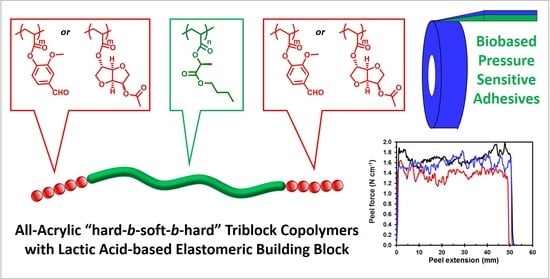
Biosourced All-Acrylic ABA Block Copolymers with Lactic Acid-Based Soft Phase
Abstract
1. Introduction
2. Results and Discussion
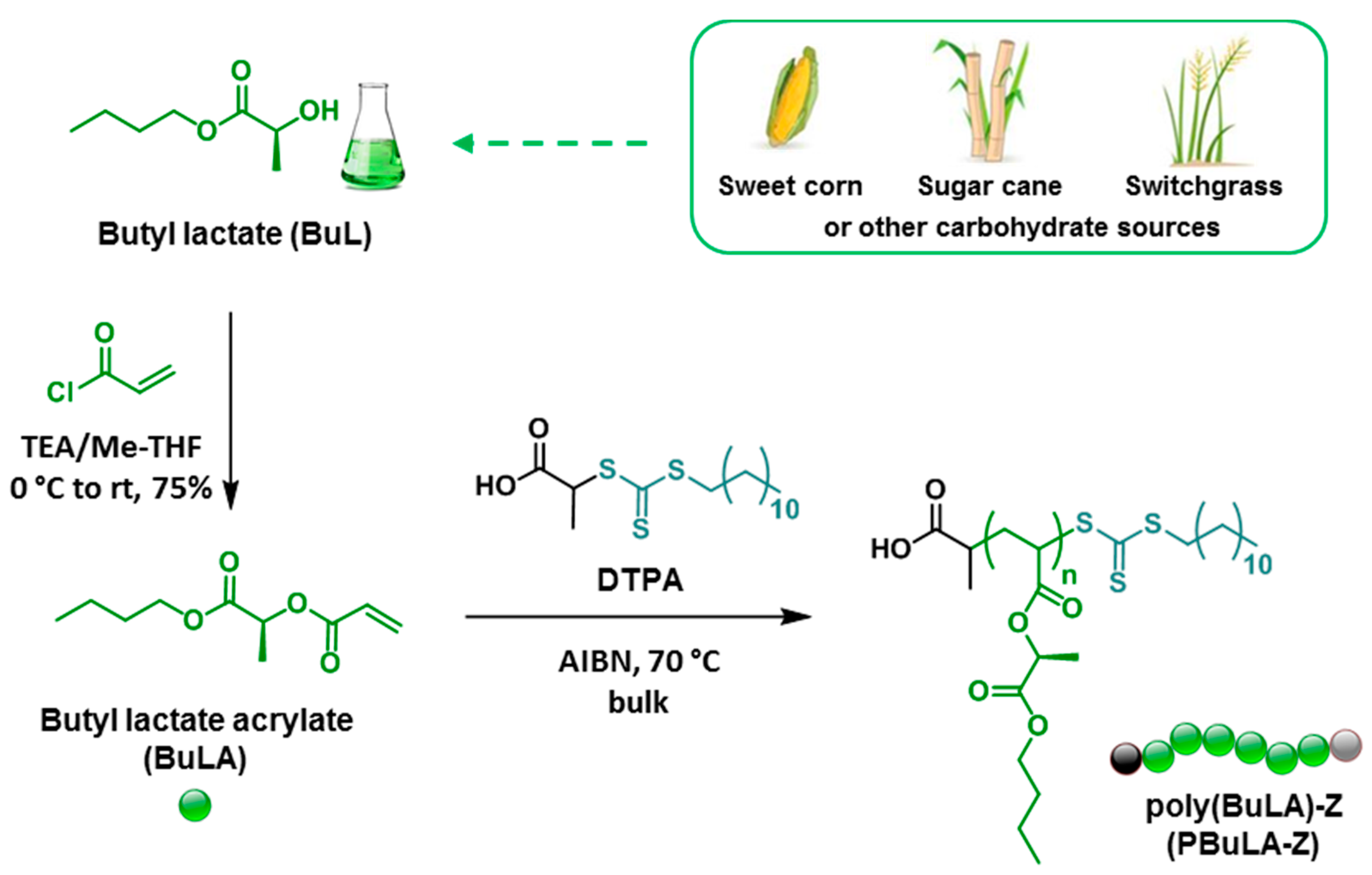
2.1. BuLA Monomer Synthesis and RAFT Polymerization Model Studies
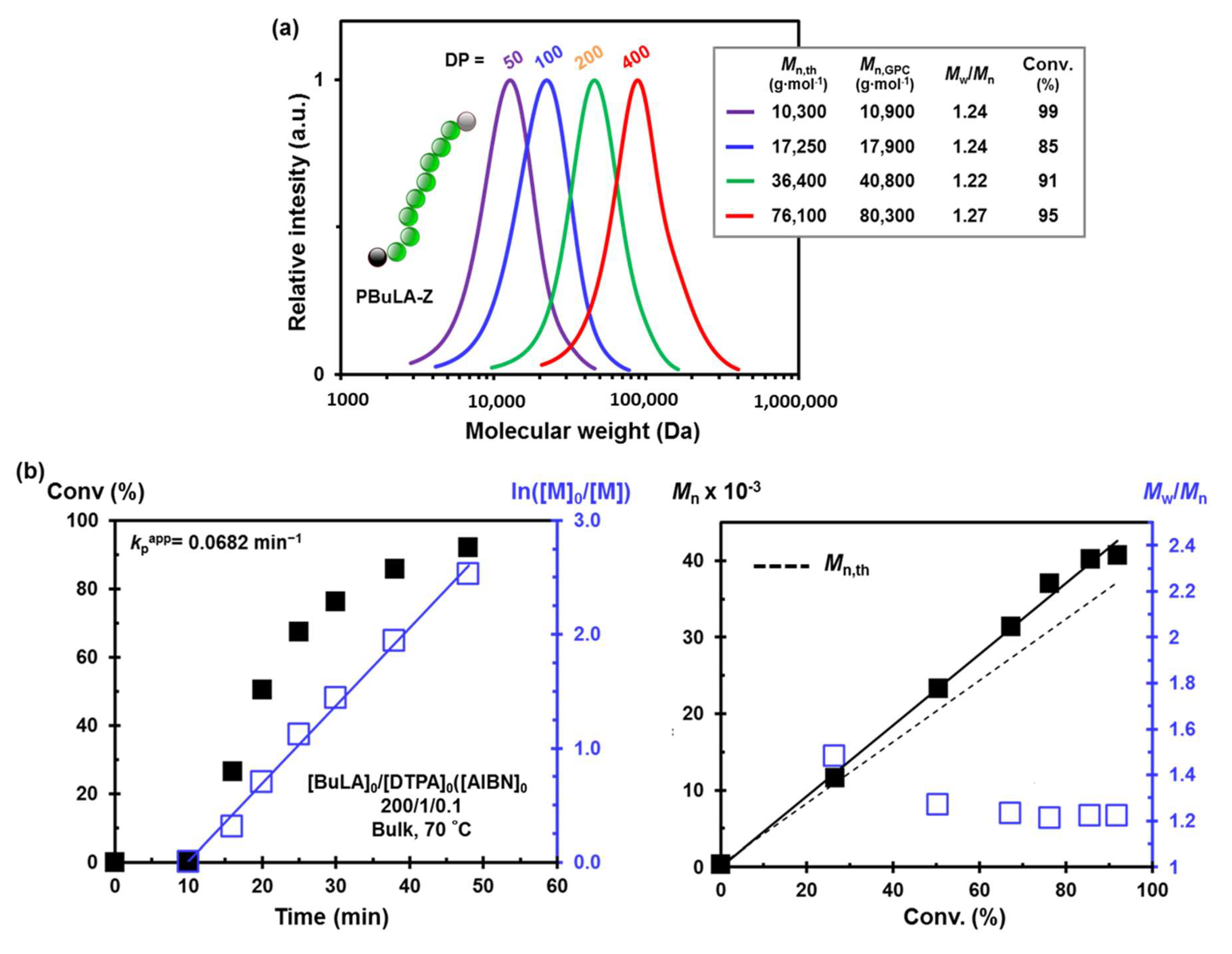
2.2. Bifunctional PBuLA-2Z macro-CTA via bulk RAFT Polymerization
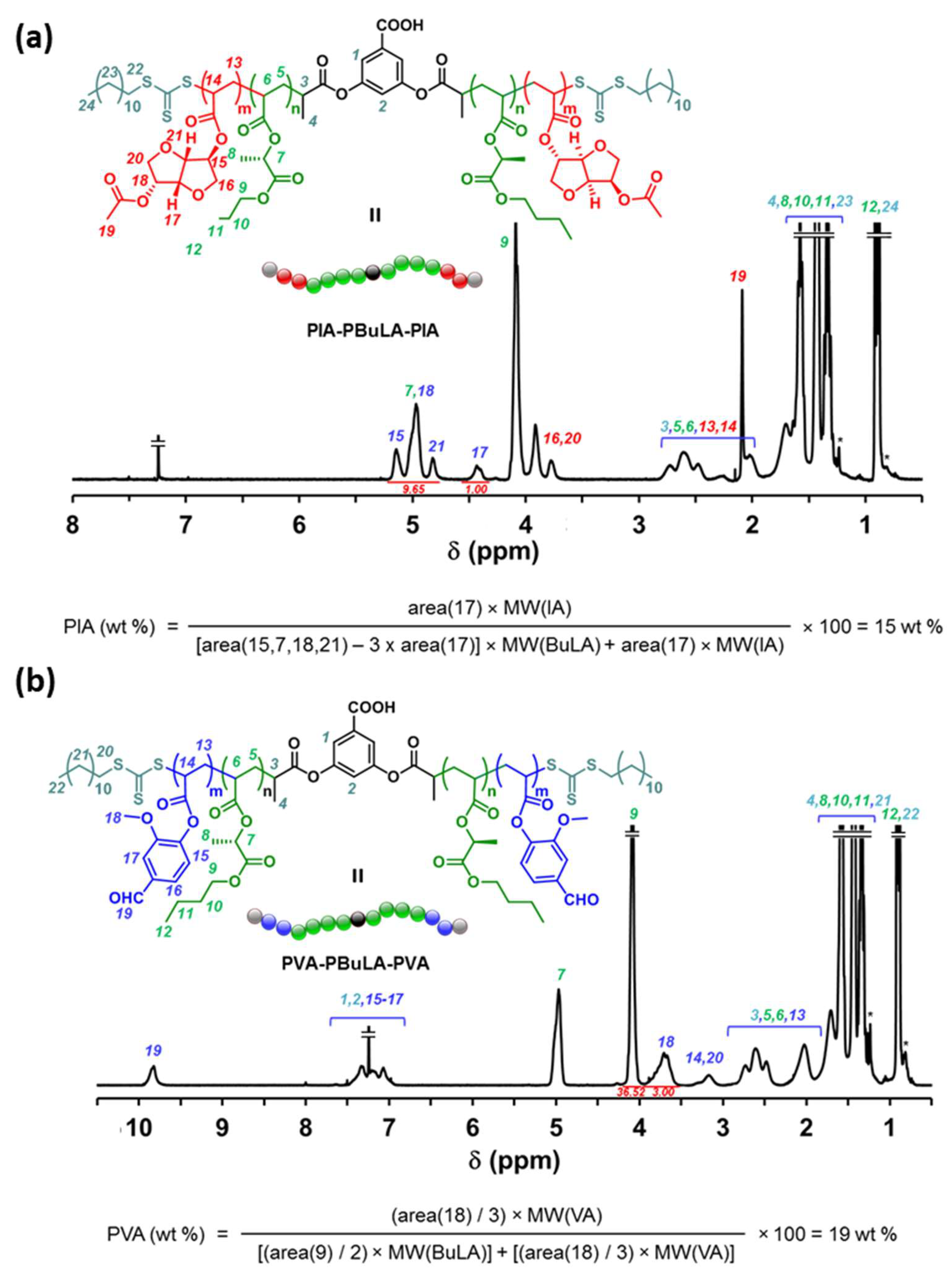
2.3. ABA Block Copolymers via RAFT in Rhodiasolv® PolarClean Solvent
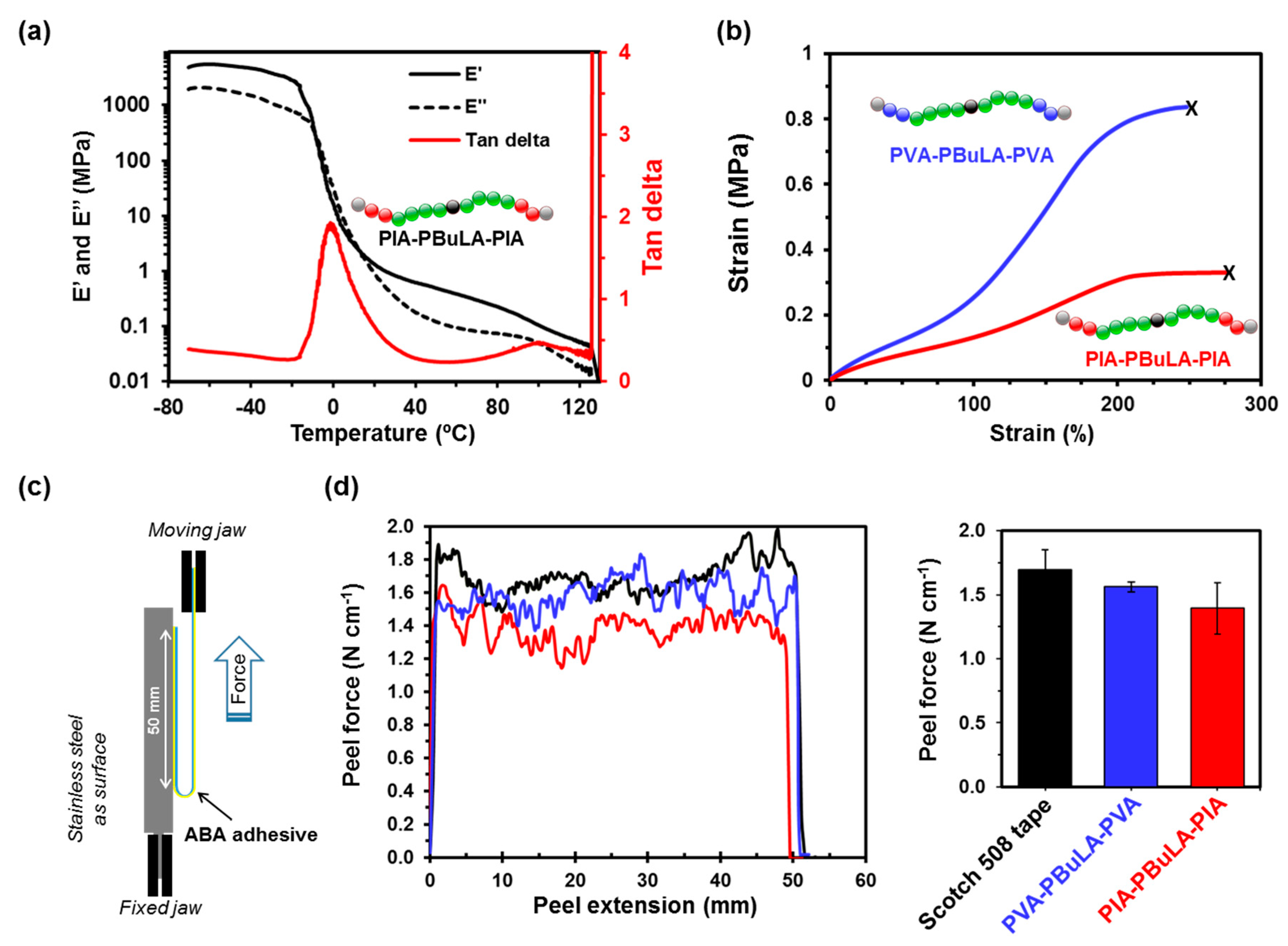
2.4. ABA Block Copolymers Characterization: Thermal, Mechanical, and Ahesive Properties
3. Materials and Methods
3.1. Materials
3.2. Methods
3.3. Synthesis of BuLA Monomer
3.4. General Procedure for the RAFT Polymerization of BuLA Monomer
3.5. General Procedure for RAFT Block Copolymerization Experiments
3.6. Cell Cultures
3.7. Cell Viability Assays
3.8. General Procedure for 180° Peel Adhesion Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References and Note
- Bates, C.M.; Bates, F.S. 50th Anniversary Perspective: Block Polymers-Pure Potential. Macromolecules 2017, 50, 3–22. [Google Scholar] [CrossRef]
- Epps, T.H., III; O’Reilly, R.K. Block Copolymers: Controlling Nanostructures to Generate Functional Materials—Synthesis, Characterization, and Engineering. Chem. Sci. 2016, 7, 1674–1689. [Google Scholar] [CrossRef]
- Ruzette, A.V.; Leibler, L. Block Copolymers in Tomorrow’s Plastics. Nat. Mater. 2005, 4, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Holden, G.; Kricheldorf, H.R.; Quirk, R.P. Thermoplastic Elastomers, 3rd ed.; Hanser Publishers: Munich, Germany, 2004. [Google Scholar]
