Biobased Polymers via Radical Homopolymerization and Copolymerization of a Series of Terpenoid-Derived Conjugated Dienes with exo-Methylene and 6-Membered Ring
Abstract
1. Introduction
2. Results and Discussion
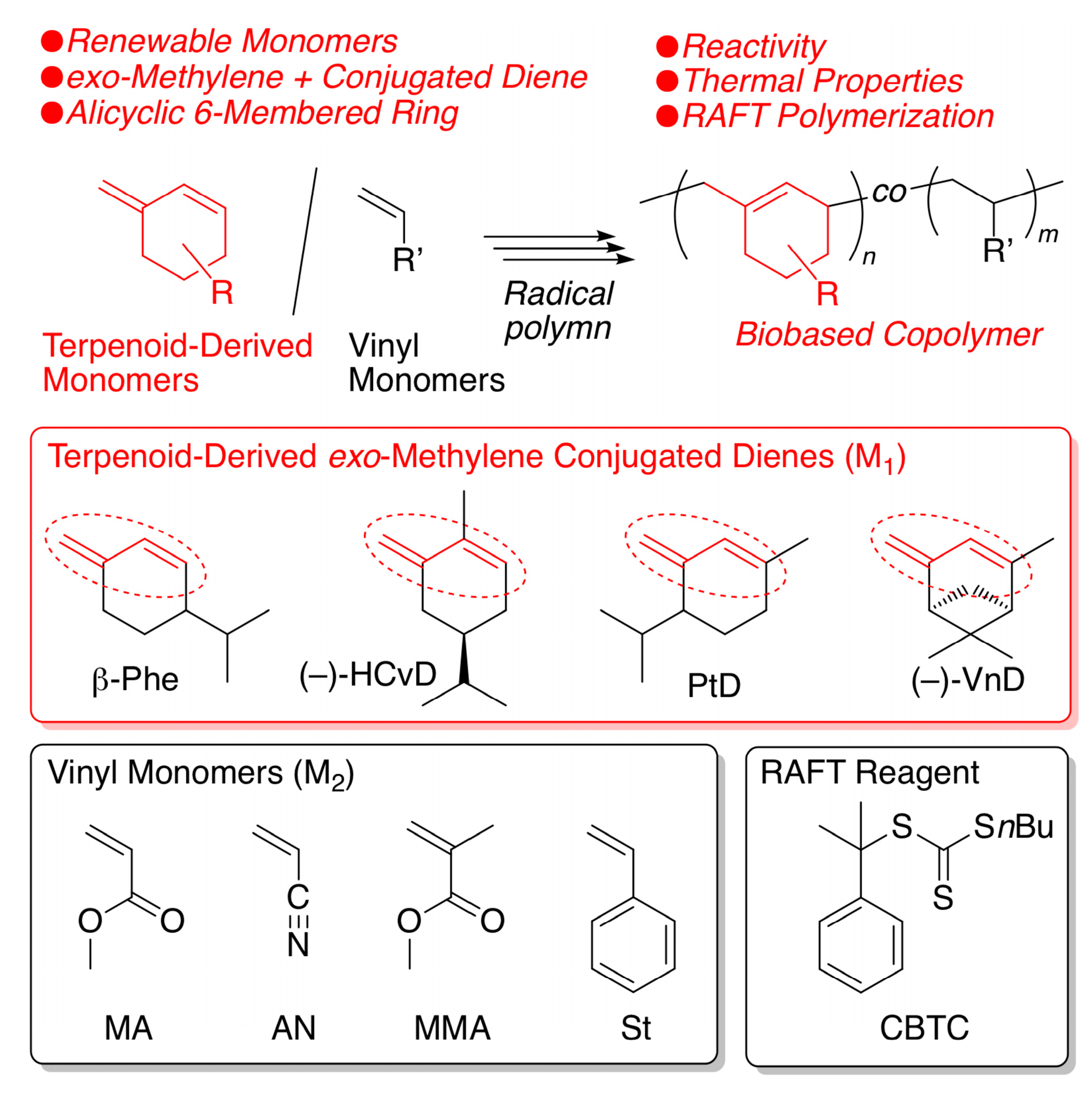
2.1. Radical Homopolymerization
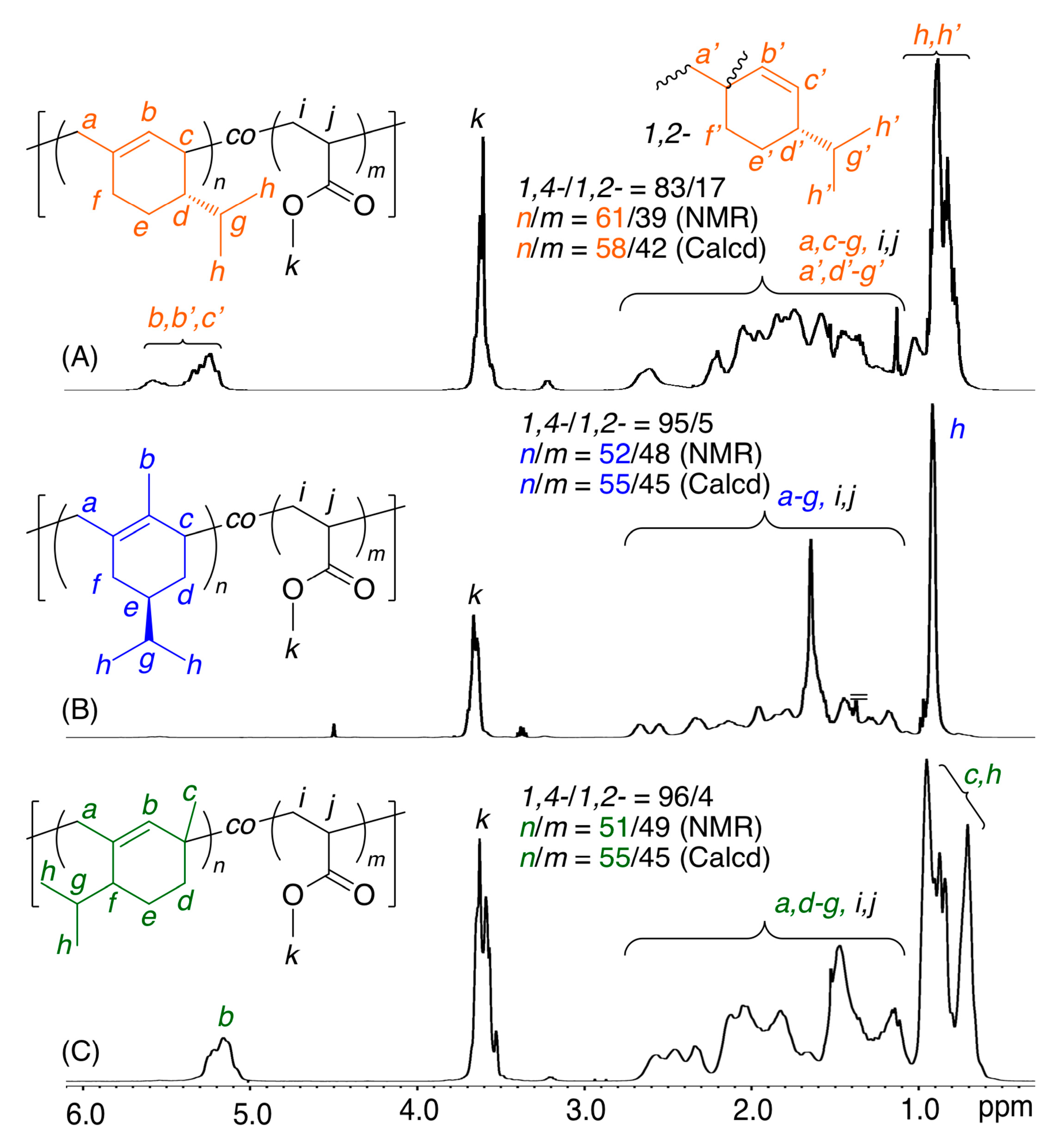
2.2. Radical Copolymerization with Various Common Vinyl Monomers
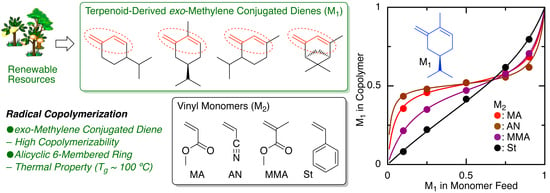
2.3. Monomer Reactivity Ratio
2.4. Thermal Properties of Copolymers
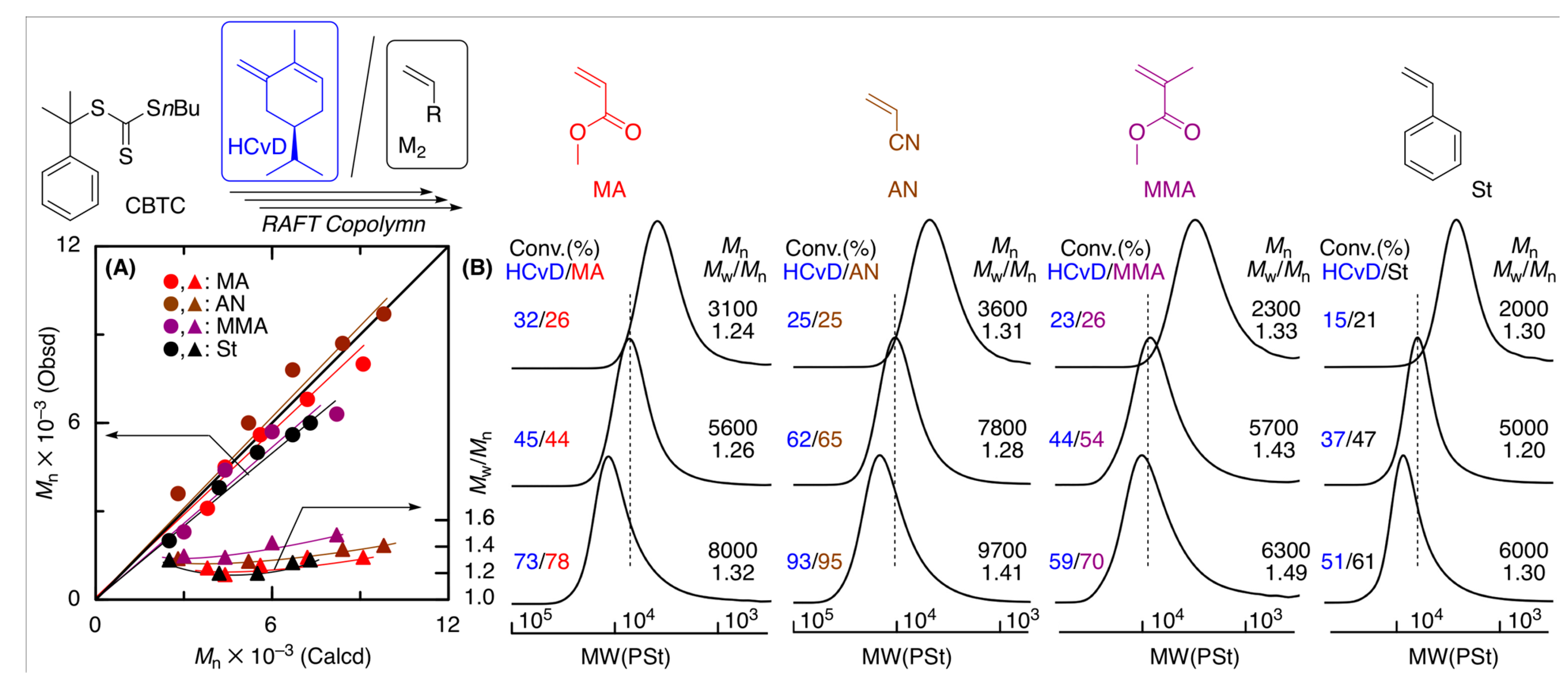
2.5. Reversible Addition Fragmentation Chain-Transfer (RAFT) Copolymerization of (–)-HCvD and Various Common Vinyl Monomers
3. Materials and Methods
3.1. Materials
3.2. Purification of β-Phellandrene (β-Phe)
3.3. Synthesis of (–)-VnD
3.4. Synthesis of PtD
3.5. RAFT Copolymerization
3.6. Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Moad, G.; Solomon, D.H. The Chemistry of Radical Polymerization: Second Fully Revised Edition; Elsevier: Oxford, UK, 2006. [Google Scholar]
- Hawker, C.J.; Bosman, A.W.; Harth, E. New Polymer Synthesis by Nitroxide Mediated Living Radical Polymerizations. Chem. Rev. 2001, 101, 3661–3688. [Google Scholar] [CrossRef] [PubMed]
- Matyjaszewski, K.; Xia, J.H. Atom Transfer Radical Polymerization. Chem. Rev. 2001, 101, 2921–2990. [Google Scholar] [CrossRef] [PubMed]
- Kamigaito, M.; Ando, T.; Sawamoto, M. Metal-Catalyzed Living Radical Polymerization. Chem. Rev. 2001, 101, 3689–3745. [Google Scholar] [CrossRef] [PubMed]
- Moad, G.; Rizzardo, E.; Thang, S.H. Toward Living Radical Polymerization. Acc. Chem. Res. 2008, 41, 1133–1142. [Google Scholar] [CrossRef] [PubMed]
- Matyjaszewski, K. Atom Transfer Radical Polymerization (ATRP): Current Status and Future Perspectives. Macromolecules 2012, 45, 4015–4039. [Google Scholar] [CrossRef]
- McKenzie, T.G.; Fu, Q.; Uchiyama, M.; Satoh, K.; Xu, J.; Boyer, C.; Kamigaito, M.; Qiao, G.G. Beyond Traditional RAFT: Alternative Activation of Thiocarbonylthio Compounds for Controlled Polymerization. Adv. Sci. 2016, 3, 1500394. [Google Scholar] [CrossRef]
- Ouchi, M.; Sawamoto, M. 50th Anniversary Perspective: Metal-Catalyzed Living Radical Polymerization: Discovery and Perspective. Macromolecules 2017, 50, 2603–2614. [Google Scholar] [CrossRef]
