Possibilities for Optimization of Industrial Alkaline Steeping of Wood-Based Cellulose Fibers
Abstract
1. Introduction
2. Results
2.1. The Change in Yield of the Pulp Caused by Alkalization
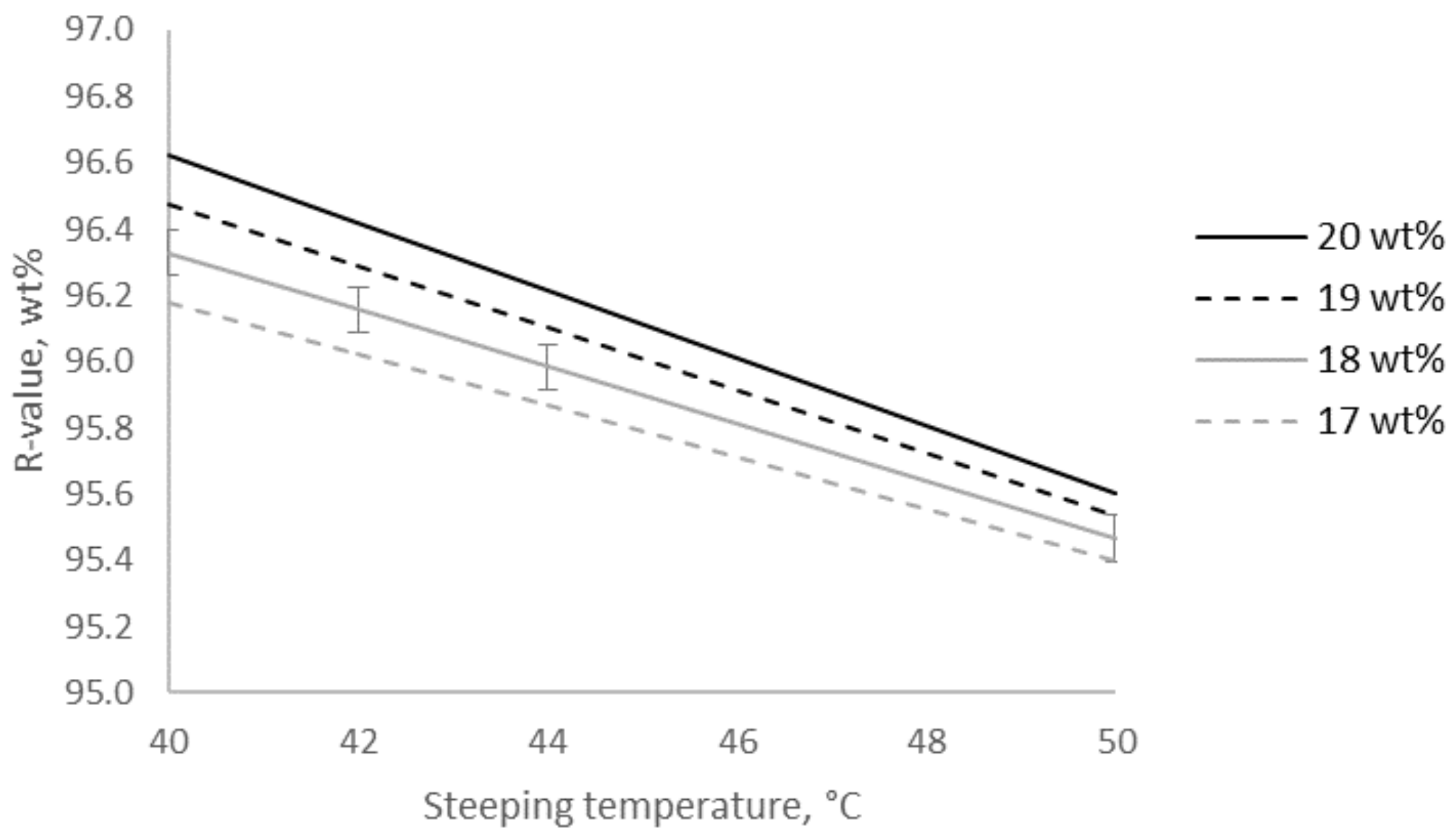
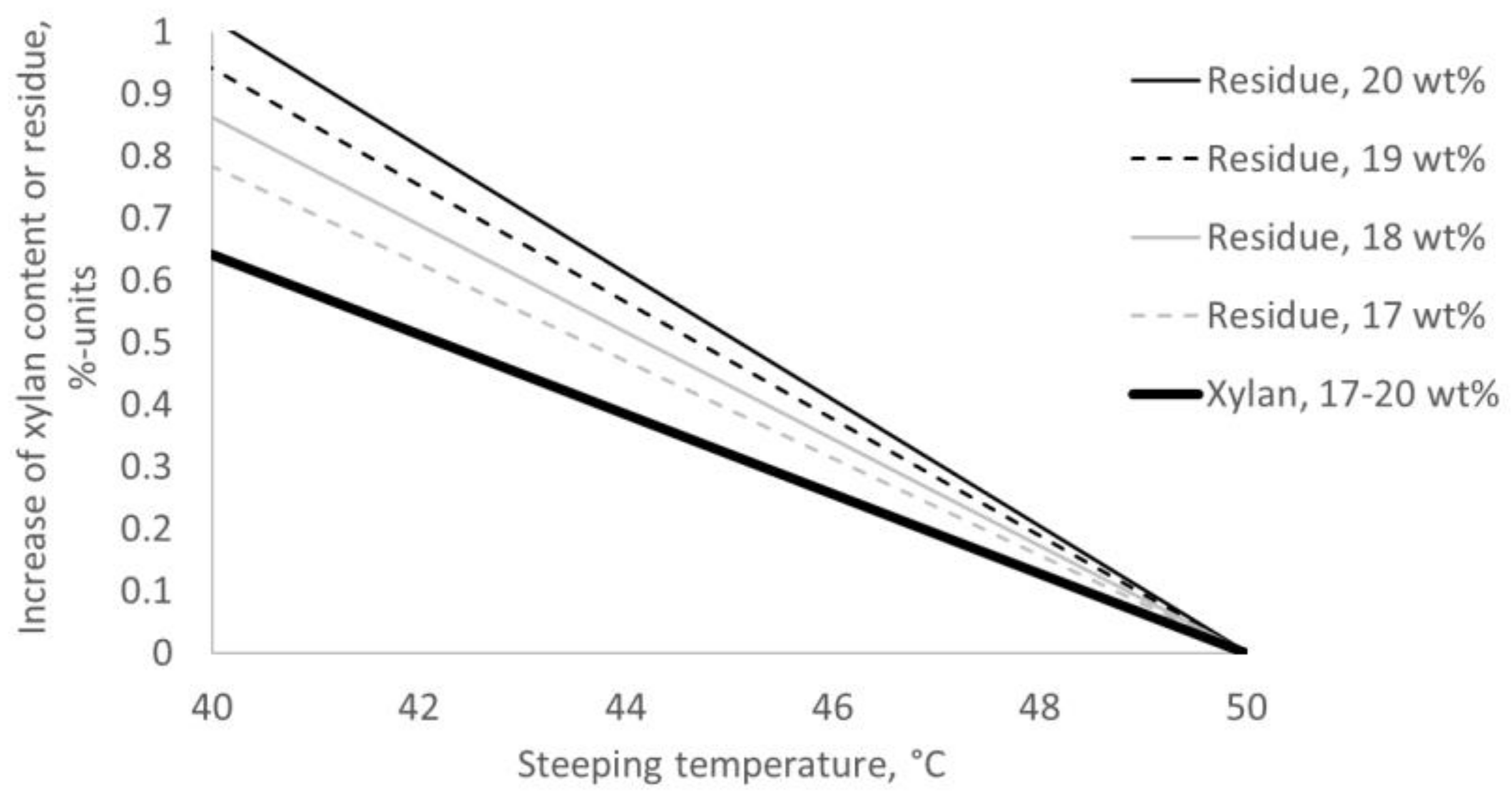
2.2. The Change in Purity of the Pulp Caused by Alkalization
2.3. Molecular Weight
2.4. Transformation of Cellulose I to Cellulose II
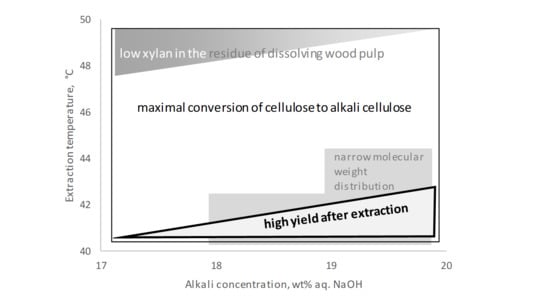
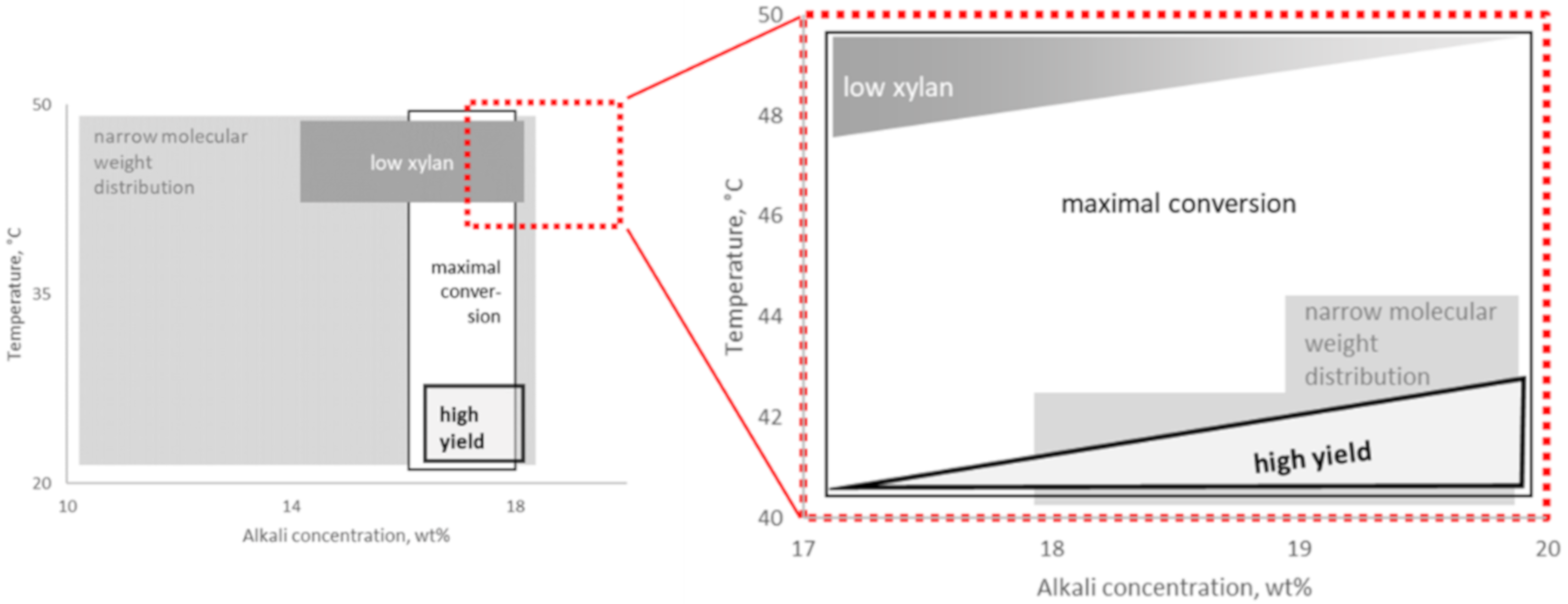
3. Discussion
4. Materials and Methods
4.1. Material
4.2. Methods
4.2.1. Preparation of Residues (R) and Determination of the R-Value
4.2.2. Analysis of Pulp and Its Residues
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Götze, K. Chemiefasern Nach Dem Viskoseverfahren, 3rd ed.; Springer: Berlin, Germany, 1967; p. 1282. [Google Scholar]
- Woodings, C. Regenerated Cellulose Fibres; Woodhead Publishing: Cambridge, UK, 2001; p. 336. [Google Scholar]
- Young, R. World Dissolving Pulp Monitor; Fastmarkets RISI—Market research provider for forest product industry, RISI, Inc.: Burlington, MA, USA, 2018. [Google Scholar]
- Rydholm, S. Carbohydrate removing methods. In Pulping Processes; John Wiley & Sons, Inc.: New York, NJ, USA, 1965; pp. 992–1023. [Google Scholar]
- Kolpak, F.J.; Weih, M.; Blackwell, J. Mercerization of cellulose: 1. Determination of the structure of mercerized cotton. Polymer 1978, 19, 123–131. [Google Scholar] [CrossRef]
