93.5, 55.5, 46.0, 22.2; IR (KBr) (cm−1) = 3506, 2928, 1628, 1134; MS (EI) m/z (relative intensity, %) = 270 [M+•] (72), 225 (100), 182 (30), 142 (20), 83 (57); HRMS (EI) calcd. for C15H14N2O3: 270.1005; found 270.0970; purity: 100% (λ = 210 nm), 99% (λ = 210 nm) (by HPLC).
2-(7-Methoxy-1-methyl-9H-pyrido[3,4-b]indol-9-yl)-N-acetamide (AnnH28). To harmine (103 mg, 0.486 mmol) in dry DMF (3.0 mL) was added sodium hydride (22 mg, 0.54 mmol). The mixture was stirred at 40 °C for 30 min. Then, it was cooled to room temperature and 2-bromoacetamide (67 mg, 0.49 mmol) was added. The mixture was stirred at room temperature for 24 h, then poured into ice water (25 mL) and extracted with ethyl acetate (3 × 20 mL). The combined organic layers were washed with water (2 × 60 mL), dried over magnesium sulfate and evaporated to dryness. The residue was purified by column chromatography (silica gel, iso-hexane/ethyl acetate/ethanol/triethylamine 4:4:3 +1%) to afford AnnH28 as a beige solid (18 mg, 14%). m.p. 291–297 °C; 1H-NMR (400 MHz, DMSO-d6) δ = 8.21 (d, J = 5.4 Hz, 1 H, 3-H), 8.16 (d, J = 8.6 Hz, 1 H, 5-H), 8.04 (d, J = 5.4 Hz, 1 H, 4-H), 7.80 (s, 1 H, NH2), 7.42 (s, 1 H, NH2), 7.21 (d, J = 2.2 Hz, 1 H, 8-H), 6.92 (dd, J = 8.5, 2.0 Hz, 1 H, 6-H), 5.23 (s, 2 H, 1′-H), 3.90 (s, 3 H, OCH3), 2.91 (s, 3 H, 1-CH3); 13C-NMR (100 MHz, DMSO-d6) δ = 170.0, 161.2, 144.3, 140.0, 136.0, 135.2, 129.5, 122.7, 114.0, 112.7, 110.0, 93.6, 55.7, 47.1, 21.4; IR (KBr) (cm−1) = 3423, 3013, 1683, 1196; MS (EI) m/z (relative intensity, %) = 269 [M+•] (32), 225 (100), 182 (25); HRMS (EI) calcd. for C15H15N3O2: 269.1164; found 264.1164; purity: 100% (λ = 210 nm), 100% (λ = 254 nm) (by HPLC).
2-(7-Methoxy-1-methyl-9H-pyrido[3,4-b]indol-9-yl)-N-methylacetamide (AnnH30). Prepared from harmine (150 mg, 0.708 mmol), sodium hydride (76 mg, 1.9 mmol), dry DMF (5.0 mL) and 2-chloro-N-methylacetamide (81 mg, 0.75 mmol). The mixture was stirred at 40 °C for 6 h and over night at room temperature. Then, additional 2-chloro-N-methylacetamide (81 mg, 0.75 mmol) was added and the mixture was stirred at 40 °C for 5 h, then poured into ice water (25 mL) and extracted with ethyl acetate (3 × 20 mL). The combined organic layers were washed with water (2 × 60 mL), dried over magnesium sulfate, and evaporated to dryness. The residue was purified by column chromatography (silica gel, iso-hexane/ethyl acetate/ethanol/triethylamine 2:2:1 + 1%) to afford AnnH30 as a beige solid (65 mg, 33%). m.p. 265–272 °C; 1H-NMR (400 MHz, DMSO-d6) δ = 8.21 (d, J = 4.6 Hz, 1 H, NH), 8.17 (d, J = 5.2 Hz, 1 H, 3-H), 8.10 (d, J = 8.6 Hz, 1 H, 5-H), 7.90 (d, J = 5.2 Hz, 1 H, 4 H), 7.17 (d, J = 2.0 Hz, 1 H, 8-H), 6.88 (dd, J = 8.6, 2.1 Hz, 1 H, 6-H), 5.20 (s, 2 H, 1′-H), 3.88 (s, 3 H, OCH3), 2.84 (s, 3 H, 1-CH3), 2.64 (d, J = 4.6 Hz, 3 H, N-CH3); 13C-NMR (100 MHz, DMSO-d6) δ = 168.6, 160.6, 143.6, 140.9, 137.7, 135.4, 128.7, 122.4, 114.3, 112.3, 109.4, 93.6, 55.7, 40.1, 25.7, 22,5; IR (KBr) (cm−1) = 3424, 2925, 1656, 1193; MS (EI) m/z (relative intensity, %) = 283 [M+•] (28), 225 (100), 182 (24); HRMS (EI) calcd. for C16H17N3O2: 283.1321; found 283.1322; purity: 100%(λ = 210 nm), 92% (λ = 254 nm) (by HPLC).
2-(7-Methoxy-1-methyl-9H-pyrido[3,4-b]indol-9-yl)-N,N-dimethylacetamide (
AnnH34). The preparation of
AnnH34 has been reported in in a patent [
35] without presenting spectroscopic data.
1H-NMR (500 MHz, CDCl
3)
δ = 8.27 (d,
J = 5.2 Hz, 1 H, 3-H), 7.96 (d,
J = 8.6 Hz, 1 H, 5-H), 7.72 (d,
J = 5.2 Hz, 1 H, 4-H), 6.87 (dd,
J = 8.6, 2.1 Hz, 1 H, 6-H), 6.67 (d,
J = 2.0 Hz, 1 H, 8-H), 5.19 (s, 2 H, 1′-H), 3.90 (s, 3 H, OCH
3), 3.19 (s, 3 H, 1-CH
3), 3.03 (s, 3 H, N-CH
3), 2.87 (s, 3 H, N-CH
3);
13C-NMR (100 MHz, CDCl
3)
δ = 167.2, 161.0, 143.8, 140.3, 138.6, 136.1, 129.7, 122.5, 115.4, 112.5, 108.9, 93.2, 55.7, 46.5, 36.4, 36.1, 22.9; IR (KBr)
(cm
−1) = 3496, 2959, 1650, 1177; MS (EI)
m/z (relative intensity, %) = 297 [M
+•] (23), 225 (100), 182 (27); HRMS (EI) calcd. for C
17H
19N
3O
2: 297.1477; found 297.147; purity: 100%, 100% (λ = 210 nm) (by HPLC).
2-(7-Methoxy-1-methyl-9H-pyrido[3,4-b]indol-9-yl)-acetonitrile (
AnnH31). The preparation of
AnnH31 has been reported previously in a patent [
35] without spectroscopic data.
1H-NMR (500 MHz, CDCl
3)
δ = 8.36 (d,
J = 5.2 Hz, 1 H, 3-H), 7.97 (d,
J = 8.6 Hz, 1 H, 5- H), 7.72 (d,
J = 5.2 Hz, 1 H, 4-H), 6.96 (dd,
J = 8.6, 2.0 Hz, 1 H, 6-H), 6.86 (d,
J = 1.9 Hz, 1 H, 8-H), 5.35 (s, 2 H, 1′-H), 3.96 (s, 3 H, OCH
3), 3.08 (s, 3 H, 1-CH
3);
13C-NMR (125 MHz, CDCl
3)
δ = 161.5, 142.4, 140.4, 140,3, 134.8, 130.5, 122.9, 115.7, 114.7, 112.6, 110.3, 93.2, 55.8, 33.6, 23.0; IR (KBr)
(cm
−1) = 3441, 2924, 2208, 1629; MS (EI)
m/z (relative intensity, %) = 251 [M
+•] (100), 225 (10), 211 (68), 168 (23); HRMS (EI) calcd. for C
15H
13N
3O: 251.1059; found 251.1059.
3-(7-Methoxy-1-methyl-9H-pyrido[3,4-b]indol-9-yl)propanenitrile (AnnH42). A suspension of harmine (102 mg, 0.482 mmol) in acrylonitrile (2.0 mL, 30 mmol) was stirred in an ice-bath for 15 min. Then, benzyltriethylammonium hydroxide (40% in methanol, 3.5 µL, 0.0062 mmol) was added dropwise and the mixture was stirred at room temperature for 3 h and at 70 °C for 1h. The crude product that precipitated in the flask upon cooling was recrystallized from acetonitrile to afford AnnH42 as white solid (35 mg, 27%). m.p. 190 °C; 1H-NMR (400 MHz, CDCl3) δ = 8.34 (d, J = 5.2 Hz, 1 H, 3-H), 7.99 (d, J = 8.6 Hz, 1 H, 5-H), 7.74 (d, J = 5.2 Hz, 1 H, 4-H), 6.94 (dd, J = 8.6, 2.1 Hz, 1 H, 6-H), 6.88 (d, J = 2.0 Hz, 1 H, 8-H), 4.92–4.79 (m, 2 H, 1′-H), 3.97 (s, 3 H, OCH3), 3.03 (s, 3 H, 1-CH3), 2.89–2.76 (m, 2 H, 2′-H); 13C-NMR (400 MHz, CDCl3) δ = 161.3, 142.2, 140.1, 139.5, 134.7, 130.1, 122.8, 116.7, 115.7, 112.5, 109.6, 93.2, 55.8, 40.6, 23.53, 18.4; IR (KBr) (cm−1) = 3442, 2990, 2345, 1627, 1173; MS (ESI) m/z = 266 [M++ H], 225, 231, 150; HRMS (EI) calcd. for C16H15N3O: 265.1215; found 265.1216; purity: 100% (λ = 210 nm), 100% (λ = 254 nm) (by HPLC).
