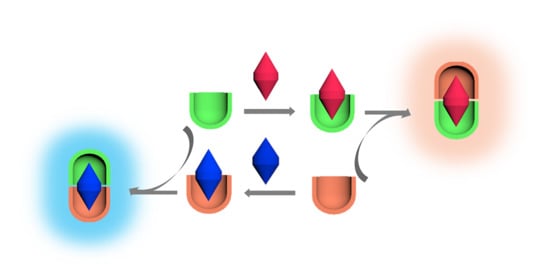
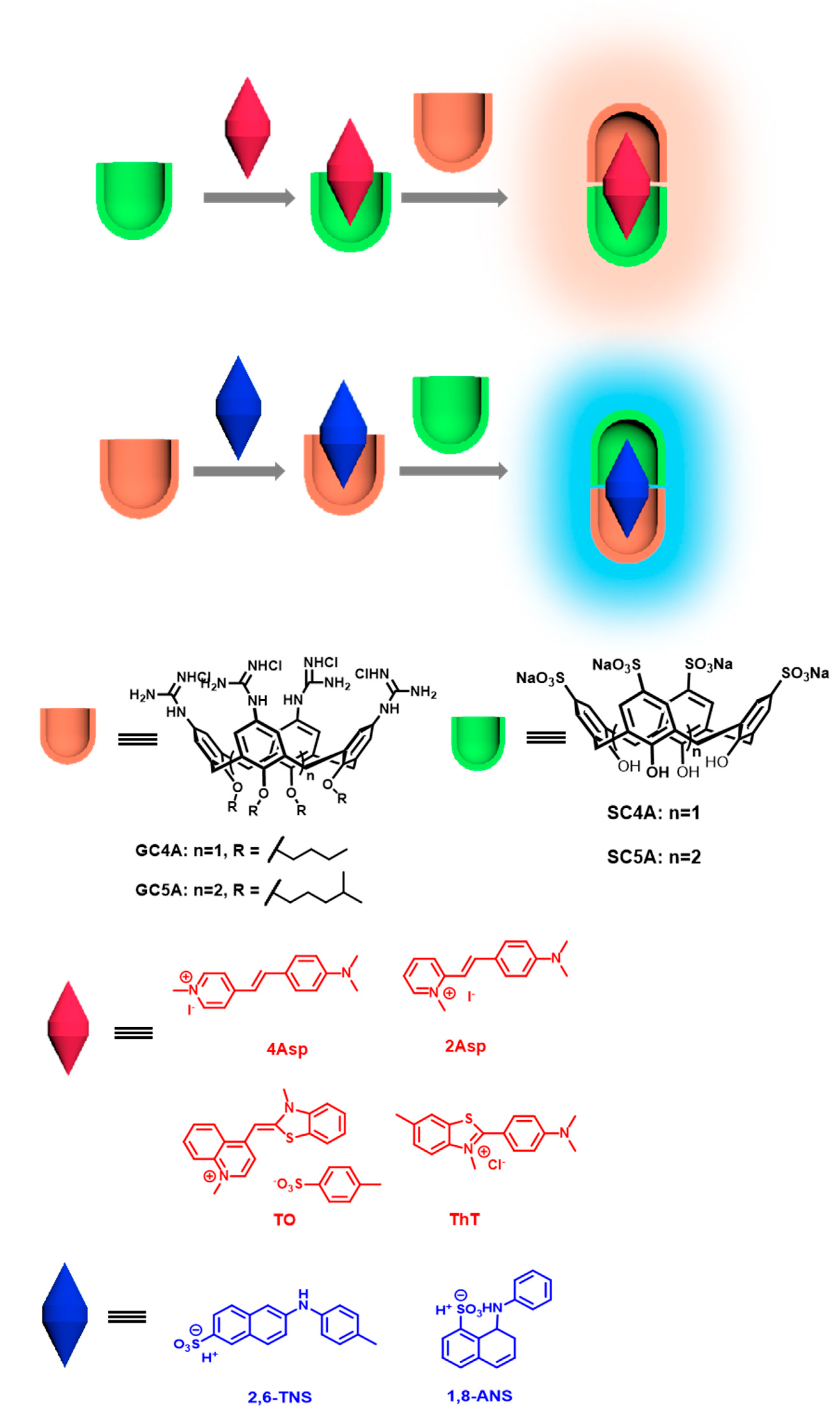
Fluorescence Enhancement by Calixarene Supramolecular Aggregate
Abstract
1. Introduction
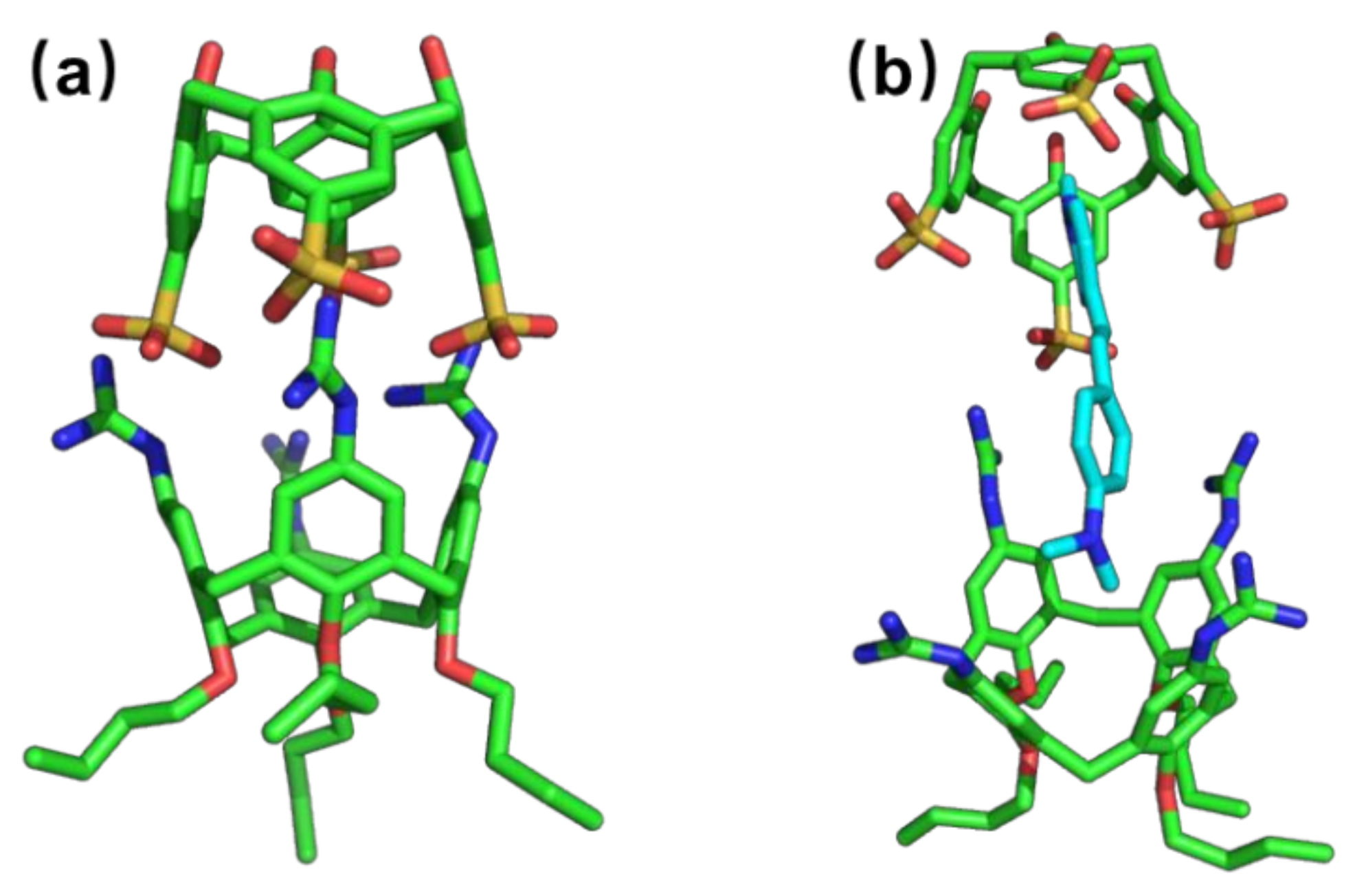
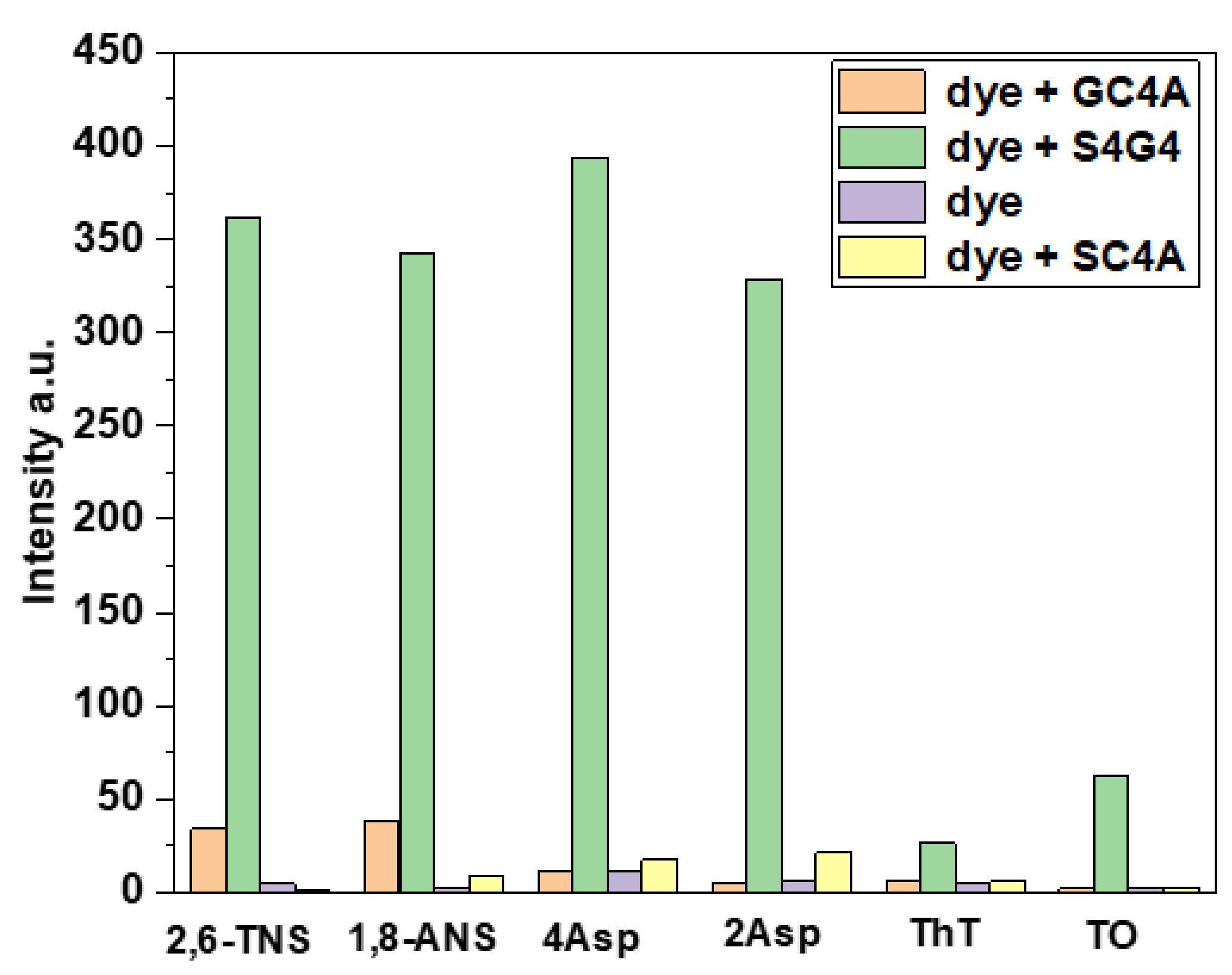
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Instruments
3.3. ITC Experiment
3.4. Fluorescence Quantum Yield Measurements
3.5. Molecular Simulation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- You, L.; Zha, D.; Anslyn, E.V. Recent Advances in Supramolecular Analytical Chemistry Using Optical Sensing. Chem. Rev. 2015, 115, 7840–7892. [Google Scholar] [CrossRef] [PubMed]
- Rebek, J., Jr. Simultaneous Encapsulation: Molecules Held at Close Range. Angew. Chem. Int. Ed. 2005, 44, 2068–2078. [Google Scholar] [CrossRef] [PubMed]
- Ogoshi, T.; Harada, A. Chemical Sensors Based on Cyclodextrin Derivatives. Sensors 2008, 8, 4961–4982. [Google Scholar] [CrossRef] [PubMed]
- Berberan-Santos, M.N.; Choppinet, P.; Fedorov, A.; Jullien, L.; Valeur, B. Multichromophoric Cyclodextrins. 8. Dynamics of Homo- and Heterotransfer of Excitation Energy in Inclusion Complexes with Fluorescent Dyes. J. Am. Chem. Soc. 2000, 122, 11876–11886. [Google Scholar] [CrossRef]
- Xu, Z.; Peng, S.; Wang, Y.-Y.; Zhang, J.-K.; Lazar, A.I.; Guo, D.-S. Broad-Spectrum Tunable Photoluminescent Nanomaterials Constructed from a Modular Light-Harvesting Platform Based on Macrocyclic Amphiphiles. Adv. Mater. 2016, 28, 7666–7671. [Google Scholar] [CrossRef] [PubMed]
