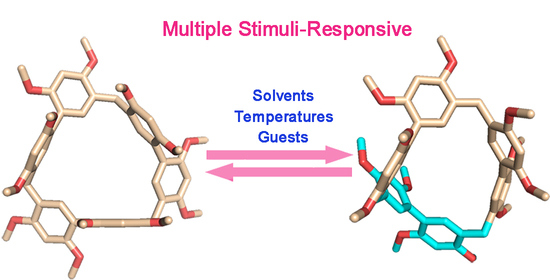
Multiple Stimuli-Responsive Conformational Exchanges of Biphen[3]arene Macrocycle
Abstract
1. Introduction
2. Results
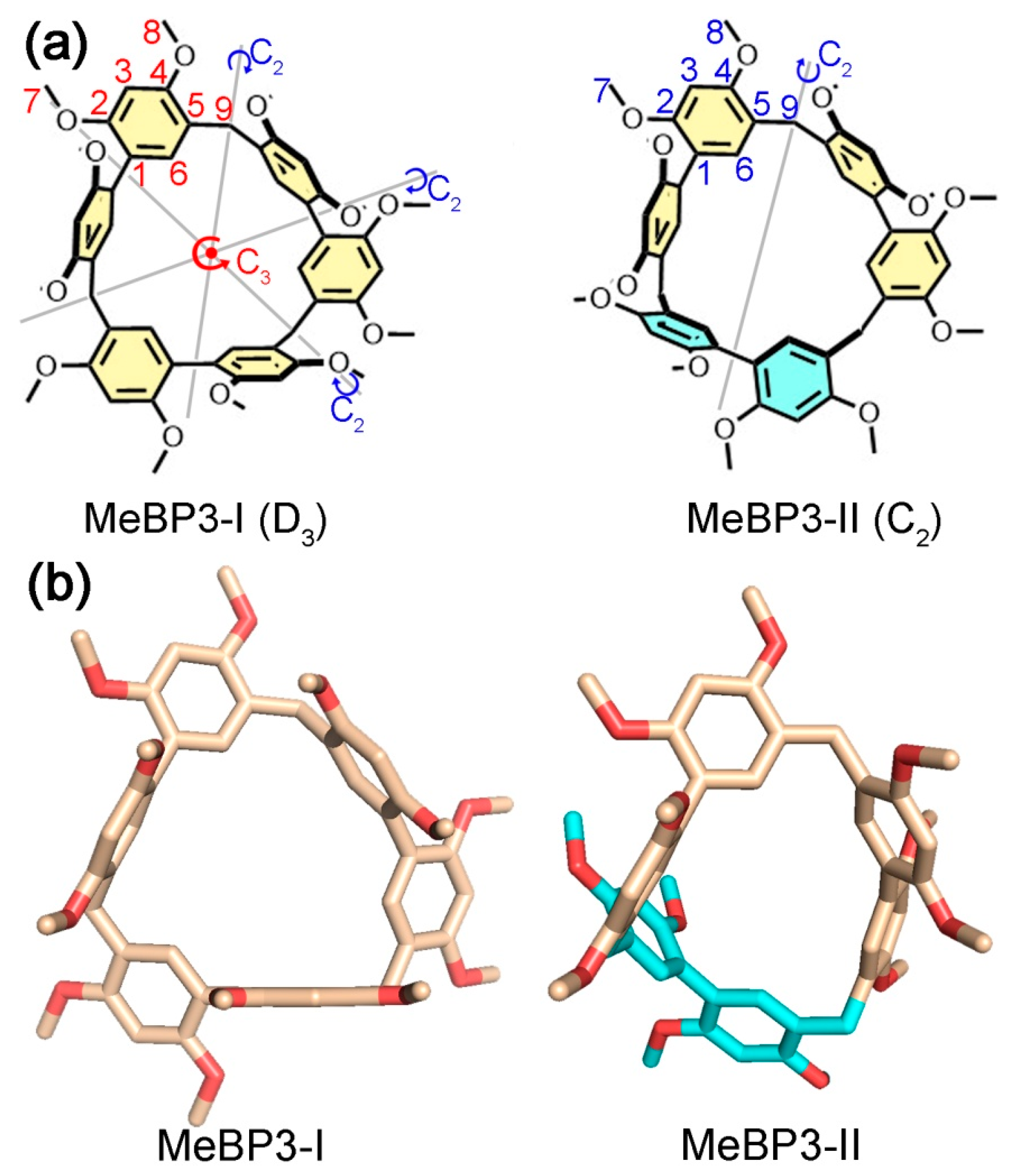
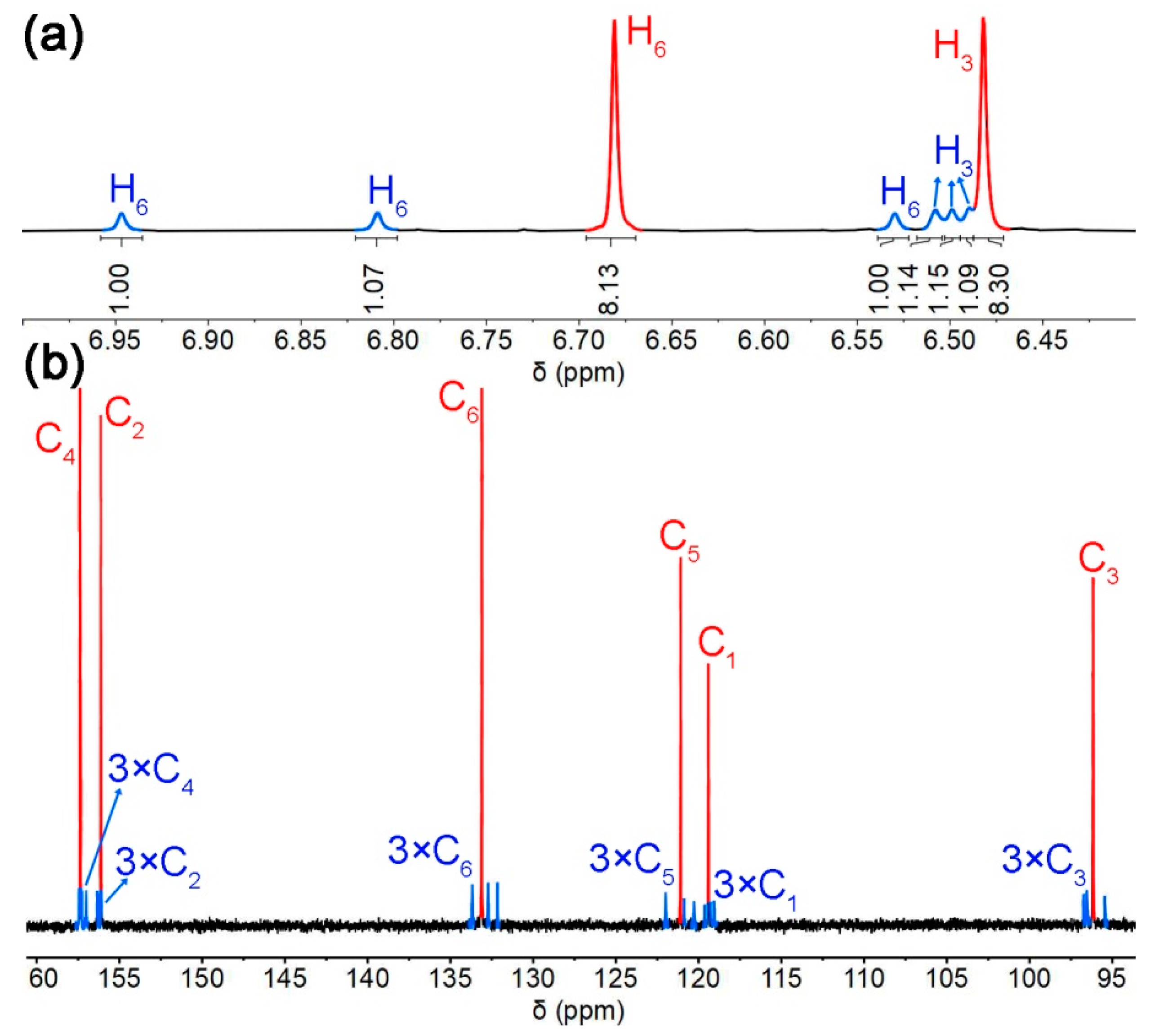
2.1. Conformations Analysis
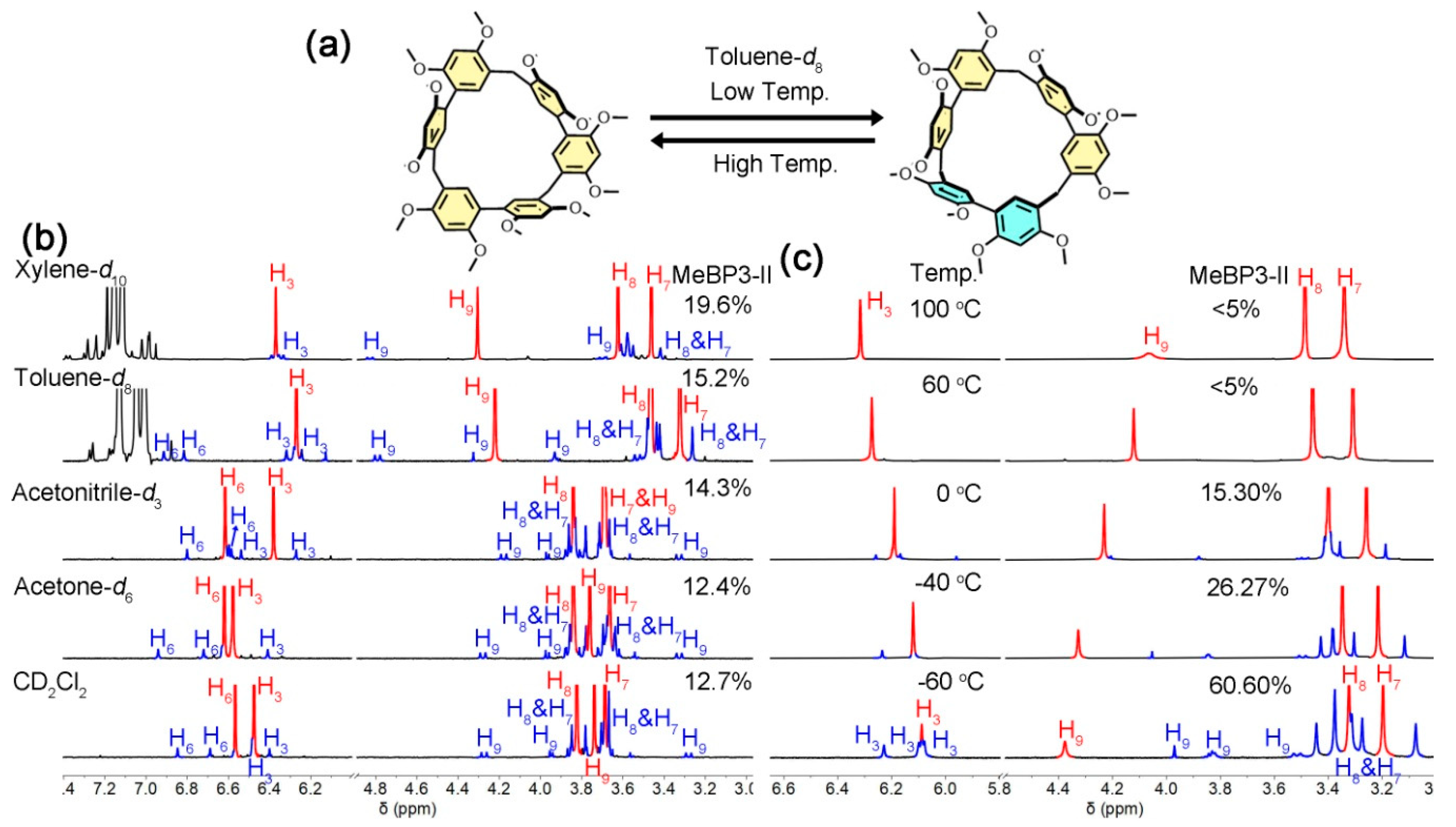
2.2. Solvent and Temperature Effects in Conformational Exchanges
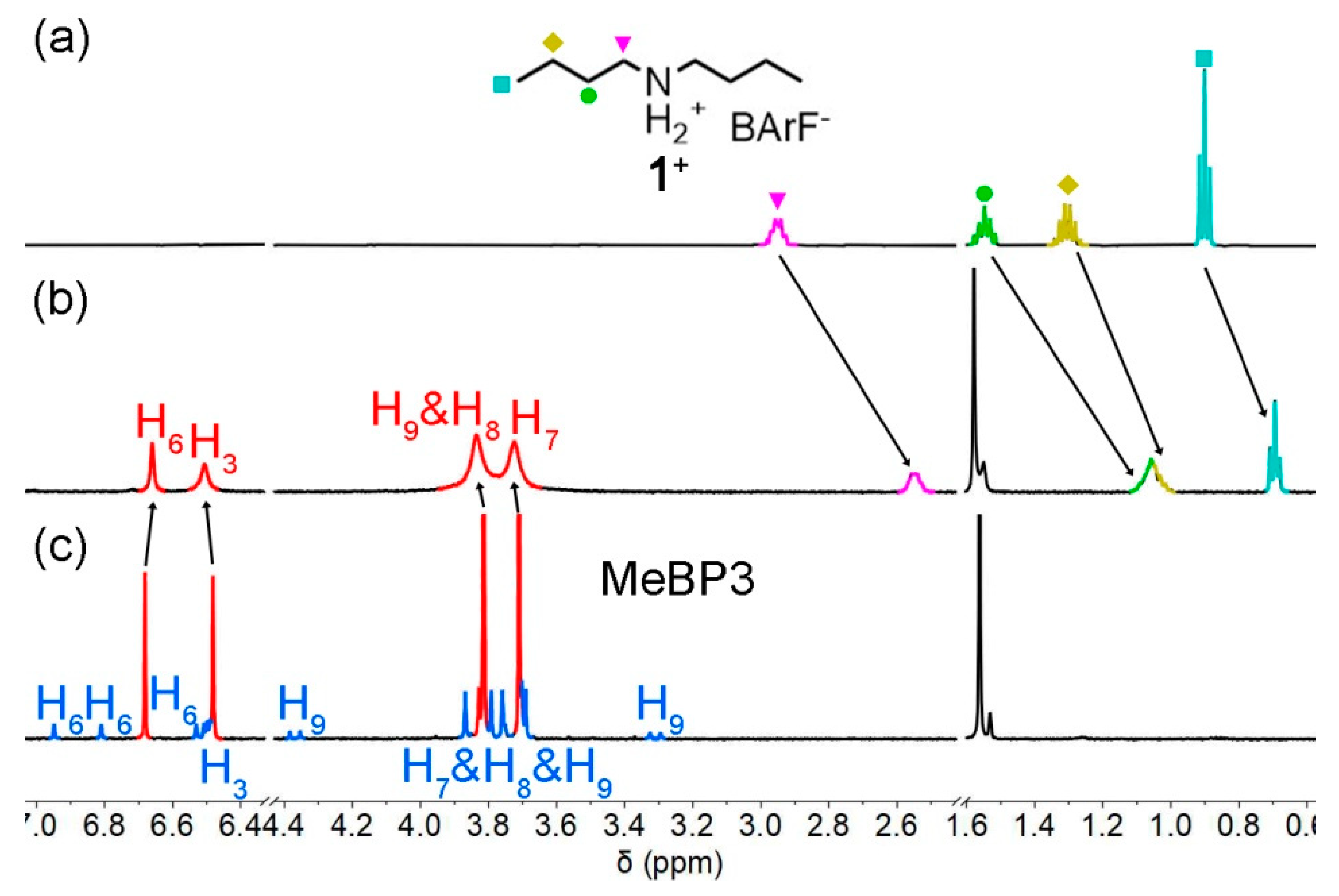
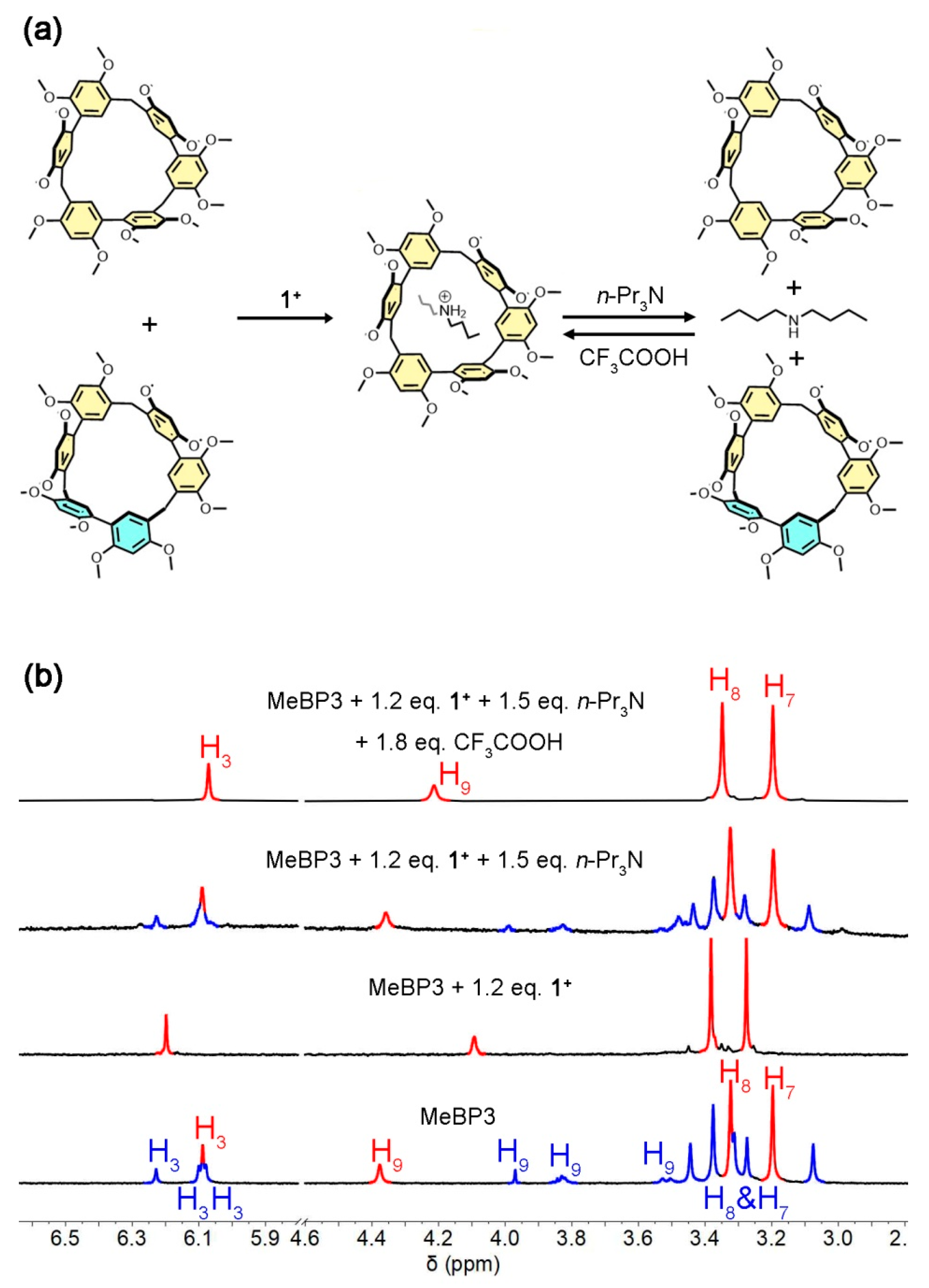
2.3. Guest-Binding and Acid/Base Addition in Conformational Exchanges
3. Discussion
4. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Han, K.-L.; Zhang, X.; Yang, M.-J. (Eds.) Protein Conformational Dynamics; Springer: Basel, Switzerland, 2014. [Google Scholar]
- Kay, E.R.; Leigh, D.A.; Zerbetto, F. Synthetic molecular motors and mechanical machines. Angew. Chem. Int. Ed. 2007, 46, 72–191. [Google Scholar] [CrossRef] [PubMed]
- Feringa, B.L.; van Delden, R.A.; Koumura, N.; Geertsema, E.M. Chiroptical Molecular Switches. Chem. Rev. 2000, 100, 1789–1816. [Google Scholar] [CrossRef]
- Browne, W.R.; Pollard, M.M.; de Lange, B.; Meetsma, A.; Feringa, B.L. Reversible Three-State Switching of Luminescence: A New Twist to Electro- and Photochromic Behavior. J. Am. Chem. Soc. 2006, 128, 12412–12413. [Google Scholar] [CrossRef] [PubMed]
- Ivashenko, O.; Logtenberg, H.; Areephong, J.; Coleman, A.C.; Wesenhagen, P.V.; Geertsema, E.M.; Heureux, N.; Feringa, B.L.; Rudolf, P.; Browne, W.R. Remarkable Stability of High Energy Conformers in Self-Assembled Monolayers of a Bistable Electro- and Photoswitchable Overcrowded Alkene. J. Phys. Chem. C 2011, 115, 22965–22975. [Google Scholar] [CrossRef]