(±)-2-(7-Methoxy-1-methyl-9H-pyrido[3,4-b]indol-9-yl)propanenitrile (AnnH43). Prepared from harmine (150 mg, 0.706 mmol), sodium hydride (40 mg, 1.0 mmol), dry DMF (5.0 mL) and (±)-2-bromopropionitrile (0.060 mL, 0.69 mmol). The mixture was stirred at 40 °C for 7 h. Beige solid (39 mg, 21%). m.p. 132–134 °C; 1H-NMR (500 MHz, CDCl3) δ = 8.35 (d, J = 5.2 Hz, 1 H, 3-H), 8.00 (d, J = 8.6 Hz, 1 H, 5-H), 7.74 (d, J = 5.2 Hz, 1 H, 4-H), 7.26 (d, J = 2.1 Hz, 1 H, 8-H), 6.98 (dd, J = 8.6, 2.1 Hz, 1 H, 6-H), 6.25 (q, J = 7.2 Hz, 1 H, CH(CH3)), 3.98 (s, 3 H, OCH3), 3.01 (s, 3 H, 1-CH3), 1.95 (d, J = 7.3 Hz, 3 H, CH(CH3)); 13C-NMR (100 MHz, CDCl3) δ = 161.0, 140.7, 139.8, 139.6, 134.6, 130.4, 122.7, 117.2, 116.4, 112.6, 110.2, 95.6, 55.8, 41.9, 23.9, 19.3; IR (KBr) (cm−1) = 3428, 2918, 2369, 1628, 1211; MS (EI) m/z (relative intensity, %) = 265 [M+•] (78), 211 (100), 197 (20), 169 (38); HRMS (EI) calcd. for C16H15N3O: 265.1215; found 265.1209; purity: 100% (λ = 210 nm), 100% (λ = 254 nm) (by HPLC).
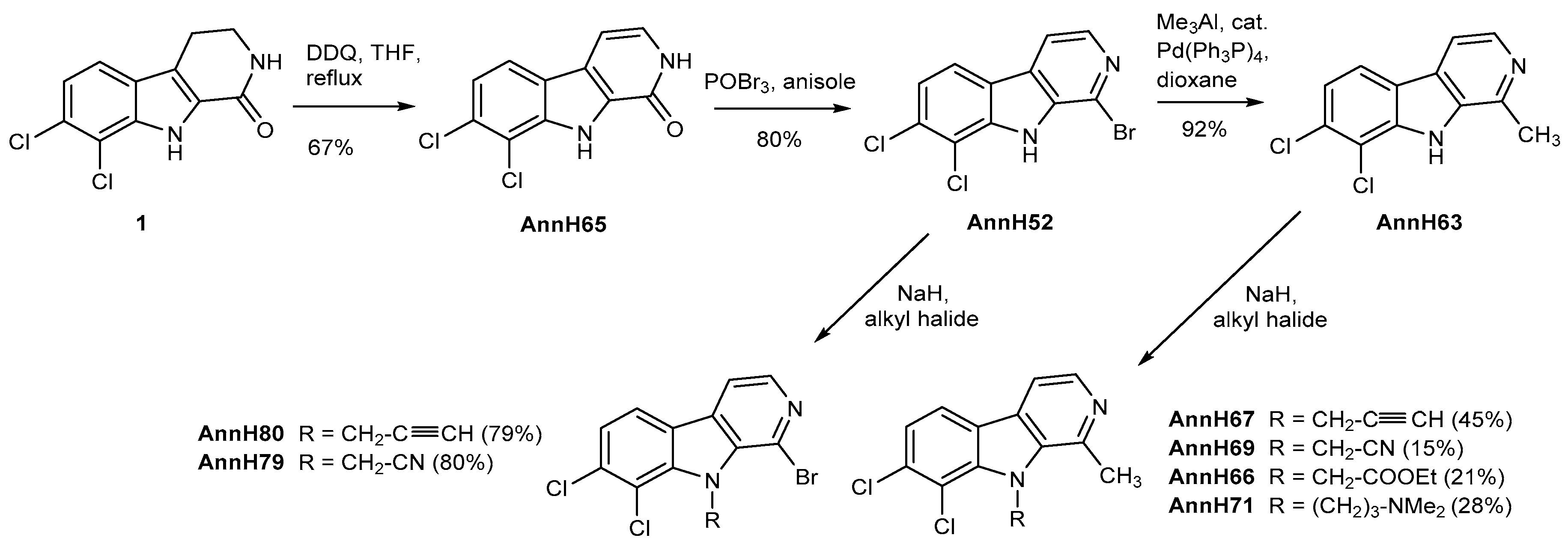
7,8-Dichloro-2,9-dihydro-1H-pydrido[3,4-b]indol-1-one (
AnnH65). To a solution of 6,7-dichloro-2,3,4,9-tetrahydro-1
H-pyrido[3,4-
b]indol-1-one (
1) [
36] (1.94 g, 7.61 mmol) in 120 mL of dry THF was added 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (3.46 g, 15.0 mmol) under a N
2 atmosphere. The mixture was stirred under reflux for 15 h and then cooled to room temperature. After addition of ethyl acetate (55 mL), the reaction mixture was washed with 1 N sodium hydroxide solution (4 × 100 mL). The combined aqueous layers were extracted with ethyl acetate (2 × 100 mL). The combined organic layers were dried over sodium sulfate and evaporated to dryness. The beige residue was washed with hot ethyl acetate, methanol and DCM to afford
AnnH65 as a beige solid (1.27 g, 67%). m.p. 200 °C;
1H-NMR (500 MHz, DMSO-
d6)
δ = 12.52 (s, 1 H, 9-H), 11.58 (s, 1 H, 2-H), 8.07 (d,
J = 8.2 Hz, 1 H, 5-H), 7.40 (d,
J = 8.2 Hz, 1 H, 6-H), 7.19 (d,
J = 6.4 Hz, 1 H, 3-H), 7.03 (d,
J = 6.4 Hz, 1 H, 4-H);
13C-NMR (100 MHz, DMSO-
d6)
δ = 155.3, 137.0, 129.3, 128.7, 126.0, 125.1, 122.4, 121.2, 121.1, 115.2, 99.2; IR (KBr)
(cm
−1) = 3434, 3114, 1739, 1644, 1161; MS (EI)
m/z (relative intensity, %)
= 256 (6), 254 (66), 252 [M
+•] (100); HRMS (EI) calcd. for C
11H
6N
2Cl
2O: 251.9857; found 251.9847; purity: 100% (λ = 210 nm), 100% (λ = 254 nm) (by HPLC).
1-Bromo-7,8-dichloro-9H-pyrido[3,4-b]indole(AnnH52). To a solution of 7,8-dichloro-2,9-dihydro-1H-pyrido[3,4-b]indol-1-one (AnnH65) (1.27 g, 5.04 mmol) in anisole (20 mL) was added phosphorus oxybromide (10.0 g, 35.0 mmol). The mixture was stirred at 120 °C for 4 h, then cooled to room temperature, and saturated aqueuos sodium carbonate solution (130 mL) was added. The mixture was extracted with ethyl acetate (4 × 200 mL). The combined organic layers were dried over sodium sulfate and evaporated to dryness. The residue was purified by column chromatography (silica gel, iso-hexane/ethyl acetate 1:1) to afford AnnH52 as a beige solid (1.27 g, 80%). m.p. 200 °C; 1H-NMR (500 MHz, DMSO-d6) δ = 12.15 (s, 1 H, NH), 8.29 (d, J = 8.4 Hz, 1 H, 5-H), 8.26 (d, J = 5.2 Hz, 1 H, 4-H), 8.22 (d, J = 5.2 Hz, 1 H, 3-H), 7.54 (d, J = 8.4 Hz, 1 H, 6-H); 13C-NMR (125 MHz, DMSO-d6) δ = 140.9, 139.2, 136.2, 131.8, 130.2, 125.0, 122.6, 122.4, 122.3, 115.9, 115.8; IR (KBr): (cm−1) = 3423, 1624, 798; MS (ESI): m/z = 319, 317, 315 [M++H], 280, 248, 113; HRMS (EI) calcd. for C11H5N2Cl2Br: 313.9013; found 313.9005; purity: 96% (λ = 254 nm), 100% (λ = 254 nm) (by HPLC).