- Kamigaito, M.; Sawamoto, M. Synergistic Advances in Living Cationic and Radical Polymerizations. Macromolecules 2020, 53, 6749–6753. [Google Scholar] [CrossRef]
- Nothling, M.D.; Fu, Q.; Reyhani, A.; Allison-Logan, S.; Jung, K.; Zhu, J.; Kamigaito, M.; Boyer, C.; Qiao, G.G. Progress and Perspective Beyond Traditional RAFT Polymerization. Adv. Sci. 2020, 7, 2001656. [Google Scholar] [CrossRef]
- Yao, K.; Tang, C. Controlled Polymerization of Next-Generation Renewable Monomers and Beyond. Macromolecules 2013, 46, 1689–1712. [Google Scholar] [CrossRef]
- Iwata, T. Biodegradable and Bio-Based Polymers: Future Prospects of Eco-Friendly Plastics. Angew. Chem. Int. Ed. 2015, 54, 3210–3215. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Romain, C.; Williams, C.K. Sustainable polymers from renewable resources. Nature 2016, 540, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Froidevaux, V.; Negrell, C.; Caillol, S.; Pascault, J.-P.; Boutevin, B. Biobased Amines: From Synthesis to Polymers; Present and Future. Chem. Rev. 2016, 116, 14181–14224. [Google Scholar] [CrossRef] [PubMed]
- Llevot, A.; Dannecker, P.-K.; von Czapiewski, M.; Over, L.C.; Söyer, Z.; Meier, M.A.R. Renewability is not Enough: Recent Advances in the Sustainable Synthesis of Biomass-Derived Monomers and Polymers. Chem. Eur. J. 2016, 22, 11510–11521. [Google Scholar] [CrossRef]
- Thomsett, M.R.; Storr, T.E.; Monaghan, O.R.; Stockman, R.A.; Howdle, S.M. Progress in the sustainable polymers from terpenes and terpenoids. Green Mater. 2016, 4, 115–134. [Google Scholar] [CrossRef]
- Llevot, A.; Grau, E.; Carlotti, S.; Grelier, S.; Cramail, H. From Lignin-Derived Aromatic Compounds to Novel Biobased Polymers. Macromol. Rapid Commun. 2016, 37, 9–28. [Google Scholar] [CrossRef]
- Schneiderman, D.K.; Hillmyer, M.A. There is a Great Future in Sustainable Polymers. Macromolecules 2017, 50, 3733–3749. [Google Scholar] [CrossRef]
- Nguyen, H.T.H.; Rostagno, P.; Qi, M.; Feteha, A.; Miller, S.A. The quest for high glass transition temperature bioplastics. J. Mater. Chem. A 2018, 6, 9298–9331. [Google Scholar] [CrossRef]
- Tang, X.; Chen, E.Y.-X. Toward Infinity Recyclable Plastics Derived from Renewable Cyclic Esters. Chem 2019, 5, 284–312. [Google Scholar] [CrossRef]