- Fink, H.P.; Fanter, D.; Loth, F. Röntgen-Weitwinkeluntersuchungen zur Phasenumwandlung bei der Alkalisierung von Cellulose. Acta Polym. 1982, 33, 241–245. [Google Scholar] [CrossRef]
- Fengel, D.; Wegener, G. Wood—Chemistry, Ultrastructure, Reactions; Walter de Gruyter: Berlin, Germany; New York, NJ, USA, 1989; p. 482. [Google Scholar]
- Krässig, H.A. Cellulose: Structure, accessibility and reactivity; methods of activation. In Polymer Monographs; Gordon and Breach Science Publishers: Yverdon, Switzerland, 1993; Volume 11, p. 89. [Google Scholar]
- Sixta, H. Pulp purification. In Handbook of Pulp; Wiley-VCH: Weinheim, Germany, 2006; Volume 2, pp. 933–966. [Google Scholar]
- Mozdyniewicz, D.; Schild, G.; Sixta, H. Alkaline steeping of dissolving pulp. Part I: Soluble compounds in the press lye. Cellulose 2014, 21, 2889–2900. [Google Scholar] [CrossRef]
- Reyes, D.; Skoglund, N.; Svedberg, A.; Eliasson, B.; Sundman, O. The influence of different parameters on the mercerisation of cellulose for viscose production. Cellulose 2016, 23, 1061–1072. [Google Scholar] [CrossRef]
- Fechter, C.; Fischer, S.; Reimann, F.; Brelid, H.; Heinze, T. Influence of pulp characteristics on the properties of alkali cellulose. Cellulose 2020, 27, 7227–7241. [Google Scholar] [CrossRef]
- Ellefsen, Ö. Nitration as an analytical tool in the study of wood pulps VI. Properties of rayon pulps and influence on filterability. Norsk Skogsindustri 1955, 9, 399–406. [Google Scholar]