7,8-Dichloro-1-methyl-9H-pyrido[3,4-b]indole (AnnH63). 1-Bromo-7,8-dichloro-9H-[3,4-b] indole (AnnH52) (69 mg, 0.22 mmol) and tetrakis(triphenylphosphine)palladium(0) (15 mg, 0.013 mmol) were dissolved under a N2 atmosphere in 5 mL of dioxane. To this reaction trimethylaluminium (0.12 mL of 2 M solution in hexane, 0.24 mmol) was added. The mixture was stirred at reflux for 3 h and then cooled to room temperature. After the addition of water (25 mL) the mixture was extracted with ethyl acetate (3 × 50 mL). The combined organic layers were dried over magnesium sulfate and evaporated to dryness. The white solid was purified by precipitation with using of DCM and n-hexane to afford AnnH63 as a white solid (51 mg, 92%). m.p. 226–227 °C; 1H-NMR (400 MHz, CDCl3) δ = 8.65 (s, 9-H), 8.41 (d, J = 5.4 Hz, 1 H, 3-H), 7.91 (d, J = 8.4 Hz, 1 H, 5-H), 7.76 (d, J = 5.4 Hz, 1 H, 4-H), 7.36 (d, J = 8.4 Hz, 1 H, 6-H), 2.87 (s, 3 H, 1-CH3); 13C-NMR (100 MHz, CDCl3) δ = 142.6, 139.7, 138.2, 134.6, 131.5, 128.4, 122.1, 121.7, 120.6, 115.6, 113.0, 20.4; IR (KBr) (cm−1) = 3425, 2926, 1618, 942; MS (ESI) m/z = 255, 253, 251 [M++H], 102; HRMS (ESI) calcd. for C12H8Cl2N2+ H+: 251.0143; found 251.0136; purity: 100% (λ = 210 nm), 100% (λ = 254 nm) (by HPLC).
Ethyl 2-(7,8-Dichloro-1-methyl-9H-pyrido[3,4-b]-9-yl)acetate (AnnH66). Prepared from AnnH63 (81 mg, 0.32 mmol), sodium hydride (17 mg, 0.41 mmol), dry DMF (3.0 mL) and ethyl bromoacetate (0.040 mL, 0.36 mmol). The mixture was stirred at 40 °C for 4 h. Beige solid (22 mg, 21%). m.p. 110–111 °C; 1H-NMR (400 MHz, CDCl3) δ = 8.40 (d, J = 5.2 Hz, 1 H, 3-H), 7.94 (d, J = 8.3 Hz, 1 H, 5-H), 7.78 (d, J = 5.3 Hz, 1 H, 4-H), 7.41 (d, J = 8.3 Hz, 1 H, 6-H), 6.08–5.27 (m, 2 H, 1′-H), 4.29 (q, J = 7.1 Hz, 2 H, O-CH2), 2.96 (s, 3 H, 1-CH3), 1.30–1.26 (m, 3 H, CH2-CH3); 13C-NMR (100 MHz, CDCl3) δ = 169.3, 142.0, 139.9, 138.5, 137.4, 133.7, 129.0, 123.4, 122.8, 120.2, 115.8, 112.7, 62.0, 48.5, 23.7, 14.2; IR (KBr) (cm−1) = 3430, 3059, 1743, 1655, 1165; MS (APCI) m/z = 341, 339, 337 [M++ H], 251; HRMS (ESI) calcd. for C16H14N2O2Cl2 + H+: 337.0510; found 337.0508; purity: 92% (λ = 210 nm), 97% (λ = 254 nm) (by HPLC).
2-(7,8-Dichloro-1-methyl-9H-pyrido[3,4-b]indol-9-yl)acetonitrile (AnnH69). Prepared from AnnnH63 (102 mg, 0.404 mmol), sodium hydride (18 mg, 0.45 mmol), dry DMF (2.5 mL) and bromoacetonitrile (0.030 mL, 0.44 mmol). The mixture was stirred at 40 °C for 4 h and overnight at room temperature. Then, additional bromoacetonitrile (0.030 mL, 0.44 mmol) was added and the mixture was stirred at 40 °C for 2 h. Brown solid (17 mg, 15%). m.p. 180–181 °C; 1H-NMR (500 MHz, CDCl3) δ = 8.36 (d, J = 5.3 Hz, 1 H, 3-H), 7.87 (d, J = 8.3 Hz, 1 H, 5-H), 7.73 (d, J = 5.3 Hz, 1 H, 4-H), 7.43 (d, J = 8.3 Hz, 1 H, 6-H), 5.75 (s, 2 H, 1′-H), 3.04 (s, 3 H, 1-CH3); 13C-NMR (100 MHz, CDCl3) δ = 142.5, 141.2, 138.5, 137.3, 134.6, 130.5, 124.5, 124.2, 120.6, 116.6, 115.5, 113.1, 35.6, 23.0; IR (KBr) (cm−1) = 3433, 2983, 2364, 1654, 1066; MS (APCI) m/z = 294, 292, 290 [M++ H], 136; HRMS (ESI) calcd. for C14H9N3Cl2 + H+: 290.0252; found 290.0249; purity: 97% (λ = 210 nm), 100% (λ = 254 nm) (by HPLC).
7,8-Dichloro-1-methyl-9-(prop-2-yn-1-yl)-9H-pyrido[3,4-b]indole (AnnH67). Prepared from AnnnH63 (79 mg, 0.31 mmol), sodium hydride (15 mg, 0.37 mmol), dry DMF (2.5 mL) and propargyl bromide (80 wt % in toluene, 0.040 mL, 0.35 mmol). The mixture was stirred at 40 °C for 3 h. Beige solid (41 mg, 45%). m.p. 174–175 °C; 1H-NMR (500 MHz, CDCl3) δ = 8.38 (d, J = 5.2 Hz, 1 H, 3-H), 7.88 (d, J = 8.3 Hz, 1 H, 5-H), 7.78 (d, J = 5.3 Hz, 1 H, 4-H), 7.38 (d, J = 8.3 Hz, 1 H, 6-H), 5.67 (d, J = 2.2 Hz, 2 H, 1′-H), 3.13 (s, 3 H, 1-CH3), 2.38 (t, J = 2.4 Hz, 1 H, 3′-H); 13C-NMR (100 MHz, CDCl3) δ = 142.9, 140.1, 138.1, 137.1, 133.8, 129.3, 123.7, 122.9, 120.0, 116.3, 112.5, 79.4, 74.4, 36.7, 23.6; IR (KBr) (cm−1) = 3426, 3160, 2107, 1613, 1062; MS (APCI) m/z = 293, 291, 289 [M++ H], 250; HRMS (ESI) calcd. for C15H10N2Cl2 + H+: 289.0299; found 289.0296; purity: 100% (λ = 210 nm), 99% (λ = 254 nm) (by HPLC).
3-(7,8-Dichloro-1-methyl-9H-pyrido[3,4-b]-9-yl)-N,N-dimethylpropan-1-amine (AnnH71). Prepared from 7,8-dichloro-1-methyl-9H-pyrido[3,4-b]indole (AnnH63) (92 mg, 0.37 mmol), sodium hydride (57 mg, 1.4 mmol), dry THF (5 mL) and N,N-dimethyl-3-chloropropylamine hydrochloride (133 mg, 0.843 mmol). Beige solid (35 mg, 28%). m.p. 126–128 °C; 1H-NMR (500 MHz, CDCl3) δ = 8.35 (d, J = 5.2 Hz, 1 H, 3-H), 7.90 (d, J = 8.3 Hz, 1 H, 5-H), 7.74 (d, J = 5.3 Hz, 1 H, 4-H), 7.36 (d, J = 8.3 Hz, 1 H, 6-H), 5.00–4.90 (m, 2 H, 1′-H), 3.06 (s, 3 H, 1-CH3), 2.19 (t, J = 7.0 Hz, 2 H, 3′-H), 2.15 (s, 6 H, N(CH3)2), 1.96–1.81 (m, 2 H, 2′-H); 13C-NMR (100 MHz, CDCl3) δ = 142.7, 139.2, 138.1, 137.1, 133.5, 128.7, 123.6, 122.2, 120.0, 116.0, 112.4, 56.2, 45.4 (2 C), 44.3, 30.0, 24.3; IR (KBr) (cm−1) = 3426, 2932, 1628, 1051; MS (APCI) m/z = 340, 338, 336 [M++ H], 149; HRMS (ESI) calcd. for C17H19N3Cl2 + H+: 336.1034; found 336.1031; purity: 100% (λ = 210 nm), 100% (λ = 254 nm) (by HPLC).
2-(1-Bromo-7,8-dichloro-9H-pyrido[3,4-b]indol-9-yl)-acetonitrile (AnnH79). Prepared from AnnnH52 (102 mg, 0.404 mmol), sodium hydride (13 mg, 0.34 mmol), dry DMF (2.5 mL) and bromoacetonitrile (0.020 mL, 0.40 mmol). The mixture was stirred at 40 °C for 3 h. The residue was purified by column chromatography (silica gel, iso-hexane/ethyl acetate 3:2) to afford AnnH79 as a white solid (92 mg, 80%). m.p. 208–209 °C; 1H-NMR (500 MHz, CDCl3) δ = 8.35 (d, J = 5.1 Hz, 1 H, 3-H), 7.95 (d, J = 8.3 Hz, 1 H, 5-H), 7.88 (d, J = 5.1 Hz, 1 H, 4-H), 7.53 (d, J = 8.3 Hz, 1 H, 6-H), 6.12 (s, 2 H, 1′-H); 13C-NMR (125 MHz, CDCl3) δ = 141.9, 138.5, 135.5, 135.3, 132.6, 124.9, 123.8, 123.3, 120.4, 116.9, 115.2, 114.3, 35.1; IR (KBr) (cm−1) = 3423, 3062, 2361, 1615, 1168; MS (ESI) m/z = 362, 360, 358, 356 [M+ + H], 238, 102; HRMS (ESI) calcd. for C13H6N3BrCl2 + H+: 353.9200; found 353.9194; purity: 100% (λ = 210 nm), 100% (λ = 254 nm) (by HPLC).