- Koumura, N.; Zijlstra, R.W.J.; van Delden, R.A.; Harada, N.; Feringa, B.L. Light-driven monodirectional molecule rotor. Nature 1999, 401, 152–155. [Google Scholar] [CrossRef] [PubMed]
- Stoddart, J.F. Mechanically Interlocked Molecules (MIMs)-Molecular Shuttles, Switches, and Machines (Nobel Lecture). Angew. Chem. Int. Ed. 2017, 56, 11094–11125. [Google Scholar] [CrossRef]
- Zhang, M.; Yan, X.; Huang, F.; Niu, Z.; Gibson, H.W. Stimuli-Responsive Host-Guest Systems Based on the Recognition of Cryptands by Organic Guests. Acc. Chem. Res. 2014, 47, 1995–2005. [Google Scholar] [CrossRef]
- Yang, H.; Yuan, B.; Zhang, X.; Scherman, O.A. Supramolecular Chemistry at Interfaces: Host-Guest Interactions for Fabricating Multifunctional Biointerfaces. Acc. Chem. Res. 2014, 47, 2106–2115. [Google Scholar] [CrossRef]
- Hu, J.; Liu, S. Engineering Responsive Polymer Building Blocks with Host-Guest Molecular Recognition for Functional Applications. Acc. Chem. Res. 2014, 47, 2084–2095. [Google Scholar] [CrossRef]
- Kremer, C.; Letzen, A. Artificial Allosteric Receptors. Chem. Eur. J. 2013, 19, 6162–6196. [Google Scholar] [CrossRef]
- Jia, F.; He, Z.; Yang, L.-P.; Pan, Z.-S.; Yi, M.; Jiang, R.-W.; Jiang, W. Oxatub[4]arene: A smart macrocyclic receptor with multiple interconvertible cavities. Chem. Sci. 2015, 6, 6731–6738. [Google Scholar] [CrossRef] [PubMed]
