Novel 3-Acetyl-2,5-disubstituted-1,3,4-oxadiazolines: Synthesis and Biological Activity
Abstract
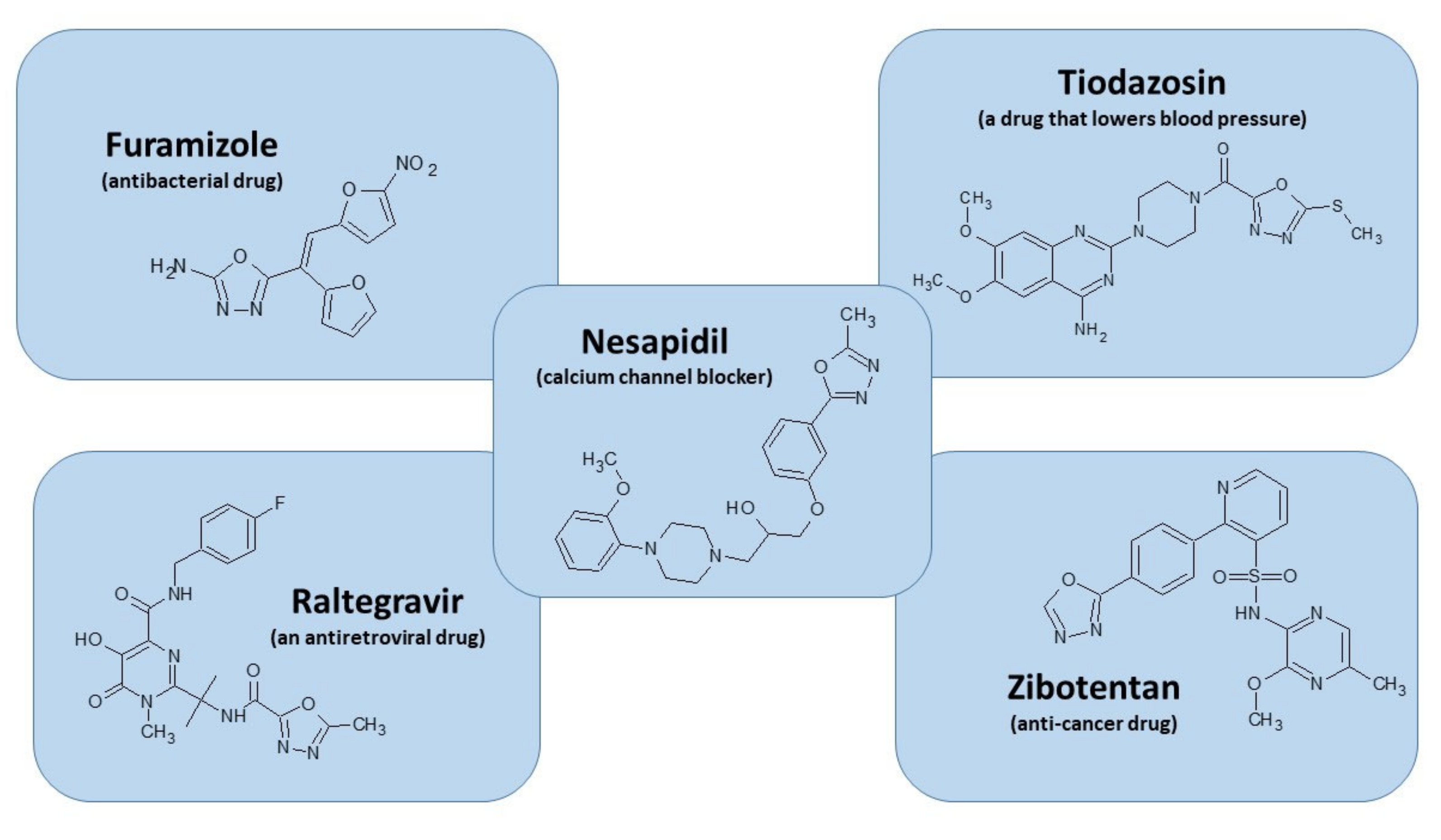
1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Microbiology
2.3. Cytotoxicity Studies
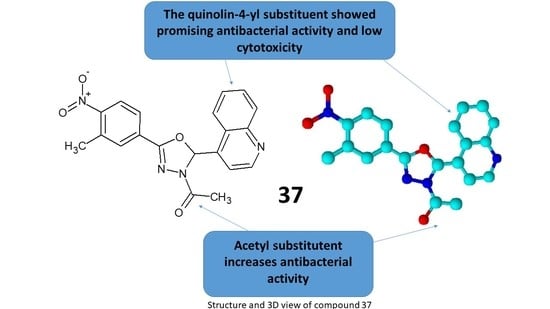
2.4. SAR Analysis
3. Material and Methods
3.1. Chemistry
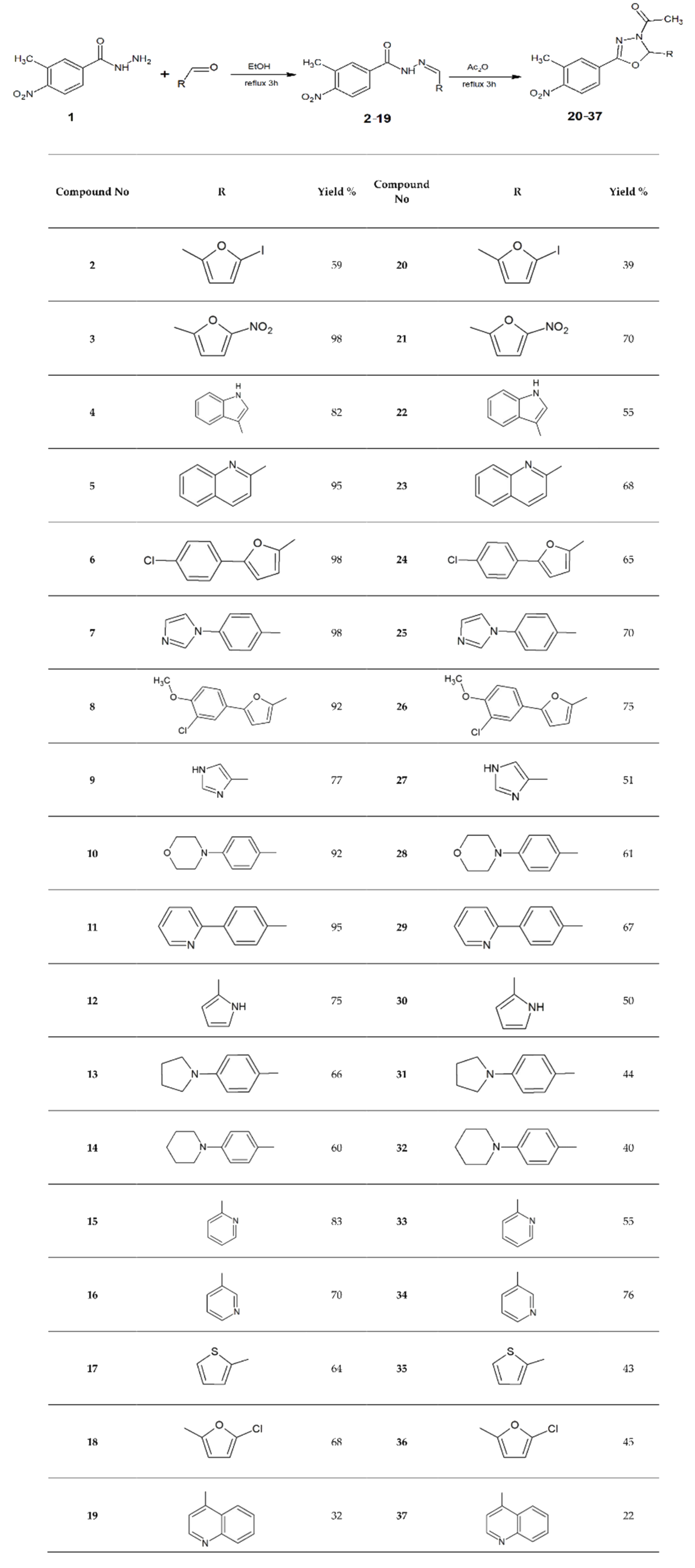
3.2. Synthesis of Hydrazide–Hydrazones of 3-Methyl-4-nitrobenzoic Acid (2–19)
3.3. Synthesis of 3-Acetyl-2,5-disubstituted-1,3,4-oxadiazolines (20–37)
3.4. Microbiology
3.5. Cytotoxicity Studies
3.5.1. Cell Lines
3.5.2. MTT Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- European Centre for Disease Prevention and Control (ECDC); European Food Safety Authority (EFSA); European Medicines Agency (EMA). Joint Interagency Antimicrobial Consumption and Resistance Analysis (JIACRA) Report. EFSA J. 2017, 15, 4872. [Google Scholar]
- Mohammad, H.; Mayhoub, A.S.; Ghafoor, A.; Soofi, M.; Alajlouni, R.A.; Cushman, M.; Seleem, M.N. Discovery and Characterization of Potent Thiazoles versus Methicillin- and Vancomycin-Resistant Staphylococcus aureus. J. Med. Chem. 2014, 57, 1609–1615. [Google Scholar] [CrossRef] [PubMed]
- Carbon, C. MRSA and MRSE: Is there an answer? Clin. Microbiol. Infect. 2000, 6 (Suppl. 2), 17–22. [Google Scholar] [CrossRef] [PubMed]
- Weber, D.J.; Raasch, R.; Rutala, W.A. Nosocomial infections in the ICU. The growing importance of antibiotic-resistant pathogens. Chest 1999, 115 (Suppl. 3), 34–41. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.J.; Xie, C.B.; Sun, F.H.; Guo, L.J.; Dai, M.; Cheng, X.; Ma, Y.X. Molecular Characteristics of Methicillin-Resistant Staphylococcus epidermidis on the Abdominal Skin of Females before Laparotomy. Int. J. Mol. Sci. 2016, 17, 992. [Google Scholar] [CrossRef]
- Watanabe, K.; Nakaminami, H.; Azuma, C.; Tanaka, I.; Nakase, K.; Matsunaga, N.; Okuyama, K.; Yamada, K.; Utsumi, K.; Fujii, T.; et al. Methicillin-Resistant Staphylococcus epidermidis Is Part of the Skin Flora on the Hands of Both Healthy Individuals and Hospital Workers. Biol. Pharm. Bull. 2016, 39, 1868–1875. [Google Scholar] [CrossRef] [PubMed]
- Nowakowicz-Dębek, B.; Wlazło, Ł.; Kasela, M.; Ossowski, M. Epidemiologia wielolekoopornych szczepów Staphylococcus aureus. Probl. Hig. Epidemiol. 2016, 97, 106–112. [Google Scholar]
- Popiołek, Ł.; Biernasiuk, A.; Paruch, K.; Malm, A.; Wujec, M. Synthesis and in vitro Antimicrobial Activity Screening of New 3-Acetyl-2,5-disubstituted-1,3,4-oxadiazoline derivatives. Chem. Biodivers. 2019, 16, e1900082. [Google Scholar] [CrossRef]
- Paruch, K.; Popiołek, Ł.; Wujec, M. Antimicrobial and antiprotozoal activity of 3-acetyl-2,5-disubstituted-1,3,4-oxadiazolines: A review. Med. Chem. Res. 2020, 29, 1–16. [Google Scholar] [CrossRef]