- Shipp, D.A.; Wang, J.L.; Matyjaszewski, K. Synthesis of Acrylate and Methacrylate Block Copolymers using Atom Transfer Radical Polymerization. Macromolecules 1998, 31, 8005–8008. [Google Scholar] [CrossRef]
- Jeusette, M.; Leclère, P.; Lazzaroni, R.; Simal, F.; Vaneecke, J.; Lardot, T.; Roose, P. New “All-Acrylate” Block Copolymers: Synthesis and Influence of the Architecture on the Morphology and the Mechanical Properties. Macromolecules 2007, 40, 1055–1065. [Google Scholar] [CrossRef]
- Nakamura, Y.; Adachi, M.; Tachibana, Y.M.; Sakai, Y.; Nakano, S.; Fujii, S.; Sasaki, M.; Urahama, Y. Tack and Viscoelastic Properties of an Acrylic Block Copolymer/Tackifier System. Int. J. Adhes. Adhes. 2009, 29, 806–811. [Google Scholar] [CrossRef]
- Corrigan, N.; Jung, K.; Moad, G.; Hawker, C.J.; Matyjaszewski, K.; Boyer, C. Reversible-Deactivation Radical Polymerization (Controlled/Living Radical Polymerization): From Discovery to Materials Design and Applications. Prog. Polym. Sci. 2020, 111, 101311. [Google Scholar] [CrossRef]
- Wang, S.; Ding, W.; Yang, G.; Robertson, M.L. Biorenewable Thermoplastic Elastomeric Triblock Copolymers Containing Salicylic Acid-Derived End-Blocks and a Fatty Acid-Derived Midblock. Macromol. Chem. Phys. 2016, 217, 292–303. [Google Scholar] [CrossRef]
- Nasiria, M.; Reineke, T.M. Sustainable Glucose-based Block Copolymers Exhibit Elastomeric and Adhesive Behavior. Polym. Chem. 2016, 7, 5233–5240. [Google Scholar] [CrossRef]