- Rebek, J., Jr.; Trend, J.E.; Wattley, R.V.; Chakravorti, S. Allosteric effects in organic chemistry. Site-specific binding. J. Am. Chem. Soc. 1979, 101, 4333–4337. [Google Scholar] [CrossRef]
- Petitjean, A.; Khoury, R.G.; Kyritsakas, N.; Lehn, J.-M. Dynamic Devices. Shape Switching and Substrate Binding in Ion-Controlled Nanomechanical Molecular Tweezers. J. Am. Chem. Soc. 2004, 126, 6637–6647. [Google Scholar] [CrossRef]
- De Namor, A.F.D.; Cleverley, R.M.; Zapata-Ormachea, M.L. Thermodynamics of Calixarene Chemistry. Chem. Rev. 1998, 98, 2495–2525. [Google Scholar] [CrossRef]
- Park, J.S.; Sessler, J.L. Tetrathiafulvalene (TTF)-Annulated Calix[4]pyrroles: Chemically Switchable Systems with Encodable Allosteric Recognition and Logic Gate Functions. Acc. Chem. Res. 2018, 51, 2400–2410. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Yang, Y.; Chi, X.; Yan, X.; Huang, F. Development of Pseudorotaxanes and Rotaxanes: From Synthesis to Stimuli-Responsive Motions to Applications. Chem. Rev. 2015, 115, 7398–7501. [Google Scholar] [CrossRef] [PubMed]
- Knipe, P.C.; Thompson, S.; Hamilton, A.D. Ion-mediated conformational switches. Chem. Sci. 2015, 6, 1630–1639. [Google Scholar] [CrossRef] [PubMed]
- Schneider, H.-J. Strain effects determine the performance of artificial allosteric systems: Calixarenes as models. Chem. Commun. 2019, 55, 3433–3444. [Google Scholar] [CrossRef]
- Tong, S.; Li, J.-T.; Liang, D.-D.; Zhang, Y.-E.; Feng, Q.-Y.; Zhang, X.; Zhu, J.; Wang, M.-X. Catalytic Enantioselective Synthesis and Switchable Chiroptical Property of Inherently Chiral Macrocycles. J. Am. Chem. Soc. 2020, 142, 14432–14436. [Google Scholar] [CrossRef]