- Zheng, Z.; Liu, Q.; Kim, W.; Tharmalingam, N.; Fuchs, B.B.; Mylonakis, E. Antimicrobial activity of 1,3,4-oxadiazole derivatives against planktonic cells and biofilm of Staphylococcus aureus. Future Med. Chem. 2018, 10, 283–296. [Google Scholar] [CrossRef]
- Rollas, S.; Gulerman, N.; Erdeniz, H. Synthesis and antimicrobial activity of some new hydrazones of 4-fluorobenzoic acid hydrazide and 3-acetyl-2,5-disubstituted-1,3,4-oxadiazolines. IL Farm. 2018, 57, 171–174. [Google Scholar] [CrossRef]
- Joshi, S.D.; More, U.A.; Pansuriya, K.; Aminabhavi, T.M.; Gadad, A.K. Synthesis and molecular modeling studies of novel pyrrole analogs as antimycobacterial agents. J. Saudi Chem. Soc. 2017, 21, 42–57. [Google Scholar] [CrossRef]
- Fuloria, N.K.; Singh, V.; Shaharyar, M.; Ali, M. Synthesis and Antimicrobial Evaluation of Some New Oxadiazoles Derived from Phenylpropionohydrazides. Molecules 2009, 14, 1898–1903. [Google Scholar] [CrossRef] [PubMed]
- Chawla, R.; Arora, A.; Parameswaran, M.K.; Sharma, P.C.; Michael, S.; Ravi, T.K. Synthesis of novel 1,3,4-oxadiazole derivatives as potential antimicrobial agents. Acta Pol. Pharm. 2010, 67, 247–253. [Google Scholar] [PubMed]
- Kumar, S.G.V.; Rajendraprasad, Y.; Mallikarjuna, B.P.; Chandrashekar, S.M.; Kistayya, C. Synthesis of some novel 2-substituted-5-[isopropylthiazole]clubbed 1,2,4-triazole and 1,3,4-oxadiazoles as potential antimicrobial and antitubercular agents. Eur. J. Med. Chem. 2010, 45, 2063–2074. [Google Scholar] [CrossRef] [PubMed]
- Ke, S.; Liu, F.; Wang, N.; Yang, Q.; Qian, X. 1,3,4-Oxadiazoline derivatives as novel potential inhibitors targeting chitin biosynthesis: Design, synthesis and biological evaluation. Bioorg. Med. Chem. Lett. 2009, 19, 332–335. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-F.; Cao, H.; Liu, H.; Li, B.-Q.; Ma, Y.-M. Synthesis and Bioactivity of Novel Bis-heterocyclic Compounds Containing Pyrazole and Oxadiazoline. J. Chin. Chem. Soc. 2011, 58, 369–375. [Google Scholar] [CrossRef]
- Koçyiğit-Kaymakçıoğlu, B.; Oruc-Emre, E.E.; Unsalan, S.; Tabanca, N.; Khan, S.I.; Wedge, D.E.; Iscan, G.; Demirci, F.; Rollas, S. Synthesis and biological activity of hydrazide-hydrazones and their corresponding 3-acetyl-2,5-disubstituted-2,3-dihydro-1,3,4-oxadiazoles. Med. Chem. Res. 2012, 21, 3499–3508. [Google Scholar] [CrossRef]
- Shyma, P.C.; Kalluraya, B.; Peethambar, S.K.; Telkar, S.; Arulmoi, T. Synthesis, characterization and molecular docking studies of some new 1,3,4-oxadiazolines bearing 6-methylpyridine moiety for antimicrobial property. Eur. J. Med. Chem. 2013, 68, 394–404. [Google Scholar] [CrossRef]