- O’Dea, R.M.; Willie, J.A.; Epps, T.H., III. 100th Anniversary of Macromolecular Science Viewpoint: Polymers from Lignocellulosic Biomass. Current Challenges and Future Opportunities. ACS Macro Lett. 2020, 9, 476–493. [Google Scholar]
- Erman, W.F. Chemistry of the Monoterpenes: An Encyclopedia Handbook; Marcel Dekker, Inc.: New York, NY, USA, 1985. [Google Scholar]
- Connolly, J.D.; Hill, R.A. Dictionary of Terpenoids; Chapman & Hall: London, UK, 1991. [Google Scholar]
- Breitmaier, E. Terpenes: Flavors, Fragrances, Pharmaca, Pheromones; Wiley-VCH: Weinheim, Germany, 2006. [Google Scholar]
- Handbook of Essential Oils: Science, Technology, and Applications; Başer, K.H.C., Buchbouer, G., Eds.; CRC Press: Boca Baton, FL, USA, 2016. [Google Scholar]
- Satoh, K.; Kamigaito, M. New Polymerization Methods for Biobased Polymers. In Bio-Based Polymers; Kimura, Y., Ed.; CMC: Tokyo, Japan, 2013; pp. 95–111. [Google Scholar]
- Satoh, K. Controlled/Living Polymerization of renewable vinyl monomers into bio-based polymers. Polym. J. 2015, 47, 527–536. [Google Scholar] [CrossRef]
- Kamigaito, M.; Satoh, K. Bio-Based Hydrocarbon Polymers, In Encyclopedia of Polymeric Nanomaterials.; Kobayashi, S., Müllen, K., Eds.; Springer: Heidelberg, Germany, 2015; Volume 1, pp. 109–118. [Google Scholar]
- Kamigaito, M.; Satoh, K. Sustainable Vinyl Polymers via Controlled Polymerization of Terpenes. In Sustainable Polymers from Biomass; Tang, C., Ryu, C.Y., Eds.; Wiley-VCH: Weinheim, Germany, 2017; pp. 55–90. [Google Scholar]
- Akkapeddi, M.K. Poly(α-methylene-γ-butyrolactone) Synthesis, Configurational Structure, and Properties. Macromolecules 1979, 12, 546–551. [Google Scholar] [CrossRef]
- Akkapeddi, M.K. The free radical copolymerization characteristics of α-methylene γ-butyrolactone. Polymer 1979, 20, 1215–1216. [Google Scholar] [CrossRef]
- Mosnacek, J.; Matyjaszewski, K. Atom Transfer Radical Polymerization of Tulipalin A: A Naturally Renewable Monomer. Macromolecules 2008, 41, 5509–5511. [Google Scholar] [CrossRef]
- Mosnacek, J.; Yoon, J.A.; Juhari, A.; Koynov, K.; Matyjaszewski, K. Synthesis, morphology and mechanical properties of linear triblock copolymers based on poly(α-methylene-γ-butyrolactone). Polymer 2009, 50, 2087–2094. [Google Scholar] [CrossRef]