1-Bromo-7,8-dichloro-9-(prop-2-yn-1-yl)-9H-pyrido[3,4-b]indole (AnnH80). Prepared from AnnnH52 (101 mg, 0.320 mmol), sodium hydride (17 mg, 0.43 mmol), dry DMF (2.5 mL) and propargyl bromide (80 wt % in toluene, 0.040 mL, 0.36 mmol). The mixture was stirred at 40 °C for 3 h. The residue was purified by column chromatography (silica gel, iso-hexane/ethyl acetate 3:2) to afford AnnH80 as a beige solid (90 mg, 79%). m.p. 190–193 °C; 1H-NMR (500 MHz, CDCl3) δ = 8.30 (d, J = 5.0 Hz, 1 H, 3-H), 7.93 (d, J = 8.3, 1 H, 5-H), 7.87 (d, J = 5.0 Hz, 1 H, 4-H), 7.48 (d, J = 8.3 Hz, 1 H, 6-H), 6.07 (d, J = 2.4 Hz, 2 H, 1′-H), 2.33 (t, J = 2.4 Hz, 1 H, 3′-H); 13C-NMR (125 MHz, CDCl3) δ = 140.7, 138.8, 135.5, 135.0, 132.3, 124.0, 123.8, 123.1, 120.0, 117.0, 114.0, 79.0, 74.1, 36.1; IR (KBr) (cm−1) = 3433, 3293, 2125, 1614, 1053; MS (APCI) m/z = 361, 359, 357, 355 [M++ H], 353, 279, 277, 275, 238, 102; HRMS (ESI) calcd. for C14H7N2BrCl2 + H+: 352.9247; found 352.9243; purity: 100% (λ = 210 nm), 100% (λ = 254 nm) (by HPLC).
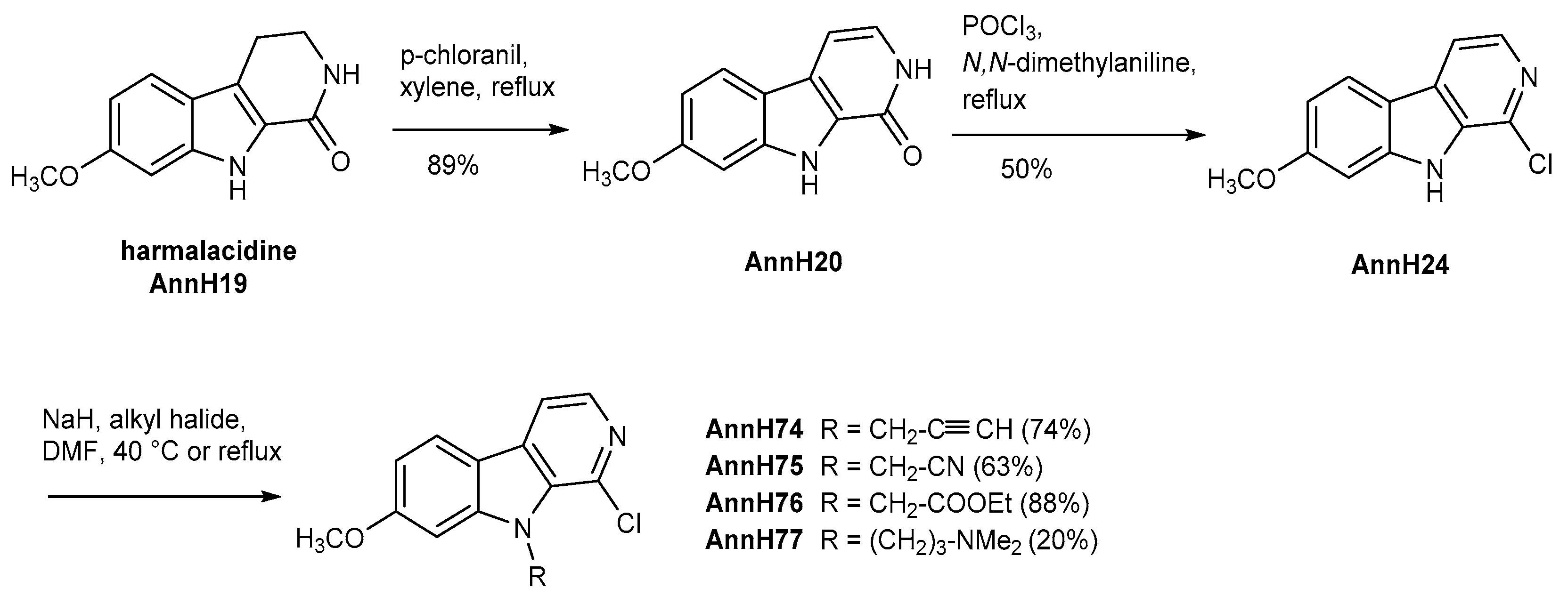
7-Methoxy-2,9-dihydro-1H-pyrido[3,4-b]indol-1-one (
AnnH20). To 7-methoxy-2,3,4,9-tetrahydro-1
H-pyrido[3,4-
b]indol-1-one (harmalacidine;
AnnH19) [
40] (440 mg, 2.03 mmol) in 20 mL of xylene was added
p-chloranil (747 mg, 3.04 mmol). The mixture was stirred at reflux for 23 h, then cooled to room temperature and evaporated to dryness. The residue was purified by column chromatography (silica gel, iso-hexane/ethyl acetate/ethanol 2:2:1) to afford
AnnH20 as a beige solid (388 mg, 89%).
1H-NMR (500 MHz, DMSO-
d6)
δ = 11.80 (s, 1 H, NH), 11.31 (s, 1 H, NH), 7.88 (d,
J = 8.7 Hz, 1 H, 5-H), 7.04 (d,
J = 6.8 Hz, 1 H, 3-H), 6.93 (d,
J = 2.2 Hz, 1 H, 8-H), 6.91 (d,
J = 6.8 Hz, 1 H, 4-H), 6.80 (dd,
J = 8.7, 2.3 Hz, 1-H, 6-H), 3.82 (s, 3 H, CH
3);
13C-NMR (100 MHz, DMSO-
d6)
δ = 158.9, 155.3, 140.4, 127.3, 124.7 (2 C), 122.1, 116.0, 110.3, 99.4, 94.3, 55.2; IR (KBr)
(cm
−1) = 3430, 1639, 1560, 1262; MS (EI)
m/z (relative intensity, %) = 214 (20) [M
+•], 149 (18), 84 (40), 73 (100); HRMS (EI) calcd. for C
12H
10N
2O
2: 214.0742; found 214.0736; purity: 100% (λ = 210 nm), 100% (λ = 254 nm) (by HPLC).
1-Chloro-7-methoxy-9-(prop-2-yn-1-yl)-9H-pyrido[3,4-b]indole (AnnH74). Prepared from AnnnH24 (61 mg, 0.26 mmol), sodium hydride (17 mg, 0.42 mmol), dry DMF (2.5 mL) and propargyl bromide (80 wt % in toluene, 0.030 mL, 0.26 mmol). The mixture was stirred at 40 °C for 4 h. The residue was purified by column chromatography (silica gel, iso-hexane/ethyl acetate 3:2) to afford AnnH74 as a beige solid (53 mg, 74%). mp 141–144 °C; 1H-NMR (400 MHz, CDCl3) δ = 8.19 (d, J = 5.1 Hz, 1 H, 3-H), 7.97 (d, J = 8.4 Hz, 1 H, 5-H), 7.79 (d, J = 5.1 Hz, 1 H, 4-H), 7.00–6.97 (m, 2 H, 6-H, 8-H), 5.50 (d, J = 2.5 Hz, 2 H, 1′-H), 3.98 (s, 3 H, OCH3), 2.33 (t, J = 2.5 Hz, 1 H, 3′-H); 13C-NMR (125 MHz, CDCl3) δ = 161.7, 143.2, 139.0, 132.7, 132.6, 131.8, 122.6, 114.9, 113.5, 110.6, 93.6, 78.1, 73.2, 55.8, 34.2; IR (KBr) (cm−1) = 3428, 3159, 2108, 1627, 1168; MS (APCI) m/z = 273, 271 [M++ H]; HRMS (ESI) calcd. for C15H11N2OCl + H+: 271.0638; found 271.0632; purity: 100% (λ = 210 nm), 99% (λ = 254 nm) (by HPLC).
