Abstract
Chitin is a long-chain polymer of N-acetyl-glucosamine, which is regularly found in the exoskeleton of arthropods including insects, shellfish and the cell wall of fungi. It has been known that chitin can be used for biological and biomedical applications, especially as a biomaterial for tissue repairing, encapsulating drug for drug delivery. However, chitin has been postulated as an inducer of proinflammatory cytokines and certain diseases including asthma. Likewise, chitosan, a long-chain polymer of N-acetyl-glucosamine and d-glucosamine derived from chitin deacetylation, and chitosan oligosaccharide, a short chain polymer, have been known for their potential therapeutic effects, including anti-inflammatory, antioxidant, antidiarrheal, and anti-Alzheimer effects. This review summarizes potential utilization and limitation of chitin, chitosan and chitosan oligosaccharide in a variety of diseases. Furthermore, future direction of research and development of chitin, chitosan, and chitosan oligosaccharide for biomedical applications is discussed.
1. Introduction
1.1. Chitin
Chitin is long-chain polymers of N-acetylglucosamine, presence of which has been experimentally confirmed in unicellular (diatoms, protists, fungi) as well as in multicellular (sponges, corals, mollusks, worms and arthropods) organisms [1]. Chitin has been used as supplementary for nutraceutical food, pharmaceutical products as well as 3D scaffolds for biomedicine [2,3,4] and technological applications [5,6,7,8]. Chitin contains thermostability which can be synthesized in the high temperature process [9]. Moreover, chitin has a high tolerance to high chemical concentration that generates the deposition of metals such as copper into chitin via electrochemical procedure at room temperature [10]. The linear polymer of chitin contains β-(1,4)-N-acetyl-d-glucosamines, which are linked by glycosidic bond. There are three isoforms of chitin including α-chitin, β-chitin and γ-chitin [11]. The different forms of chitin depend on the arrangement of side chain of backbone. For instance, the structure of α-chitin is parallel chain arrangement, whereas the structure of β-chitin is antiparallel chain arrangement. α-chitin is frequently found in nature including exoskeleton of arthropods. α-chitin has been frequently applied for tissue engineering [12,13]. In contrast to α-chitin, β-chitin is mostly found in squid pen. β-chitin has been also applied for biomaterial usage such as wound healing [14,15], preservative agents for methylene blue [16], and biological application such as an enhancer of saltiness perception [17]. γ-chitin has been gathered from cocoon of the moth (Orgyia dubia), and it is found that the structure of γ-chitin is similar to α-chitin rather than β-chitin, but the surface morphology of γ-chitin is consisted of microfibers, whereas α-chitin and β-chitin are composed of nanofibers [18].
1.2. Chitosan
Chitosan is derived from chitin by deacetylation. The major component of chitosan is the mixture between N-acetyl-d-glucosamine and β-(1,4)-linked-d-glucosamine. Chitosan has amounts of N-acetyl-d-glucosamine less than β-(1,4)-linked-d-glucosamine [19]. Chitosan is generally applied as biomaterials, especially for drug delivery system and use in combination with other substances for improving their therapeutic effects [20,21].
1.3. Chitosan Oligosaccharide
Chitosan oligosaccharide or chitooligosaccharide is oligomers of chitosan. Chitosan oligosaccharide has the degree of polymerization of <55 and molecular weight of <10 kDa [22]. Chitosan oligosaccharides have a variety of biomedical applications such as drug delivery system [20], functional food [23], as well as the drug against acne vulgaris [24].
In this review, we emphasize the potential application of chitin, chitosan and their oligosaccharides as therapeutics and useful biomaterials in a variety of human diseases. Furthermore, we discuss the limitation of chitin and chitosan oligosaccharide, which will be helpful in guiding future direction of research and development based on these molecules.
2. Potential Applications of Chitin and Chitosan Oligosaccharides
2.1. Neurological and Musculoskeletal Diseases
Chitin has been proposed as biomaterials for neural treatment. For instance, chitin was utilized with carbon nanotube as a scaffold for neural growth [25]. Chitin as a biological absorbable tube or catheter was effectively used as the bridge for sural nerve grafts in a rat sciatic nerve defect model [26,27]. Chitin hydrogel repaired cartilage injury by protecting chondrocytes from apoptosis and promoting immunomodulation of macrophage and chondrogenesis [28]. The combination of chitin as 2,2,6,6-Tetramethylpiperidine-1-oxyl (TEMPO)-oxidized sacchachitin nanofibers (TOSCNFs) and chitosan-activated platelet-rich plasma (cPRP) induced healing effect in corneal damage by promoting cell proliferation and cell migration in Statens Seruminstitut rabbit corneal (SIRC) epithelial cells [29]. Interestingly, chitin is not only proposed as potential therapy, but also claimed as a molecular marker for neurological diseases. In Alzheimer’s disease, chitin is elevated and accumulated within the brain and facilitates a scaffolding for amyloid-β deposition [30,31,32]. Fungal chitin was also detected in brain tissues from Alzheimer’s disease patients [33]. Moreover, chitin accumulation was found in multiple sclerosis patients [34]. Interestingly, both microglia and neurons produce N-acetylglucosamine polymerization, which causes neurotoxicity in Alzheimer’s disease [35]. Furthermore, chitin derived from demosponge Aplysina aerophoba was used as 3D scaffold for human bone marrow-derived stromal cells, and it promoted cell proliferation, cell bridging formation and metabolic activity with no toxicity [36]. Interestingly, chitin derived from demosponge Ianthella basta was applied as a cryopreservative agent that effectively retained adipogenic differentiation in human mesenchymal stromal cells [4].
In contrast to chitin, chitosan and chitosan oligosaccharide have been widely studied for the beneficial effect on neurological diseases, especially Alzheimer’s disease. A water-soluble form of chitosan inhibited the production of proinflammatory cytokines including TNFα and IL-6 as well as inducible nitric oxide synthase (iNOS) in human astrocytoma cell line CCF-STTG1 stimulated with IL-1β and Aβ fragments (25–35) [37]. It is known that acetylcholinesterase inhibition is the target for Alzheimer’s disease therapy. Chitosan oligosaccharide with 90% deacetylation and low molecular weight (1 to 5 kDa) inhibited the protein expression of acetylcholinesterase and Aβ fragment (25–35)-induced acetylcholinesterase activity in PC12 cell lines [38]. Furthermore, caffeic acid conjugated chitosan oligosaccharide effectively inhibited β-site amyloid precursor protein-cleaving enzyme activity, which was the rate limiting step of Aβ peptide formation in Alzheimer’s disease [39]. Interestingly, chitosan oligosaccharide at 500 µg/mL inhibited β-site amyloid precursor protein cleaving enzyme 1 (BACE1) activity and protein expression in HEK293 APPswe cells [40]. Chitosan oligosaccharide inhibited αβ aggregation, inhibited Aβ1–42 fibrils formation, and induced fibril destabilization in oligomeric Aβ-induced neurotoxicity and oxidative stress in rat hippocampal neurons [41]. In addition, 0.1% of chitosan oligosaccharide injected into the spatium intermusculare around the biceps femoris muscle inhibited scar formation and promoted regeneration of axons, as well as sensory and motor function in a mouse model of sciatic nerve injury [42]. Interestingly, ingestion of chitosan oligosaccharide (10 mg/kg/day) alleviated inflammatory signal including COX-2 expression in the synovium of an anterior cruciate ligament (ACL) transection-induced osteoarthritis rabbit model [43]. Ingestion of chitosan oligosaccharide at least 200 mg/kg/day recovered cognitive deficiency in Aβ1–42-induced learning and memory loss rats [44]. Chitosan oligosaccharide also protected hippocampal neuron from Aβ peptide [45]. Furthermore, low molecular weight chitosan activated mitogenic response to platelet-derived growth factor (PDGF) in vascular smooth muscle cells [46].
Chitosan has been applied as biomaterials for neural therapy. For instance, amphiphatic carboxymethyl-hexanoyl chitosan hydrogel increased cell viability and maintained stem-cell-like gene expression of induced pluripotent stem cells applied for corneal reconstruction [47]. Chitosan-polylactide fiber was utilized for nerve growth factor in PC12 cell lines [48]. Chitosan oligosaccharide with calcium silicate and gelatin was applied for implantation of cortical bone repair and bone fracture fixation [49]. Chitosan was coated into nanoparticles for delivering antiamyloid antibody as a drug for Alzheimer’s disease. It was found that chitosan coating improved aqueous dispersibility and stability of vehicle during lyophilization [50]. Chitosan in the form of chitosan beads effectively interfered with amyloid-β aggregation [51]. Chitosan was utilized as nanocapsules for delivering p38 inhibitor to the brain by nasal administration [52]. Chitosan with polyvinyl alcohol nanofibrous scaffold promoted skeletal muscle regeneration by increasing cell viability, cell adhesion, cell growth, and cell spread on the scaffold [53]. Chitosan with laminin and poly (lactic-co-glycolic acid) effectively repaired nerve injury by promoting nerve regeneration [54]. Chitosan combined to hyaluronate regenerated nerve function defect in parotidectomy rabbit model by promoting scar formation, increasing nerve fibers, thickening myelin sheath, and promoting nerve conduction velocity [55]. Furthermore, chitosan, as chitosan tubes or incorporated to mesenchymal stem cells or keratin, was also utilized for nerve repairing [56,57,58].
2.2. Cardiovascular and Hematological Diseases
Chitin has been incorporated with other substances for treatment of cardiovascular disease. For instance, chitin with glucan and polyphenols from pomegranate recovered endothelial dysfunction by reducing inflammatory marker in the liver and adipose tissues and promoting NO synthase in apolipoprotein E deficient mice (apoE−/−) with high fat diet [59]. Furthermore, chitin combined to graphene oxide as aerogel beads effectively absorbed excessive bilirubin in the blood [60]. Chitin nanogel with rectorite nanocomposite stopped bleeding within 121 s in rat tail vein, and promoted higher hemostatic activity compared to chitosan-based hemostatic products [61]. Furthermore, Chitin derived from demosponge Ianthella labyrinthus and chitin derived from spider Caribena versicolor were effectively served as a 3D scaffold for culturing induced pluripotent stem-cell-derived cardiomyocytes as well as commercial extracellular matrix [62,63].
It has been known that orally intake of 5% chitosan in the diet reduces serum cholesterol and atherogenesis inhibition in apolipoprotein E-deficient mouse model [64]. Chitosan as a supplemental diet downregulated the markers involving obesity such as leptin in high fat diet rats [65]. Chitosan oligosaccharide decreased serum cholesterol by promoting accumulation of cholesterol in liver, bile, and feces with reverse cholesterol transport pathway [66]. It is known that chitosan oligosaccharide reduces serum cholesterol. Chitosan oligosaccharide increased cell surface expression of low-density lipoprotein receptor (LDLR) and increased lipid droplets in HepG2 cells, suggesting that chitosan oligosaccharide effectively reduced serum lipids by facilitating the accumulation of lipids into the cells [67]. Furthermore, chitosan oligosaccharide downregulated mRNA expression of LPS-induced E-selectin and intercellular adhesion molecule-1, which are a part of the inflammatory responses in endothelial cells via MAPK inhibition [68]. Oral intake of chitosan oligosaccharide also reduced the marker of atherosclerosis including the lesion area in aorta or plaque area in aortic roots, and greatly reduced cholesterol and triglyceride in apolipoprotein E deficient mice (apoE−/−) [69]. The combination of chitosan oligosaccharide ingestion and exercise such as running improved immune system by increasing spleen to body weight ratio and lung to body weight ratio compared to water gavage only in Sprague-Dawley (SD) rats [70]. Interestingly, chitosan improved blood perfusion and promoted neovascularization by modulation of gut microbiota in a mouse hindlimb ischemia model [71]. Furthermore, chitosan was used as a vehicle for drug delivery for transportation of doxorubicin to improve the treatment of blood malignancies [72]. Chitosan in the form of synthetic CD47 antibody-chitosan/hyaluronic acid polyelectrolyte complex inhibited atherosclerotic plaques with downregulated NLRP3 inflammasome expression in apolipoprotein E deficient mice (apoE−/−) [73].
2.3. Respiratory Diseases
It is well known that penetration of chitin into human bodies causes the production of chitinase, the enzyme that modulates immune response, and chitinase-like proteins YKL-40. YKL-40 was associated with increased severity in asthmatic patients [74]. YKL-40 level was also positively correlated with neutrophils, sputum IL-1β, and plasma IL-6 [75]. Moreover, chitin from fungi induced eosinophilic infiltration in a mouse model [76]. Chitin also promoted proinflammatory response by inducing proinflammatory cytokine release including IL-25 and IL-33 in human bronchial epithelial cells [77]. Chitin has been known as an adjuvant for immune response. It was demonstrated that chitin from house dust mite promoted airway hypersensitivity in ovalbumin-induced airway inflammation via a TLR-2 dependent pathway [78]. Furthermore, it is known that chitin is the major component of fungi in their cell wall. Chitin exposure induced macrophage activation which upregulates the expression of chitin degrading enzyme chitotriosidase [79]. Chitotriosidase was also involved in lung diseases such as tuberculosis, chronic obstructive lung diseases [80]. Apart from chitotriosidase, acute exposure to the fungal pathogen Aspergillus fumigatus promoted the function of acidic mammalian chitinase, which determined the severity of fungal asthma [81]. Therefore, inhibition of chitotriosidase and acidic mammalian chitinase is regarded as a drug target for respiratory diseases [82]. However, it has been shown that a chitin analog AVR-25 partially alleviated pulmonary dysregulation in a hyperoxia-induced experimental mouse model of bronchopulmonary dysplasia by suppressing inflammation [83]. These findings indicate that chitin may have both beneficial and detrimental effects.
In contrast to chitin, chitosan oligosaccharides have been demonstrated to have potential beneficial effects on respiratory diseases. Oral intake of chitosan oligosaccharide (500 mg/kg) at a single dose alleviated particulate matter (PM) 2.5-induced lung inflammation by decreasing lactate dehydrogenase, IL-8, and TNF-α in PM 2.5-induced rat model [84]. Chitosan oligosaccharide (100 kDa and 90% deacetylation) prevented inflammation, oxidative stress and apoptosis in the lung tissues of blast injury-induced mice by diminishing protein expression of p38 and ADMA (an inhibitor of endogenous nitric oxide synthase that positively correlated with hypertension) and recovering dimethylarginine dimethylaminohydrolase 1 (DDAH1), a hydrolase of ADMA [85]. Furthermore, oral intake of 16 mg/kg/day of low molecular weight chitosan oligosaccharide reduced IgE-induced airway inflammation in mice by downregulating both protein level and mRNA level of proinflammatory cytokines including IL-4, IL-5, IL-13, and TNF-α [86].