- Dsouza, R.N.; Pischel, U.; Nau, W.M. Fluorescent dyes and their supramolecular host/guest complexes with macrocycles in aqueous solution. Chem. Rev. 2011, 111, 7941–7980. [Google Scholar] [CrossRef] [PubMed]
- Barooah, N.; Mohanty, J.; Pal, H.; Bhasikuttan, A.C. Non-covalent interactions of coumarin dyes with cucurbit[7]uril macrocycle: Modulation of ICT to TICT state conversion. Org. Biomol. Chem. 2012, 10, 5055–5062. [Google Scholar] [CrossRef]
- Choudhury, S.D.; Mohanty, J.; Pal, H.; Bhasikuttan, A.C. Cooperative Metal Ion Binding to a Cucurbit[7]uril−Thioflavin T Complex: Demonstration of a Stimulus-Responsive Fluorescent Supramolecular Capsule. J. Am. Chem. Soc. 2010, 132, 1395–1401. [Google Scholar] [CrossRef]
- Hua, B.; Zhou, W.; Yang, Z.; Zhang, Z.; Shao, L.; Zhu, H.; Huang, F. Supramolecular Solid-State Microlaser Constructed from Pillar[5]arene-Based Host–Guest Complex Microcrystals. J. Am. Chem. Soc. 2018, 140, 15651–15654. [Google Scholar] [CrossRef]
- Xia, Y.; Chen, S.; Ni, X.-L. White Light Emission from Cucurbituril-Based Host-Guest Interaction in the Solid State: New Function of the Macrocyclic Host. ACS Appl. Mater. Interfaces 2018, 10, 13048–13052. [Google Scholar] [CrossRef]