- Kolboe, A. A study of systematic errors in the filtration stage during filterability determinations of viscose. Part I: Errors caused by non-constant pressure and viscosity. Norsk Skogsindustri 1960, 14, 348–353. [Google Scholar]
- Treiber, E.; Rehnström, J.; Ameen, C.; Kolos, F. Ueber eine Laboratoriums-Viskose-Kleinstanlage zur Testung von Chemiezellstoffen. Das Pap. 1962, 16, 85–94. [Google Scholar]
- Kyrklund, B.; Sihtola, H. On the conductrometic determination of the particle size distribution in viscose. Pap. Tim. 1963, 45, 119–129. [Google Scholar]
- Sihtola, H.; Nizovsky, B. The yield in the viscose process compared with the alkali solubility of the pulp. Pap. Tim. 1963, 45, 299–304. [Google Scholar]
- Wyatt, W. Optimum viscose process studies I. Steeping and pressing. Tappi 1966, 49, 464–468. [Google Scholar]
- Richter, G.; Glidden, K. Cellulose sheet swelling. Effect of temperature and concentration of sodium hydroxide solutions. Ind. Eng. Chem. 1940, 32, 480–486. [Google Scholar] [CrossRef]
- Sisson, W.A.; Saner, W.R. The effect of the temperature and the concentration of sodium hydroxide on the X-ray diffraction behavior of raw and of degraded cotton. J. Phys. Chem 1941, 45, 717–730. [Google Scholar] [CrossRef]
- Okano, T.; Sarko, A. Mercerization of cellulose. II. Alkali–cellulose intermediates and a possible mercerization mechanism. J. Appl. Polym. Sci. 1985, 30, 325–332. [Google Scholar] [CrossRef]