- Baquero, E.; Quinones, W.; Ribon, W.; Caldas, M.L.; Sarmiento, L.; Echeverri, F. Effect of an Oxadiazoline and a Lignan on Mycolic Acid Biosynthesis and Ultrastructural Changes of Mycobacterium tuberculosis. Tuberc. Res. Treat. 2011. [Google Scholar] [CrossRef]
- Bhat, M.A. Synthesis and anti-mycobacterial activity of new 4-thiazolidinone and 1,3,4-oxadiazole derivatives of isoniazid. Acta Pol. Pharm. 2014, 71, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.D.; Vagdevi, H.M.; Vaidya, V.P.; Gadaginamath, G.S. Synthesis of new 4-pyrrol-1-yl benzoic acid hydrazide analogs and some derived oxadiazole, triazole and pyrrole ring systems: A novel class of potential antibacterial and antitubercular agents. Eur. J. Med. Chem. 2008, 43, 1989–1996. [Google Scholar] [CrossRef] [PubMed]
- Ishii, M.; Jorge, S.D.; de Oliveira, A.A.; Palace-Berl, F.; Sonehara, I.Y.; Pasqualoto, K.F.M.; Tavares, L.C. Synthesis, molecular modeling and preliminary biological evaluation of a set of 3-acetyl-2,5-disubstituted-2,3-dihydro-1,3,4-oxadiazole as potential antibacterial, anti-Trypanosoma cruzi and antifungal agents. Bioorg. Med. Chem. 2011, 19, 6292–6301. [Google Scholar] [CrossRef] [PubMed]
- Palace-Berl, F.; Jorge, S.D.; Pasqualoto, K.F.M.; Ferreira, A.K.; Maria, D.A.; Zorzi, R.R.; de Sá Bortolozzo, L.; Lindoso, J.A.L.; Tavares, L.C. 5-Nitro-2-furfuriliden derivatives as potential anti-Trypanosoma cruzi agents: Design, synthesis, bioactivity evaluation, cytotoxicity and exploratory data analysis. Bioorg. Med. Chem. 2013, 21, 5395–5406. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.N.; Han, D.; Xu, F.F.; Meng, X.-B.; Li, Z.-J. Microwave-assisted efficient synthesis of glucose-based 3-acetyl-5-alkyl-2,3-dihydro-1,3,4-oxadiazole derivatives catalyzed by sodium acetate. Carbohydr. Res. 2009, 344, 2113–2119. [Google Scholar] [CrossRef] [PubMed]
- Chaaban, I.; Khawass, E.L.; El Razik, H.A.A.; Elsalamouny, N.; Redondo-Horcajo, M.; Barasoain, I.; Díaz, J.F.; Yli-Kauhaluoma, J.; Moreira, M. Synthesis and biological evaluation of new oxadiazoline-substituted naphthalenyl acetates as anticancer agents. Eur. J. Med. Chem. 2014, 87, 805–813. [Google Scholar] [CrossRef]
- Jin, L.; Chen, J.; Song, B.; Chen, Z.; Yang, S.; Li, Q.; Hu, D.; Xu, R. Synthesis, structure, and bioactivity of N′-substituted benzylidene-3,4,5-trimethoxybenzohydrazide and 3-acetyl-2-substituted phenyl-5-(3,4,5-trimethoxyphenyl)-2,3-dihydro-1,3,4-oxadiazole derivatives. Bioorg. Med. Chem. Lett. 2006, 16, 5036–5040. [Google Scholar] [CrossRef]
- Salum, L.; Mascarello, A.; Canevarolo, R.; Altei, W.F.; Laranjeira, A.; Neuenfeldt, P.; Stumpf, T.; Chiaradia-Delatorre, L.; Vollmer, L.; Daghestani, H.; et al. N-(1′-naphthyl)-3,4,5-trimethoxybenzohydrazide as microtubule destabilizer: Synthesis, cytotoxicity, inhibition of cell migration and in vivo activity against acute lymphoblastic leukemia. Eur. J. Med. Chem. 2015, 96, 504–518. [Google Scholar] [CrossRef]