- Azov, V.A.; Beeby, A.; Cacciarini, M.; Cheetham, A.G.; Diederich, F.; Frei, M.; Gimzewski, J.K.; Gramlich, V.; Hecht, B.; Jaun, B.; et al. Resorcin[4]arene cavitand-based molecular switches. Adv. Funct. Mater. 2006, 16, 147–156. [Google Scholar] [CrossRef]
- Thondorf, I. Conformations and Stereodynamics of Calixarenes; Asfari, Z., Ed.; Springer: Dordrecht, Holand, 2001. [Google Scholar]
- Groenen, L.C.; van Loon, J.-D.; Verboom, W.; Harkema, S.; Casnati, A.; Ungaro, R.; Pochini, A.; Ugozzoll, F.; Reinhoudt, D.N. The 1,2-alternate conformation of calix[4]arenes: A rare conformation? Dynamic 1H NMR studies of flexible tetraalkylated calix[4]arenes. J. Am. Chem. Soc. 1991, 113, 2385–2392. [Google Scholar] [CrossRef]
- Matthews, S.E.; Cecioni, S.; O’Brien, J.E.; MacDonald, C.J.; Hughes, D.L.; Jones, G.A.; Ashworth, S.H.; Vidal, S. Fixing the Conformation of Calix[4]arenes: When Are Three Carbons Not Enough? Chem. Eur. J. 2018, 24, 4436–4444. [Google Scholar] [CrossRef] [PubMed]
- Gutsche, C.D.; Bauer, L.J. Calixarenes. 13. The conformational properties of calix[4]arenes, calix[6]arenes, calix[8]arenes, and oxacalixarenes. J. Am. Chem. Soc. 1985, 107, 6052–6059. [Google Scholar] [CrossRef]
- Gutsche, C.D.; Bauer, L.J. Calixarenes. 14. The conformational properties of the ethers and esters of the calix[6]arenes and the calix[8]arenes. J. Am. Chem. Soc. 1985, 107, 6059–6063. [Google Scholar] [CrossRef]