- Gallagher, J.J.; Hillmyer, M.A.; Reineke, T.M. Acrylic Triblock Copolymers Incorporating Isosorbide for Pressure Sensitive Adhesives. ACS Sustain. Chem. Eng. 2016, 4, 3379–3387. [Google Scholar] [CrossRef]
- Satoh, K.; Lee, D.H.; Nagai, K.; Kamigaito, M. Precision Synthesis of Bio-Based Acrylic Thermoplastic Elastomer by RAFT Polymerization of Itaconic Acid Derivatives. Macromol. Rapid Commun. 2014, 35, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Wang, S.; Yao, K.; Ganewatta, M.S.; Tang, C.; Robertson, M.L. Physical Behavior of Triblock Copolymer Thermoplastic Elastomers Containing Sustainable Rosin-Derived Polymethacrylate End Blocks. ACS Sustain. Chem. Eng. 2017, 5, 11470–11480. [Google Scholar] [CrossRef]
- Wang, S.; Shuai, L.; Saha, B.; Vlachos, D.G.; Epps, T.H. From Tree to Tape: Direct Synthesis of Pressure Sensitive Adhesives from Depolymerized Raw Lignocellulosic Biomass. ACS Cent. Sci. 2018, 4, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Vendamme, R.; Schüwer, N.; Eevers, W. Recent Synthetic Approaches and Emerging Bio-Inspired Strategies for the Development of Sustainable Pressure-Sensitive Adhesives derived from Renewable Building Blocks. J. Appl. Polym. Sci. 2014, 131, 40669. [Google Scholar] [CrossRef]
- Sajjad, H.; Tolman, W.B.; Reineke, T.M. Block Copolymer Pressure-Sensitive Adhesives Derived from Fatty Acids and Triacetic Acid Lactone. ACS Appl. Polym. Mater. 2020, 2, 2719–2728. [Google Scholar] [CrossRef]