- Hamdi, N.; Passarelli, V.; Romerosa, A. Synthesis, spectroscopy and electrochemistry of new 4-(4-acetyl-5-substituted-4,5-dihydro-1,3,4-oxodiazol-2-yl)methoxy)-2H-chromen-2-ones as a novel class of potential antibacterial and antioxidant derivatives. Comptes Rendus Chim. 2011, 14, 548–555. [Google Scholar] [CrossRef]
- Malhotra, M.; Rawal, R.K.; Malhotra, D.; Dhingra, R.; Deep, A.; Sharma, P.C. Synthesis, characterization and pharmacological evaluation of (Z)-2-(5-(biphenyl-4-yl)-3-(1-(imino)ethyl)-2,3-dihydro-1,3,4-oxadiazol-2-yl)phenol derivatives as potent antimicrobial and antioxidant agents. Arab. J. Chem. 2017, 10, 1022–1031. [Google Scholar] [CrossRef]
- Patil, P.O.; Bari, S.B. Nitrogen heterocycles as potential monoamine oxidase inhibitors: Synthetic aspects. Arab. J. Chem. 2014, 7, 857–884. [Google Scholar] [CrossRef]
- Maccioni, E.; Alcaro, S.; Cirilli, R.; Vigo, S.; Cardia, M.C.; Sanna, M.L.; Meleddu, R.; Yanez, M.; Costa, G.; Casu, L.; et al. 3-Acetyl-2,5-diaryl-2,3-dihydro-1,3,4-oxadiazoles: A New Scaffold for the Selective Inhibition of Monoamine Oxidase, B. J. Med. Chem. 2011, 54, 6394–6398. [Google Scholar] [CrossRef] [PubMed]
- Ke, S.; Li, Z.; Qian, X. 1,3,4-Oxadiazole-3(2H)-carboxamide derivatives as potential novel class of monoamine oxidase (MAO) inhibitors: Synthesis, evaluation, and role of urea moiety. Bioorg. Med. Chem. 2008, 16, 7565–7572. [Google Scholar] [CrossRef] [PubMed]
- Distinto, S.; Meleddu, R.; Yáñez, M.; Cirilli, R.; Bianco, G.; Sanna, M.; Arridu, A.; Cossu, P.; Cottiglia, F.; Faggi, C.; et al. Drug design, synthesis, in vitro and in silico evaluation of selective monoaminoxidase B inhibitors based on 3-acetyl-2-dichlorophenyl-5-aryl-2,3-dihydro-1,3,4-oxadiazole chemical scaffold. Eur. J. Med. Chem. 2016, 108, 542–552. [Google Scholar] [CrossRef] [PubMed]
- Bala, S.; Kamboj, S.; Kumar, A. Heterocyclic 1,3,4-oxadiazole compounds with diverse biological activities: A comprehensive review. J. Pharm. Res. 2010, 3, 2993–2997. [Google Scholar]
- Schlecker, R.; Thieme, P.C. The synthesis of antihypertensive 3-(1,3,4-oxadiazol-2-yl)phenoxypropanolahines. Tetrahedron 1998, 44, 3289–3294. [Google Scholar] [CrossRef]
- Cocohoba, J.; Dong, B.J. Raltegravir: The First HIV Integrase Inhibitor. Clin. Ther. 2008, 30, 1747–1765. [Google Scholar] [CrossRef]
- Vardan, S.; Smulyan, H.; Mookherjee, S.; Eich, R. Effects of tiodazosin, a new antihypertensive, hemodynamics and clinical variables. Clin. Pharm. Ther. 1983, 34, 290–296. [Google Scholar] [CrossRef]