- Pochorovski, I.; Diederich, F. Development of Redox-Switchable Resorcin[4]arene Cavitands. Acc. Chem. Res. 2014, 47, 2096–2105. [Google Scholar] [CrossRef] [PubMed]
- Neri, P.; Sessler, J.L.; Wang, M.-X. Calixarenes and Beyond; Springer International Publishing: Basel, Switzerland, 2016. [Google Scholar]
- Kim, D.S.; Sessler, J.L. Calix[4]pyrroles: Versatile molecular containers with ion transport, recognition, and molecular switching functions. Chem. Soc. Rev. 2015, 44, 532–546. [Google Scholar] [CrossRef]
- Rosa, M.D.; Talotta, C.; Gaeta, C.; Soriente, A.; Neri, P.; Pappalardo, S.; Gattuso, G.; Notti, A.; Parisi, M.F.; Pisagatti, I. Calix[5]arene Through-the-Annulus Threading of Dialkylammonium Guests Weakly Paired to the TFPB Anion. J. Org. Chem. 2017, 82, 5162–5168. [Google Scholar] [CrossRef]
- Ogoshi, T.; Akutsu, T.; Yamafuji, D.; Aoki, T.; Yamagishi, T.-A. Solvent- and Achiral-Guest-Triggered Chiral Inversion in a Planar Chiral pseudo[1]Catenane. Angew. Chem. Int. Ed. 2013, 52, 8111–8115. [Google Scholar] [CrossRef]
- Chen, Y.; Fu, L.; Sun, B.; Qian, C.; Wang, R.; Jiang, J.; Lin, C.; Ma, J.; Wang, L. Competitive Selection of Conformation Chirality of Water-Soluble Pillar[5]arene Induced by Amino Acid Derivatives. Org. Lett. 2020, 22, 2266–2270. [Google Scholar] [CrossRef]
- Xiao, C.; Wu, W.; Liang, W.; Zhou, D.; Kanagaraj, K.; Cheng, G.; Su, D.; Zhong, Z.; Chruma, J.J.; Yang, C. Redox-Triggered Chirality Switching and Guest-Capture/Release with a Pillar[6]arene-Based Molecular Universal Joint. Angew. Chem. Int. Ed. 2020, 59, 8094–8098. [Google Scholar] [CrossRef]