- Zhang, Y.; Miyake, G.M.; Chen, E.Y.-X. Alane-Based Classical and Frustrated Lewis Pairs in Polymer Synthesis: Rapid Polymerization of MMA and Naturally Renewable Methylene Butyrolactones to High Molecular Weight Polymers. Angew. Chem. Int. Ed. 2010, 49, 10158–10162. [Google Scholar] [CrossRef]
- Schmitt, M.; Falivene, L.; Caporaso, L.; Cavallo, L.; Chen, E.Y.-X. High-speed organocatalytic polymerization of a renewable methylene butyrolactone by a phospazene superbase. Polym. Chem. 2014, 5, 3261–3270. [Google Scholar] [CrossRef]
- Pfelifer, V.F.; Vojnovich, C.; Heger, E.N. Itaconic Acid by Fermentation with Aspergillus Terreus. Ind. Chem. Eng. 1952, 44, 2975–2980. [Google Scholar] [CrossRef]
- Kertes, A.S.; King, C.J. Extraction chemistry of fermentation product carboxylic acids. Biotechnol. Bioeng. 1986, 28, 269–282. [Google Scholar] [CrossRef]
- Tate, B.E. Polymerization of itaconic acid and derivatives. Adv. Polym. Sci. 1967, 5, 214–232. [Google Scholar]
- Ishida, S.; Saito, S. Polymerization of Itaconic Acid Derivatives. J. Polym. Sci. Part. A-1 Polym. Chem. 1967, 5, 689–705. [Google Scholar] [CrossRef]
- Satoh, K.; Lee, D.-H.; Nagai, K.; Kamigaito, M. Precision Synthesis of Bio-Based Acrylic Thermoplastic Elastomer by RAFT Polymerization of Itaconic Acid Derivatives. Macromol. Rapid Commun. 2014, 35, 161–167. [Google Scholar] [PubMed]
- Gowda, R.R.; Chen, E.Y.-X. Synthesis of β-methyl-α-methylene-γ-butyrolactone from biorenewable itaconic acid. Org. Chem. Fornt. 2014, 1, 230–234. [Google Scholar] [CrossRef]
- Nonoyama, Y.; Satoh, K.; Kamigaito, M. Renewable β-methylstyrenes for bio-based heat-resistant styrenic copolymers: Radical copolymerization enhanced by fluoroalcohol and controlled/living copolymerization by RAFT. Polym. Chem. 2014, 5, 3182–3189. [Google Scholar]
- Terao, Y.; Satoh, K.; Kamigaito, M. Controlled Radical Copolymerization of Cinnamic Derivatives as Renewable Vinyl Monomers with Both Acrylic and Styrenic Substituents: Reactivity, Regioselectivity, Properties, and Functions. Biomacromolecules 2019, 20, 192–203. [Google Scholar] [CrossRef]
- Terao, Y.; Satoh, K.; Kamigaito, M. 1:3 ABAA sequence-regulated substituted polymethylenes via alternating radical copolymerization of methyl cinnamate and maleic anhydride followed by post-polymerization reactions. Eur. Polym. J. 2019, 120, 109225. [Google Scholar] [CrossRef]