- Henniges, U.; Kostic, M.; Borgards, A.; Rosenau, T.; Potthast, A. Dissolution behavior of different celluloses. Biomacromolecules 2011, 12, 871–879. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, U.; Atalla, R.; Isogai, A. Chapter 4: Raman spectroscopy in the analysis of cellulose nanomaterials. In Nanocelluloses: Their Preparation, Properties, and Applications; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2017; pp. 75–90. [Google Scholar]



| NaOH- Conc. | wt% | - | 17 | 17 | 18 | 18 | 18 | 18 | 19 | 19 | 20 | 20 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Extraction Temp. | °C | - | 40 | 44 | 40 | 42 | 44 | 50 | 42 | 44 | 40 | 50 |
| Residue 1 | wt% | - | 96.1 | 95.8 | 96.5 | 96.2 | 96.0 | 95.5 | 96.2 | 96.1 | 96.6 | 95.6 |
| Glucan 2 | mg g−1 | 953 | 978 | 980 | 976 | 977 | 979 | 983 | 978 | 978 | 976 | 982 |
| Xylan 2 | mg g−1 | 39 | 17 | 14 | 18 | 17 | 15 | 11 | 17 | 16 | 18 | 13 |
| Mannan 2 | mg g−1 | 4 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Mn | kg mol−1 | 65 | 57 | 49 | 72 | 71 | 61 | 65 | 68 | 71 | 66 | 46 |
| Mw | kg mol−1 | 215 | 182 | 166 | 178 | 189 | 187 | 185 | 183 | 189 | 179 | 158 |
| Mz | kg mol−1 | 466 | 371 | 355 | 332 | 374 | 367 | 355 | 353 | 369 | 334 | 322 |
| PDI 3 | 3.3 | 3.2 | 3.4 | 2.5 | 2.7 | 3.1 | 2.8 | 2.7 | 2.7 | 2.7 | 3.4 | |
| DP 4 | 1327 | 1123 | 1025 | 1099 | 1169 | 1153 | 1141 | 1130 | 1167 | 1107 | 975 | |
| DP < 50 | % | 0.9 | 1.0 | 1.6 | 0.2 | 0.4 | 1.0 | 0.7 | 0.4 | 0.4 | 0.5 | 1.4 |