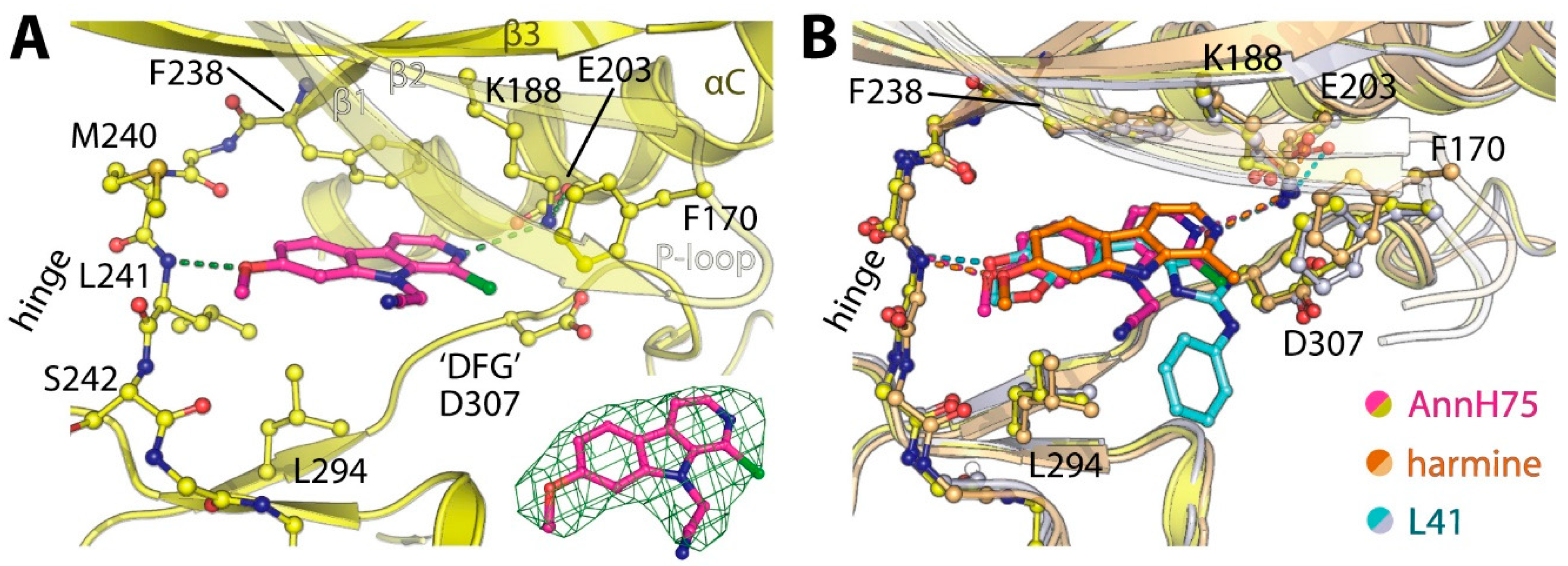
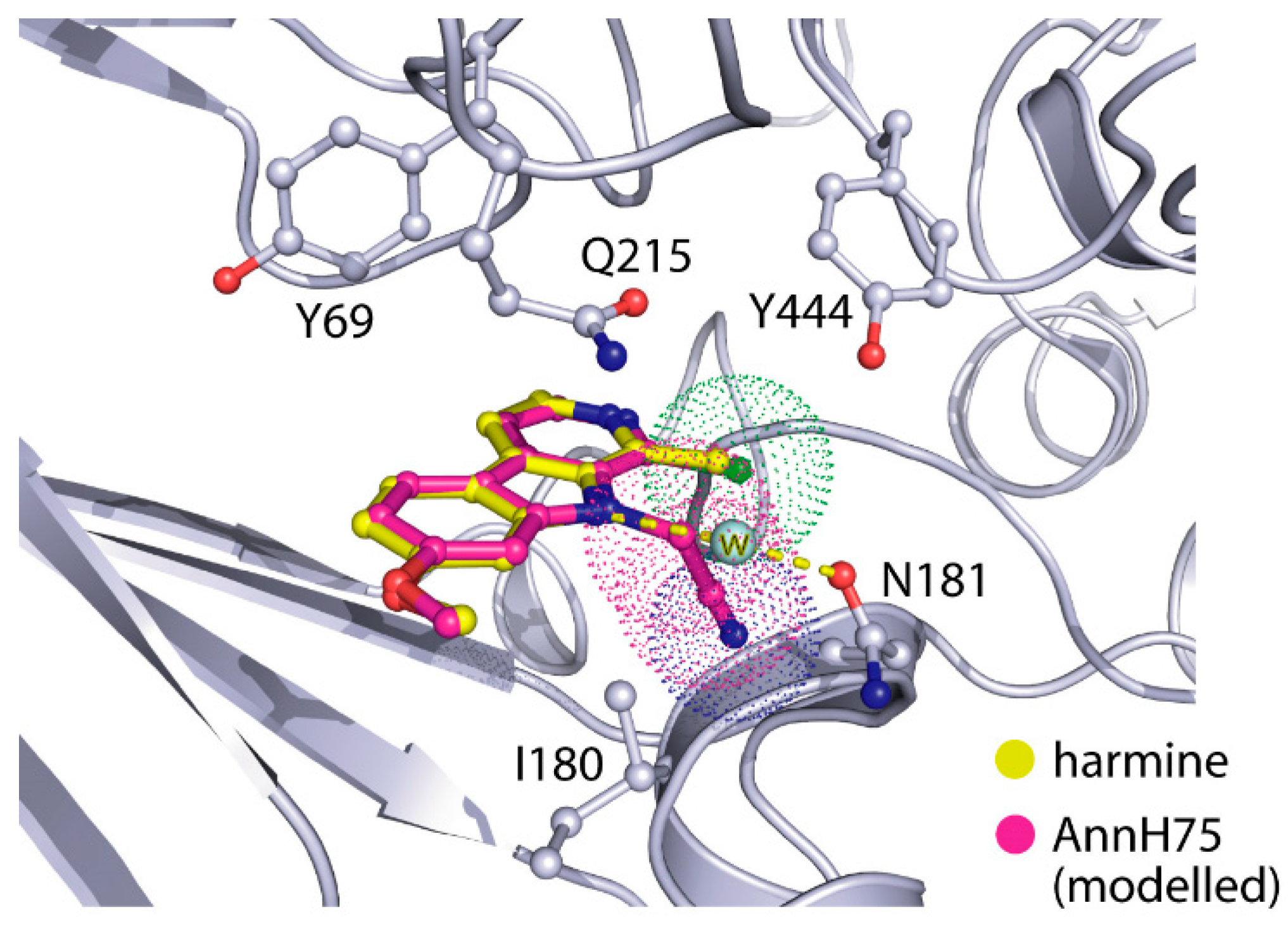
2-(1-Chloro-7-methoxy-9H-pyrido[3,4-b]indol-9-yl)acetonitrile (AnnH75). Prepared from AnnnH24 (96 mg, 0.41 mmol), sodium hydride (16 mg, 0.40 mmol), dry DMF (2.5 mL) and bromoacetonitrile (0.030 mL, 0.43 mmol). The mixture was stirred at 40 °C for 4 h. The residue was purified by column chromatography (silica gel, iso-hexane/ethyl acetate 3:2) to afford AnnH75 as a beige solid (71 mg, 63%). m.p. 163–164 °C; 1H-NMR (400 MHz, CDCl3) δ = 8.25 (d, J = 5.1 Hz, 1 H, 3-H), 7.99 (d, J = 8.7 Hz, 1 H, 5-H), 7.81 (d, J = 5.1 Hz, 1 H, 4-H), 7.02 (dd, J = 8.1, 2.1 Hz, 1 H, 6-H), 6.92 (d, J = 2.1 Hz, 1 H, 8-H), 5.67 (s, 2 H, 1′-H), 3.99 (s, 3 H, OCH3); 13C-NMR (125 MHz, CDCl3) δ = 162.1, 142.7, 140.1, 133.3, 132.4, 131.5, 123.0, 115.1, 114.5, 113.8, 111.3, 93.3, 55.9, 32.9; IR (KBr) (cm−1) = 3434, 2926, 2363, 1624, 1204; MS (APCI) m/z = 274, 272 [M++ H]; HRMS (ESI) calcd. for C14H10N3OCl + H+: 272.0590; found 272.0584; purity: 100% (λ = 210 nm), 99% (λ = 254 nm) (by HPLC).
Ethyl 2-(1-Chloro-7-methoxy-9H-pyrido[3,4-b]indol-9-yl)acetate (AnnH76). Prepared from AnnnH24 (64 mg, 0.28 mmol), sodium hydride (20 mg, 0.50 mmol), dry DMF (2.5 mL) and ethyl bromoacetate (0.030 mL, 0.30 mmol). The mixture was stirred at 40 °C for 3 h. The residue was purified by column chromatography (silica gel, iso-hexane/ethyl acetate 3:2) to afford AnnH76 as a beige solid (78 mg, 88%). m.p. 152–153 °C; 1H-NMR (500 MHz, CDCl3) δ = 8.18 (d, J = 5.1 Hz, 1 H, 3-H), 7.98 (d, J = 8.6, 1 H, 5-H), 7.80 (d, J = 5.1 Hz, 1 H, 4-H), 6.95 (dd, J = 8.6, 2.1 Hz, 1 H, 6-H), 6.78 (d, J = 2.1 Hz, 1 H, 8-H), 5.42 (s, 2 H, 1′-H), 4.25 (q, J = 7.1 Hz, 2 H, OCH2), 3.94 (s, 3 H, OCH3), 1.26 (t, J = 7.1 Hz, 3 H, CH2-CH3); 13C-NMR (125 MHz, CDCl3) δ = 168.6, 161.6, 143.7, 138.9, 132.6 (2 C), 122.8, 114.8 (2 C), 113.6, 110.3, 92.9, 61.9, 55.7, 46.1, 14.1; IR (KBr) (cm−1) = 3442, 3043, 1733, 1626, 1168; MS (APCI) m/z = 321, 319 [M+ + H]; HRMS (ESI) calcd. for C16H15N2O3Cl + H+: 319.0849; found 319.0844; purity: 98% (λ = 210 nm), 99% (λ = 254 nm) (by HPLC).