2.4. Renal Diseases
There are reports demonstrating the effect of chitin on renal diseases. Oral ingestion of chitin as surface-deacetylated chitin nano-fiber (40 mg/kg/day) reduced uremic toxins including oxidants in nephrectomized rats [87]. However, upregulated YKL-40 level in urine and plasma represents the biomarker of acute kidney injury [88,89]. In contrast, chitosan and chitosan oligosaccharide have potential therapeutic effects on kidney diseases. The combination of chitosan with gynostemma and motherwort was used for protecting chronic renal failure by inhibiting inflammation in adenine-induced rat chronic renal failure [90]. Chitosan incorporated with gallic acid reduced the formation of calcium oxalate crystal, which was mainly a kidney stone, and had antioxidative effects [91]. Chitosan as a cat diet improved kidney function and quality of life in elderly cats with 3 and 4 International Renal Interest Society (IRIS) stages [92]. It was also shown that chitosan oligosaccharide improved kidney function in streptozotocin-induced diabetic rats [93]. It was found that 0.1% chitosan oligosaccharide with more than 90% deacetylation prevented glycerol-induced acute renal failure in rats by decreasing renal dipeptidase activity, a diagnostic marker of acute renal failure [94]. Furthermore, chitosan oligosaccharide with carboxymethyl group relieved renal injury induced by doxorubicin and promoted antioxidative effects in rats [95]. Interestingly, chitosan oligosaccharide triggered G2/M phase arrest, promoted endoplasmic reticulum stress pathway, and inhibited tumor growth in human renal carcinoma cells and xenograft tumor models [96]. Moreover, chitosan oligosaccharide had a chelating property which detoxified depleted uranium cytotoxicity in human renal proximal tubular epithelial cells [97]. Recently, chitosan oligosaccharide at the concentration of 50 and 100 µg/mL was shown to reduce renal cyst growth via CaMKKβ-induced AMPK activation without cytotoxicity [98]. Furthermore, the detoxification property of chitosan has been applied in hemodialysis patients. The ingestion of chitosan decreased the level of indoxyl sulfate and phosphate by binding to these molecules [99]. Chitosan has also been applied as siRNA delivery system targeting kidney. For instance, chitosan nanoplex effectively covered siRNA for knocking down PDGF-B and PDGFR-beta [100]. Chitosan was also used as biomaterials in combination with collagen for culturing human renal proximal tubular cells [101]. The potential beneficial effects of chitosan oligosaccharide on kidney diseases have been also reviewed elsewhere [102].
2.5. Gastrointestinal Diseases and Gut Microbiota
Many previous studies demonstrated the potential effects of chitin and chitosan oligosaccharide on gastrointestinal disease. It was shown that chitin protected intestinal barrier function in DSS-induced colitis in a protochordate model [103]. Chitin combined with glucan (chitin-glucan complex) was used as a prebiotic. This chitin-glucan complex improved the growth of Bifidobacterium, a probiotic, in a rat model [104]. Interestingly, intake of 4.5 g/day of chitin-glucan in food for 3 weeks increased beneficial microbiota metabolites including butyric, iso-valeric and caproic acids without major changes in gut microbiota composition [105]. Chitin has also been applied as surface-deacetylated chitin nanofiber. It was shown that oral intake of 80 mg/kg/day of surface-deacetylated chitin nanofiber decreased hepatic injury and oxidative stress in a nonalcoholic steatohepatitis model of rats [106].
Chitosan and chitosan oligosaccharide have beneficial effects on gastrointestinal tract, especially as nutritional supplements for animals and food supplements for human. In livestock, chitosan oligosaccharide has been used as feed additives in animal diet. It was shown that 100 mg/kg of dietary chitosan oligosaccharide supplementation promoted growth performance, reduction of diarrhea, nutrient digestibility and attenuation of E.coli K88 infection in weaning pigs [107,108]. The combination of chitosan and zinc at the dose of 100 mg/kg as a feed additive in diet also promoted the activities of digestive enzymes such as amylase, reduced diarrhea and improved growth performance in weaning pigs [109]. However, chitosan mixed with probiotic such as Enterococcus faecalis did not significantly reduce severity of diarrhea and affect growth performance in E.coli K88-inoculated weaning pigs [110]. Moreover, high molecular weight chitosan oligosaccharide (20 to 30 kDa) just only increased ZO-1 expression and decreased the mRNA expression of IL-1β and TNFα in weaning pigs without affecting diarrhea, average dairy gain, gain to feed ratio, and antioxidant capacity [111], suggesting that specific forms of chitosan oligosaccharide gives the therapeutic effects in weaning pig. Furthermore, low molecular weight chitosan oligosaccharide (8 kDa) with 90% deacetylation improved gut absorption, increased villus length and promoted intestinal cell proliferation in weaning pig [112]. Oral intake of 200 mg/kg/day of chitosan oligosaccharide in drinking water also protected gut from the modulation of glucose metabolism and gut dysbiosis in diabetic mice [113]. At a cellular level, 100 µg/mL of low molecular wight chitosan oligosaccharide (approximately 5 kDa) accelerated tight junction assembly and inhibited cholera toxin-induced intestinal fluid secretion via CaSR-PLC-IP3 receptor channel-mediated AMPK activation in intestinal epithelial cells [114]. Chitosan oligosaccharide suppressed mRNA expression of proinflammatory cytokines, and inhibited the downregulation of PPARγ in palmitic acid-induced HepG2 cells and high fat diet mice [115]. Many reports reveal that chitosan and chitosan oligosaccharide modulate gut microbiota. Chitosan prevented gut dysbiosis and inhibited the activation of toll-like receptor and nod-like receptor signaling pathway in high fat diet rats [116]. However, molecular weight and degree of deacetylation of chitosan oligosaccharide are major determinants of effects on human gut microbiota composition as well as therapeutic effects. For instance, a highly deacetylated chitosan oligosaccharide decreased Bifidobacterium spp., E. rectale/C. coccoides, C. histolyticum and Bacteroides/Prevotella populations in human gut [117]. Chitosan oligosaccharide with >95% deacetylation reduced Lactobacillus, Bifidobacterium and Desulfovibrio, deleterious bacteria that were correlated with inflammatory bowel disease [118], and increased abundance of Akkermansia that was a good bacteria [119]. The 3 kDa chitosan oligosaccharide diminished gut dysbiosis and downregulated mRNA expression of proinflammatory cytokines in azoxymethane and dextran sulfate sodium-induced mouse model of colorectal cancer [120]. Moreover, chitosan oligosaccharide ameliorated hepatic steatosis and liver injury, and reduced triglyceride and free fatty acid in diet-induced obese mice by downregulating inflammatory genes and modulation of gut microbiota [121]. Furthermore, chitosan protected liver from ischemia-reperfusion injury via regulating Bcl-2/Bax, TNF-α and TGF-β expression [122], prevented lipid metabolic disorder by combination with Ganoderma polysaccharide [123], alleviated menopausal symptoms [124], and protected the gut from ischemic symptoms [71]. High molecular weight chitosan was also prepared as nanoparticles for drug delivery in gut. For instance, 400 kDa chitosan integrated to insulin loaded chitosan nanoparticles prolonged bioavailability of insulin release [125]. Interestingly, chitosan nanoparticle was also used as a food supplement for improvement of growth performance and immunity in weaning pigs [126].
Apart from chitin, chitosan, and chitosan oligosaccharide, there are other polysaccharides that have therapeutic effects on gut. For instance, mannan oligosaccharide (10 µM) promoted intestinal barrier function in T84 cells via AMPK activation [127,128]. Fructo-oligosaccharide (0.1 mg/mL), a prebiotic, accelerated intestinal tight junction reassembly via AMPK activation in T84 cells [129].
2.6. Endocrinological Diseases and Diabetes Mellitus
Chitin combined with other compounds has been shown to possess therapeutic effects. For instance, oral intake of 4.5 g/day of chitin combined with glucan reduced oxidized low-density lipoprotein in human subjects [130]. Furthermore, chitin has been applied as a biomaterial for prolonging bioavailability. For instance, injectable thermo-sensitive hydrogel based on hydroxypropyl chitin was incorporated to salmon calcitonin to extend long-term sustained salmon calcitonin release [131].
Likewise, chitosan has been combined with other compounds that possess antidiabetic effects. For instance, 3-O-sulfochitosan was reported to reduce blood glucose in diabetic rats [132]. Chitosan combined with metformin, a type 2 diabetic drug, synergistically enhanced drug efficacy and reduced lethal effects of drug overdose [133]. Furthermore, chitosan oligosaccharide has been applied for drug delivery system in diabetes. For instance, chitosan-microcapsulated insulin, chitosan-stabilized selenium nanoparticles, chitosan encapsulated resveratrol, chitosan coating of TiO2 nanotube arrays for metformin, chitosan nanoparticle and chitosan hydrogel were used for improving diabetic therapy [134,135,136,137,138,139,140,141,142]. Interestingly, the development of chitosan oligosaccharide as a drug or supplement for diabetic treatment has been studied for a decade. The proteomic data demonstrated the antidiabetic effects and anti-obesity of orally intake chitosan oligosaccharide in ob/ob mice [143]. Oral intake of chitosan oligosaccharide (>90% deacetylation; 500 mg/kg) promoted insulin sensitivity in streptozotocin-induced diabetic rats. Chitosan oligosaccharide (100 mg/L) also promoted cell proliferation in primary culture islet cells and pancreatic β-cell lines [144]. Moreover, the low molecular weight chitosan oligosaccharide (~1.2 kDa) promoted cell proliferation in primary culture islet cells and pancreatic β-cell lines as well as improving insulin sensitivity greater than the high molecular weight chitosan oligosaccharide [145]. Moreover, chitosan oligosaccharide with a molecular weight of 1.3 kDa and 55% deacetylation was used as an oral insulin delivery system that showed the highest effect of glucose reduction [146]. Furthermore, chitosan oligosaccharide has been used as a food supplement for diabetic treatment. For instance, GO2KA1, a commercial chitosan oligosaccharide supplement, had a beneficial effect on glucose control in subjects with prediabetes by regulating postprandial glucose [147]. GO2KA1 promoted glucose uptake into intestinal epithelial cells, enhanced adipocyte differentiation, and upregulated PPARγ expression [148]. GO2KA1 has been used in clinical trials. It was found that GO2KA1 effectively reduced postprandial blood glucose levels in subjects with impaired glucose tolerance and impaired fasting glucose [149]. However, it remains unclear whether chitosan oligosaccharide has a direct or indirect antidiabetic effect. It was also shown that chitosan oligosaccharide exerted antidiabetic effects via gut microbiota modulation [116]. Chitosan oligosaccharide was used as a supplementary drug for improving the glycemic control of sitagliptin in type 2 diabetes mellitus (T2DM) by reducing insulin resistance and proinflammatory cytokines, and increasing insulin sensitivity [150]. Chitosan oligosaccharide combined with xanthine derivatives improved liver and kidney functions compared to pioglitazone, a standard antidiabetic drug [151]. Chitosan oligosaccharide also upregulated the expression of browning genes in white adipose tissues and thermogenesis of brown adipose tissues, which consequently reduced obesity in obese rats [152]. Chitosan oligosaccharide was applied in the form of tablet that had therapeutic effects on the regulation of serum lipid level and downregulation of cholesterol excretion genes including CYP7A1, LXR, PPAR-α, and LDLR in high fat diet-induced hyperlipidemic rats [153]. Moreover, chitosan oligosaccharide reduced endoplasmic reticulum stress in HepG2 cell lines [154]. Interestingly, chitosan oligosaccharide did not induce any hepatotoxic effects or lipid metabolism disorders in normal Sprague-Dawley rats [155].
2.7. Inflammatory Diseases
Inflammation is generally a crucial defense mechanism of human body against pathogens, injuries, or toxins. In addition, there are several diseases related to hyperinflammatory responses such as inflammatory bowel disease and systemic lupus erythematosus. Chitin has been applied as a biomaterial for suppressing inflammation. For instance, chitin nanofibril was used for inhibiting skin inflammation in the experimental atopic dermatitis mouse model by suppression of NF-κB [156]. However, many reports have demonstrated that chitin is an inflammatory inducer. For instance, chitin induced inflammation in peripheral blood mononuclear cells from obese subjects ex vivo [157]. Chitin triggered inflammatory responses via type 2 innate lymphoid cells and γδ T cell activation [158]. Furthermore, chitin enhanced IL-33 secretion and consequently IL-1β secretion by dendritic cells in ovalbumin-induced asthmatic mice [159]. However, Wagener el al demonstrated that fungal chitin triggered anti-inflammatory cytokines including IL-10 via NOD2 and TLR-9 activation, indicating that chitin exposure triggered inflammatory responses together with anti-inflammatory responses as negative feedback to regulate the inflammatory process. Interestingly, low size chitin (1-10 µm) induced secretion of IL-10, an anti-inflammatory cytokine, at low concentrations, but induced secretion of TNFα, a proinflammatory cytokine, at high concentrations [160]. However, chitin nanofibrils, nanorods structure of chitin, downregulated proinflammatory cytokines including TNF-α, IL-1α, IL-1β, IL-6, and IL-8, and concomitantly upregulated antimicrobial peptide β-defensin 2 in human keratinocytes (HaCaT cells) [161].
Chitosan and chitosan oligosaccharide have been reported as anti-inflammatory agents. Chitosan recovered intestinal barrier function in DSS-induced colitis by stimulating expression of tight junction proteins such as claudin-1, occludin, and ZO-1 [162]. Chitosan downregulated chitinase enzyme YKL-40 in primary human macrophages [163]. Carboxymethyl chitosan was shown to have anti-inflammatory effects in mice [164]. Likewise, it was shown that an oral intake of chitosan oligosaccharide (20 mg/kg/day) alleviated DDS-induced acute and chronic colitis in mice by inhibiting an NF-κB pathway [165]. Moreover, chitosan oligosaccharide downregulated NF-κB downstream targets such as COX-2 and upstream targets such as TLR-4 in lipopolysaccharide-induced inflammation in intestinal epithelial cells [166]. Furthermore, 50-200 µg/mL of a highly N-acetylated chitosan oligosaccharide inhibited protein expression of PI3K/Akt signaling pathway, which was involved in proinflammatory cytokine production in RAW 264.7 macrophage cells [167]. The physiochemical properties as well as preparation processes of chitosan oligosaccharide influence its anti-inflammatory effect. It was shown that 42% fully deacetylated oligomers plus 54% monoacetylated oligomers of chitosan oligosaccharide alleviated inflammation, whereas 50% fully deacetylated oligomers plus 27% monoacetylated oligomers promoted inflammation in RAW 264.7 macrophage cells [168]. Furthermore, chitosan oligosaccharide protected against shrimp tropomyosin-induced food allergy by downregulation of IL-4, IL-5, and IL-13 and upregulation of IFN-γ in sensitized mice [169]. Chitosan oligosaccharide (200 mg/kg) prevented heat stress-induced inflammatory responses by decreasing liver IL-1β concentration [170]. Apart from chitin and chitosan oligosaccharide, fructooligosaccharide and yeast polysaccharide had an inhibitory effects on TNF-α-induced GLP-1 secretion in L cells and DSS-induced colitis in mice, respectively [171,172].
2.8. Cancer
Chitin has promise for development as an anti-cancer agent and a vehicle for anticancer drug delivery. It was shown that chitinase-3 like protein-1 (CHI3L1), which was upregulated and promoted proinflammatory mediators in breast cancer cells, was inhibited by chitin [173,174]. Chitin downregulated vascular endothelial growth factor C (VEGF-C) synthesis that was related to tumor angiogenesis [175]. In addition, chitin has been prepared in various forms that can counteract cancer. For instance, silver embedded chitin nanocomposites promoted cytotoxicity in human breast cancer (MCF-7) cells [176]. Chitin-glucan-aldehyde-quercetin conjugation induced cytotoxicity in a macrophage cancer cell line (J774) with no toxic effect on peripheral blood mononuclear cells (PBMCs) [177]. Furthermore, chitin has been used for anticancer drug delivery. For instance, chitin with poly L lactic acid composite nanogel containing doxorubicin induced cytotoxicity in liver cancer HepG2 cells and enhanced anticancer drug efficacy [178]. Chitin nanoparticles were loaded with anticancer natural product ellagic acid, which inhibited breast cancer cell growth [179].