- Imada, M.; Takenaka, Y.; Hatanaka, H.; Tsuge, T.; Abe, H. Unique acrylic resins with aromatic side chains by homopolymerization of cinnamic monomers. Commun. Chem. 2019, 2, 109. [Google Scholar] [CrossRef]
- Takeshima, H.; Satoh, K.; Kamigaito, M. Bio-Based Functional Styrene Monomers Derived from Naturally Occurring Ferulic Acid for Poly(vinylcatechol) and Poly(vinylguaicol) via Controlled Radical Polymerization. Macromolecules 2017, 50, 4206–4216. [Google Scholar] [CrossRef]
- Takeshima, H.; Satoh, K.; Kamigaito, M. Scalable Synthesis of Bio-Based Functional Styrene: Protected Vinyl Catechol from Caffeic Acid and Controlled Radical and Anionic Polymerizations Thereof. ACS Sustainable Chem. Eng. 2018, 6, 13681–13686. [Google Scholar] [CrossRef]
- Takeshima, H.; Satoh, K.; Kamigaito, M. Bio-Based Vinylphenol Family: Synthesis via Decarboxylation of Naturally Occurring Cinnamic Acids and Living Radical Polymerization for Functionalized Polystyrenes. J. Polym. Sci. 2020, 58, 91–100. [Google Scholar] [CrossRef]
- Satoh, K.; Matsuda, M.; Nagai, K.; Kamigaito, M. AAB-Sequence Living Radical Chain Copolymerization of Naturally-Occurring Limonene with Maleimide: An End-to-End Sequence-Regulated Copolymer. J. Am. Chem. Soc. 2010, 132, 10003–10005. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, M.; Satoh, K.; Kamigaito, M. Periodically Functionalized and Grafted Copolymers via 1:2-Sequence-Regulated Radical Copolymerization of Naturally Occurring Functional Limonene and Maleimide Derivatives. Macromolecules 2013, 46, 5473–5482. [Google Scholar] [CrossRef]
- Matsuda, M.; Satoh, K.; Kamigaito, M. Controlled Radical Copolymerization of Naturally-Occurring Terpenes with Acrylic Monomers in Fluorinated Alcohol. KGK Kaut. Gummi Kunstst. 2013, 66, 51–56. [Google Scholar]
- Matsuda, M.; Satoh, K.; Kamigaito, M. 1:2-Sequence-Regulated Radical Copolymerization of Naturally Occurring Terpenes with Maleimide Derivatives in Fluorinated Alcohol. J. Polym. Sci. Part. A Polym. Chem. 2013, 51, 1774–1785. [Google Scholar] [CrossRef]
- Miyaji, H.; Satoh, K.; Kamigaito, M. Bio-Based Polyketones by Selective Ring-Opening Radical Polymerization of α-Pinene Derived Pinocarvone. Angew. Chem. Int. Ed. 2016, 55, 1372–1376. [Google Scholar] [CrossRef]