- Teran, N.B.; He, G.S.; Baev, A.; Shi, Y.; Swihart, M.T.; Prasad, P.N.; Marks, T.J.; Reynolds, J.R. Twisted Thiophene-Based Chromophores with Enhanced Intramolecular Charge Transfer for Cooperative Amplification of Third-Order Optical Nonlinearity. J. Am. Chem. Soc. 2016, 138, 6975–6984. [Google Scholar] [CrossRef] [PubMed]
- Biedermann, F.; Elmalem, E.; Ghosh, I.; Nau, W.M.; Scherman, O.A. Strongly Fluorescent, Switchable Perylene Bis(diimide) Host–Guest Complexes with Cucurbit[8]uril In Water. Angew. Chem. Int. Ed. 2012, 51, 7739–7743. [Google Scholar] [CrossRef] [PubMed]
- Jana, P.; Mukherjee, T.; Khurana, R.; Barooah, N.; Soppina, V.; Mohanty, J.; Kanvah, S. Fluorescence enhancement of cationic styrylcoumarin-cucurbit[7]uril complexes: Enhanced stability and cellular membrane localization. J. Photochem. Photobiol. A 2019, 384, 112062. [Google Scholar] [CrossRef]
- Sowmiya, M.; Purkayastha, P.; Tiwari, A.K.; Jaffer, S.S.; Saha, S.K. Characterization of guest molecule concentration dependent nanotubes of β-cyclodextrin and their secondary assembly: Study with trans-2-[4(dimethylamino)styryl]benzothiazole, a TICT-fluorescence probe. J. Photochem. Photobiol. A 2009, 205, 186–196. [Google Scholar] [CrossRef]
- Singh, P.K.; Kumbhakar, M.; Pal, H.; Nath, S. Confined ultrafast torsional dynamics of Thioflavin-T in a nanocavity. Phys. Chem. Chem. Phys. 2011, 13, 8008–8014. [Google Scholar] [CrossRef]