- Shi, J.; Luo, N.; Ding, M.; Bao, X. Synthesis, in vitro antibacterial and antifungal evaluation of novel 1,3,4-oxadiazole thioether derivatives bearing the 6-fluoroquinazolinylpiperidinyl moiety. Chin. Chem. Lett. 2020, 31, 434–438. [Google Scholar] [CrossRef]
- James, N.D.; Growcott, J.W. Zibotentan endothelin ETA receptor antagonist oncolytic. Drugs Future 2009, 34, 624–633. [Google Scholar] [CrossRef]
- Fizazi, K.; Higano, C.; Nelson, J.B.; Gleave, M.; Miller, K.; Morris, T.; Nathan, F.E.; McIntosh, S.; Pemberton, K.; Moul, J.W. Phase III, Randomized, Placebo-Controlled Study of Docetaxel in Combination with Zibotentan in Patients with Metastatic Castration-Resistant Prostate Cancer. J. Clin. Oncol. 2013, 31, 1740–1747. [Google Scholar] [CrossRef] [PubMed]
- Desai, N.C.; Dodiya, A.M. Conventional and microwave techniques for the synthesis and antimicrobial studies of novel 1-[2-(2-chloro-6-methyl(3-quinolyl))-5-(4-nitrophenyl)-(1,3,4-oxadiazolin-3-yl)]-3-(aryl)prop-2-en-1-ones. Arab. J. Chem. 2016, 9, 379–387. [Google Scholar] [CrossRef]
- Zorzi, R.R.; Jorge, S.D.; Palace-Berl, F.; Pasqualoto, K.F.M.; de Sá Bortolozzo, L.; de Castro Siqueira, A.M.; Tavares, L.C. Exploring 5-nitrofuran derivatives against nosocomial pathogens: Synthesis, antimicrobial activity and chemometric analysis. Bioorg. Med. Chem. 2014, 22, 2844–2854. [Google Scholar] [CrossRef] [PubMed]
- Ghaisas, D.S.; Patel, N.B. Design, Synthesis and Evaluation of Newer Diaryl Ether Analogues Integrated with 1,3,4 Oxadiazole Core. IJPPR Hum. 2018, 12, 57–67. [Google Scholar]
- Jadhav, G.R.; Deshmukh, D.G.; Medhane, V.J.; Gaikwad, V.B.; Bholay, A.D. 2,5-Disubstituted 1,3,4-oxadiazole derivatives of chromeno [4,3-b]pyridine: Synthesis and study of antimicrobial potency. Heterocycl. Commun. 2016, 22, 123–130. [Google Scholar] [CrossRef]
- Dewangan, D.; Pandey, A.; Sivakumar, T.; Rajavel, R.; Dubey, R. Synthesis of some Novel 2, 5-Disubstituted 1, 3, 4-Oxadiazole and its Analgesic, Anti-Inflammatory, Anti-Bacterial and Anti-Tubercular Activity. Int. J. Chem. Tech. Res. 2010, 2, 1397–1412. [Google Scholar]
- Popiołek, Ł.; Biernasiuk, A.; Berecka, A.; Gumieniczek, A.; Malm, A.; Wujec, M. New hydrazide-hydrazones of isonicotinic acid: Synthesis, lipophilicity and in vitro antimicrobial screening. Chem. Biol. Drug Des. 2018, 91, 915–923. [Google Scholar] [CrossRef]
- Popiołek, Ł.; Bienasiuk, A. Design, synthesis and in vitro antimicrobial activity of hydrazide-hydrazones of 2-substituted acetic acid. Chem. Biol. Drug Des. 2016, 88, 873–883. [Google Scholar] [CrossRef]
- European Committee for Antimicrobial Susceptibility Testing (EUCAST). Determination of minimum inhibitory concentrations (MICS) of antibacterial agents by broth dilution, EUCAST discussion document E. Dis 5.1. Clin. Microbiol. Infect. 2003, 9, 1–7. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts, M27-S4; Clinical and Laboratory Standards Institute (CLSI): Wayne, PA, USA, 2012. [Google Scholar]