- Ojika, M.; Satoh, K.; Kamigaito, M. BAB-random-C Monomer Sequence via Radical Terpolymerization of Limonene (A), Maleimide (B), and Methacrylate (C): Terpene Polymers with Randomly Distributed Periodic Sequences. Angew. Chem. Int. Ed. 2017, 56, 1789–1793. [Google Scholar] [CrossRef]
- Hashimoto, H.; Takeshima, H.; Nagai, T.; Uchiyama, M.; Satoh, K.; Kamigaito, M. Valencene as a naturally occurring sesquiterpene monomer for radical copolymerization with maleimide to induce concurrent 1:1 and 1:2 propagation. Polym. Degrad. Stab. 2019, 161, 183–190. [Google Scholar] [CrossRef]
- Runckel, W.J.; Goldblatt, L.A. Inhibition of Myrcene Polymerization during Storage. Ind. Eng. Chem. 1946, 38, 749–751. [Google Scholar] [CrossRef]
- Johanson, A.J.; McKennon, F.L.; Goldblatt, L.A. Emulsion Polymerization of Myrcene. Ind. Eng. Chem. 1948, 401, 500–502. [Google Scholar] [CrossRef]
- Marvel, C.S.; Hwa, C.C.L. Polymyrcene. J. Polym. Sci. 1960, 45, 25–34. [Google Scholar] [CrossRef]
- Cawse, J.L.; Stanford, J.L.; Still, R.H. Polymers from Renewable Resources. III. Hydroxy-Terminated Myrcene Polymers. J. Appl. Polym. Sci. 1986, 31, 1963–1975. [Google Scholar] [CrossRef]
- Trumbo, D.L. Free radical copolymerization behavior of myrcene I. Copolymers with styrene, methyl methacrylate or p-fluorostyrene. Polym. Bull. 1993, 31, 629–636. [Google Scholar] [CrossRef]
- Sarkar, P.; Bhowmick, A.K. Synthesis, characterization and properties of a bio-based elastomer: Polymyrcene. RSC Adv. 2014, 41, 61343–61354. [Google Scholar] [CrossRef]
- Hilschmann, J.; Kali, G. Bio-based polymyrcene with highly ordered structure via solvent free controlled radical polymerization. Eur. Polym. J. 2015, 73, 363–373. [Google Scholar] [CrossRef]
- Kamigaito, M.; Satoh, K.; Suzuki, S.; Kori, Y.; Eguchi, Y.; Iwasa, K.; Shiroto, H. β-Phellandrene polymer, production method for same, and molded article. WO 2015/060310 A1; filed 21 October 2014 and issued 30 April 2015,
- Nishida, T.; Satoh, K.; Nagano, S.; Seki, T.; Tamura, M.; Li, Y.; Tomishige, K.; Kamigaito, M. Biobased Cycloolefin Polymers: Carvone-Derived Cyclic Conjugated Diene with Reactive exo-Methylene Group for Regioselective and Stereospecific Living Cationic Polymerization. ACS Macro Lett. 2020, 9, 1178–1183. [Google Scholar] [CrossRef]