- Schoder, S.; Schröder, H.V.; Cera, L.; Puttreddy, R.; Güttler, A.; Resch-Genger, U.; Rissanen, K.; Schalley, C.A. Strong Emission Enhancement in pH-Responsive 2:2 Cucurbit[8]uril Complexes. Chem. Eur. J. 2019, 25, 3257–3261. [Google Scholar] [CrossRef]
- Bhasikuttan, A.C.; Mohanty, J.; Nau, W.M.; Pal, H. Efficient fluorescence enhancement and cooperative binding of an organic dye in a supra-biomolecular host-protein assembly. Angew. Chem. Int. Ed. 2007, 46, 4120–4122. [Google Scholar] [CrossRef]
- Kitagawa, H.; Kobori, Y.; Yamanaka, M.; Yoza, K.; Kobayashi, K. Molecular recognition and self-assembly special feature: Encapsulated-guest rotation in a self-assembled heterocapsule directed toward a supramolecular gyroscope. Proc. Natl. Acad. Sci. USA 2009, 106, 10444–10448. [Google Scholar] [CrossRef]
- Biros, S.M.; Rebek, J., Jr. Structure and binding properties of water-soluble cavitands and capsules. Chem. Soc. Rev. 2007, 36, 93–104. [Google Scholar] [CrossRef]
- Ajami, D.; Rebek, J., Jr. More Chemistry in Small Spaces. Acc. Chem. Res. 2013, 46, 990–999. [Google Scholar] [CrossRef]
- Rebek, J., Jr. Host–guest chemistry of calixarene capsules. Chem. Commun. 2000, 637–643. [Google Scholar] [CrossRef]
- Schalley, C.A.; Castellano, R.K.; Brody, M.S.; Rudkevich, D.M.; Siuzdak, G.; Rebek, J., Jr. Investigating Molecular Recognition by Mass Spectrometry: Characterization of Calixarene-Based Self-Assembling Capsule Hosts with Charged Guests. J. Am. Chem. Soc. 1999, 121, 4568–4579. [Google Scholar] [CrossRef]