- Noppalit, S.; Simula, A.; Ballard, N.; Callies, X.; Asua, J.M.; Billon, L. Renewable Terpene Derivative as a Biosourced Elastomeric Building Block in the Design of Functional Acrylic Copolymers. Biomacromolecules 2019, 20, 2241–2251. [Google Scholar] [CrossRef]
- Wang, Y.; Deng, W.; Wang, B.; Zhang, Q.; Wan, X.; Tang, Z.; Wang, Y.; Zhu, C.; Cao, Z.; Wang, G.; et al. Chemical Synthesis of Lactic Acid from Cellulose Catalysed by Lead(II) Ions in Water. Nat. Commun. 2013, 4, 2141. [Google Scholar] [CrossRef]
- Cubas-Cano, E.; González-Fernández, C.; Ballesteros, M.; Tomás-Pejó, E. Biotechnological Advances in Lactic Acid Production by Lactic Acid Bacteria: Lignocellulose as Novel Substrate. Biofuels Bioprod. Bioref. 2018, 12, 290–303. [Google Scholar] [CrossRef]
- Yee, G.M.; Hillmyer, M.A.; Tonks, I.A. Bioderived Acrylates from Alkyl Lactates via Pd-Catalyzed Hydroesterification. ACS Sustain. Chem. Eng. 2018, 6, 9579–9584. [Google Scholar] [CrossRef]
- Kim, H.J.; Jin, K.; Shim, J.; Dean, W.; Hillmyer, M.A.; Ellison, C.J. Sustainable Triblock Copolymers as Tunable and Degradable Pressure Sensitive Adhesives. ACS Sustain. Chem. Eng. 2020, 8, 12036–12044. [Google Scholar] [CrossRef]
- Galaster™ EL 98.5 FCC, Galasolv™ 003, PURASOLV® ELECT/BL/ML, and VertecBio™ EL are examples of commercial ethyl lactate solvents produced by Galactic, Corbion, or Vertec Biosolvents
- Purushothaman, M.; Krishnan, P.S.G.; Nayak, S.K. Poly(alkyl lactate acrylate)s Having Tunable Hydrophilicity. J. Appl. Polym. Sci. 2014, 131, 40962. [Google Scholar] [CrossRef]