| Species/Compound No. | 2 | 4 | 11 | 13 | 14 | 15 | 18 | 19 | CIP/NY * | NIT | CFX | APC | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gram-positive bacteria | Staphylococcus aureus ATCC 25923 | 31.25 (62.5) {2} | 250 (>1000) {>4} | 500 (>1000) {>2} | 125 (>1000) {>8} | 250 (>1000) {>4} | 125 (>1000) {>8} | 500 (1000) {2} | 500 (1000) {2} | 0.48 (0.48) {1} | 15.62 (15.62) | 0.49 | nd |
| Staphylococcus aureus ATCC 6538 | 62.5 (125) {2} | 500 (>1000) {>2} | 500 (>1000) {>2} | 250 (>1000) {>4} | 250 (>1000) {>4} | 1000 (>1000) {>1} | 500 (1000) {2} | 500 (1000) {2} | 0.24 (0.24) {1} | 15.62 (15.62) | 0.98 | nd | |
| Staphylococcus aureus ATCC 43300 | 125 (500) {4} | 500 (>1000) {>2} | 500 (>1000) {>2} | 1000 (>1000) {>1} | 1000 (>1000) {>1} | 1000 (>1000) {>1} | 500 (1000) {2} | 500 (1000) {2} | 0.24 (0.24) {1} | 7.81 (15.62) | nd | nd | |
| Staphylococcus aureus ATCC 29213 | 125 (500) {4} | 500 (>1000) {>2} | 500 (>1000) {>2} | 500 (>1000) {>2} | 500 (>1000) {>2} | 1000 (>1000) {>1} | 500 (1000) {2} | 500 (1000) {2} | 0.48 (0.48) {1} | nd | nd | nd | |
| Staphylococcus epidermidis ATCC 12228 | 15.62 (15.62) {1} | 250 (>1000) {>4} | 250 (>1000) {>4} | 500 (>1000) {>2} | 250 (>1000) {>4} | 500 (>1000) {>2} | 500 (1000) {2} | 500 (1000) {2} | 0.12 (0.12) {1} | 3.91 (7.81) | 0.24 | nd | |
| Enterococcus faecalis ATCC 29212 | 1000 (>1000) {>1} | 1000 (>1000) {>1} | 500 (>1000) {>1} | 500 (>1000) {>2} | 500 (>1000) {>2} | 1000 (>1000) {>1} | 500 (1000) {2} | 500 (1000) {2} | 0.98 (1.95) {2} | nd | nd | nd | |
| Micrococcus luteus ATCC 10240 | 1000 (>1000) {>1} | 125 (>1000) {>8} | 250 (>1000) {>4} | 250 (>1000) {>4} | 31.25 (>1000) {>32} | 500 (>1000) {>2} | 500 (1000) {2} | 500 (1000) {2} | 0.98 (1.95) {2} | 62.5 (62.5) | 0.98 | nd | |
| Bacillus subtilis ATCC 6633 | 62.5 (125) {2} | 500 (>1000) {>2} | 125 (>1000) {>8} | 250 (>1000) {>4} | 31.25 (>1000) {>32} | 125 (>1000) {>8} | 500 (500) {1} | 500 (500) {1} | 0.03 (0.03) {1} | 3.91 (3.91) | 15.62 | 62.5 | |
| Bacillus cereus ATCC 10876 | 1000 (>1000) {>1} | 1000 (>1000) {>1} | 500 (>1000) {>2} | 500 (>1000) {>2} | 1000 (>1000) {>1} | 1000 (>1000) {>1} | 500 (1000) {2} | 500 (1000) {2} | 0.06 (0.12) {2} | 7.81 (15.62) | 31.25 | nd | |
| Gram-negative bacteria | Bordetella bronchiseptica ATCC 4617 | - | 1000 (>1000) {>1} | 500 (>1000) {>2} | - | - | - | 500 (1000) {2} | 500 (1000) {2} | 0.98 (0.98) {1} | 125 (>1000) | nd | nd |
| Fungi | Candida albicans ATCC 2091 | 1000 (>1000) {>1} | - | 1000 (>1000) {>1} | - | - | - | 125 (500) {4} | 125 (500) {4} | 0.24 * (0.24) {1} | na | na | na |