- Matsumoto, A.; Yamamoto, D. Radical Copolymerization of N-Phenylmaleimide and Diene Monomers in Competition with Diels–Alder Reaction. J. Polym. Sci., Part. A Polym. Chem. 2016, 54, 3616–3625. [Google Scholar] [CrossRef]
- Li, Y.; Padias, A.B.; Hall, H.K. Evidence for 2-Hexene-1,6-diyl Diradicals Accompanying the Concerted Diels-Alder Cycloaddition of Acrylonitrile with Nonpolar 1,3-Dienes. J. Org. Chem. 1993, 58, 7049–7058. [Google Scholar] [CrossRef]
- Trumbo, D.L. Synthesis and Polymerization of 1-Methyl-4-isopropenyl-6-methylene-1-cyclohexene. J. Polym. Sci.: Part. A Polym. Chem. 1995, 33, 599–601. [Google Scholar] [CrossRef]
- Kobayashi, S.; Lu, C.; Hoye, T.R.; Hillmyer, M.A. Controlled Polymerization of a Cyclic Diene Prepared from the Ring-Closing Metathesis of a Naturally Occurring Monoterpene. J. Am. Chem. Soc. 2009, 131, 7960–7961. [Google Scholar] [CrossRef]
- Yamamoto, D.; Matsumoto, A. Controlled Radical Polymerization of 3-Methylenecyclopentene with N-Substituted Maleimides To Yield Highly Alternating and Regiospecific Copolymers. Macromolecules 2013, 46, 9526–9536. [Google Scholar] [CrossRef]
- Polymer Handbook, 4th ed.; Brandrup, J., Immergut, E.H., Grulke, E.A., Eds.; John Wiley & Sons: New York, NY, USA, 1999. [Google Scholar]
- Thang, S.H.; Chong, Y.K.; Mayadunne, R.T.A.; Moad, G.; Rizzardo, E. A novel synthesis of functional dithioesters, dithiocarbamates, xanthates, and trithiocarbonates. Tetrahedron Lett. 1999, 40, 2435–2438. [Google Scholar] [CrossRef]

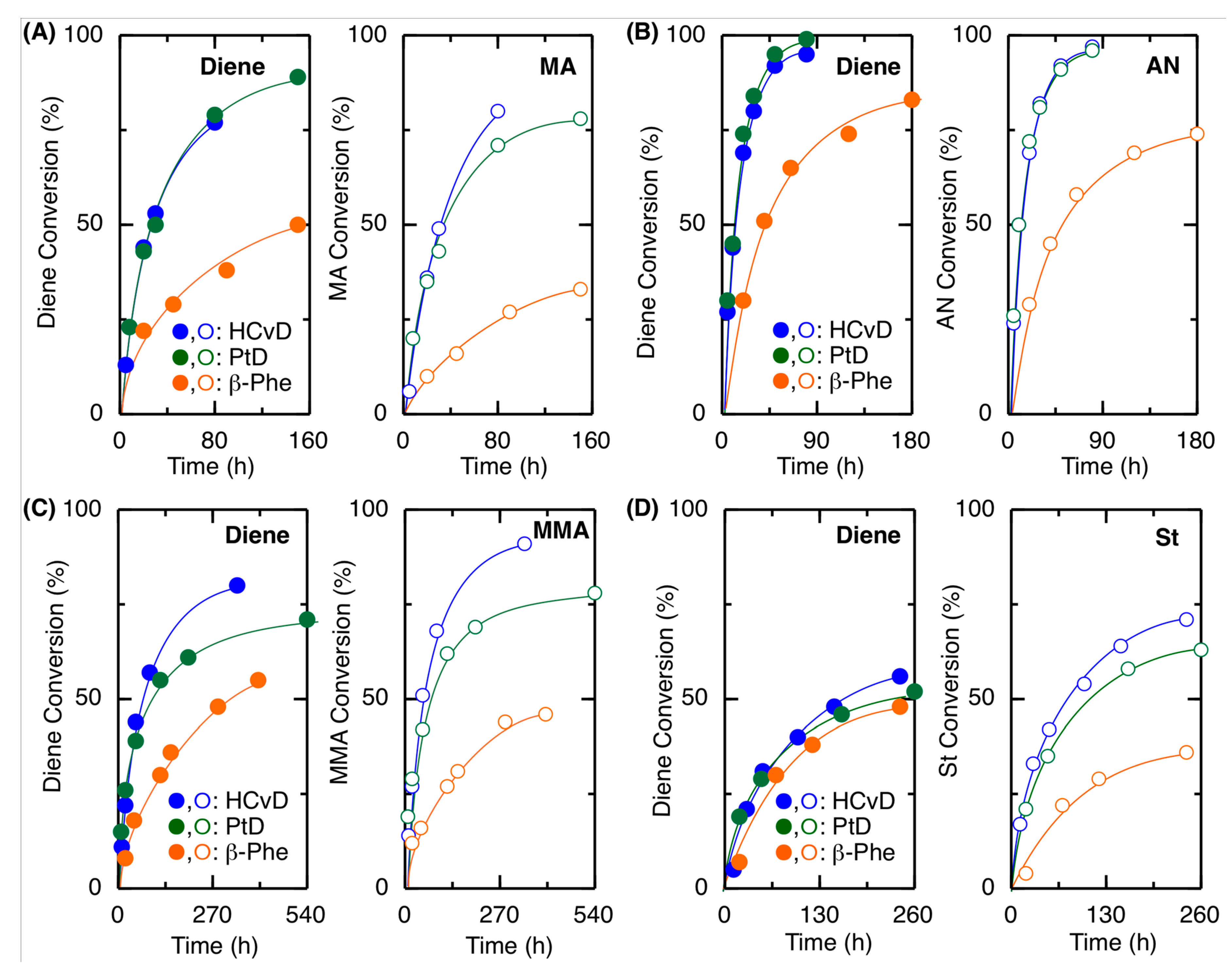

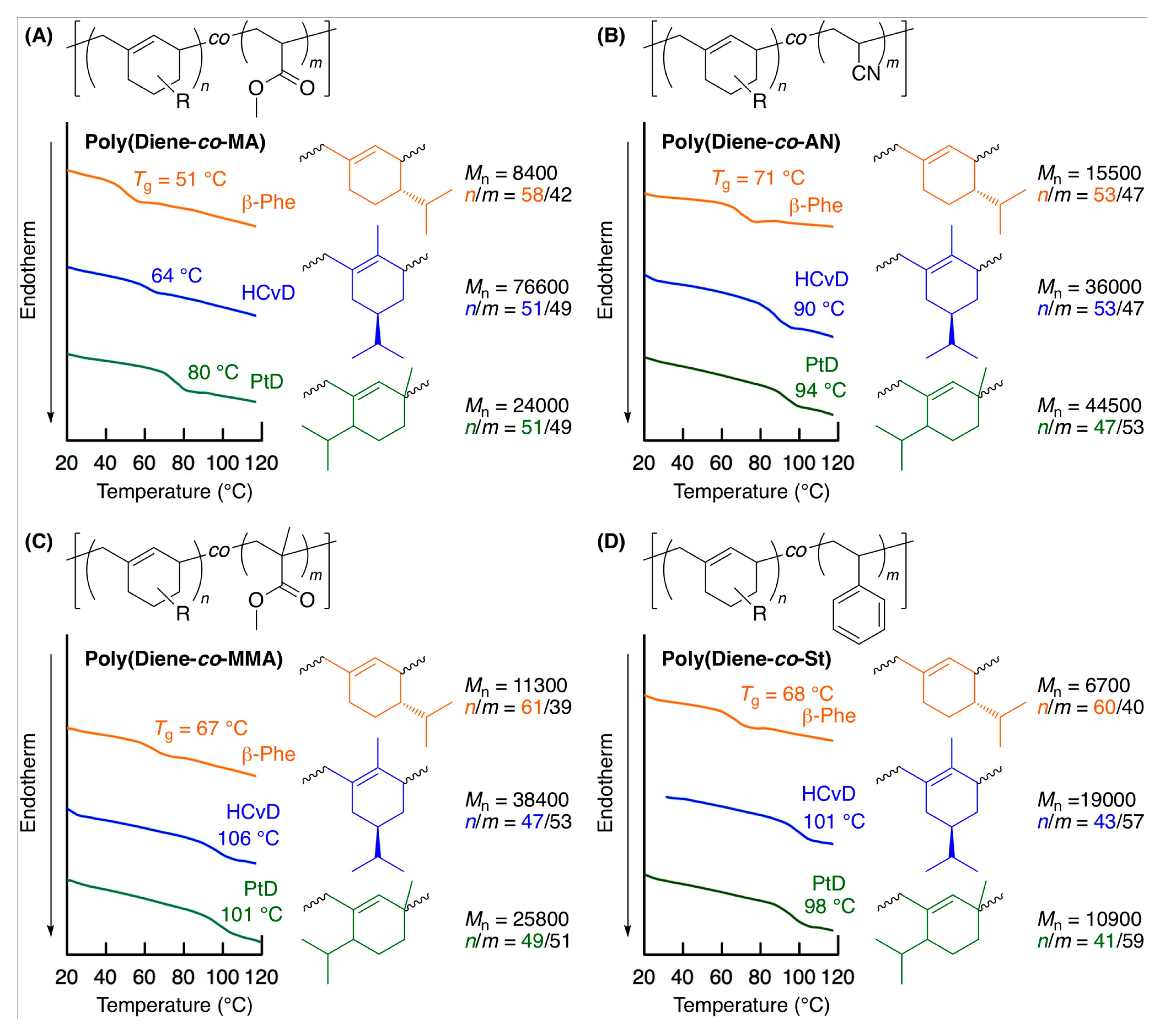
| Entry | Monomer | Temp. (°C) | Conv. (%) b | Mn(SEC) c | Mw/Mnc | 1,4-/1,2- d | Tg (°C) e |
|---|---|---|---|---|---|---|---|
| 1 | β-Phe | 60 | 2 | 3700 | 2.82 | 93/7 | n.d. f |
| 2 | β-Phe | 100 | 27 | 17,700 | 1.79 | 83/17 | 66 |
| 3 | HCvD | 60 | 0 | 10,800 | 3.03 | >99/0 | n.d. f |
| 4 | HCvD | 100 | 32 | 34,400 | 1.89 | >99/0 | 105 |
| 5 | PtD | 60 | 0 | 3200 | 2.47 | >99/0 | n.d. f |
| 6 | PtD | 100 | 29 | 10,700 | 1.69 | >99/0 | 113 |
| 7 | VnD | 60 | 0 | 240 | 1.10 | n.d. | n.d. f |
| 8 | VnD | 100 | 2 | 300 | 1.15 | n.d. | n.d. f |