- Purushothaman, M.; Krishnan, P.S.G.; Nayak, S.K. Tunable Hydrophilicity of Poly(ethyl lactate acrylate-co-acrylic acid). J. Renew. Mater. 2015, 3, 292–301. [Google Scholar] [CrossRef]
- Purushothaman, M.; Krishnan, P.S.G.; Nayak, S.K. Effect of Isoalkyl Lactates as Pendant Group on Poly(acrylic acid). J. Macromol. Sci. A 2014, 51, 470–480. [Google Scholar] [CrossRef]
- Purushothaman, M.; Krishnan, P.; Santhana, G.; Nayak, S.K. Effect of Nano Sepiolite Fiber on the Properties of Poly(ethyl lactate acrylate): Hydrophilicity and Thermal Stability. Mater. Focus 2018, 7, 101–107. [Google Scholar] [CrossRef]
- Bensabeh, N.; Moreno, A.; Roig, A.; Monaghan, O.R.; Ronda, J.C.; Cádiz, V.; Galià, M.; Howdle, S.M.; Lligadas, G.; Percec, V. Polyacrylates Derived from Biobased Ethyl Lactate Solvent via SET-LRP. Biomacromolecules 2019, 20, 2135–2147. [Google Scholar] [CrossRef]
- Moreno, A.; Bensabeh, N.; Parve, J.; Ronda, J.C.; Cádiz, V.; Galià, M.; Vares, L.; Lligadas, G.; Percec, V. SET-LRP of Bio- and Petroleum-Sourced Methacrylates in Aqueous Alcoholic Mixtures. Biomacromolecules 2019, 20, 1816–1827. [Google Scholar] [CrossRef]
- Bensabeh, N.; Moreno, A.; Roig, A.; Rahimzadeh, M.; Rahimi, K.; Ronda, J.C.; Cádiz, V.; Galià, M.; Percec, V.; Rodriguez-Emmenegger, C.; et al. Photoinduced Upgrading of Lactic Acid-Based Solvents to Block Copolymer Surfactants. ACS Sustain. Chem. Eng. 2020, 8, 1276–1284. [Google Scholar] [CrossRef]