| Candida albicans ATCC 10231 | 1000 (>1000) {>1} | 500 (>1000) {>2} | 500 (>1000) {>2} | - | - | - | 62.5 (250) {4} | 62.5 (250) {4} | 0.48 * (0.48) {1} | na | na | na | |
| Candida parapsilosis ATCC 22019 | 1000 (>1000) {>1} | 1000 (>1000) {>1} | 500 (>1000) {>2} | 1000 (>1000) {>1} | - | - | 125 (250) {2} | 125 (250) {2} | 0.24 * (0.48) {2} | na | na | na | |
| Species/Compound No | 20 | 24 | 25 | 30 | 31 | 32 | 37 | CIP/VA */NY ** | NIT | CFX | APC | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gram-positive bacteria | Staphylococcus aureus ATCC 25923 | 15.62 (15.62) {1} | 1000 (>2000) {>2} | - | 1000 (>2000) {>2} | 1000 (2000) {2} | 500 (1000) {2} | 7.81 (7.81) {1} | 0.48 (0.48) {1} | 15.62 (15.62) | 0.49 | nd |
| Staphylococcus aureus ATCC 6538 | 15.62 (15.62) {1} | 1000 (>2000) {>2} | 125 (>2000) {>16} | - | 1000 (2000) {2} | 500 (2000) {4} | 7.81 (7.81) {1} | 0.24 (0.24) {1} | 15.62 (15.62) | 0.98 | nd | |
| Staphylococcus aureus ATCC 43300 | 15.62 (15.62) {1} | 1000 (>2000) {>2} | - | - | - | 1000 (2000) {2} | 15.62 (15.62) {1} | 0.24 (0.24) {1} | 7.81 (15.62) | nd | nd | |
| Staphylococcus aureus ATCC 29213 | 15.62 (15.62) {1} | 500 (>2000) {>4} | - | 1000 (>2000) {>2} | - | 500 (500) {1} | 15.62 (15.62) {1} | 0.48 (0.48) {1} | Nd | nd | nd | |
| Staphylococcus epidermidis ATCC 12228 | 1.95 (3.91) {2} | 250 (250) {1} | 62.5 (125) {2} | 7.81 (15.62) {2} | 31.25 (125) {4} | 62.5 (125) {2} | 0.48 (0.48) {1} | 0.12 (0.12) {1} | 3.91 (7.81) | 0.24 | nd | |
| Enterococcus faecalis ATCC 29212 | 125 (500) {4} | 500 (>2000) {>4} | - | 1000 (>2000) {>2} | - | 500 (>2000) {>4} | 500 (1000) {2} | 0.98* (1.95) {2} | Nd | nd | nd | |
| Micrococcus luteus ATCC 10240 | 125 (250) {2} | 1000 (2000) {2} | 250 (2000) {8} | 1000 (>2000) {>2} | 500 (1000) {2} | 250 (500) {2} | 62.5 (62.5) {1} | 0.98 (1.95) {2} | 62.5 (62.5) | 0.98 | nd | |
| Bacillus subtilis ATCC 6633 | 31.25 (62.5) {2} | 125 (2000) {16} | - | 1000 (2000) {2} | - | 1000 (2000) {2} | 31.25 (31.25) {1} | 0.03 (0.03) {1} | 3.91 (3.91) | 15.62 | 62.5 | |
| Bacillus cereus ATCC 10876 | 31.25 (31.25) {1} | 125 (>2000) {>16} | - | 1000 (>2000) {>2} | 1000 (>2000) {>2} | 500 (>2000) {>4} | 31.25 (62.5) {2} | 0.06 (0.12) {2} | 7.81 (15.62) | 31.25 | nd | |
| Gram-negative bacteria | Bordetella bronchiseptica ATCC 4617 | 125 (500) {4} | 1000 (>2000) {>2} | - | 1000 (2000) {2} | 1000 (1000) {1} | 1000 (2000) {2} | 1000 (2000) {2} | 0.98 (0.98) {1} | 125 (>1000) | nd | nd |
| Fungi | Candida albicans ATCC 2091 | 31.25 (62.5) {2} | 250 (2000) {8} | 1000 (2000) {2} | 1000 (2000) {2} | 250 (500) {2} | 500 (500) {1} | 15.62 (15.62) {1} | 0.24 ** (0.24) {1} | na | na | na |