- Shimizu, K.D.; Rebek, J., Jr. Synthesis and assembly of self-complementary calix[4]arenes. Proc. Natl. Acad. Sci. USA 1995, 92, 12403–12407. [Google Scholar] [CrossRef] [PubMed]
- Brewster, R.E.; Shuker, S.B. Molecular recognition in methanol: The first example of hydrogen-bond-mediated self-association of a calix[4]arene in polar. J. Am. Chem. Soc. 2002, 124, 7902–7903. [Google Scholar] [CrossRef] [PubMed]
- Corbellini, F.; Di Costanzo, L.; Crego-Calama, M.; Geremia, S.; Reinhoudt, D.N. Guest encapsulation in a water-soluble molecular capsule based on ionic interactions. J. Am. Chem. Soc. 2003, 125, 9946–9947. [Google Scholar] [CrossRef]
- Bonaccorso, C.; Sgarlata, C.; Grasso, G.; Zito, V.; Sciotto, D.; Arena, G. A gemini guest triggers the self-assembly of a calixarene capsule in water at neutral pH. Chem. Commun. 2011, 47, 6117–6119. [Google Scholar] [CrossRef]
- Steed, J.W.; Johnson, C.P.; Barnes, C.L.; Juneja, R.K.; Atwood, J.L.; Reilly, S.; Hollis, R.L.; Smith, P.H.; Clark, D.L. Supramolecular Chemistry of p-Sulfonatocalix[5]arene: A Water-Soluble, Bowl-Shaped Host with a Large Molecular Cavity. J. Am. Chem. Soc. 1995, 117, 11426–11433. [Google Scholar] [CrossRef]
- Zheng, Z.; Geng, W.-C.; Gao, J.; Wang, Y.-Y.; Sun, H.; Guo, D.-S. Ultrasensitive and specific fluorescence detection of a cancer biomarker via nanomolar binding to a guanidinium-modified calixarene. Chem. Sci. 2018, 9, 2087–2091. [Google Scholar] [CrossRef]