- Semsarilar, M.; Perrier, S. ‘Green’ Reversible Addition-Fragmentation Chain-Transfer (RAFT) Polymerization. Nat. Chem. 2010, 2, 811–820. [Google Scholar] [CrossRef]
- Holmberg, A.L.; Reno, K.H.; Wool, R.P.; Epps, T.H., III. Biobased Building Blocks for the Rational Design of Renewable Block Polymers. Soft Matter 2014, 10, 7405–7424. [Google Scholar] [CrossRef]
- Matt, L.; Parve, J.; Parve, O.; Pehk, T.; Pham, T.H.; Liblikas, I.; Vares, L.; Jannasch, P. Enzymatic Synthesis and Polymerization of Isosorbide-Based Monomethacrylates for High-Tg Plastics. ACS Sustain. Chem. Eng. 2018, 6, 17382–17390. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, T.; Xin, J.; Zhang, J. Biobased Miktoarm Star Copolymer from Soybean Oil, Isosorbide, and Caprolactone. J. Appl. Polym. Sci. 2020, 137, 48281. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, H.; Deng, J.; Wu, Y. High Glass-Transition Temperature Acrylate Polymers Derived from Biomasses, Syringaldehyde, and Vanillin. Macromol. Chem. Phys. 2016, 217, 2402–2408. [Google Scholar] [CrossRef]
- Noppalit, S.; Simula, A.; Billon, L.; Asua, J.M. Paving the Way to Sustainable Waterborne Pressure-Sensitive Adhesives Using Terpene-Based Triblock Copolymers. ACS Sustain. Chem. Eng. 2019, 7, 17990–17998. [Google Scholar] [CrossRef]
- Cseri, L.; Szekely, G. Towards Cleaner PolarClean: Efficient Synthesis and Extended Applications of the Polar Aprotic Solvent Methyl 5-(dimethylamino)-2-methyl-5-oxopentanoate. Green. Chem. 2019, 21, 4178–4188. [Google Scholar] [CrossRef]