Interestingly, there are several reports demonstrating the potential antitumor effect of chitosan and chitosan oligosaccharide. For instance, chitosan decreased cell proliferation, stimulated apoptotic effects, and decreased cell adhesion in human melanoma cell lines including SKMEL38 cells, RPMI7951 cells, and A375 cells, respectively [180]. Oral intake of 500 mg/kg/day of chitosan oligosaccharide abolished tumor progression in colitis-associated colorectal cancer via NF-κB inhibition and AMPK activation [181]. Chitosan oligosaccharide modulated cell autophagy that inhibited cell proliferation of A549 lung cancer cell line [182]. Low molecular weight chitosan oligosaccharide induced cytotoxic effects, cell cycle arrest and apoptosis in oral squamous cell carcinoma (SCC) cells without any effects on noncancerous keratinocyte (HaCaT) cell lines [183]. Chitosan also had anticancer activity in various types of cancer such as human ovarian cancer, breast cancer and cervical carcinoma [184,185,186]. Chitosan was combined with other compounds to enhance anticancer effects. For instance, carboxymethyl chitosan inhibited tumor growth in mouse hepatocarcinoma by abolishing tumor angiogenesis [187]. Chitosan selenate inhibited cancer cell viability and promoted cancer cell apoptosis in lung cancer A549 cells [188]. Furthermore, 5-fluorouracil-conjugated chitosan oligosaccharide plus vanillin, indomethacin-conjugated chitosan oligosaccharide nanoparticles, and thioguanine-conjugated chitosan graphene oxide were applied for cancer drug delivery systems [189,190,191].
2.9. Aging
The world population is beginning to age. Antiaging agents have been developed to support the aging society. Oxidative stress is a major promoting factor of aging. Antioxidant compounds are recognized as therapeutic agents for delay aging. Chitin had a scavenging activity to chelate 1,1-diphenyl-2-picrylhydrazyl radicals [192]. Chitin was also used as a biomaterial for antioxidant agent container. For instance, chitin nano-crystal complex containing melatonin, vitamin E, and β-glucan reduced wrinkle and yielded a better skin appearance in human subjects [193]. Chitin-glucan-aldehyde-quercetin conjugates also had a potent antioxidant activity [177]. Interestingly, chitin nanofibrils and nanochitin can mimic the extracellular matrix, so these agents have been applied as cosmeceuticals against aging [194].
Chitosan oligosaccharide has widely been used as antioxidative agents. Chitosan supplementation reduced oxidative stress in the heart tissues and maintained glutathione reductase, glutathione peroxidase, and reduced glutathione in young and aged rats [195]. Chitosan oligosaccharide restored redox balance in LPS-induced oxidative stress [196]. Chitosan oligosaccharide with 90% deacetylation potently inhibited oxidative stress in rats [197]. Several studies confirmed the therapeutic effects of chitosan and chitosan oligosaccharide on oxidative stress in many types of animal models including aging mice, weaning pig, hydrogen peroxide-induced rats, and heat-stressed rats [198,199,200,201]. Interestingly, chitosan oligosaccharide recovered aging-induced liver dysfunction via the upregulation of Nrf2 antioxidant signaling [202]. Chitosan-gallic acid, synthetic carboxymethyl chitosan, chitosan-ellagic acid and selenide chitosan sulfate have also been demonstrated to have an antioxidant activity [203,204,205,206].
2.10. Infectious Disease
Generally, chitin has no antipathogenic effects [207]. Although, chitin derived from demosponges Ianthella flabelliformis was applied as drug delivery material for antibiotic drug including decamethoxine [208]. Additionally, many reports have demonstrated the antibacterial effects and antifungal effects of chitosan oligosaccharide. Chitosan oligosaccharide (10 kDa) had the highest antimicrobial effect with minimum inhibitory concentration values of 32–64 μg/mL on Propionibacterium acnes [24]. In addition, low-molecular-weight chitosan oligosaccharide inhibited bacterial activity, biofilm formation and hemolytic activity of Staphylococcus aureus [209]. In vivo and in vitro models showed that chitosan reduced Cryptosporidium parvum oocyst viability and Cryptosporidium parvum multiplication, respectively [210,211]. Furthermore, chitosan oligosaccharide killed Candida auris in both nonaggregative form (NCPF 8973) and aggregative form (NCPF 8978) [212]. Interestingly, chitosan oligosaccharide with an averaged degree of polymerization of 32 and a fraction of acetylation of 0.15 inhibited Candida spp. growth [213]. Interestingly, chitosan oligosaccharide had antifungal effects against Ceratocystis fimbriata by promoting fungal apoptotic cascades including ROS accumulation, mitochondrial dysfunction, and caspase activation [214]. Furthermore, N,N,N-trimethyl-O-(ureidopyridinium) acetyl chitosan derivatives, chitosan oligosaccharide functionalized silver nanoparticles, chitosan oligosaccharide-capped gold nanoparticles, and chitosan oligosaccharide-N-chlorokojic acid mannich base polymer were shown to hold promise for antibacterial application [215,216,217,218].
2.11. Trauma/Wound
Chitin has been applied as a major component of biomaterials for wound healing. Chitin was used for suturing because of its safety and rapid tissue recovery [219]. Chitin derived from demosponges Ianthella labyrinthus and Aplysina archeri was applied as alternative gauze fabrics, and it effectively absorbed blood into chitinous microtubes [62,220]. Additionally, several studies improved efficacy of chitin for wound healing by integration with other components. For instance, diacetyl chitin, a novel absorbable surgical suture, was ultimately absorbed within 42 days after suturing without tissue reaction, and promoted faster skin regeneration in vivo [221]. Chitin hydrogel, acrylamide-modified-β-chitin with alginate dialdehyde, showed promising properties including biocompatibility, biodegradability and injectability, and effectively accelerated wound healing [222]. In addition, sacchachitin nanofibers accelerated blood clotting times by 30 s and significantly promoted wound healing in streptozotocin-induced diabetic rats [223]. Interestingly, chitin accelerated wound healing via a MyD88-dependent pathway, followed by a TGF-β/Smad pathway [224]. Furthermore, Pseudomonas aeruginosa-infected wounds in db/db diabetic mice were diminished by cleaning with cleansing agent hypochlorous acid and covering with silver nanoparticle/chitin in the form of nanofiber sheet [225]. Chitin-amphiphilic ion/quaternary ammonium salt having antibacterial and antipollution effects was also used for wound healing [226]. Chitin-lignin gels, as an extracellular matrix-like scaffold, were applied as wound dressing material that had a property of sustainable drug release especially antibiotics [227]. Recently, chitin has been applied as a tissue adhesive. Chitin nanowhiskers with a Schiff base crosslinking hydrogel of carboxymethyl chitosan and dextran dialdehyde enhanced tissue adhesive strength with no cytotoxicity and with antibacterial effects [228]. Furthermore, chitin nanofibrils have been also applied as biomaterials. For instance, addition of 0.5% chitin nanofibrils into chitosan sponges promoted the stopping of arterial bleeding faster than commercial hemostatic agents [229].
Likewise, chitosan and chitosan oligosaccharide have been demonstrated as a biomaterial for promoting wound healing. For instance, chitosan oligosaccharide incorporated with silver nanoparticles accelerated wound healing by activating TGF-β/Smad pathway [230,231]. Chitosan was further used as a nanoparticle for carrying drugs including pioglitazone, heparin and bemiparin for wound healing application especially for diabetic wounds [232,233]. Chitosan-polyurethane hydrogel membrane in combination with mononuclear bone marrow fraction cells was used for wound healing in a diabetic rat model [234]. Furthermore, chitosan-curcumin complex, quaternary ammonium chitosan nanoparticles, carboxymethyl chitosan plus alginate were developed as biomaterials with high potential for wound healing application [235,236,237].
3. Limitation
The limitation in utilization of chitin is its potential toxicity in the human body, especially in the respiratory tract. According to previous studies, chitin exposure in the respiratory tract induced chitinase production, which was positively correlated to asthma [76]. In addition, chitin exposure to the respiratory tract can trigger innate immune response, especially macrophage and eosinophil activation. Furthermore, chitin is insoluble in water. However, oral intake of 5% chitin in a diet for 13 weeks showed no apparent toxicity in the gastrointestinal tract in rats [238]. Therefore, it appears that the adverse effects of chitin depend on the route of exposure.
In contrast to chitin, several studies have reported that oral ingestion of chitosan and chitosan oligosaccharide shown minimal toxicity [239,240]. Furthermore, chitosan oligosaccharide can dissolve in water. Chitosan and chitosan oligosaccharide have been approved as food additives by the American Food and Drug Administration (FDA). The potential systemic toxic effects of chitosan oligosaccharide are minimal.
4. Conclusions and Future Perspectives
Currently, chitin and chitosan have been used as a biomaterial for wound healing and drug delivery. Moreover, chitin and chitosan have been integrated with other chemicals to improve efficacy in therapeutic applications. Interestingly, the thermostability of chitin, chemical tolerance of chitin, and accessible natural source of chitin shed light on various procedures for biomaterial generation. Based on these advantageous properties, further development of chitin is needed to provide better biomaterials applicable for various human diseases. However, the development of chitin for medical applications requires further steps to improve safety and efficacy. A summary of potential applications and adverse effects of chitin is shown in Figure 1.
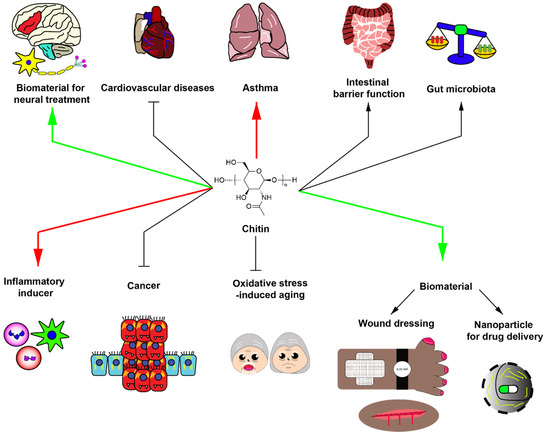
Figure 1.
Potential roles of chitin in biomedical applications. Green arrows indicate potential beneficial applications of chitin with strong evidence. Red arrows demonstrate potential detrimental effect of chitin with strong evidence. Black arrows delineate the potential effects of chitin with slight evidence. Chitin has been generally utilized as biomaterials for neural treatment, wound dressing and nanoparticle component for drug delivery. In addition, chitin has been used for improving intestinal barrier function, increasing beneficial gut microbiota, partially inhibiting cardiovascular diseases, inhibiting cancer growth, and inhibiting oxidative stress-induced aging. However, chitin may have detrimental effects. For instance, chitin exposure can induce asthma and acts as an inflammatory inducer.
Chitosan oligosaccharide has been applied for treatment of several types of human diseases or pathological conditions. Chitosan oligosaccharide with low molecular weight (<5 kDa) and >90% degree of deacetylation inhibits inflammatory responses by promoting anti-inflammatory pathways. Furthermore, chitosan oligosaccharide has antioxidative and anticancer effects. Interestingly, chitosan oligosaccharide, as a prebiotic for gut microbiota, modulates gut microbiota leading to the alleviation of systemic diseases, especially atherosclerosis. Biological effects of these compounds are determined by degree of deacetylation and polymerization, which remains the challenge in the development of chitosan oligosaccharide as an effective food supplement. Further investigations are needed to reveal detailed molecular/cellular mechanisms of biological effects of these polymers especially the role of their prebiotic effect and their direct effect on disease-specific cells or drug targets. Finally, since chitosan oligosaccharide possesses antioxidative effects, its potential application as an anti-aging agent should be further investigated. Potential applications and adverse effects of chitosan and chitosan oligosaccharide are summarized in Figure 2.
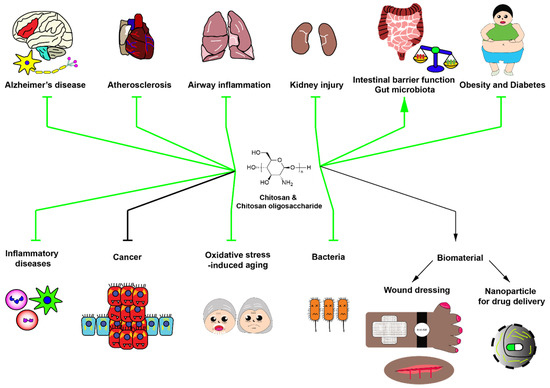
Figure 2.
Potential roles of chitosan and chitosan oligosaccharide in biomedical applications. Green arrows indicate the beneficial applications of chitosan and chitosan oligosaccharide with strong evidence. Black arrows indicate the potential effects of chitosan and chitosan oligosaccharide with slight evidence. Chitosan and chitosan oligosaccharide have numerous beneficial effects including anti-Alzheimer’s disease, anti-atherosclerosis, anti-inflammatory diseases, kidney injury alleviation, improvement of intestinal barrier function, enhancement of beneficial gut microbiota, alleviation of obesity and type II diabetes, prevention of inflammatory diseases, anticancer activity, antiaging activity and antibacterial activity, and serve as valuable biomaterials for wound dressing and drug delivery.