- Park, J.; Choi, Y.; Lee, S.S.; Jung, J.H. Critical Role of Achiral Guest Molecules in Planar Chirality Inversion of Alanine-Appender Pillar[5]arenes. Org. Lett. 2019, 21, 1232–1236. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.-B.; Wang, Z.; Zhao, X.-L.; Liu, Y.A.; Li, J.-S.; Jiang, B.; Wen, K. A Diaminopillar[5]arene-Based Macrobicyclic Molecules: Synthesis, Characterization and A Lock-Key Story. Chem. Eur. J. 2019, 25, 2189–2194. [Google Scholar] [PubMed]
- Lee, E.; Ju, H.; Park, I.-H.; Jung, J.H.; Ikeda, M.; Kuwahara, S.; Habata, Y.; Lee, S.S. pseudo[1]Catenane-Type Pillar[5]thiacrown Whose Planar Chiral Inversion is Triggered by Metal Cation and Controlled by Anion. J. Am. Chem. Soc. 2018, 140, 9669–9677. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.-P.; Zhang, L.; Quan, M.; Ward, J.S.; Ma, Y.-L.; Zhou, H.; Rissanen, K.; Jiang, W. A supramolecular system that strictly follows the binding mechanism of conformational selection. Nat. Commun. 2020, 11, 2740. [Google Scholar] [CrossRef]
- Hong, C.M.; Kaphan, D.M.; Bergman, R.G.; Raymond, K.N.; Toste, F.D. Conformational Selection as the Mechanism of Guest Binding in a Flexible Supramolecular Host. J. Am. Chem. Soc. 2017, 139, 8013–8021. [Google Scholar] [CrossRef]
- Ajami, D.; Liu, L.; Rebek, J., Jr. Soft templates in encapsulation complexes. Chem. Soc. Rev. 2015, 44, 490–499. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, K.; Li, B.; Cui, L.; Li, J.; Jia, X.; Zhao, H.; Fang, J.; Li, C. Efficient Separation of cis- and trans-1,2-Dichloroethene Isomers by Adaptive Biphen[3]arene Crystals. Angew. Chem. Int. Ed. 2019, 58, 10281–10284. [Google Scholar] [CrossRef]