- Lu, W.; Wang, Y.; Wang, W.; Cheng, S.; Zhu, J.; Xu, Y.; Hong, K.; Kang, N.G.; Mays, J. All Acrylic—based Thermoplastic Elastomers with Upper Service Temperature and Superior Mechanical Properties. Polym. Chem. 2017, 8, 5741–5748. [Google Scholar] [CrossRef]
Sample Availability: Samples of the monomeric compounds, macroinitiators and block copolymers are available from the authors. |





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bensabeh, N.; Jiménez-Alesanco, A.; Liblikas, I.; Ronda, J.C.; Cádiz, V.; Galià, M.; Vares, L.; Abián, O.; Lligadas, G. Biosourced All-Acrylic ABA Block Copolymers with Lactic Acid-Based Soft Phase. Molecules 2020, 25, 5740. https://doi.org/10.3390/molecules25235740
Bensabeh N, Jiménez-Alesanco A, Liblikas I, Ronda JC, Cádiz V, Galià M, Vares L, Abián O, Lligadas G. Biosourced All-Acrylic ABA Block Copolymers with Lactic Acid-Based Soft Phase. Molecules. 2020; 25(23):5740. https://doi.org/10.3390/molecules25235740
Chicago/Turabian StyleBensabeh, Nabil, Ana Jiménez-Alesanco, Ilme Liblikas, Juan C. Ronda, Virginia Cádiz, Marina Galià, Lauri Vares, Olga Abián, and Gerard Lligadas. 2020. "Biosourced All-Acrylic ABA Block Copolymers with Lactic Acid-Based Soft Phase" Molecules 25, no. 23: 5740. https://doi.org/10.3390/molecules25235740
APA StyleBensabeh, N., Jiménez-Alesanco, A., Liblikas, I., Ronda, J. C., Cádiz, V., Galià, M., Vares, L., Abián, O., & Lligadas, G. (2020). Biosourced All-Acrylic ABA Block Copolymers with Lactic Acid-Based Soft Phase. Molecules, 25(23), 5740. https://doi.org/10.3390/molecules25235740