Author Contributions
Conceptualization, S.S. and C.M.; resources, S.S.; data curation, S.S.; writing—original draft preparation, S.S.; writing—review and editing, C.M.; funding acquisition, S.S. and C.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Thailand Research Fund, National Research Council of Thailand and Mahidol University, TRG6280002 (S.S.) and Thailand Research Fund and National Research Council of Thailand, grant number DBG6180029 (C.M.).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tsurkan, M.V.; Voronkina, A.; Khrunyk, Y.; Wysokowski, M.; Petrenko, I.; Ehrlich, H. Progress in chitin analytics. Carbohydr. Polym. 2020, 252, 117204. [Google Scholar] [CrossRef] [PubMed]
- Wysokowski, M.; Machalowski, T.; Petrenko, I.; Schimpf, C.; Rafaja, D.; Galli, R.; Zietek, J.; Pantovic, S.; Voronkina, A.; Kovalchuk, V.; et al. 3D Chitin Scaffolds of Marine Demosponge Origin for Biomimetic Mollusk Hemolymph-Associated Biomineralization Ex-Vivo. Mar. Drugs 2020, 18, 123. [Google Scholar] [CrossRef] [PubMed]
- Tsurkan, D.; Wysokowski, M.; Petrenko, I.; Voronkina, A.; Khrunyk, Y.; Fursov, A.; Ehrlich, H. Modern scaffolding strategies based on naturally pre-fabricated 3D biomaterials of poriferan origin. Appl. Phys. A 2020, 126, 382. [Google Scholar] [CrossRef]
- Mutsenko, V.V.; Gryshkov, O.; Lauterboeck, L.; Rogulska, O.; Tarusin, D.N.; Bazhenov, V.V.; Schutz, K.; Bruggemeier, S.; Gossla, E.; Akkineni, A.R.; et al. Novel chitin scaffolds derived from marine sponge Ianthella basta for tissue engineering approaches based on human mesenchymal stromal cells: Biocompatibility and cryopreservation. Int. J. Biol. Macromol. 2017, 104, 1955–1965. [Google Scholar] [CrossRef]
- Petrenko, I.; Khrunyk, Y.; Voronkina, A.; Kovalchuk, V.; Fursov, A.; Tsurkan, D.; Ivanenko, V. Poriferan chitin: 3D scaffolds from nano-to macroscale. A review. Lett. Appl. Nanobiosci. 2020, 9, 1004–1014. [Google Scholar]
- Machałowski, T.; Wysokowski, M.; Petrenko, I.; Fursov, A.; Rahimi-Nasrabadi, M.; Amro, M.d.M.; Meissner, H.; Joseph, Y.; Fazilov, B.; Ehrlich, H.; et al. Naturally pre-designed biomaterials: Spider molting cuticle as a functional crude oil sorbent. J. Environ. Manag. 2020, 261, 110218. [Google Scholar] [CrossRef] [PubMed]
- Machałowski, T.; Amemiya, C.; Jesionowski, T. Chitin of Araneae origin: Structural features and biomimetic applications: A review. Appl. Phys. A 2020, 126, 1–17. [Google Scholar] [CrossRef]
- Khrunyk, Y.; Lach, S.; Petrenko, I.; Ehrlich, H. Progress in Modern Marine Biomaterials Research. Mar. Drugs 2020, 18, 589. [Google Scholar] [CrossRef]
- Wysokowski, M.; Petrenko, I.; Stelling, A.L.; Stawski, D.; Jesionowski, T.; Ehrlich, H. Poriferan chitin as a versatile template for extreme biomimetics. Polymers 2015, 7, 235–265. [Google Scholar] [CrossRef]
- Petrenko, I.; Bazhenov, V.V.; Galli, R.; Wysokowski, M.; Fromont, J.; Schupp, P.J.; Stelling, A.L.; Niederschlag, E.; Stöker, H.; Kutsova, V.Z.; et al. Chitin of poriferan origin and the bioelectrometallurgy of copper/copper oxide. Int. J. Biol. Macromol. 2017, 104, 1626–1632. [Google Scholar] [CrossRef]
- Moussian, B. Chitin: Structure, Chemistry and Biology. Adv. Exp. Med. Biol. 2019, 1142, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.T.S.; Srinivasan, S.; Lakshmanan, V.K.; Tamura, H.; Nair, S.V.; Jayakumar, R. Synthesis, characterization and cytocompatibility studies of α-chitin hydrogel/nano hydroxyapatite composite scaffolds. Int. J. Biol. Macromol. 2011, 49, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, R.; Divya Rani, V.V.; Shalumon, K.T.; Kumar, P.T.S.; Nair, S.V.; Furuike, T.; Tamura, H. Bioactive and osteoblast cell attachment studies of novel α- and β-chitin membranes for tissue-engineering applications. Int. J. Biol. Macromol. 2009, 45, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Zhao, Y.; Yuan, X.; Zhang, J. β-Chitin nanofiber hydrogel as a scaffold to in situ fabricate monodispersed ultra-small silver nanoparticles. Colloids Surf. A 2019, 574, 36–43. [Google Scholar] [CrossRef]
- Jung, H.S.; Kim, M.H.; Shin, J.Y.; Park, S.R.; Jung, J.Y.; Park, W.H. Electrospinning and wound healing activity of β-chitin extracted from cuttlefish bone. Carbohydr. Polym. 2018, 193, 205–211. [Google Scholar] [CrossRef]
- Mulongo-Masamba, R.; El Hazzat, M.; El Hamidi, A.; Halim, M.; Arsalane, S. New functional β-chitin/calcium phosphate as promising support of copper nanocatalyst for the reductive degradation of methylene blue. Int. J. Environ. Sci. Technol. 2019, 16, 8117–8128. [Google Scholar] [CrossRef]
- Somsak, P.; Sriwattana, S.; Prinyawiwatkul, W. Ultrasonic-assisted chitin nanoparticle and its application as saltiness enhancer. Int. J. Food Sci. Technol. 2020. [Google Scholar] [CrossRef]
- Kaya, M.; Mujtaba, M.; Ehrlich, H.; Salaberria, A.M.; Baran, T.; Amemiya, C.T.; Galli, R.; Akyuz, L.; Sargin, I.; Labidi, J. On chemistry of γ-chitin. Carbohydr. Polym. 2017, 176, 177–186. [Google Scholar] [CrossRef]
- Qin, Z.; Zhao, L. The History of Chito/Chitin Oligosaccharides and Its Monomer. In Oligosaccharides of Chitin and Chitosan: Bio-manufacture and Applications; Zhao, L., Ed.; Springer: Singapore, 2019; pp. 3–14. [Google Scholar]
- Li, J.; Cai, C.; Li, J.; Li, J.; Li, J.; Sun, T.; Wang, L.; Wu, H.; Yu, G. Chitosan-Based Nanomaterials for Drug Delivery. Molecules 2018, 23, 2661. [Google Scholar] [CrossRef]
- Jiang, T.; James, R.; Kumbar, S.G.; Laurencin, C.T. Chitosan as a Biomaterial: Structure, Properties, and Applications in Tissue Engineering and Drug Delivery. In Natural and Synthetic Biomedical Polymers; Kumbar, S.G., Laurencin, C.T., Deng, M., Eds.; Elsevier: Oxford, UK, 2014; pp. 91–113. [Google Scholar]
- Muanprasat, C.; Chatsudthipong, V. Chitosan oligosaccharide: Biological activities and potential therapeutic applications. Pharmacol. Ther. 2017, 170, 80–97. [Google Scholar] [CrossRef]
- Gallo, M.; Naviglio, D.; Armone Caruso, A.; Ferrara, L. Applications of chitosan as a functional food. In Novel Approaches of Nanotechnology in Food; Grumezescu, A.M., Ed.; Academic Press: London, UK, 2016; pp. 425–464. [Google Scholar]
- Kim, S.H.; Eom, S.H.; Yu, D.; Lee, M.S.; Kim, Y.M. Oligochitosan as a potential anti-acne vulgaris agent: Combined antibacterial effects against Propionibacterium acnes. Food Sci. Biotechnol. 2017, 26, 1029–1036. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Chen, J.; Koziol, K.K.; Hallam, K.R.; Janas, D.; Patil, A.J.; Strachan, A.; Hanley, J.G.; Rahatekar, S.S. Chitin and carbon nanotube composites as biocompatible scaffolds for neuron growth. Nanoscale 2016, 8, 8288–8299. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Y.; Wang, J.W.; Qin, L.H.; Zhang, W.G.; Zhang, P.X.; Jiang, B.G. Chitin biological absorbable catheters bridging sural nerve grafts transplanted into sciatic nerve defects promote nerve regeneration. CNS Neurosci. Ther. 2018, 24, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Fan, J.; Yang, X.; Zhang, W.; Zhang, P.; Jiang, B. The neural regeneration effect of chitin biological absorbable tubes bridging sciatic nerve defects with sural nerve grafts. Am. J. Transl. Res. 2018, 10, 2362–2371. [Google Scholar] [PubMed]
- Ji, X.; Lei, Z.; Yuan, M.; Zhu, H.; Yuan, X.; Liu, W.; Pu, H.; Jiang, J.; Zhang, Y.; Jiang, X.; et al. Cartilage repair mediated by thermosensitive photocrosslinkable TGFβ1-loaded GM-HPCH via immunomodulating macrophages, recruiting MSCs and promoting chondrogenesis. Theranostics 2020, 10, 2872–2887. [Google Scholar] [CrossRef]
- Lin, H.L.; Wu, T.H.; Ho, H.O.; Chao, F.C.; Meng, H.; Liu, W.D.Z.; Chen, L.C.; Sheu, M.T. TEMPO-oxidized sacchachitin nanofibers (TOSCNFs) combined with platelet-rich plasma (PRP) for management of dry eye syndrome. Int. J. Nanomed. 2020, 15, 1721–1730. [Google Scholar] [CrossRef]
- Lomiguen, C.; Vidal, L.; Kozlowski, P.; Prancan, A.; Stern, R. Possible role of chitin-like proteins in the etiology of Alzheimer’s disease. J. Alzheimer’s Dis. 2018, 66, 439–444. [Google Scholar] [CrossRef]
- Castellani, R.J.; Perry, G.; Smith, M.A. The role of novel chitin-like polysaccharides in Alzheimer disease. Neurotox. Res. 2007, 12, 269–274. [Google Scholar] [CrossRef]
- Castellani, R.J.; Siedlak, S.L.; Fortino, A.E.; Perry, G.; Ghetti, B.; Smith, M.A. Chitin-like polysaccharides in Alzheimer’s disease brains. Curr. Alzheimer. Res. 2005, 2, 419–423. [Google Scholar] [CrossRef]
- Pisa, D.; Alonso, R.; Rábano, A.; Horst, M.N.; Carrasco, L. Fungal enolase, β-tubulin, and chitin are detected in brain tissue from Alzheimer’s disease patients. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef]
- Sotgiu, S.; Musumeci, S.; Marconi, S.; Gini, B.; Bonetti, B. Different content of chitin-like polysaccharides in multiple sclerosis and Alzheimer’s disease brains. J. Neuroimmunol. 2008, 197, 70–73. [Google Scholar] [CrossRef] [PubMed]
- Turano, E.; Busetto, G.; Marconi, S.; Guzzo, F.; Farinazzo, A.; Commisso, M.; Bistaffa, E.; Angiari, S.; Musumeci, S.; Sotgiu, S.; et al. Neurotoxicity and synaptic plasticity impairment of N-acetylglucosamine polymers: Implications for Alzheimer’s disease. Neurobiol. Aging 2015, 36, 1780–1791. [Google Scholar] [CrossRef] [PubMed]
- Mutsenko, V.V.; Bazhenov, V.V.; Rogulska, O.; Tarusin, D.N.; Schütz, K.; Brüggemeier, S.; Gossla, E.; Akkineni, A.R.; Meißner, H.; Lode, A.; et al. 3D chitinous scaffolds derived from cultivated marine demosponge Aplysina aerophoba for tissue engineering approaches based on human mesenchymal stromal cells. Int. J. Biol. Macromol. 2017, 104, 1966–1974. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Sung, M.J.; Seo, S.B.; Yoo, S.J.; Lim, W.K.; Kim, H.M. Water-soluble chitosan inhibits the production of pro-inflammatory cytokine in human astrocytoma cells activated by amyloid beta peptide and interleukin-1beta. Neurosci. Lett. 2002, 321, 105–109. [Google Scholar] [CrossRef]
- Lee, S.H.; Park, J.S.; Kim, S.K.; Ahn, C.B.; Je, J.Y. Chitooligosaccharides suppress the level of protein expression and acetylcholinesterase activity induced by Aβ25-35 in PC12 cells. Bioorganic Med. Chem. Lett. 2009, 19, 860–862. [Google Scholar] [CrossRef]
- Eom, T.K.; Ryu, B.; Lee, J.K.; Byun, H.G.; Park, S.J.; Kim, S.K. β-secretase inhibitory activity of phenolic acid conjugated chitooligosaccharides. J. Enzym. Inhib. Med. Chem. 2013, 28, 214–217. [Google Scholar] [CrossRef]
- Dai, X.; Chang, P.; Li, X.; Gao, Z.; Sun, Y. The inhibitory effect of chitosan oligosaccharides on beta-site amyloid precursor protein cleaving enzyme 1 (BACE1) in HEK293 APPswe cells. Neurosci. Lett. 2018, 665, 80–85. [Google Scholar] [CrossRef]
- Dai, X.; Hou, W.; Sun, Y.; Gao, Z.; Zhu, S.; Jiang, Z. Chitosan oligosaccharides inhibit/disaggregate fibrils and attenuate amyloid β-mediated neurotoxicity. Int. J. Mol. Sci. 2015, 16, 10526–10536. [Google Scholar] [CrossRef]
- Hou, H.; Zhang, L.; Ye, Z.; Li, J.; Lian, Z.; Chen, C.; He, R.; Peng, B.; Xu, Q.; Zhang, G.; et al. Chitooligosaccharide Inhibits Scar Formation and Enhances Functional Recovery in a Mouse Model of Sciatic Nerve Injury. Mol. Neurobiol. 2016, 53, 2249–2257. [Google Scholar] [CrossRef]
- Kunanusornchai, W.; Witoonpanich, B.; Tawonsawatruk, T.; Pichyangkura, R.; Chatsudthipong, V.; Muanprasat, C. Chitosan oligosaccharide suppresses synovial inflammation via AMPK activation: An in vitro and in vivo study. Pharm. Res. 2016, 113, 458–467. [Google Scholar] [CrossRef]
- Jia, S.; Lu, Z.; Gao, Z.; An, J.; Wu, X.; Li, X.; Dai, X.; Zheng, Q.; Sun, Y. Chitosan oligosaccharides alleviate cognitive deficits in an amyloid-beta1-42-induced rat model of Alzheimer’s disease. Int. J. Biol. Macromol. 2016, 83, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Chang, P.; Zhu, Q.; Liu, W.; Sun, Y.; Zhu, S.; Jiang, Z. Chitosan oligosaccharides protect rat primary hippocampal neurons from oligomeric beta-amyloid 1-42-induced neurotoxicity. Neurosci. Lett. 2013, 554, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Inui, H.; Tsujikubo, M.; Hirano, S. Low molecular weight chitosan stimulation of mitogenic response to platelet-derived growth factor in vascular smooth muscle cells. Biosci. Biotechnol. Biochem. 1995, 59, 2111–2114. [Google Scholar] [CrossRef]
- Chien, Y.; Liao, Y.W.; Liu, D.M.; Lin, H.L.; Chen, S.J.; Chen, H.L.; Peng, C.H.; Liang, C.M.; Mou, C.Y.; Chiou, S.H. Corneal repair by human corneal keratocyte-reprogrammed iPSCs and amphiphatic carboxymethyl-hexanoyl chitosan hydrogel. Biomaterials 2012, 33, 8003–8016. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Liu, J.; Fang, Q.; Xiao, B.; Wan, Y. Establishment of nerve growth factor gradients on aligned chitosan-polylactide /alginate fibers for neural tissue engineering applications. Colloids Surf. B 2017, 160, 598–609. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.K.; Ding, S.J. Acid-resistant calcium silicate-based composite implants with high-strength as load-bearing bone graft substitutes and fracture fixation devices. J. Mech. Behav. Biomed. Mater. 2016, 62, 366–383. [Google Scholar] [CrossRef] [PubMed]