- Zhao, H.-X.; Guo, D.-S.; Liu, Y. Binding Behaviors of p-Sulfonatocalix[4]arene with Gemini Guests. J. Phys. Chem. B 2013, 117, 1978–1987. [Google Scholar] [CrossRef]
- Grabowski, Z.R.; Rotkiewicz, K.; Rettig, W. Structural changes accompanying intramolecular electron transfer: Focus on twisted intramolecular charge-transfer states and structures. Chem. Rev. 2003, 103, 3899–4032. [Google Scholar] [CrossRef]
- Huang, Y.; Cheng, T.; Li, F.; Huang, C.-H.; Wang, S.; Huang, W.; Gong, Q. Studies on the Mono- and Dichromophoric Hemicyanine Dyes III. Ultrafast Fluorescence Up-conversion in Methanol: Twisting Intramolecular Charge Transfer and “Two-State Three-Mode” Model. J. Phys. Chem. B 2002, 106, 10041–10050. [Google Scholar] [CrossRef]
- Haidekker, M.A.; Theodorakis, E.A. Environment-sensitive behavior of fluorescent molecular rotors. Mapping viscosity in cells using molecular rotors. J. Biol. Eng. 2010, 4, 11. [Google Scholar] [CrossRef] [PubMed]
- Kuimova, M.K. Mapping viscosity in cells using molecular rotors. Phys. Chem. Chem. Phys. 2012, 14, 12671–12686. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Dong, H.; Wei, C.; Zhang, W.; Yan, Y.; Zhao, Y.S. Wavelength-Tunable Microlasers Based on the Encapsulation of Organic Dye in Metal-Organic Frameworks. Adv. Mater. 2016, 28, 7424–7429. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.-C.; Wang, H.; Xu, X.; Tian, H.-W.; Zhao, H.; Hu, X.-Y.; Zhao, Y.; Liu, Y.; Ding, G.; Meng, Q.; et al. Coassembly of macrocyclic amphiphiles for anti-β-amyloid therapy of Alzheimer’s disease. CCS Chem. 2020. [Google Scholar] [CrossRef]
- Lacroix, P.G.; Nakatani, K. Multiproperty molecular materials combining conductivity and second-order optical nonlinearities. Adv. Mater. 1997, 9, 1105–1108. [Google Scholar] [CrossRef]
- Valeur, B. Molecular Fluorescence; Wiley-VCH: Weinheim, Germany, 2002; p. 104. [Google Scholar]
- Hatcher, E.; Guvench, O.; MacKerell, A., Jr. CHARMM Additive All-Atom Force Field for Acyclic Polyalcohols, Acyclic Carbohydrates, and Inositol. J. Chem. Theory Comput. 2009, 5, 1315–1329. [Google Scholar] [CrossRef]
- Vanommeslaeghe, K.; Hatcher, E.; Acharya, C.; Kundu, S.; Zhong, S.; Shim, J.; Darian, E.; Guvench, O.; Lopes, P.; Vorobyov, I.; et al. CHARMM general force field: A force field for drug-like molecules compatible with the CHARMM all-atom additive biological force fields. J. Comput. Chem. 2010, 31, 671–690. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Chandrasekhar, J.; Madura, J.D.; Impey, R.W.; Klein, M.L. Comparison of Simple Potential Functions for Simulating Liquid Water. Journal of Chemical Physics. J. Chem. Phys. 1983, 79, 926–935. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. Model. 1996, 14, 33–38. [Google Scholar] [CrossRef]