3-(1-Chloro-7-methoxy-9H-pyrido[3,4-b]indol-9-yl)-N,N-dimethylpropan-1-amine (AnnH77). Prepared from 1-chloro-7-methoxy-9H-pyrido[3,4-b]indole (AnnH24) (66 mg, 0.28 mmol), sodium hydride (41 mg, 1.0 mmol), dry THF (3 mL) and N,N-dimethyl-3-chloropropylamine hydrochloride (82 mg, 0.52 mmol). Beige solid (18 mg, 20%). m.p. 86–87 °C; 1H-NMR (500 MHz, CDCl3) δ = 8.15 (d, J = 5.1 Hz, 1 H, 3-H), 7.91 (d, J = 8.7 Hz, 1 H, 5-H), 7.78 (d, J = 5.1 Hz, 1 H, 4-H), 7.21 (d, J = 2.0 Hz, 1 H, 8-H), 6.91 (dd, J = 8.7, 2.1 Hz, 1 H, 6-H), 4.92 (t, J = 7.4 Hz, 2 H, 1′-H), 4.02 (s, 3 H, OCH3), 3.20–3.03 (m, 2 H, 3′-H), 2.80 (s, 6 H, N(CH3)2), 2.56–2.42 (m, 2 H, 2′-H); 13C-NMR (125 MHz, CDCl3) δ = 161.4, 143.7, 138.0, 132.4 (2 C), 132.2, 122.4, 114.5, 113.3, 110.0, 93.4, 56.4, 55.6, 45.5 (2 C), 42.3, 28.9; IR (KBr) (cm−1) = 3425, 2932, 1611, 1061; MS (APCI) m/z = 320, 318 [M++ H], 282, 268; HRMS (ESI) calcd. for C17H20N3OCl + H+: 318.1373; found 318.1369; purity: 100% (λ = 210 nm), 100% (λ = 254 nm) (by HPLC).
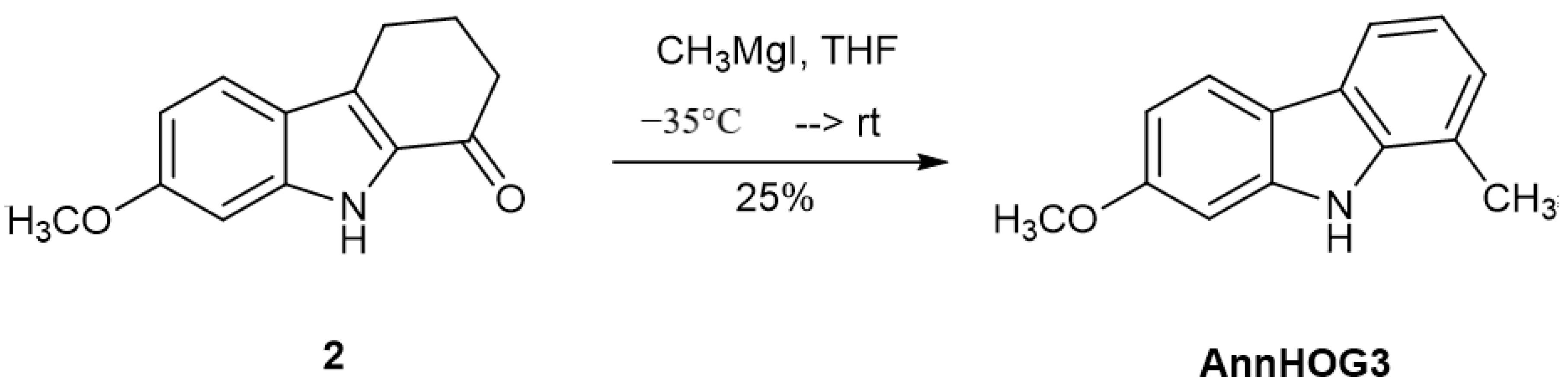
7-Methoxy-1-methyl-9H-carbazole(AnnHOG3). A solution of 100 mg (0.465 mmol) 7-methoxy-2,3,4,9-tetrahydro-1
H-carbazol-1-one (
2) [
42] in 3 mL anhydrous THF under nitrogen was cooled to −35 °C and treated slowly under stirring with 0.620 mL of a 3M solution of methylmagnesium iodide (1.86 mmol). The solution was allowed to come to room temperature over 2 h and stirred for another 14 h, then quenched with 5 mL water and stirred for 30 min. The mixture was extracted with ethyl acetate (3 × 10 mL), the combined organic layers dried over sodium sulfate and evaporated. Purification by column chromatography (silica gel, iso-hexane/ethyl acetate 19:1) afforded the target compound as a pale brown solid (25 mg, 25%). m.p. 120 °C.
1H-NMR (DMSO-D
6, 400 MHz):
δ = 7.99 (s, 1 H, NH), 7.91 (d, J = 8.4 Hz, 1 H, 5-H), 7.82 (d, J = 6.4 Hz, 1 H, 3-H), 7.18 − 7.10 (m, 2 H, 2-H, 4-H), 6.97–6.95 (m, 1 H, 8-H), 6.86 (dd, J = 1.6 Hz, 8.8 Hz, 1 H, 6-H), 3.90 (s, 3 H, OCH
3), 2.55 (s, 3 H, CH
3).
13C-NMR (DMSO-D
6, 125 MHz):
δ = 158.92 (C-7), 140.72 (C-8a), 138.84 (C-1), 125.25 (C-2), 122.99 (C-4a), 121.17 (C-5), 119.65 (C-4), 119.44 (C-9a), 117.76 (C-4b), 117.13 (C-3), 108.12 (C-6), 94.83 (C-8), 55.60 (OCH
3), 16.87 (CH
3). IR (KBr):
(cm
−1) = 3439, 2925, 2854, 1628, 1610, 1500, 1448, 1280, 827, 780, 747. HR-MS (ESI -):
m/z = 211.1003 (calcd. for für C
14H
13NO: 211.0997); purity: 96% (λ = 210 nm), 100% (λ = 254 nm) (by HPLC).
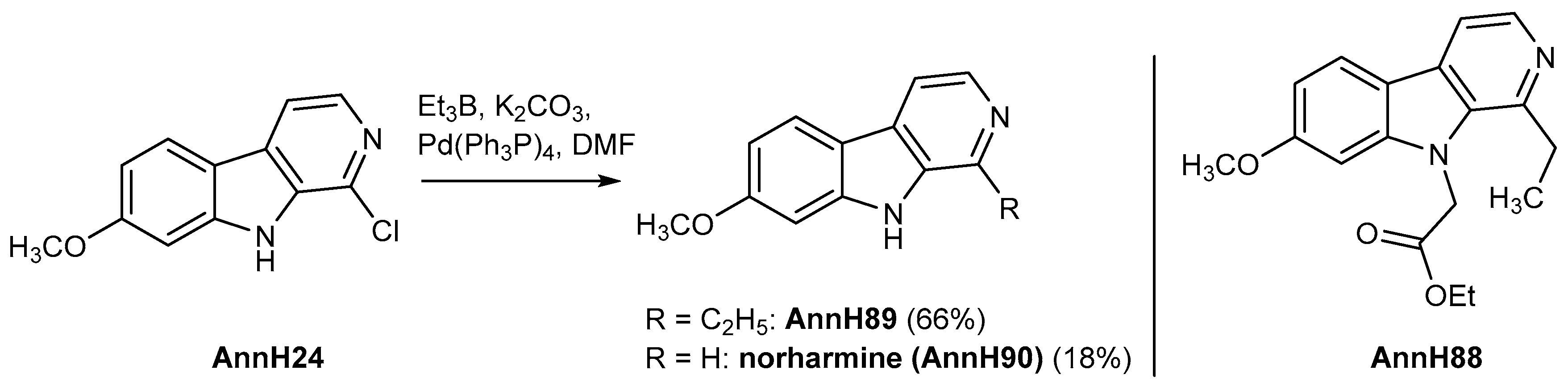
1-Ethyl-7-methoxy-9H-pyrido[3,4-b]indole (AnnH89). A dispersion of AnnH24 (57 mg, 0.24 mmol), tetrakis(triphenylphosphine)palladium(0) (13.2 mg, 0.011 mmol) and potassium carbonate (68 mg) in 1.8 mL of dry DMF under a N2 atmosphere was treated with triethylborane (0.46 mL of a 1 M solution in hexane, 4.6 mmol) and stirred at reflux for 50 min. The mixture was worked up in the same manner as described for AnnH88 to afford AnnH89 as a beige solid (37 mg, 66%); as a by-product we obtained the dechlorination product norharmine (AnnH90) (9 mg, 18%) as a red–brown solid. Data for AnnH89: m.p. 109–117 °C; 1H-NMR (500 MHz, CDCl3) δ = 9.13 (s, NH), 8.36 (d, J = 5.3 Hz, 1 H, 3-H), 7.97 (d, J = 8.6 Hz, 1 H, 5-H), 7.73 (d, J = 5.3 Hz, 1 H, 4-H), 6.94 (d, J = 2.2 Hz, 1 H, 8-H), 6.89 (dd, J = 8.6, 2.2 Hz, 1 H, 6-H), 3.87 (s, 3 H, OCH3), 3.12 (q, J = 7.6 Hz, 2 H, 1′-H), 1.43 (t, J = 7.6 Hz, 3 H, 2′-H); 13C-NMR (125 MHz, CDCl3) δ = 161.0, 146.1, 141.8, 138.7, 134.0, 128.8, 122.5, 115.8, 112.2, 109.5, 94.7, 55.5, 27.2, 12.8; IR (KBr) (cm−1) = 3374, 3009, 1568, 1161; MS (ESI) m/z = 227 [M++ H], 212; HRMS (ESI) calcd. for C14H14N2O+ H+: 227.1184; found 227.1177; purity: 61% (λ = 210 nm), 99% (λ = 210 nm) (by HPLC).