- Jaruszewski, K.M.; Ramakrishnan, S.; Poduslo, J.F.; Kandimalla, K.K. Chitosan enhances the stability and targeting of immuno-nanovehicles to cerebro-vascular deposits of Alzheimer’s disease amyloid protein. Nanomedicine 2012, 8, 250–260. [Google Scholar] [CrossRef]
- Mahl, C.R.A.; Taketa, T.B.; Rocha-Neto, J.B.M.; Almeida, W.P.; Beppu, M.M. Copper Ion Uptake by Chitosan in the Presence of Amyloid-beta and Histidine. Appl. Biochem. Biotechnol. 2020, 190, 949–965. [Google Scholar] [CrossRef]
- Casadome-Perales, A.; Matteis, L.; Alleva, M.; Infantes-Rodriguez, C.; Palomares-Perez, I.; Saito, T.; Saido, T.C.; Esteban, J.A.; Nebreda, A.R.; de la Fuente, J.M.; et al. Inhibition of p38 MAPK in the brain through nasal administration of p38 inhibitor loaded in chitosan nanocapsules. Nanomedicine 2019, 14, 2409–2422. [Google Scholar] [CrossRef]
- Kheradmandi, M.; Vasheghani-Farahani, E.; Ghiaseddin, A.; Ganji, F. Skeletal muscle regeneration via engineered tissue culture over electrospun nanofibrous chitosan/PVA scaffold. J. Biomed. Mater. Res. A 2016, 104, 1720–1727. [Google Scholar] [CrossRef]
- Li, Y.; Yu, Z.; Men, Y.; Chen, X.; Wang, B. Laminin-chitosan-PLGA conduit co-transplanted with Schwann and neural stem cells to repair the injured recurrent laryngeal nerve. Exp. Med. 2018, 16, 1250–1258. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Huang, H.; Bi, W.; Tan, X.; Li, R.; Wen, W.; Song, W.; Zhang, Y.; Zhang, F.; Hu, M. Effect of chitosan combined with hyaluronate on promoting the recovery of postoperative facial nerve regeneration and function in rabbits. Exp. Med. 2018, 16, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Crosio, A.; Fornasari, B.E.; Gambarotta, G.; Geuna, S.; Raimondo, S.; Battiston, B.; Tos, P.; Ronchi, G. Chitosan tubes enriched with fresh skeletal muscle fibers for delayed repair of peripheral nerve defects. Neural. Regen. Res. 2019, 14, 1079–1084. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, C.R.; Costa, J.B.; Costa, L.; Silva-Correia, J.; Moay, Z.K.; Ng, K.W.; Reis, R.L.; Oliveira, J.M. Enhanced performance of chitosan/keratin membranes with potential application in peripheral nerve repair. Biomater. Sci. 2019, 7, 5451–5466. [Google Scholar] [CrossRef]
- Moattari, M.; Kouchesfehani, H.M.; Kaka, G.; Sadraie, S.H.; Naghdi, M.; Mansouri, K. Chitosan-film associated with mesenchymal stem cells enhanced regeneration of peripheral nerves: A rat sciatic nerve model. J. Chem. Neuroanat. 2018, 88, 46–54. [Google Scholar] [CrossRef]
- Neyrinck, A.M.; Catry, E.; Taminiau, B.; Cani, P.D.; Bindels, L.B.; Daube, G.; Dessy, C.; Delzenne, N.M. Chitin-glucan and pomegranate polyphenols improve endothelial dysfunction. Sci. Rep. 2019, 9, 14150. [Google Scholar] [CrossRef]
- Song, X.; Huang, X.; Li, Z.; Li, Z.; Wu, K.; Jiao, Y.; Zhou, C. Construction of blood compatible chitin/graphene oxide composite aerogel beads for the adsorption of bilirubin. Carbohydr. Polym. 2019, 207, 704–712. [Google Scholar] [CrossRef]
- Zhang, J.; Xue, S.; Zhu, X.; Zhao, Y.; Chen, Y.; Tong, J.; Shi, X.; Du, Y.; Zhong, Z.; Ye, Q. Emerging chitin nanogels/rectorite nanocomposites for safe and effective hemorrhage control. J. Mater. Chem. B 2019, 7, 5096–5103. [Google Scholar] [CrossRef]
- Schubert, M.; Binnewerg, B.; Voronkina, A.; Muzychka, L.; Wysokowski, M.; Petrenko, I.; Kovalchuk, V.; Tsurkan, M.; Martinovic, R.; Bechmann, N.; et al. Naturally Prefabricated Marine Biomaterials: Isolation and Applications of Flat Chitinous 3D Scaffolds from Ianthella labyrinthus (Demospongiae: Verongiida). Int. J. Mol. Sci. 2019, 20, 5105. [Google Scholar] [CrossRef]
- Machałowski, T.; Wysokowski, M.; Żółtowska-Aksamitowska, S.; Bechmann, N.; Binnewerg, B.; Schubert, M.; Guan, K.; Bornstein, S.R.; Czaczyk, K.; Pokrovsky, O.; et al. Spider Chitin. The biomimetic potential and applications of Caribena versicolor tubular chitin. Carbohydr. Polym. 2019, 226, 115301. [Google Scholar] [CrossRef]
- Ormrod, D.J.; Holmes, C.C.; Miller, T.E. Dietary chitosan inhibits hypercholesterolaemia and atherogenesis in the apolipoprotein E-deficient mouse model of atherosclerosis. Atherosclerosis 1998, 138, 329–334. [Google Scholar] [CrossRef]
- Bahijri, S.M.; Alsheikh, L.; Ajabnoor, G.; Borai, A. Effect of Supplementation With Chitosan on Weight, Cardiometabolic, and Other Risk Indices in Wistar Rats Fed Normal and High-Fat/High-Cholesterol Diets Ad Libitum. Nutr. Metab. Insights 2017, 10, 1178638817710666. [Google Scholar] [CrossRef] [PubMed]
- Zong, C.; Yu, Y.; Song, G.; Luo, T.; Li, L.; Wang, X.; Qin, S. Chitosan oligosaccharides promote reverse cholesterol transport and expression of scavenger receptor BI and CYP7A1 in mice. Exp. Biol. Med. (Maywood) 2012, 237, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhang, J.; Chen, L.; Wu, Q.; Yu, C. Chitosan oligosaccharides enhance lipid droplets via down-regulation of PCSK9 gene expression in HepG2 cells. Exp. Cell. Res. 2018, 366, 152–160. [Google Scholar] [CrossRef]
- Li, Y.; Xu, Q.; Wei, P.; Cheng, L.; Peng, Q.; Li, S.; Yin, H.; Du, Y. Chitosan oligosaccharides downregulate the expression of E-selectin and ICAM-1 induced by LPS in endothelial cells by inhibiting MAP kinase signaling. Int. J. Mol. Med. 2014, 33, 392–400. [Google Scholar] [CrossRef]
- Yu, Y.; Luo, T.; Liu, S.; Song, G.; Han, J.; Wang, Y.; Yao, S.; Feng, L.; Qin, S. Chitosan Oligosaccharides Attenuate Atherosclerosis and Decrease Non-HDL in ApoE-/- Mice. J. Atheroscler. Thromb. 2015, 22, 926–941. [Google Scholar] [CrossRef]
- Xiong, Y.; Xiong, M.; Li, Y.; Qian, J.; Li, Y.; Han, X.; Tan, J.; Luo, Y.; Wang, Q.; Qin, C. Chitosan oligosaccharide combined with running benefited the immune status of rats. Int. Immunopharmacol. 2020, 88, 106915. [Google Scholar] [CrossRef]
- Zhu, L.; Hu, B.; Guo, Y.; Yang, H.; Zheng, J.; Yao, X.; Hu, H.; Liu, H. Effect of Chitosan oligosaccharides on ischemic symptom and gut microbiota disbalance in mice with hindlimb ischemia. Carbohydr. Polym. 2020, 240, 116271. [Google Scholar] [CrossRef]
- Termsarasab, U.; Yoon, I.S.; Park, J.H.; Moon, H.T.; Cho, H.J.; Kim, D.D. Polyethylene glycol-modified arachidyl chitosan-based nanoparticles for prolonged blood circulation of doxorubicin. Int. J. Pharm. 2014, 464, 127–134. [Google Scholar] [CrossRef]
- Yu, J.; Ruan, Q.; Nie, X.; Yu, L.; Huang, B. Synthetic CD47 antibody-chitosan/hyaluronic acid polyelectrolyte complex mediates targeted inhibition of atherosclerotic plaques by exogenous foam-like cells via the NLRP3 pathway. J. Biomater. Appl. 2020, 34, 1381–1394. [Google Scholar] [CrossRef]
- Tang, H.; Fang, Z.; Sun, Y.; Li, B.; Shi, Z.; Chen, J.; Zhang, T.; Xiu, Q. YKL-40 in asthmatic patients, and its correlations with exacerbation, eosinophils and immunoglobulin E. Eur. Respir. J. 2010, 35, 757–760. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhang, X.; Liu, Y.; Zhang, L.; Zheng, J.; Wang, J.; Hansbro, P.M.; Wang, L.; Wang, G.; Hsu, A.C.Y. Chitinase-like protein YKL-40 correlates with inflammatory phenotypes, anti-asthma responsiveness and future exacerbations. Respir. Res. 2019, 20, 95. [Google Scholar] [CrossRef] [PubMed]
- Van Dyken, S.J.; Garcia, D.; Porter, P.; Huang, X.; Quinlan, P.J.; Blanc, P.D.; Corry, D.B.; Locksley, R.M. Fungal chitin from asthma-associated home environments induces eosinophilic lung infiltration. J. Immunol. 2011, 187, 2261–2267. [Google Scholar] [CrossRef] [PubMed]
- Khosravi, A.R.; Erle, D.J. Chitin-Induced Airway Epithelial Cell Innate Immune Responses Are Inhibited by Carvacrol/Thymol. PLoS ONE 2016, 11, e0159459. [Google Scholar] [CrossRef]
- Choi, J.P.; Lee, S.M.; Choi, H.I.; Kim, M.H.; Jeon, S.G.; Jang, M.H.; Jee, Y.K.; Yang, S.; Cho, Y.J.; Kim, Y.K. House Dust Mite-Derived Chitin Enhances Th2 Cell Response to Inhaled Allergens, Mainly via a TNF-alpha-Dependent Pathway. Allergy Asthma Immunol. Res. 2016, 8, 362–374. [Google Scholar] [CrossRef]
- Boot, R.G.; Renkema, G.H.; Strijland, A.; van Zonneveld, A.J.; Aerts, J.M. Cloning of a cDNA encoding chitotriosidase, a human chitinase produced by macrophages. J. Biol. Chem. 1995, 270, 26252–26256. [Google Scholar] [CrossRef]
- Cho, S.J.; Weiden, M.D.; Lee, C.G. Chitotriosidase in the pathogenesis of inflammation, interstitial lung diseases and COPD. Allergy Asthma Immunol. Res. 2014, 7, 14–21. [Google Scholar] [CrossRef]
- Garth, J.M.; Mackel, J.J.; Reeder, K.M.; Blackburn, J.P.; Dunaway, C.W.; Yu, Z.; Matalon, S.; Fitz, L.; Steele, C. Acidic Mammalian Chitinase Negatively Affects Immune Responses during Acute and Chronic Aspergillus fumigatus Exposure. Infect. Immun. 2018, 86. [Google Scholar] [CrossRef]
- Mazur, M.; Dymek, B.; Koralewski, R.; Sklepkiewicz, P.; Olejniczak, S.; Mazurkiewicz, M.; Piotrowicz, M.; Salamon, M.; Jȩdrzejczak, K.; Zagozdzon, A.; et al. Development of Dual Chitinase Inhibitors as Potential New Treatment for Respiratory System Diseases. J. Med. Chem. 2019, 62, 7126–7145. [Google Scholar] [CrossRef]
- Das, P.; Acharya, S.; Shah, D.; Agarwal, B.; Prahaladan, V.; Bhandari, V. Chitin Analog AVR-25 Prevents Experimental Bronchopulmonary Dysplasia. J. Pediatr. Intensive Care 2020, 9, 225–232. [Google Scholar] [CrossRef]
- Zhao, Y.; Xu, G.; Wang, S.; Yi, X.; Wu, W. Chitosan oligosaccharides alleviate PM2.5-induced lung inflammation in rats. Environ. Sci. Pollut. Res. Int. 2018, 25, 34221–34227. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.E.; Tong, C.C.; Zhang, Y.B.; Cong, P.F.; Shi, X.Y.; Liu, Y.; Shi, L.; Tong, Z.; Jin, H.X.; Hou, M.X. Chitosan oligosaccharide ameliorates acute lung injury induced by blast injury through the DDAH1/ADMA pathway. PLoS ONE 2018, 13, e0192135. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.J.; Park, J.K.; Park, Y.I. Anti-inflammatory effects of low-molecular weight chitosan oligosaccharides in IgE-antigen complex-stimulated RBL-2H3 cells and asthma model mice. Int. Immunopharmacol. 2012, 12, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Anraku, M.; Tabuchi, R.; Ifuku, S.; Nagae, T.; Iohara, D.; Tomida, H.; Uekama, K.; Maruyama, T.; Miyamura, S.; Hirayama, F.; et al. An oral absorbent, surface-deacetylated chitin nano-fiber ameliorates renal injury and oxidative stress in 5/6 nephrectomized rats. Carbohydr. Polym. 2017, 161, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Conroy, A.L.; Hawkes, M.T.; Elphinstone, R.; Opoka, R.O.; Namasopo, S.; Miller, C.; John, C.C.; Kain, K.C. Chitinase-3-like 1 is a biomarker of acute kidney injury and mortality in paediatric severe malaria. Malar. J. 2018, 17, 82. [Google Scholar] [CrossRef]
- Schmidt, I.M.; Hall, I.E.; Kale, S.; Lee, S.; He, C.-H.; Lee, Y.; Chupp, G.L.; Moeckel, G.W.; Lee, C.G.; Elias, J.A.; et al. Chitinase-Like Protein Brp-39/YKL-40 Modulates the Renal Response to Ischemic Injury and Predicts Delayed Allograft Function. J. Am. Soc. Nephrol. 2013, 24, 309. [Google Scholar] [CrossRef]
- Bai, W.; Wang, S.; An, S.; Guo, M.; Gong, G.; Liu, W.; Ma, S.; Li, X.; Fu, J.; Yao, W. Combination therapy of chitosan, gynostemma, and motherwort alleviates the progression of experimental rat chronic renal failure by inhibiting STAT1 activation. Oncotarget 2018, 9, 15498–15511. [Google Scholar] [CrossRef]
- Queiroz, M.F.; Melo, K.R.T.; Sabry, D.A.; Sassaki, G.L.; Rocha, H.A.O.; Costa, L.S. Gallic Acid-Chitosan Conjugate Inhibits the Formation of Calcium Oxalate Crystals. Molecules 2019, 24. [Google Scholar] [CrossRef]
- Biasibetti, E.; Martello, E.; Bigliati, M.; Biasato, I.; Cocca, T.; Bruni, N.; Capucchio, M.T. A long term feed supplementation based on phosphate binders in Feline Chronic Kidney Disease. Vet. Res. Commun. 2018, 42, 161–167. [Google Scholar] [CrossRef]
- Liu, B.; Qin, Z.K.; Lin, X.M.; Mei, L.; Liu, W.S.; Han, B.Q. Effects of chitooligosaccharides and its derivatives on antioxygenization and renal function in experimental diabetes rats. J. Clin. Rehabil. Tissue Eng. Res. 2008, 12, 9651–9654. [Google Scholar]
- Yoon, H.J.; Moon, M.E.; Park, H.S.; Kim, H.W.; Im, S.Y.; Lee, J.H.; Kim, Y.H. Effects of chitosan oligosaccharide (COS) on the glycerol-induced acute renal failure in vitro and in vivo. Food Chem. Toxicol. 2008, 46, 710–716. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; Liu, Y.; Jiang, Z.; Yang, Y.; Liu, W.; Han, B. Preparation and renoprotective effects of carboxymethyl chitosan oligosaccharide on adriamycin nephropathy. Carbohydr. Polym. 2018, 201, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Yuan, S.; Yang, X.; Zou, P.; Li, L.; Li, G.; Shao, Y.; Abd El-Aty, A.M.; Hacimuftuoglu, A.; Wang, J. Chitosan Oligosaccharides Induce Apoptosis in Human Renal Carcinoma via Reactive-Oxygen-Species-Dependent Endoplasmic Reticulum Stress. J. Agric. Food Chem. 2019, 67, 1691–1701. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.F.; Ding, C.L.; Liu, H.; Liu, L.H.; Zhao, C.Q. Protective effects of ion-imprinted chitooligosaccharides as uranium-specific chelating agents against the cytotoxicity of depleted uranium in human kidney cells. Toxicology 2011, 286, 75–84. [Google Scholar] [CrossRef]