- Chen, H.; Fan, J.; Hu, X.; Ma, J.; Wang, S.; Li, J.; Yu, Y.; Jia, X.; Li, C. Biphen[n]arenes. Chem. Sci. 2015, 6, 197–202. [Google Scholar] [CrossRef]
- Dai, L.; Ding, Z.-J.; Cui, L.; Li, J.; Jia, X.; Li, C. 2,2′-Biphen[n]arenes (n = 4–8): One-step, high-yield synthesis, and host-guest properties. Chem. Commun. 2017, 53, 12096–12099. [Google Scholar] [CrossRef]
- Li, B.; Wang, B.; Huang, X.; Dai, L.; Cui, L.; Li, J.; Jia, X.; Li, C. Terphen[n]arenes and Quaterphen[n]arenes (n = 3–6): One-Pot Synthesis, Self-Assembly into Supramolecular Gels and Iodine Capture. Angew. Chem. Int. Ed. 2019, 58, 3885–3889. [Google Scholar] [CrossRef]
- Xu, K.; Zhang, Z.-Y.; Yu, C.; Wang, B.; Dong, M.; Zeng, X.; Gou, R.; Cui, L.; Li, C. A Modular Synthetic Strategy for Functional Macrocycles. Angew. Chem. Int. Ed. 2020, 59, 7214–7218. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wang, Y.; Li, C. Biphen[n]arenes: Synthesis and Host–Guest Properties. In Handbook of Macrocyclic Supramolecular Assembly; Liu, Y., Chen, Y., Zhang, H.Y., Eds.; Springer Nature Singapore Pte Ltd.: Singapore, 2019. [Google Scholar]
- CCDC 1885453 and 1885657 (CH3CN@MeBP3, cis-DCE@MeBP3). These Data Can be Obtained Free of Charge from The Cambridge Crystallographic Data Centre. Available online: www.ccdc.cam.ac.uk/data_request/cif (accessed on 17 December 2018).