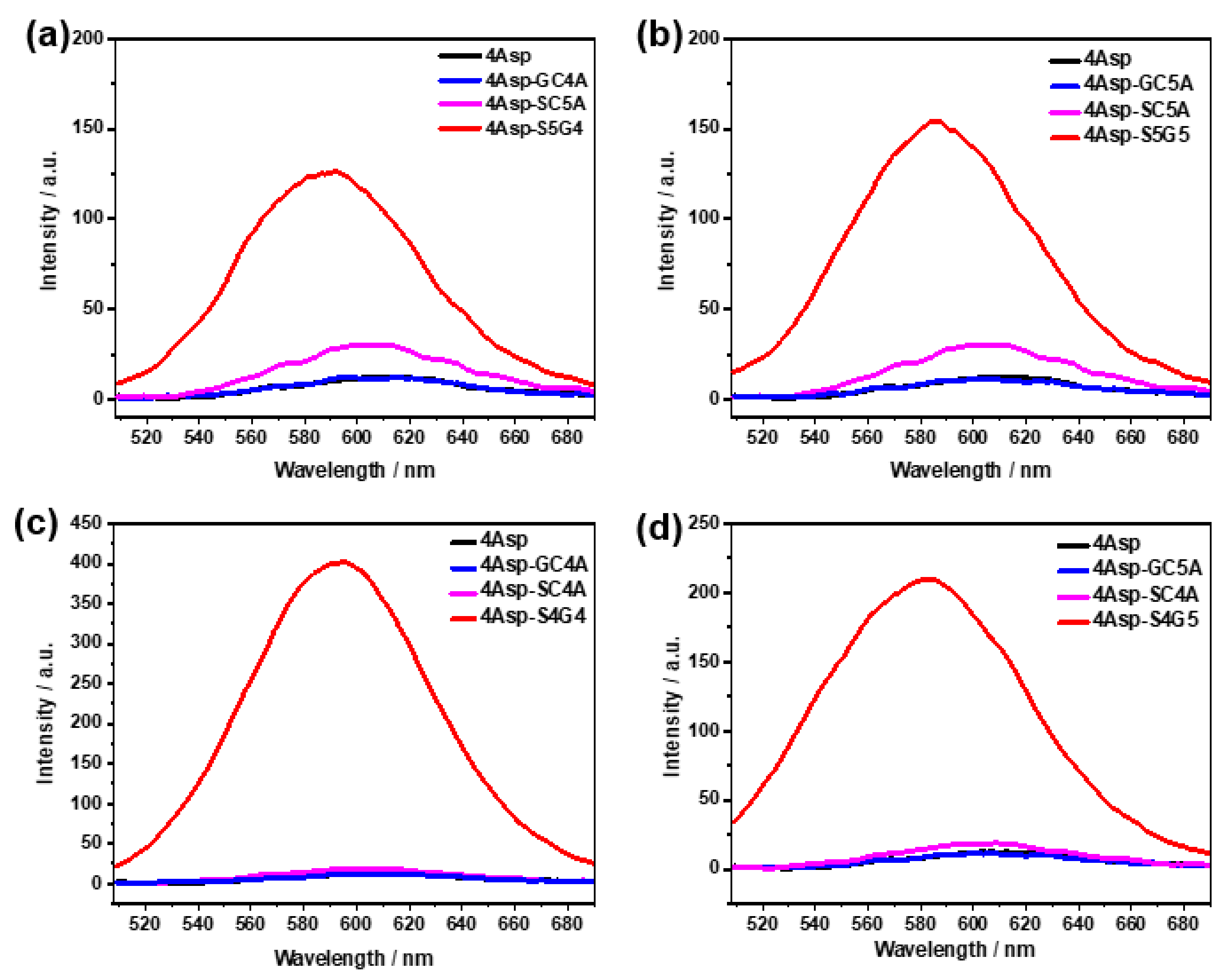
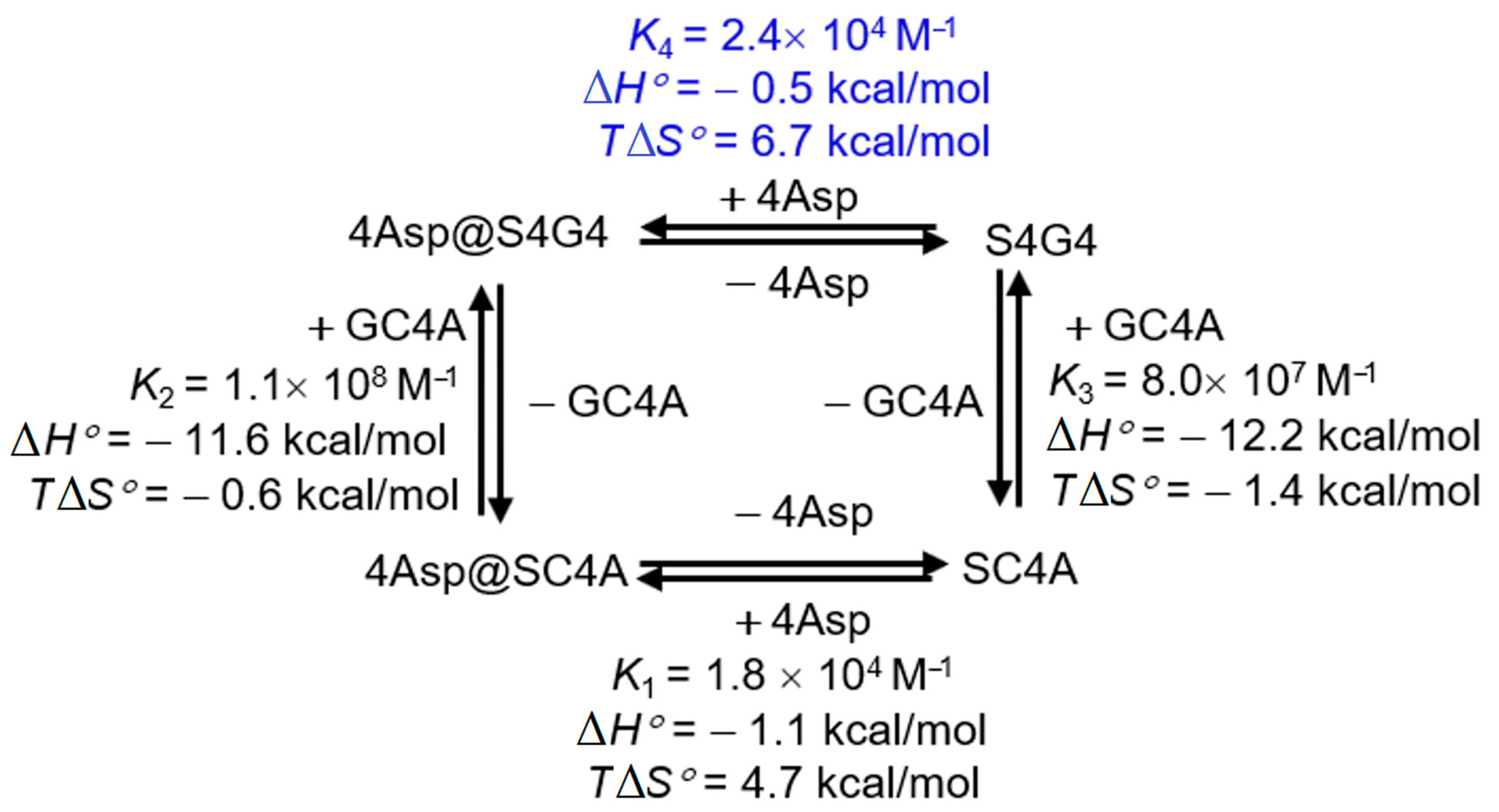
| ΔH° (kcal/mol) | TΔS° (kcal/mol) | ΔG° (kcal/mol) | Ka (M−1) | |
|---|---|---|---|---|
| S4G4 | −12.2 ± 0.2 | −1.4 ± 0.1 | −10.8 ± 0.2 | (8.0 ± 0.2) × 107 |
| S4G5 | −16.6 ± 0.2 | −7.0 ± 0.2 | −9.6 ± 0.2 | (1.0 ± 0.2) × 107 |
| S5G4 | −19.2 ± 0.1 | −8.8 ± 0.1 | −10.3 ± 0.1 | (3.7 ± 0.1) × 107 |
| S5G5 | −26.9 ± 0.3 | −17.5 ± 0.2 | −9.4 ± 0.2 | (7.2 ± 0.2) × 107 |
Sample Availability: Samples of the compounds GC4A, GC5A, SC4A, SC5A are available from the authors. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, X.-Y.; Wang, Y.-Y.; Li, H.-B.; Guo, D.-S. Fluorescence Enhancement by Calixarene Supramolecular Aggregate. Molecules 2020, 25, 5912. https://doi.org/10.3390/molecules25245912
Hu X-Y, Wang Y-Y, Li H-B, Guo D-S. Fluorescence Enhancement by Calixarene Supramolecular Aggregate. Molecules. 2020; 25(24):5912. https://doi.org/10.3390/molecules25245912
Chicago/Turabian StyleHu, Xin-Yue, Yu-Ying Wang, Hua-Bin Li, and Dong-Sheng Guo. 2020. "Fluorescence Enhancement by Calixarene Supramolecular Aggregate" Molecules 25, no. 24: 5912. https://doi.org/10.3390/molecules25245912
APA StyleHu, X.-Y., Wang, Y.-Y., Li, H.-B., & Guo, D.-S. (2020). Fluorescence Enhancement by Calixarene Supramolecular Aggregate. Molecules, 25(24), 5912. https://doi.org/10.3390/molecules25245912