- Pathomthongtaweechai, N.; Soodvilai, S.; Pichyangkura, R.; Muanprasat, C. Novel Potential Application of Chitosan Oligosaccharide for Attenuation of Renal Cyst Growth in the Treatment of Polycystic Kidney Disease. Molecules 2020, 25, 5589. [Google Scholar] [CrossRef]
- Anraku, M.; Tanaka, M.; Hiraga, A.; Nagumo, K.; Imafuku, T.; Maezaki, Y.; Iohara, D.; Uekama, K.; Watanabe, H.; Hirayama, F.; et al. Effects of chitosan on oxidative stress and related factors in hemodialysis patients. Carbohydr. Polym. 2014, 112, 152–157. [Google Scholar] [CrossRef]
- Salva, E.; Turan, S.O.; Akbuga, J. Inhibition of Glomerular Mesangial Cell Proliferation by siPDGF-B- and siPDGFR-beta-Containing Chitosan Nanoplexes. AAPS PharmSciTech. 2017, 18, 1031–1042. [Google Scholar] [CrossRef]
- Chiang, I.N.; Huang, W.C.; Huang, C.Y.; Pu, Y.S.; Young, T.H. Development of a chitosan-based tissue-engineered renal proximal tubule conduit. J. Biomed. Mater. Res. B 2018, 106, 9–20. [Google Scholar] [CrossRef]
- Sutthasupha, P.; Lungkaphin, A. The potential roles of chitosan oligosaccharide in prevention of kidney injury in obese and diabetic conditions. Food Funct. 2020, 11, 7371–7388. [Google Scholar] [CrossRef]
- Liberti, A.; Zucchetti, I.; Melillo, D.; Skapura, D.; Shibata, Y.; De Santis, R.; Pinto, M.R.; Litman, G.W.; Dishaw, L.J. Chitin protects the gut epithelial barrier in a protochordate model of DSS-induced colitis. Biol. Open 2018, 7. [Google Scholar] [CrossRef]
- Alessandri, G.; Milani, C.; Duranti, S.; Mancabelli, L.; Ranjanoro, T.; Modica, S.; Carnevali, L.; Statello, R.; Bottacini, F.; Turroni, F.; et al. Ability of bifidobacteria to metabolize chitin-glucan and its impact on the gut microbiota. Sci. Rep. 2019, 9, 5755. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.; Neyrinck, A.M.; Zhang, Z.; Seethaler, B.; Nazare, J.A.; Robles Sanchez, C.; Roumain, M.; Muccioli, G.G.; Bindels, L.B.; Cani, P.D.; et al. Metabolite profiling reveals the interaction of chitin-glucan with the gut microbiota. Gut. Microbes 2020, 12, 1810530. [Google Scholar] [CrossRef] [PubMed]
- Goto, M.; Iohara, D.; Michihara, A.; Ifuku, S.; Azuma, K.; Kadowaki, D.; Maruyama, T.; Otagiri, M.; Hirayama, F.; Anraku, M. Effects of surface-deacetylated chitin nanofibers on non-alcoholic steatohepatitis model rats and their gut microbiota. Int. J. Biol. Macromol. 2020, 164, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Piao, X.S.; Thacker, P.A.; Zeng, Z.K.; Li, P.F.; Wang, D.; Kim, S.W. Chito-oligosaccharide reduces diarrhea incidence and attenuates the immune response of weaned pigs challenged with Escherichia coli K88. J. Anim. Sci. 2010, 88, 3871–3879. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Piao, X.S.; Kim, S.W.; Wang, L.; Shen, Y.B.; Lee, H.S.; Li, S.Y. Effects of chito-oligosaccharide supplementation on the growth performance, nutrient digestibility, intestinal morphology, and fecal shedding of Escherichia coli and Lactobacillus in weaning pigs. J. Anim. Sci. 2008, 86, 2609–2618. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Yue, X.; Hu, L.; Ma, Y.; Han, X. Changes in diarrhea, nutrients apparent digestibility, digestive enzyme activities of weaned piglets in response to chitosan-zinc chelate. Anim. Sci. J. 2016, 87, 564–569. [Google Scholar] [CrossRef] [PubMed]
- Aluko, K.; Velayudhan, D.E.; Khafipour, E.; Li, A.; Yin, Y.; Nyachoti, M. Combined effects of chitosan and microencapsulated Enterococcus faecalis CG1.0007 probiotic supplementation on performance and diarrhea incidences in enterotoxigenic Escherichia coli K88(+) challenged piglets. Anim. Nutr. 2017, 3, 366–371. [Google Scholar] [CrossRef]
- Hu, S.; Wang, Y.; Wen, X.; Wang, L.; Jiang, Z.; Zheng, C. Effects of low-molecular-weight chitosan on the growth performance, intestinal morphology, barrier function, cytokine expression and antioxidant system of weaned piglets. Bmc. Vet. Res. 2018, 14, 215. [Google Scholar] [CrossRef]
- Thongsong, B.; Suthongsa, S.; Pichyangkura, R.; Kalandakanond-Thongsong, S. Effects of chito-oligosaccharide supplementation with low or medium molecular weight and high degree of deacetylation on growth performance, nutrient digestibility and small intestinal morphology in weaned pigs. Livest. Sci. 2018, 209, 60–66. [Google Scholar] [CrossRef]
- Zheng, J.; Yuan, X.; Cheng, G.; Jiao, S.; Feng, C.; Zhao, X.; Yin, H.; Du, Y.; Liu, H. Chitosan oligosaccharides improve the disturbance in glucose metabolism and reverse the dysbiosis of gut microbiota in diabetic mice. Carbohydr. Polym. 2018, 190, 77–86. [Google Scholar] [CrossRef]
- Muanprasat, C.; Wongkrasant, P.; Satitsri, S.; Moonwiriyakit, A.; Pongkorpsakol, P.; Mattaveewong, T.; Pichyangkura, R.; Chatsudthipong, V. Activation of AMPK by chitosan oligosaccharide in intestinal epithelial cells: Mechanism of action and potential applications in intestinal disorders. Biochem. Pharm. 2015, 96, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Zheng, J.; Yuan, X.; Jiao, S.; Feng, C.; Du, Y.; Liu, H.; Zheng, L. Chitosan oligosaccharides improve glucolipid metabolism disorder in liver by suppression of obesity-related inflammation and restoration of peroxisome proliferator-activated receptor gamma (PPARγ). Mar. Drugs 2018, 16. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, B.; Rajput, P.; Jena, P.K.; Seshadri, S. Investigation of Chitosan for Prevention of Diabetic Progression Through Gut Microbiota Alteration in Sugar Rich Diet Induced Diabetic Rats. Curr. Pharm. Biotechnol. 2015, 17, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Mateos-Aparicio, I.; Mengibar, M.; Heras, A. Effect of chito-oligosaccharides over human faecal microbiota during fermentation in batch cultures. Carbohydr. Polym. 2016, 137, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Rowan, F.; Docherty, N.G.; Murphy, M.; Murphy, B.; Calvin Coffey, J.; O’Connell, P.R. Desulfovibrio bacterial species are increased in ulcerative colitis. Dis. Colon. Rectum. 2010, 53, 1530–1536. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Jiao, S.; Wang, Z.A.; Du, Y. Exploring Effects of Chitosan Oligosaccharides on Mice Gut Microbiota in in vitro Fermentation and Animal Model. Front. Microbiol. 2018, 9, 2388. [Google Scholar] [CrossRef]
- Wu, M.; Li, J.; An, Y.; Li, P.; Xiong, W.; Li, J.; Yan, D.; Wang, M.; Zhong, G. Chitooligosaccharides Prevents the Development of Colitis-Associated Colorectal Cancer by Modulating the Intestinal Microbiota and Mycobiota. Front. Microbiol. 2019, 10, 2101. [Google Scholar] [CrossRef]
- Qian, M.; Lyu, Q.; Liu, Y.; Hu, H.; Wang, S.; Pan, C.; Duan, X.; Gao, Y.; Qi, L.W.; Liu, W.; et al. Chitosan Oligosaccharide Ameliorates Nonalcoholic Fatty Liver Disease (NAFLD) in Diet-Induced Obese Mice. Mar. Drugs 2019, 17. [Google Scholar] [CrossRef]
- Saleh, H.; El-Shorbagy, H.M. Chitosan protects liver against ischemia-reperfusion injury via regulating Bcl-2/Bax, TNF-alpha and TGF-beta expression. Int. J. Biol. Macromol. 2020, 164, 1565–1574. [Google Scholar] [CrossRef]
- Tong, A.J.; Hu, R.K.; Wu, L.X.; Lv, X.C.; Li, X.; Zhao, L.N.; Liu, B. Ganoderma polysaccharide and chitosan synergistically ameliorate lipid metabolic disorders and modulate gut microbiota composition in high fat diet-fed golden hamsters. J. Food. Biochem. 2020, 44, e13109. [Google Scholar] [CrossRef]
- Wu, X.; Kim, M.J.; Yang, H.J.; Park, S. Chitosan alleviated menopausal symptoms and modulated the gut microbiota in estrogen-deficient rats. Eur. J. Nutr. 2020, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Kong, M.; Zhou, Z.; Yan, D.; Yu, X.; Cheng, X.; Feng, C.; Liu, Y.; Chen, X. Mechanism of surface charge triggered intestinal epithelial tight junction opening upon chitosan nanoparticles for insulin oral delivery. Carbohydr. Polym. 2017, 157, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Mao, H.; Yang, C.; Du, H.; Wang, H.; Tu, J. Effects of chitosan nanoparticle supplementation on growth performance, humoral immunity, gut microbiota and immune responses after lipopolysaccharide challenge in weaned pigs. J. Anim. Physiol. Anim. Nutr. (Berl) 2020, 104, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Nopvichai, C.; Pongkorpsakol, P.; Wongkrasant, P.; Wangpaiboon, K.; Charoenwongpaiboon, T.; Ito, K.; Muanprasat, C.; Pichyangkura, R. Galactomannan Pentasaccharide Produced from Copra Meal Enhances Tight Junction Integration of Epithelial Tissue through Activation of AMPK. Biomedicines 2019, 7. [Google Scholar] [CrossRef] [PubMed]
- Nopvichai, C.; Charoenwongpaiboon, T.; Luengluepunya, N.; Ito, K.; Muanprasat, C.; Pichyangkura, R. Production and purification of mannan oligosaccharide with epithelial tight junction enhancing activity. PeerJ 2019, 7, e7206. [Google Scholar] [CrossRef] [PubMed]
- Wongkrasant, P.; Pongkorpsakol, P.; Ariyadamrongkwan, J.; Meesomboon, R.; Satitsri, S.; Pichyangkura, R.; Barrett, K.E.; Muanprasat, C. A prebiotic fructo-oligosaccharide promotes tight junction assembly in intestinal epithelial cells via an AMPK-dependent pathway. Biomed. Pharm. 2020, 129, 110415. [Google Scholar] [CrossRef] [PubMed]
- Bays, H.E.; Evans, J.L.; Maki, K.C.; Evans, M.; Maquet, V.; Cooper, R.; Anderson, J.W. Chitin-glucan fiber effects on oxidized low-density lipoprotein: A randomized controlled trial. Eur. J. Clin. Nutr. 2013, 67, 2–7. [Google Scholar] [CrossRef]
- Yu, P.; Xie, J.; Chen, Y.; Liu, J.; Liu, Y.; Bi, B.; Luo, J.; Li, S.; Jiang, X.; Li, J. A thermo-sensitive injectable hydroxypropyl chitin hydrogel for sustained salmon calcitonin release with enhanced osteogenesis and hypocalcemic effects. J. Mater. Chem. B 2020, 8, 270–281. [Google Scholar] [CrossRef]
- Xing, R.; He, X.; Liu, S.; Yu, H.; Qin, Y.; Chen, X.; Li, K.; Li, R.; Li, P. Antidiabetic activity of differently regioselective chitosan sulfates in alloxan-induced diabetic rats. Mar. Drugs 2015, 13, 3072–3090. [Google Scholar] [CrossRef]
- Arun, G.; Rajaram, R.; Kaleshkumar, K.; Gayathri, N.; Sivasudha, T.; Kandasamy, S. Synergistic effect of novel chitosan combined metformin drug on streptozotocin-induced diabetes mellitus rat. Int. J. Biol. Macromol. 2020, 153, 1335–1349. [Google Scholar] [CrossRef]
- Yan, H.; Rong, L.; Xiao, D.; Zhang, M.; Sheikh, S.P.; Sui, X.; Lu, M. Injectable and self-healing hydrogel as a stem cells carrier for treatment of diabetic erectile dysfunction. Mater. Sci. Eng. C 2020, 116, 111214. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.Y.; Al-Salami, H.; Dass, C.R. Lyophilisation Improves Bioactivity and Stability of Insulin-Loaded Polymeric-Oligonucleotide Nanoparticles for Diabetes Treatment. Aaps. Pharmscitech. 2020, 21, 108. [Google Scholar] [CrossRef]
- Trinh, T.A.; Duy Le, T.M.; Ho, H.G.V.; To, T.C.T.; Nguyen, V.V.L.; Huynh, D.P.; Lee, D.S. A novel injectable pH-temperature sensitive hydrogel containing chitosan-insulin electrosprayed nanosphere composite for an insulin delivery system in type I diabetes treatment. Biomater. Sci. 2020, 8, 3830–3843. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, A.; Ezati, M.; Mohammadnejad, J.; Houshmand, B.; Faghihi, S. Chitosan Coating of TiO2 Nanotube Arrays for Improved Metformin Release and Osteoblast Differentiation. Int. J. Nanomed. 2020, 15, 4471–4481. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; Lv, Y.; Li, N.; Huang, X.; Liu, X.; Li, H.; Wang, C.; Jia, Y.F. Biological investigations on therapeutic effect of chitosan encapsulated nano resveratrol against gestational diabetes mellitus rats induced by streptozotocin. Drug Deliv. 2020, 27, 953–963. [Google Scholar] [CrossRef] [PubMed]
- Bansal, A.; D’Sa, S.; D’Souza, M.J. Biofabrication of microcapsules encapsulating beta-TC-6 cells via scalable device and in-vivo evaluation in type 1 diabetic mice. Int. J. Pharm. 2019, 572, 118830. [Google Scholar] [CrossRef] [PubMed]
- Al-Nemrawi, N.K.; Alsharif, S.S.M.; Alzoubi, K.H.; Alkhatib, R.Q. Preparation and characterization of insulin chitosan-nanoparticles loaded in buccal films. Pharm. Dev. Technol. 2019, 24, 967–974. [Google Scholar] [CrossRef]
- Zeng, S.; Ke, Y.; Liu, Y.; Shen, Y.; Zhang, L.; Li, C.; Liu, A.; Shen, L.; Hu, X.; Wu, H.; et al. Synthesis and antidiabetic properties of chitosan-stabilized selenium nanoparticles. Colloids Surf. B 2018, 170, 115–121. [Google Scholar] [CrossRef]
- Xu, J.; Cao, L.; Suo, Y.; Xu, X.; Sun, H.; Xu, S.; Zhu, X.; Yu, H.; Cao, W. Chitosan-microcapsulated insulin alleviates mesenteric microcirculation dysfunction via modulating COX-2 and VCAM-1 expression in rats with diabetes mellitus. Int. J. Nanomed. 2018, 13, 6829–6837. [Google Scholar] [CrossRef]