| Candida parapsilosis ATCC 22019 | 125 (500) {4} | 62.5 (1000) {16} | 1000 (2000) {2} | 1000 (2000) {2} | 500 (500) {1} | 500 (500) {1} | 250 (2000) {8} | 0.24 ** (0.48) {2} | na | na | na | |
| Dose/Compound | 24 | 29 | 37 | |||
|---|---|---|---|---|---|---|
| After 24 h | After 48 h | After 24 h | After 48 h | After 24 h | After 48 h | |
| 200 µM | 97% | 109% | 88% | 97% | 81% | 89% |
| 150 µM | 113% | 120% | 81% | 105% | 85% | 84% |
| 100 µM | 99% | 107% | 96% | 105% | 99% | 76% |
| 50 µM | 87% | 100% | 104% | 117% | 88% | 64% |
| 25 µM | 97% | 112% | 109% | 92% | 78% | 79% |
| 12 µM | 102% | 108% | 124% | 143% | 92% | 83% |
| 6 µM | 98% | 121% | 117% | 132% | 81% | 85% |
| Dose/Compound | 24 | 29 | 37 | |||
|---|---|---|---|---|---|---|
| After 24 h | After 48 h | After 24 h | After 48 h | After 24 h | After 48 h | |
| 200 µM | 76% | 73% | 94% | 102% | 65% | 84% |
| 150 µM | 71% | 79% | 97% | 94% | 79% | 92% |
| 100 µM | 75% | 84% | 96% | 93% | 58% | 67% |
| 50 µM | 65% | 97% | 106% | 112% | 56% | 73% |
| 25 µM | 71% | 106% | 101% | 78% | 73% | 66% |
| 12 µM | 100% | 111% | 98% | 97% | 64% | 71% |
| 6 µM | 91% | 99% | 104% | 103% | 93% | 88% |
| Dose/Compound | 24 | 29 | 37 | |||
|---|---|---|---|---|---|---|
| After 24 h | After 48 h | After 24 h | After 48 h | After 24 h | After 48 h | |
| 200 µM | 92% | 107% | 101% | 98% | 106% | 104% |
| 150 µM | 86% | 132% | 97% | 111% | 96% | 110% |
| 100 µM | 105% | 121% | 103% | 118% | 99% | 98% |
| 50 µM | 117% | 113% | 94% | 118% | 91% | 87% |
| 25 µM | 112% | 106% | 115% | 124% | 83% | 93% |
| 12 µM | 124% | 138% | 128% | 127% | 108% | 117% |
| 6 µM | 126% | 147% | 115% | 136% | 114% | 120% |
Sample Availability: Samples of the compounds 2–37 are available from the authors. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paruch, K.; Popiołek, Ł.; Biernasiuk, A.; Hordyjewska, A.; Malm, A.; Wujec, M. Novel 3-Acetyl-2,5-disubstituted-1,3,4-oxadiazolines: Synthesis and Biological Activity. Molecules 2020, 25, 5844. https://doi.org/10.3390/molecules25245844
Paruch K, Popiołek Ł, Biernasiuk A, Hordyjewska A, Malm A, Wujec M. Novel 3-Acetyl-2,5-disubstituted-1,3,4-oxadiazolines: Synthesis and Biological Activity. Molecules. 2020; 25(24):5844. https://doi.org/10.3390/molecules25245844
Chicago/Turabian StyleParuch, Kinga, Łukasz Popiołek, Anna Biernasiuk, Anna Hordyjewska, Anna Malm, and Monika Wujec. 2020. "Novel 3-Acetyl-2,5-disubstituted-1,3,4-oxadiazolines: Synthesis and Biological Activity" Molecules 25, no. 24: 5844. https://doi.org/10.3390/molecules25245844
APA StyleParuch, K., Popiołek, Ł., Biernasiuk, A., Hordyjewska, A., Malm, A., & Wujec, M. (2020). Novel 3-Acetyl-2,5-disubstituted-1,3,4-oxadiazolines: Synthesis and Biological Activity. Molecules, 25(24), 5844. https://doi.org/10.3390/molecules25245844