- Böhmer, V. Calixarenes macrocycles with (almost) unlimited possibilities. Angew. Chem., Int. Ed. Engl. 1995, 34, 713–745. [Google Scholar] [CrossRef]
- Chen, C.-F.; Han, Y. Triptycene-Derived Macrocyclic Arenes: From Calixarenes to Helicarenes. Acc. Chem. Res. 2018, 51, 2093–2106. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.-X. Nitrogen and Oxygen Bridged Calixaromatics: Synthesis, Structure, Functionalization, and Molecular Recognition. Acc. Chem. Res. 2012, 45, 182–195. [Google Scholar] [CrossRef] [PubMed]
- Dondoni, A.; Marra, A. Calixarene and Calixresorcarene Glycosides: Their Synthesis and Biological Applications. Chem. Rev. 2010, 110, 4949–4977. [Google Scholar] [CrossRef]
- Zhang, D.; Martinez, A.; Dutasta, J.-P. Emergence of Hemicryptophanes: From Synthesis to Applications for Recognition, Molecular Machines, and Supramolecular Catalysis. Chem. Rev. 2017, 117, 4900–4942. [Google Scholar] [CrossRef]
- Ogoshi, T.; Yamagishi, T.-A.; Nakamoto, Y. Pillar-Shaped Macrocyclic Hosts Pillar[n]arenes: New Key Players for Supramolecular Chemistry. Chem. Rev. 2016, 116, 7937–8002. [Google Scholar] [CrossRef]
- Xue, M.; Yang, Y.; Chi, X.; Zhang, Z.; Huang, F.; Pillararenes, A. New Class of Macrocycles for Supramolecular Chemistry. Acc. Chem. Res. 2012, 45, 1294–1308. [Google Scholar] [CrossRef]
- Ogoshi, T.; Kanai, S.; Fujinami, S.; Yamagishi, T.-A.; Nakamoto, Y. Para-bridged symmetrical pillar[5]arenes: Their Lewis acid catalyzed synthesis and host-guest property. J. Am. Chem. Soc. 2008, 130, 5022–5023. [Google Scholar] [CrossRef]
- Wang, X.; Jia, F.; Yang, L.-P.; Zhou, H.; Jiang, W. Conformationally adaptive macrocycles with flipping aromatic sidewalls. Chem. Soc. Rev. 2020, 49, 4176–4188. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds MeBP3 are available from the authors. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Yang, L.-P.; Zhao, X.; Cui, L.; Li, J.; Jia, X.; Fang, J.; Li, C. Multiple Stimuli-Responsive Conformational Exchanges of Biphen[3]arene Macrocycle. Molecules 2020, 25, 5780. https://doi.org/10.3390/molecules25245780
Wang Y, Yang L-P, Zhao X, Cui L, Li J, Jia X, Fang J, Li C. Multiple Stimuli-Responsive Conformational Exchanges of Biphen[3]arene Macrocycle. Molecules. 2020; 25(24):5780. https://doi.org/10.3390/molecules25245780
Chicago/Turabian StyleWang, Yiliang, Liu-Pan Yang, Xiang Zhao, Lei Cui, Jian Li, Xueshun Jia, Jianhui Fang, and Chunju Li. 2020. "Multiple Stimuli-Responsive Conformational Exchanges of Biphen[3]arene Macrocycle" Molecules 25, no. 24: 5780. https://doi.org/10.3390/molecules25245780
APA StyleWang, Y., Yang, L.-P., Zhao, X., Cui, L., Li, J., Jia, X., Fang, J., & Li, C. (2020). Multiple Stimuli-Responsive Conformational Exchanges of Biphen[3]arene Macrocycle. Molecules, 25(24), 5780. https://doi.org/10.3390/molecules25245780