- Kumar, S.G.; Rahman, M.A.; Lee, S.H.; Hwang, H.S.; Kim, H.A.; Yun, J.W. Plasma proteome analysis for anti-obesity and anti-diabetic potentials of chitosan oligosaccharides in ob/ob mice. Proteomics 2009, 9, 2149–2162. [Google Scholar] [CrossRef]
- Liu, B.; Liu, W.S.; Han, B.Q.; Sun, Y.Y. Antidiabetic effects of chitooligosaccharides on pancreatic islet cells in streptozotocin-induced diabetic rats. World J. Gastroenterol. 2007, 13, 725–731. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Qin, Z.K.; Lin, X.M.; Mei, L.; Liu, W.S.; Han, B.Q. Antidiabetic effects of chitooligosaccharides with different molecular weights on pancreatic islet cells in streptozotocin-induced diabetic rats. World Chin. J. Dig. 2009, 17, 36–42. [Google Scholar] [CrossRef]
- Qinna, N.A.; Karwi, Q.G.; Al-Jbour, N.; Al-Remawi, M.A.; Alhussainy, T.M.; Al-So’ud, K.A.; Al Omari, M.M.; Badwan, A.A. Influence of molecular weight and degree of deacetylation of low molecular weight chitosan on the bioactivity of oral insulin preparations. Mar. Drugs 2015, 13, 1710–1725. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Ahn, H.Y.; Kwak, J.H.; Shin, D.Y.; Kwon, Y.I.; Oh, C.G.; Lee, J.H. The effects of chitosan oligosaccharide (GO2KA1) supplementation on glucose control in subjects with prediabetes. Food Funct. 2014, 5, 2662–2669. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.Y.; Kwon, Y.I.; Lee, C.; Apostolidis, E.; Kim, Y.C. Antidiabetic effect of chitosan oligosaccharide (GO2KA1) is mediated via inhibition of intestinal alpha-glucosidase and glucose transporters and PPARgamma expression. BioFactors 2017, 43, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.; Min Cho, J.; Kwon, Y.I.; Kim, S.C.; Yeob Shin, D.; Ho Lee, J. Chitosan oligosaccharide (GO2KA1) improves postprandial glycemic response in subjects with impaired glucose tolerance and impaired fasting glucose and in healthy subjects: A crossover, randomized controlled trial. Nutr. Diabetes 2019, 9, 31. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Sun, T.; Wang, L. Chitosan oligosaccharide improves the therapeutic efficacy of sitagliptin for the therapy of Chinese elderly patients with type 2 diabetes mellitus. Clin. Risk. Manag. 2017, 13, 739–750. [Google Scholar] [CrossRef]
- Lupascu, F.G.; Giusca, S.E.; Caruntu, I.D.; Anton, A.; Lupusoru, C.E.; Profire, L. The safety profile of new antidiabetic xanthine derivatives and their chitosan based formulations. Eur. J. Pharm. Sci. 2019, 127, 71–78. [Google Scholar] [CrossRef]
- Wang, J.; He, W.; Yang, D.; Cao, H.; Bai, Y.; Guo, J.; Su, Z. Beneficial Metabolic Effects of Chitosan and Chitosan Oligosaccharide on Epididymal WAT Browning and Thermogenesis in Obese Rats. Molecules 2019, 24. [Google Scholar] [CrossRef]
- Yang, D.; Hu, C.; Deng, X.; Bai, Y.; Cao, H.; Guo, J.; Su, Z. Therapeutic Effect of Chitooligosaccharide Tablets on Lipids in High-Fat Diets Induced Hyperlipidemic Rats. Molecules 2019, 24. [Google Scholar] [CrossRef]
- Deng, X.; Ye, Z.; Cao, H.; Bai, Y.; Che, Q.; Guo, J.; Su, Z. Chitosan oligosaccharide ameliorated obesity by reducing endoplasmic reticulum stress in diet-induced obese rats. Food. Funct. 2020, 11, 6285–6296. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.H.; Chen, R.Y.; Chiang, M.T. Effects of Chitosan Oligosaccharide on Plasma and Hepatic Lipid Metabolism and Liver Histomorphology in Normal Sprague-Dawley Rats. Mar. Drugs 2020, 18, 408. [Google Scholar] [CrossRef]
- Izumi, R.; Azuma, K.; Izawa, H.; Morimoto, M.; Nagashima, M.; Osaki, T.; Tsuka, T.; Imagawa, T.; Ito, N.; Okamoto, Y.; et al. Chitin nanofibrils suppress skin inflammation in atopic dermatitis-like skin lesions in NC/Nga mice. Carbohydr. Polym. 2016, 146, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.J.; Beasley, K.N.; Acevedo, E.O.; Franco, R.L.; Jones, T.L.; Mari, D.C.; Shibata, Y. Chitin enhances obese inflammation ex vivo. Hum. Immunol. 2014, 75, 41–46. [Google Scholar] [CrossRef]
- Van Dyken, S.J.; Mohapatra, A.; Nussbaum, J.C.; Molofsky, A.B.; Thornton, E.E.; Ziegler, S.F.; McKenzie, A.N.J.; Krummel, M.F.; Liang, H.E.; Locksley, R.M. Chitin activates parallel immune modules that direct distinct inflammatory responses via innate lymphoid type 2 and γδ T cells. Immunity 2014, 40, 414–424. [Google Scholar] [CrossRef] [PubMed]
- Arae, K.; Morita, H.; Unno, H.; Motomura, K.; Toyama, S.; Okada, N.; Ohno, T.; Tamari, M.; Orimo, K.; Mishima, Y.; et al. Chitin promotes antigen-specific Th2 cell-mediated murine asthma through induction of IL-33-mediated IL-1beta production by DCs. Sci. Rep. 2018, 8, 11721. [Google Scholar] [CrossRef]
- Wagener, J.; Malireddi, R.K.S.; Lenardon, M.D.; Köberle, M.; Vautier, S.; MacCallum, D.M.; Biedermann, T.; Schaller, M.; Netea, M.G.; Kanneganti, T.D.; et al. Fungal Chitin Dampens Inflammation through IL-10 Induction Mediated by NOD2 and TLR9 Activation. PloS Pathog. 2014, 10. [Google Scholar] [CrossRef]
- Danti, S.; Trombi, L.; Fusco, A.; Azimi, B.; Lazzeri, A.; Morganti, P.; Coltelli, M.B.; Donnarumma, G. Chitin Nanofibrils and Nanolignin as Functional Agents in Skin Regeneration. Int. J. Mol. Sci. 2019, 20, 2669. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, C.; Guo, C.; Li, X. Chitosan Ameliorates DSS-Induced Ulcerative Colitis Mice by Enhancing Intestinal Barrier Function and Improving Microflora. Int. J. Mol. Sci. 2019, 20, 5751. [Google Scholar] [CrossRef]
- Gudmundsdottir, S.; Lieder, R.; Sigurjonsson, O.E.; Petersen, P.H. Chitosan leads to downregulation of YKL-40 and inflammasome activation in human macrophages. J. Biomed. Mater. Res. A 2015, 103, 2778–2785. [Google Scholar] [CrossRef]
- Liu, Y.; Zong, S.; Li, J. Carboxymethyl chitosan perturbs inflammation profile and colonic microbiota balance in mice. J. Food Drug Anal. 2020, 28, 175–182. [Google Scholar] [CrossRef]
- Yousef, M.; Pichyangkura, R.; Soodvilai, S.; Chatsudthipong, V.; Muanprasat, C. Chitosan oligosaccharide as potential therapy of inflammatory bowel disease: Therapeutic efficacy and possible mechanisms of action. Pharm. Res. 2012, 66, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Tong, Q.; Luo, H.; Huang, R.; Li, Z. Chitooligosaccharides attenuate lipopolysaccharide-induced inflammation and apoptosis of intestinal epithelial cells: Possible involvement of TLR4/NF-κB pathway. Indian J. Pharm. Educ. Res. 2016, 50, 109–115. [Google Scholar] [CrossRef]
- Xu, Q.; Liu, M.; Liu, Q.; Wang, W.; Du, Y.; Yin, H. The inhibition of LPS-induced inflammation in RAW264.7 macrophages via the PI3K/Akt pathway by highly N-acetylated chitooligosaccharide. Carbohydr. Polym. 2017, 174, 1138–1143. [Google Scholar] [CrossRef]
- Sánchez, Á.; Mengíbar, M.; Fernández, M.; Alemany, S.; Heras, A.; Acosta, N. Influence of preparation methods of chitooligosaccharides on their physicochemical properties and their anti-inflammatory effects in mice and in RAW264.7 macrophages. Mar. Drugs 2018, 16, 430. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Ji, H.; Zhang, L.; Wang, Y.; Zhou, H. Chitosan Oligosaccharide Exerts Anti-Allergic Effect against Shrimp Tropomyosin-Induced Food Allergy by Affecting Th1 and Th2 Cytokines. Int. Arch. Allergy Immunol. 2019, 180, 10–16. [Google Scholar] [CrossRef]
- Lan, R.; Li, S.; Chang, Q.; Zhao, Z. Chitosan oligosaccharides protect sprague dawley rats from cyclic heat stress by attenuation of oxidative and inflammation stress. Animals 2019, 9, 1074. [Google Scholar] [CrossRef] [PubMed]
- Wongkrasant, P.; Pongkorpsakol, P.; Chitwattananont, S.; Satianrapapong, W.; Tuangkijkul, N.; Muanprasat, C. Fructo-oligosaccharides alleviate inflammation-associated apoptosis of GLP-1 secreting L cells via inhibition of iNOS and cleaved caspase-3 expression. J. Pharm. Sci. 2020, 143, 65–73. [Google Scholar] [CrossRef]
- Sun, Y.; Shi, X.; Zheng, X.; Nie, S.; Xu, X. Inhibition of dextran sodium sulfate-induced colitis in mice by baker’s yeast polysaccharides. Carbohydr. Polym. 2019, 207, 371–381. [Google Scholar] [CrossRef]
- Libreros, S.; Garcia-Areas, R.; Iragavarapu-Charyulu, V. CHI3L1 plays a role in cancer through enhanced production of pro-inflammatory/pro-tumorigenic and angiogenic factors. Immunol. Res. 2013, 57, 99–105. [Google Scholar] [CrossRef]
- Libreros, S.; Garcia-Areas, R.; Shibata, Y.; Carrio, R.; Torroella-Kouri, M.; Iragavarapu-Charyulu, V. Induction of proinflammatory mediators by CHI3L1 is reduced by chitin treatment: Decreased tumor metastasis in a breast cancer model. Int. J. Cancer 2012, 131, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Timoshenko, A.V. Chitin hydrolysate stimulates VEGF-C synthesis by MDAMB-231 breast cancer cells. Cell Biol. Int. 2011, 35, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Solairaj, D.; Rameshthangam, P.; Arunachalam, G. Anticancer activity of silver and copper embedded chitin nanocomposites against human breast cancer (MCF-7) cells. Int. J. Biol. Macromol. 2017, 105, 608–619. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Dutta, P.K.; Kumar, H.; Kureel, A.K.; Rai, A.K. Synthesis of chitin-glucan-aldehyde-quercetin conjugate and evaluation of anticancer and antioxidant activities. Carbohydr. Polym. 2018, 193, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Arunraj, T.R.; Sanoj Rejinold, N.; Ashwin Kumar, N.; Jayakumar, R. Bio-responsive chitin-poly(l-lactic acid) composite nanogels for liver cancer. Colloids Surf. B 2014, 113, 394–402. [Google Scholar] [CrossRef]
- Pirzadeh-Naeeni, S.; Mozdianfard, M.R.; Shojaosadati, S.A.; Khorasani, A.C.; Saleh, T. A comparative study on schizophyllan and chitin nanoparticles for ellagic acid delivery in treating breast cancer. Int. J. Biol. Macromol. 2020, 144, 380–388. [Google Scholar] [CrossRef]
- Gibot, L.; Chabaud, S.; Bouhout, S.; Bolduc, S.; Auger, F.A.; Moulin, V.J. Anticancer properties of chitosan on human melanoma are cell line dependent. Int. J. Biol. Macromol. 2015, 72, 370–379. [Google Scholar] [CrossRef]
- Mattaveewong, T.; Wongkrasant, P.; Chanchai, S.; Pichyangkura, R.; Chatsudthipong, V.; Muanprasat, C. Chitosan oligosaccharide suppresses tumor progression in a mouse model of colitis-associated colorectal cancer through AMPK activation and suppression of NF-kappaB and mTOR signaling. Carbohydr. Polym. 2016, 145, 30–36. [Google Scholar] [CrossRef]
- Li, H.F.; Huang, L.F.; Chen, L.H. Chitooligosaccharides inhibit A549 lung cancer cell line proliferation by regulating cell autophagy. J. Biol. Regul. Homeost. Agents 2019, 33, 1527–1532. [Google Scholar]
- Wimardhani, Y.S.; Suniarti, D.F.; Freisleben, H.J.; Wanandi, S.I.; Siregar, N.C.; Ikeda, M.A. Chitosan exerts anticancer activity through induction of apoptosis and cell cycle arrest in oral cancer cells. J. Oral. Sci. 2014, 56, 119–126. [Google Scholar] [CrossRef]
- Abedian, Z.; Moghadamnia, A.A.; Zabihi, E.; Pourbagher, R.; Ghasemi, M.; Nouri, H.R.; Tashakorian, H.; Jenabian, N. Anticancer properties of chitosan against osteosarcoma, breast cancer and cervical cancer cell lines. Casp. J. Intern. Med. 2019, 10, 439–446. [Google Scholar] [CrossRef]
- Tan, G.; Kaya, M.; Tevlek, A.; Sargin, I.; Baran, T. Antitumor activity of chitosan from mayfly with comparison to commercially available low, medium and high molecular weight chitosans. In Vitro Cell Dev. Biol. Anim. 2018, 54, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, H.; Kanayairam, V.; Ravichandran, R. Chitin and chitosan preparation from shrimp shells Penaeus monodon and its human ovarian cancer cell line, PA-1. Int. J. Biol. Macromol. 2018, 107, 662–667. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Han, B.; Li, H.; Yang, Y.; Liu, W. Carboxymethyl chitosan represses tumor angiogenesis in vitro and in vivo. Carbohydr. Polym. 2015, 129, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Zhao, Y.; Wang, C.; Ji, H.; Yu, J.; Liu, C.; Liu, A. A novel synthetic chitosan selenate (CS) induces apoptosis in A549 lung cancer cells via the Fas/FasL pathway. Int. J. Biol. Macromol. 2020, 158, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Hasanzade, Z.; Raissi, H. Assessment of the chitosan-functionalized graphene oxide as a carrier for loading thioguanine, an antitumor drug and effect of urea on adsorption process: Combination of DFT computational and molecular dynamics simulation studies. J. Biomol. Struct. Dyn. 2019, 37, 2487–2497. [Google Scholar] [CrossRef]
- Lee, J.Y.; Termsarasab, U.; Lee, M.Y.; Kim, D.H.; Lee, S.Y.; Kim, J.S.; Cho, H.J.; Kim, D.D. Chemosensitizing indomethacin-conjugated chitosan oligosaccharide nanoparticles for tumor-targeted drug delivery. Acta. Biomater. 2017, 57, 262–273. [Google Scholar] [CrossRef]
- Li, P.W.; Wang, G.; Yang, Z.M.; Duan, W.; Peng, Z.; Kong, L.X.; Wang, Q.H. Development of drug-loaded chitosan-vanillin nanoparticles and its cytotoxicity against HT-29 cells. Drug Deliv. 2016, 23, 30–35. [Google Scholar] [CrossRef]
- Hafsa, J.; Smach, M.A.; Charfeddine, B.; Limem, K.; Majdoub, H.; Rouatbi, S. Antioxidant and antimicrobial proprieties of chitin and chitosan extracted from Parapenaeus Longirostris shrimp shell waste. Ann. Pharm. Fr. 2016, 74, 27–33. [Google Scholar] [CrossRef]