Ethyl 2-(1-ethyl-7-methoxy-9H-pyrido[3,4-b]indol-9-yl)acetate (AnnH88). A dispersion of AnnH76 (49 mg, 0.15 mmol), tetrakis(triphenylphosphine)palladium(0) (7.9 mg, 0.0068 mmol) and potassium carbonate (42 mg) in 1.0 mL of dry DMF under a N2 atmosphere was treated with triethylborane (0.28 mL of 1 M solution in hexane, 2.8 mmol) and the mixture stirred at reflux for 2 h. The mixture was cooled to room temperature and water (10 mL) was added. The reaction mixture was extracted with ethyl acetate (4 × 10 mL), the combined organic layers were dried over magnesium sulfate and evaporated to dryness. The residue was purified by column chromatography (silica gel, iso-hexane/ethyl acetate/ethanol 2:2:1) and by precipitation with using of DCM and n-hexane to afford AnnH88 as a beige solid (33 mg, 69%). m.p. 95–97 °C; 1H-NMR (500 MHz, CDCl3) δ = 8.35 (d, J = 5.1 Hz, 1 H, 3-H), 7.96 (d, J = 8.6 Hz, 1 H, 5-H), 7.72 (d, J = 5.1 Hz, 1 H, 4-H), 6.89 (dd, J = 8.6, 2.1 Hz, 1 H, 6-H), 6.75 (d, J = 2.1 Hz, 1 H, 8-H), 5.15 (s, 2 H, 1′-H), 4.22 (q, J = 7.1 Hz, 2 H, O-CH2), 3.91 (s, 3 H, OCH3), 3.17 (q, J = 7.6 Hz, 2 H, 1′′-H), 1.41 (t, J = 7.6 Hz, 3 H, 2′′-H), 1.22 (t, J = 7.1 Hz, 3 H, OCH2-CH3); 13C-NMR (125 MHz, CDCl3) δ = 168.7, 161.1, 145.7, 143.5, 139.2, 134.9, 130.2, 122.4, 115.6, 112.2, 109.3, 93.2, 61.9, 55.7, 47.0, 28.6, 14.1, 14.0; IR (KBr) (cm−1) = 3423, 2966, 1747, 1624, 1431, 1167; MS (ESI) m/z = 313 [M++ H], 279; HRMS (ESI) calcd. for C18H20N2O3+ H+: 313.1552; found 313.1548; purity: 80% (λ = 210 nm), 100% (λ = 254 nm) (by HPLC).
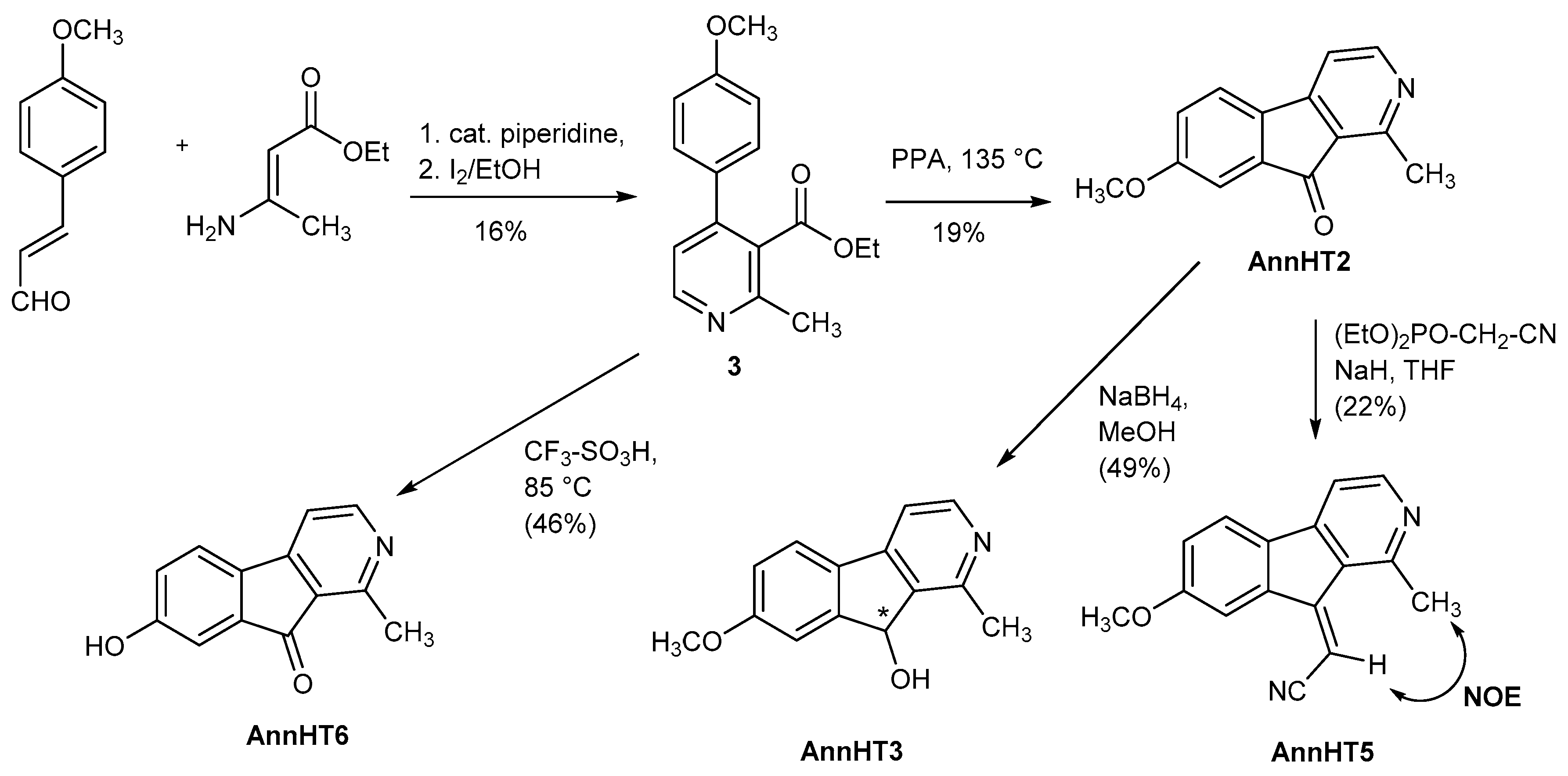
Ethyl 4-(4-methoxyphenyl)-2-methylpyridine-3-carboxylate (3). A solution of (E)-4-methoxycinnamaldehyde (3.2 g, 0.019 mol), ethyl 3-aminocrotonate (2.1 mL, 0.017 mol) and 0.080 mL piperidine in 20 mL ethanol was refluxed for 1 h, then evaporated to dryness. The residue was suspended in 70 mL ethanol and treated with 0.34 g (4.0 mmol) sodium bicarbonate. Then, a 10% ethanolic solution of iodine was added dropwise under stirring until the colour change from yellow to brown persisted for >2 min. Then, 0.1 M sodium thiosulfate solution was added until the colour changed back to yellow. The mixture was evaporated to dryness and the residue taken up in 20 mL diethyl ether. The solution was washed with 20 mL water and 20 mL brine, dried over magnesium sulfate and evaporated. The oily residue is a mixture of 3 and its less polar 6-aryl isomer. Purification by column chromatography (silica gel, iso-hexane/ethyl acetate 65:35) afforded the target compound as a brown oil. Yield: 800 mg (16%). 1H-NMR (CDCl3, 500 MHz): δ = 8.53 (d, J = 5.2 Hz, 1 H, 6-H), 7.38–7.29 (m, 2 H, 2′/6′-, 3′/5′-H), 7.14 (d, J = 5.2 Hz, 1 H, 5-H), 7.01–6.89 (m, 2 H, 2′/6′-, 3′/5′-H), 4.17 (q, J = 7.1 Hz, 2 H, CH2), 3.85 (s, 3 H, OCH3), 2.62 (s, 3 H, Ar-CH3), 1.09 (t, J = 7.1. Hz, 3 H, CH2-CH3); 13C-NMR (CDCl3, 100 MHz): δ = 168.9 (C=O), 160.0 (C-4′), 155.4 (C-2), 149.5 (C-6), 147.5 (C-4), 130.6 (C-1′), 129.2 (C-2′/6′, -3′/5′), 128.5 (C-3), 121.6 (C-5,6), 114.0 (C-2′/6′, C-3′/5′), 61.4 (CH2), 55.3 (OCH3), 22.8 (Ar-CH3), 13.8 (CH3); MS (APCI): m/z = 272 [M++ H], 102; IR (KBr): (cm−1) = 3401, 3038, 2979, 2960, 2838, 1726, 1610, 1579, 1548, 1515, 1463, 1443, 1401, 1366, 1294, 1251, 1215, 1180, 1140, 1113, 1097, 1073, 1032, 853, 829; HR-MS: m/z = 271.1207 (calcd. for C16H17NO3: 271.1208); purity: 100% (λ = 210 nm), 100% (λ = 254 nm) (by HPLC).