| Entry | M1 | M2 | Time (h) | Conv.(%) b M1/M2 | Mn (SEC) c | 1,4-/1,2- d | M1/M2 (NMR) d | M1/M2 (Calcd) e | r1f | r2f |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | β-Phe | MA | 90 | 38/27 | 7900 | 83/17 | 61/39 | 58/42 | 0.66 | 0.04 |
| 2 | HCvD | MA | 20 | 44/36 | 26,000 | 95/5 | 52/48 | 55/45 | 0.14 | 0.08 |
| 3 | PtD | MA | 20 | 43/35 | 23,800 | 96/4 | 51/49 | 55/45 | 0.11 | 0.08 |
| 4 | β-Phe | AN | 40 | 51/45 | 14,600 | 84/16 | 52/48 | 53/47 | 0.17 | 0.03 |
| 5 | HCvD | AN | 10 | 44/50 | 42,100 | 95/5 | 53/47 | 47/53 | 0.09 | 0.03 |
| 6 | PtD | AN | 10 | 45/50 | 42,300 | 99/1 | 50/50 | 47/53 | 0.02 | 0.05 |
| 7 | β-Phe | MMA | 150 | 36/31 | 7800 | 89/11 | 60/40 | 54/46 | 0.68 | 0.09 |
| 8 | HCvD | MMA | 50 | 44/51 | 12,900 | 95/5 | 47/53 | 46/54 | 0.17 | 0.31 |
| 9 | PtD | MMA | 120 | 55/62 | 13,500 | >99/0 | 46/54 | 47/53 | 0.19 | 0.25 |
| 10 | β-Phe | St | 120 | 38/29 | 6700 | 85/15 | 60/40 | 57/43 | 1.53 | 0.43 |
| 11 | HCvD | St | 100 | 40/54 | 19,000 | 95/5 | 43/57 | 43/57 | 0.40 | 1.09 |
| 12 | PtD | St | 50 | 29/35 | 10,900 | >99/0 | 41/59 | 45/55 | 0.48 | 0.96 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishida, T.; Satoh, K.; Kamigaito, M. Biobased Polymers via Radical Homopolymerization and Copolymerization of a Series of Terpenoid-Derived Conjugated Dienes with exo-Methylene and 6-Membered Ring. Molecules 2020, 25, 5890. https://doi.org/10.3390/molecules25245890
Nishida T, Satoh K, Kamigaito M. Biobased Polymers via Radical Homopolymerization and Copolymerization of a Series of Terpenoid-Derived Conjugated Dienes with exo-Methylene and 6-Membered Ring. Molecules. 2020; 25(24):5890. https://doi.org/10.3390/molecules25245890
Chicago/Turabian StyleNishida, Takenori, Kotaro Satoh, and Masami Kamigaito. 2020. "Biobased Polymers via Radical Homopolymerization and Copolymerization of a Series of Terpenoid-Derived Conjugated Dienes with exo-Methylene and 6-Membered Ring" Molecules 25, no. 24: 5890. https://doi.org/10.3390/molecules25245890
APA StyleNishida, T., Satoh, K., & Kamigaito, M. (2020). Biobased Polymers via Radical Homopolymerization and Copolymerization of a Series of Terpenoid-Derived Conjugated Dienes with exo-Methylene and 6-Membered Ring. Molecules, 25(24), 5890. https://doi.org/10.3390/molecules25245890