- Morganti, P.; Fabrizi, G.; Palombo, P.; Palombo, M.; Guarneri, F.; Cardillo, A.; Morganti, G. New chitin complexes and their anti-aging activity from inside out. J. Nutr. Health Aging 2012, 16, 242–245. [Google Scholar] [CrossRef]
- Morganti, P.; Morganti, G.; Colao, C. Biofunctional Textiles for Aging Skin. Biomedicines 2019, 7. [Google Scholar] [CrossRef] [PubMed]
- Anandan, R.; Ganesan, B.; Obulesu, T.; Mathew, S.; Asha, K.K.; Lakshmanan, P.T.; Zynudheen, A.A. Antiaging effect of dietary chitosan supplementation on glutathione-dependent antioxidant system in young and aged rats. Cell Stress Chaperones 2013, 18, 121–125. [Google Scholar] [CrossRef]
- Qiao, Y.; Bai, X.F.; Du, Y.G. Chitosan oligosaccharides protect mice from LPS challenge by attenuation of inflammation and oxidative stress. Int. Immunopharmacol. 2011, 11, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.M.; Yu, S.H.; Zhang, L.S.; Zhao, Z.Y.; Dong, L.L. Effects of several acetylated chitooligosaccharides on antioxidation, antiglycation and NO generation in erythrocyte. Bioorg. Med. Chem. Lett. 2014, 24, 4053–4057. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Li, Y.; Chang, Q.; Guo, G.; Lan, R. Effects of chitosan oligosaccharides on intestinal oxidative stress and inflammation response in heat stressed rats. Exp. Anim. 2020, 20–0085. [Google Scholar] [CrossRef] [PubMed]
- Lan, R.; Chang, Q.; An, L.; Zhao, Z. Dietary Supplementation with Chitosan Oligosaccharides Alleviates Oxidative Stress in Rats Challenged with Hydrogen Peroxide. Animals 2019, 10. [Google Scholar] [CrossRef]
- Xu, Y.Q.; Xing, Y.Y.; Wang, Z.Q.; Yan, S.M.; Shi, B.L. Pre-protective effects of dietary chitosan supplementation against oxidative stress induced by diquat in weaned piglets. Cell Stress Chaperones 2018, 23, 703–710. [Google Scholar] [CrossRef]
- Kong, S.-Z.; Li, J.-C.; Li, S.-D.; Liao, M.-N.; Li, C.-P.; Zheng, P.-J.; Guo, M.-H.; Tan, W.-X.; Zheng, Z.-H.; Hu, Z. Anti-Aging Effect of Chitosan Oligosaccharide on d-Galactose-Induced Subacute Aging in Mice. Mar. Drugs 2018, 16, 181. [Google Scholar] [CrossRef]
- Wang, Y.; Xiong, Y.; Zhang, A.; Zhao, N.; Zhang, J.; Zhao, D.; Yu, Z.; Xu, N.; Yin, Y.; Luan, X.; et al. Oligosaccharide attenuates aging-related liver dysfunction by activating Nrf2 antioxidant signaling. Food Sci. Nutr. 2020, 8, 3872–3881. [Google Scholar] [CrossRef]
- Chen, F.; Hou, L.; Zhu, L.; Chengbo, Y.; Zhu, F.; Qiu, H.; Qin, S. Effects of selenide chitosan sulfate on glutathione system in hepatocytes and specific pathogen-free chickens. Poult. Sci. 2020, 99, 3979–3986. [Google Scholar] [CrossRef]
- Chaiwong, N.; Leelapornpisid, P.; Jantanasakulwong, K.; Rachtanapun, P.; Seesuriyachan, P.; Sakdatorn, V.; Leksawasdi, N.; Phimolsiripol, Y. Antioxidant and Moisturizing Properties of Carboxymethyl Chitosan with Different Molecular Weights. Polymers 2020, 12, 1445. [Google Scholar] [CrossRef] [PubMed]
- Ahlawat, J.; Neupane, R.; Deemer, E.; Sreenivasan, S.T.; Narayan, M. Chitosan-Ellagic Acid Nanohybrid for Mitigating Rotenone-induced Oxidative Stress. ACS Appl. Mater. Interfaces 2020, 12, 18964–18977. [Google Scholar] [CrossRef] [PubMed]
- Park, H.H.; Ko, S.C.; Oh, G.W.; Jang, Y.M.; Kim, Y.M.; Park, W.S.; Choi, I.W.; Jung, W.K. Characterization and biological activity of PVA hydrogel containing chitooligosaccharides conjugated with gallic acid. Carbohydr. Polym. 2018, 198, 197–205. [Google Scholar] [CrossRef]
- Tachaboonyakiat, W.; Sukpaiboon, E.; Pinyakong, O. Development of an antibacterial chitin betainate wound dressing. Polym. J. 2014, 46, 505–510. [Google Scholar] [CrossRef]
- Kovalchuk, V.; Voronkina, A.; Binnewerg, B.; Schubert, M.; Muzychka, L.; Wysokowski, M.; Tsurkan, M.V.; Bechmann, N.; Petrenko, I.; Fursov, A.; et al. Naturally Drug-Loaded Chitin: Isolation and Applications. Mar. Drugs 2019, 17, 574. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Lee, J.W.; Pham, D.T.N.; Kim, Y.M. Chitooligosaccharides as antibacterial, antibiofilm, antihemolytic and anti-virulence agent against staphylococcus aureus. Curr. Pharm. Biotechnol. 2019, 20, 1223–1233. [Google Scholar] [CrossRef]
- Ahmed, S.A.; El-Mahallawy, H.S.; Karanis, P. Inhibitory activity of chitosan nanoparticles against Cryptosporidium parvum oocysts. Parasitol. Res. 2019, 118, 2053–2063. [Google Scholar] [CrossRef]
- Mammeri, M.; Chevillot, A.; Thomas, M.; Polack, B.; Julien, C.; Marden, J.P.; Auclair, E.; Vallee, I.; Adjou, K.T. Efficacy of chitosan, a natural polysaccharide, against Cryptosporidium parvum in vitro and in vivo in neonatal mice. Exp. Parasitol. 2018, 194, 1–8. [Google Scholar] [CrossRef]
- Arias, L.S.; Butcher, M.C.; Short, B.; McKloud, E.; Delaney, C.; Kean, R.; Monteiro, D.R.; Williams, C.; Ramage, G.; Brown, J.L. Chitosan Ameliorates Candida auris Virulence in a Galleria mellonella Infection Model. Antimicrob. Agents Chemother. 2020, 64. [Google Scholar] [CrossRef]
- Ganan, M.; Lorentzen, S.B.; Agger, J.W.; Heyward, C.A.; Bakke, O.; Knutsen, S.H.; Aam, B.B.; Eijsink, V.G.H.; Gaustad, P.; Sørlie, M. Antifungal activity of well-defined chito-oligosaccharide preparations against medically relevant yeasts. PLoS ONE 2019, 14, e0210208. [Google Scholar] [CrossRef]
- Li, T.; Zhang, Y.; Xu, M.; Liu, Y.; Zhang, C.; Zhang, Y.; Peng, X.; Li, Z.; Qin, S.; Xing, K. Novel antifungal mechanism of oligochitosan by triggering apoptosis through a metacaspase-dependent mitochondrial pathway in Ceratocystis fimbriata. Carbohydr. Polym. 2020, 245, 116574. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tan, W.; Li, Q.; Dong, F.; Guo, Z. Synthesis and Characterization of N,N,N-trimethyl-O-(ureidopyridinium)acetyl Chitosan Derivatives with Antioxidant and Antifungal Activities. Mar. Drugs 2020, 18, 163. [Google Scholar] [CrossRef] [PubMed]
- Mei, L.; Xu, Z.; Shi, Y.; Lin, C.; Jiao, S.; Zhang, L.; Li, P. Multivalent and synergistic chitosan oligosaccharide-Ag nanocomposites for therapy of bacterial infection. Sci. Rep. 2020, 10, 10011. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Lee, J.W.; Manivasagan, P.; Pham, D.T.N.; Oh, J.; Kim, Y.M. Synthesis and characterization of chitosan oligosaccharide-capped gold nanoparticles as an effective antibiofilm drug against the Pseudomonas aeruginosa PAO1. Microb. Pathog. 2019, 135. [Google Scholar] [CrossRef]
- Liu, X.; Xia, W.; Jiang, Q.; Yu, P.; Yue, L. Chitosan oligosaccharide-N-chlorokojic acid mannich base polymer as a potential antibacterial material. Carbohydr. Polym. 2018, 182, 225–234. [Google Scholar] [CrossRef]
- Nakajima, M.; Atsumi, K.; Kifune, K. Chitin is an effective material for sutures. Jpn. J. Surg. 1986, 16, 418–424. [Google Scholar] [CrossRef]
- Klinger, C.; Żółtowska-Aksamitowska, S.; Wysokowski, M.; Tsurkan, M.V.; Galli, R.; Petrenko, I.; Machałowski, T.; Ereskovsky, A.; Martinović, R.; Muzychka, L.; et al. Express Method for Isolation of Ready-to-Use 3D Chitin Scaffolds from Aplysina archeri (Aplysineidae: Verongiida) Demosponge. Mar. Drugs 2019, 17, 131. [Google Scholar] [CrossRef]
- Shao, K.; Han, B.; Gao, J.; Jiang, Z.; Liu, W.; Liu, W.; Liang, Y. Fabrication and feasibility study of an absorbable diacetyl chitin surgical suture for wound healing. J. Biomed. Mater. Res. B 2016, 104, 116–125. [Google Scholar] [CrossRef]
- Balitaan, J.N.I.; Hsiao, C.D.; Yeh, J.M.; Santiago, K.S. Innovation inspired by nature: Biocompatible self-healing injectable hydrogels based on modified-β-chitin for wound healing. Int. J. Biol. Macromol. 2020, 162, 723–736. [Google Scholar] [CrossRef]
- Chao, F.C.; Wu, M.H.; Chen, L.C.; Lin, H.L.; Liu, D.Z.; Ho, H.O.; Sheu, M.T. Preparation and characterization of chemically TEMPO-oxidized and mechanically disintegrated sacchachitin nanofibers (SCNF) for enhanced diabetic wound healing. Carbohydr. Polym. 2020, 229, 115507. [Google Scholar] [CrossRef]
- Zhong, Z.; Huang, Y.; Hu, Q.; He, W.; Duan, B.; Yan, X.; Yang, Z.; Liang, W.; Liu, Z.; Peng, Z.; et al. Elucidation of molecular pathways responsible for the accelerated wound healing induced by a novel fibrous chitin dressing. Biomater. Sci. 2019, 7, 5247–5257. [Google Scholar] [CrossRef]
- Kuwabara, M.; Sato, Y.; Ishihara, M.; Takayama, T.; Nakamura, S.; Fukuda, K.; Murakami, K.; Yokoe, H.; Kiyosawa, T. Healing of Pseudomonas aeruginosa-infected wounds in diabetic db/db mice by weakly acidic hypochlorous acid cleansing and silver nanoparticle/chitin-nanofiber sheet covering. Wound Med. 2020, 28. [Google Scholar] [CrossRef]
- Zhou, D.; Yang, R.; Yang, T.; Xing, M.; Luo, G. Preparation of chitin-amphipathic anion/ quaternary ammonium salt ecofriendly dressing and its effect on wound healing in mice. Int. J. Nanomed. 2018, 13, 4157–4169. [Google Scholar] [CrossRef] [PubMed]
- Abudula, T.; Gauthaman, K.; Mostafavi, A.; Alshahrie, A.; Salah, N.; Morganti, P.; Chianese, A.; Tamayol, A.; Memic, A. Sustainable drug release from polycaprolactone coated chitin-lignin gel fibrous scaffolds. Sci. Rep. 2020, 10, 20428. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.; Bi, S.; Kong, T.; Luo, X.; Zhou, Z.; Qiu, K.; Huang, L.; Chen, X.; Kong, M. Mechanically and functionally strengthened tissue adhesive of chitin whisker complexed chitosan/dextran derivatives based hydrogel. Carbohydr. Polym. 2020, 237, 116138. [Google Scholar] [CrossRef]
- Maevskaia, E.N.; Shabunin, A.S.; Dresvyanina, E.N.; Dobrovol’skaya, I.P.; Yudin, V.E.; Paneyah, M.B.; Fediuk, A.M.; Sushchinskii, P.L.; Smirnov, G.P.; Zinoviev, E.V.; et al. Influence of the Introduced Chitin Nanofibrils on Biomedical Properties of Chitosan-Based Materials. Nanomaterials 2020, 10, 945. [Google Scholar] [CrossRef]
- Li, Z.W.; Li, C.W.; Wang, Q.; Shi, S.J.; Hu, M.; Zhang, Q.; Cui, H.H.; Sun, J.B.; Zhou, M.; Wu, G.L.; et al. The cellular and molecular mechanisms underlying silver nanoparticle/chitosan oligosaccharide/poly(vinyl alcohol) nanofiber-mediated wound healing. J. Biomed. Nanotechnol. 2017, 13, 17–34. [Google Scholar] [CrossRef]
- Li, C.W.; Wang, Q.; Li, J.; Hu, M.; Shi, S.J.; Li, Z.W.; Wu, G.L.; Cui, H.H.; Li, Y.Y.; Zhang, Q.; et al. Silver nanoparticles/chitosan oligosaccharide/poly(vinyl alcohol) nanofiber promotes wound healing by activating TGFβ1/Smad signaling pathway. Int. J. Nanomed. 2016, 11, 373–387. [Google Scholar] [CrossRef]
- Cifuentes, A.; Gomez-Gil, V.; Ortega, M.A.; Asunsolo, A.; Coca, S.; Roman, J.S.; Alvarez-Mon, M.; Bujan, J.; Garcia-Honduvilla, N. Chitosan hydrogels functionalized with either unfractionated heparin or bemiparin improve diabetic wound healing. Biomed. Pharm. 2020, 129, 110498. [Google Scholar] [CrossRef]
- Natarajan, J.; Sanapalli, B.K.R.; Bano, M.; Singh, S.K.; Gulati, M.; Karri, V. Nanostructured Lipid Carriers of Pioglitazone Loaded Collagen/Chitosan Composite Scaffold for Diabetic Wound Healing. Adv. Wound Care (New Rochelle) 2019, 8, 499–513. [Google Scholar] [CrossRef]
- Viezzer, C.; Mazzuca, R.; Machado, D.C.; de Camargo Forte, M.M.; Gomez Ribelles, J.L. A new waterborne chitosan-based polyurethane hydrogel as a vehicle to transplant bone marrow mesenchymal cells improved wound healing of ulcers in a diabetic rat model. Carbohydr. Polym. 2020, 231, 115734. [Google Scholar] [CrossRef] [PubMed]
- Xia, G.; Zhai, D.; Sun, Y.; Hou, L.; Guo, X.; Wang, L.; Li, Z.; Wang, F. Preparation of a novel asymmetric wettable chitosan-based sponge and its role in promoting chronic wound healing. Carbohydr. Polym. 2020, 227, 115296. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.H.; Lee, S.E.; Tran, T.T.; Bui, C.B.; Nguyen, T.H.N.; Vu, N.B.D.; Tran, T.T.; Nguyen, T.H.P.; Nguyen, T.T.; Hadinoto, K. A simple strategy to enhance the in vivo wound-healing activity of curcumin in the form of self-assembled nanoparticle complex of curcumin and oligochitosan. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 98, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Liu, Y.; Song, S.; Tong, C.; Shi, X.; Zhao, Y.; Zhang, J.; Hou, M. Influence of chitosan oligosaccharide on the gelling and wound healing properties of injectable hydrogels based on carboxymethyl chitosan/alginate polyelectrolyte complexes. Carbohydr. Polym. 2019, 205, 312–321. [Google Scholar] [CrossRef]
- Niho, N.; Tamura, T.; Toyoda, K.; Uneyama, C.; Shibutani, M.; Hirose, M. A 13-week subchronic toxicity study of chitin in F344 rats. Kokuritsu Iyakuhin Shokuhin Eisei Kenkyusho Hokoku 1999, 117, 129–134. [Google Scholar]
- Mohammed, M.A.; Syeda, J.T.M.; Wasan, K.M.; Wasan, E.K. An Overview of Chitosan Nanoparticles and Its Application in Non-Parenteral Drug Delivery. Pharmaceutics 2017, 9, 53. [Google Scholar] [CrossRef]
- Bellich, B.; D’Agostino, I.; Semeraro, S.; Gamini, A.; Cesàro, A. “The Good, the Bad and the Ugly” of Chitosans. Mar. Drugs 2016, 14, 99. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).