| DP < 100 | % | 2.8 | 2.7 | 3.7 | 1.2 | 1.6 | 2.5 | 2.0 | 1.6 | 1.6 | 1.8 | 3.6 |
| DP > 2000 | % | 18.6 | 14.7 | 12.7 | 13.5 | 14.8 | 15.3 | 14.6 | 14.3 | 14.9 | 13.9 | 11.7 |
| Degree of Transformation 5 | % | - | 86 | 88 | 88 | 85 | 87 | 86 | 85 | 86 | 88 | 85 |
| 17 wt% aq. NaOH | 18 wt% aq. NaOH | 19 wt% aq. NaOH | 20 wt% aq. NaOH | |
|---|---|---|---|---|
| 40 °C | X | X | X | |
| 42 °C | X | X | ||
| 44 °C | X | X | X | |
| 50 °C | X | X |
Sample Availability: Limited amounts of samples of the compounds are available from the authors. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fechter, C.; Brelid, H.; Fischer, S. Possibilities for Optimization of Industrial Alkaline Steeping of Wood-Based Cellulose Fibers. Molecules 2020, 25, 5834. https://doi.org/10.3390/molecules25245834
Fechter C, Brelid H, Fischer S. Possibilities for Optimization of Industrial Alkaline Steeping of Wood-Based Cellulose Fibers. Molecules. 2020; 25(24):5834. https://doi.org/10.3390/molecules25245834
Chicago/Turabian StyleFechter, Catharina, Harald Brelid, and Steffen Fischer. 2020. "Possibilities for Optimization of Industrial Alkaline Steeping of Wood-Based Cellulose Fibers" Molecules 25, no. 24: 5834. https://doi.org/10.3390/molecules25245834
APA StyleFechter, C., Brelid, H., & Fischer, S. (2020). Possibilities for Optimization of Industrial Alkaline Steeping of Wood-Based Cellulose Fibers. Molecules, 25(24), 5834. https://doi.org/10.3390/molecules25245834