7-Methoxy-1-methyl-9H-indeno-[2,1-c]pyridin-9-one (AnnHT2). A mixture of 387 mg (1.43 mmol) ester 3 and 17 g polyphosphoric acid was stirred at 135 °C for 5 h. After cooling to room temperature, the brown viscous mixture was taken up in 80 mL ice-water and stirred for 30 min. The mixture was adjusted to pH 10 with 6N NaOH and extracted with ethyl acetate (4 × 200 mL). The combined organic layers were dried over magnesium sulfate and evaporated. Purification by column chromatography (silica gel, iso-hexane/ethyl acetate/ethanol 2:2:1) afforded the target compound as a yellow solid (45 mg, 14%). m.p. 140–143 °C. 1H-NMR (DMSO-D6, 400 MHz): δ = 8.55 (d, J = 4.9 Hz, 1 H, 3-H), 7.80 (d, J = 8.2 Hz, 1 H, 5-H), 7.57 (d, J = 4.9 Hz, 1 H, 4-H), 7.19 (dd, J = 8.2, 2.5 Hz, 1 H, 6-H), 7.15 (d, J = 2.4 Hz, 1 H, 8-H), 3.86 (s, 3 H, OCH3), 2.64 (s, 3 H, CH3); 13C-NMR (DMSO-D6, 125 MHz): δ = 193.3 (C=O), 162.2 (C-7), 156.1 (C-1), 155.4 (C-3), 152.2 (C-4a), 135.2 (C-8a), 133.2 (C-4b), 124.5 (C-9a), 123.9 (C-5), 120.3 (C-6/8) 113.6 (C-4), 109.1 (C-6/8), 55.9 (OCH3), 20.5 (CH3); MS (CI): m/z (rel. int. in %) = 226 [M+ + H] (28), 125 (10), 97 (12), 79 (100); MS (EI): m/z (rel. Int. in %) = 225 [M+•] (100), 210 (30), 182 (18), 154 (26), 84 (30); IR (KBr): (cm−1) = 3442, 2924, 2854, 2360, 2342, 1708, 1636, 1618, 1593, 1484, 1453, 1438, 1369, 1343, 1290, 1261, 1240, 1213, 1195, 1149, 1061, 1019, 950, 881, 827, 794, 681; HR-MS (EI): m/z = 225.0800 (calcd. for C14H11NO2: 225.0790); purity: 93% (λ = 210 nm), 96% (λ = 254 nm) (by HPLC).
7-Hydroxy-1-methyl-9H-indeno-[2,1-c]pyridin-9-one (AnnHT6). A suspension of 100 mg (0.369 mmol) of ester 3 in 1.5 mL trifluoromethanesulfonic acid was stirred under nitrogen for 4 h at 85 °C, then cooled to room temperature and neutralized carefully with ice-cooled saturated aqueous sodium carbonate solution. The mixture was extracted with ethyl acetate (3 × 50 mL), the combined organic layers were washed with brine (2 × 50 mL), dried over magnesium sulfate and evaporated. Purification by column chromatography (silica gel, iso-hexane/ethyl acetate 65:35) afforded the target compound as a yellow solid (36 mg, 46%). m.p. 275–276 °C. 1H-NMR (DMSO-D6, 400 MHz): δ (ppm) = 10.40 (s, 1 H, OH), 8.53 (d, J = 4.9 Hz, 1 H, 3-H), 7.72 (d, J = 8.7 Hz, 1 H, 5-H), 7.54 (d, J = 4.5 Hz, 1 H, 4-H), 7.03–6.99 (m, 2 H, 6-H, 8-H), 2.64 (s, 1 H, CH3); 13C-NMR (DMSO-D6, 125 MHz): δ (ppm) = 193.7 (C=O), 160.7 (C-7), 156.0 (C-1), 153.4 (C-3), 152.7 (C-4a), 135.5 (C-8a/4b), 131.6 (C-8a/4b), 124.5 (C-9a), 124.1 (C-5), 121.1 (C-6), 113.3 (C-4), 110.7 (C-8), 20.5 (CH3); MS (APCI): m/z (rel. int. in %) = 212 [M++ H], 186, 102; IR (KBr): (cm−1) = 3443, 2924, 2854, 1716, 1592, 1456, 1380, 1298, 1248, 1231, 1209, 1144, 1059, 965, 849; HR-MS (ESI): m/z = 212.0709 (calcd. for C13H10NO2: 212.0712); purity: 90% (λ = 210 nm), 100% (λ = 254 nm) (by HPLC).
(±)-7-Methoxy-1-methyl-9H-indeno-[2,1-c]pyridin-9-ol (AnnHT3). An ice-cooled suspension of 96 mg (0.42 mmol) ketone AnnHT2 in 7 mL methanol was treated with 23 mg (0.61 mmol) sodium borohydride and then stirred for 15 h at room temperature. The methanol was removed by evaporation and the residue treated with 13 mL dichloromethane and 7 mL aqueous 15% potassium hydroxide solution and stirred for 1 h. The organic layer was separated, washed with water (2 × 10 mL), dried over magnesium sulfate and evaporated. Purification by column chromatography (silica gel, iso-hexane/ethyl acetate/ethanol 2:2:1) afforded the target compound as a yellow solid (47 mg, 49%). m.p. 191–192 °C. 1H-NMR (DMSO-D6, 400 MHz): δ = 8.38 (d, J = 5.0 Hz, 1 H, 3-H), 7.78 (d, J = 8.3 Hz, 1 H, 5-H), 7.52 (d, J = 5.0 Hz, 1 H, 4-H), 7.21 (d, J = 2.4 Hz, 1 H, 8-H), 7.00 (dd, J = 8.3, 2.4 Hz, 1 H, 6-H), 5.93 (s, 1 H, OH), 3.84 (s, 3 H, OCH3), 2.58 (s, 3 H, CH3); 13C-NMR (DMSO-D6, 125 MHz): δ = 161.5 (C-7), 155.3 (C-1), 150.5 (C-8a), 149.6 (C-3), 147.8 (C-4a), 138.3 (C-9a), 130.3 (C-4b), 123.1 (C-5), 115.1 (C-6), 112.8 (C-4), 111.2 (C-8), 73.4 (C-9), 55.9 (OCH3), 21.6 (CH3); MS (APCI): m/z (rel. int. in %) = 229 [M++ H], 213, 102; IR (KBr): (cm−1) = 3423, 2921, 2836, 2599, 2361, 2343, 1708, 1592, 1483, 1438, 1369, 1342, 1290, 1239, 1212, 1148, 1103, 1060, 1019, 949, 826, 794; HR-MS (EI): m/z = 227.0953 (calcd for C14H13NO2: 227.0946); purity: 100% (λ = 210 nm), 100% (λ = 254 nm) (by HPLC).
(2E)-(7-Methoxy-1-methyl-9H-indeno[2,1c]pyridin-9-ylidene)ethanenitrile (AnnHT5). A suspension of 20 mg (0.50 mmol) sodium hydride (60% in mineral oil) in 1.25 mL THF was treated with 0.040 mL (0.30 mmol) diethyl cyanomethylphosphonate and stirred at room temperature for 15 min. Then, a solution of 56 mg (0.25 mmol) ketone AnnHT2 in 1.25 mL THF was added slowly with stirring. The mixture was stirred for another 17 h and then evaporated to dryness. The residue was taken up in 20 mL dichloromethane, the solution washed with 25 mL water, dried over magnesium sulfate, and evaporated. Purification by column chromatography (silica gel, iso-hexane/ethyl acetate/ethanol 2:2:1) afforded a yellow solid. This product was disssolved in dichloromethane and precipitated with n-hexane to give the target compound as a yellow solid (13 mg, 22%). 1H-NMR (DMSO-D6, 400 MHz): δ = 8.47 (d, J = 5.0 Hz, 1 H, 3-H), 8.08 (s, 1 H, 8-H), 7.60 (d, J = 8.4 Hz, 1 H, 5-H), 7.34 (d, J = 5.0 Hz, 1 H, 4-H), 7.04 (d, J = 8.4 Hz, 1 H, 6-H), 6.28 (s, 1 H, 1′-H), 3.92 (s, 3 H, OCH3), 2.76 (s, 1 H, CH3); 13C-NMR (DMSO-D6, 125 MHz): δ = 162.0 (C-7), 154.4 (C-1), 153.1 (CN), 151.1 (C-3), 148.8 (C-4a), 137.5 (C-8a), 131.7 (C-4b), 129.0 (C-9a), 122.4 (C-5), 118.2 (C-6), 117.1 (C-9), 112.7 (C-4), 110.2 (C-8), 93.3 (C-1′), 55.7 (OCH3), 24.5 (CH3); MS (APCI): m/z (rel. int. in %) = 249 [M++ H], 102; IR (KBr): (cm−1) = 3443, 3088, 2987, 2966, 2838, 225, 2204, 1619, 1585, 1484, 1458, 1141, 1403, 1371, 1340, 1325, 1283, 1259, 1242, 1139, 1063, 1027, 974, 856, 829, 814; HR-MS (ESI): m/z = 249.1025 (calcd. for C16H13N2O: 249.1028); purity: 100% (λ = 210 nm), 100% (λ = 254 nm) (by HPLC).