Discrimination of Lycium chinense and L. barbarum Based on Metabolite Analysis and Hepatoprotective Activity
Abstract
1. Introduction
2. Results
2.1. Hepatoprotective Activity of Extracts and Fractions of L. chinense and L. barbarum
2.2. Amino Acid and Phenolic Acid Profiling Analyses
2.3. Star Pattern Recognition Analysis
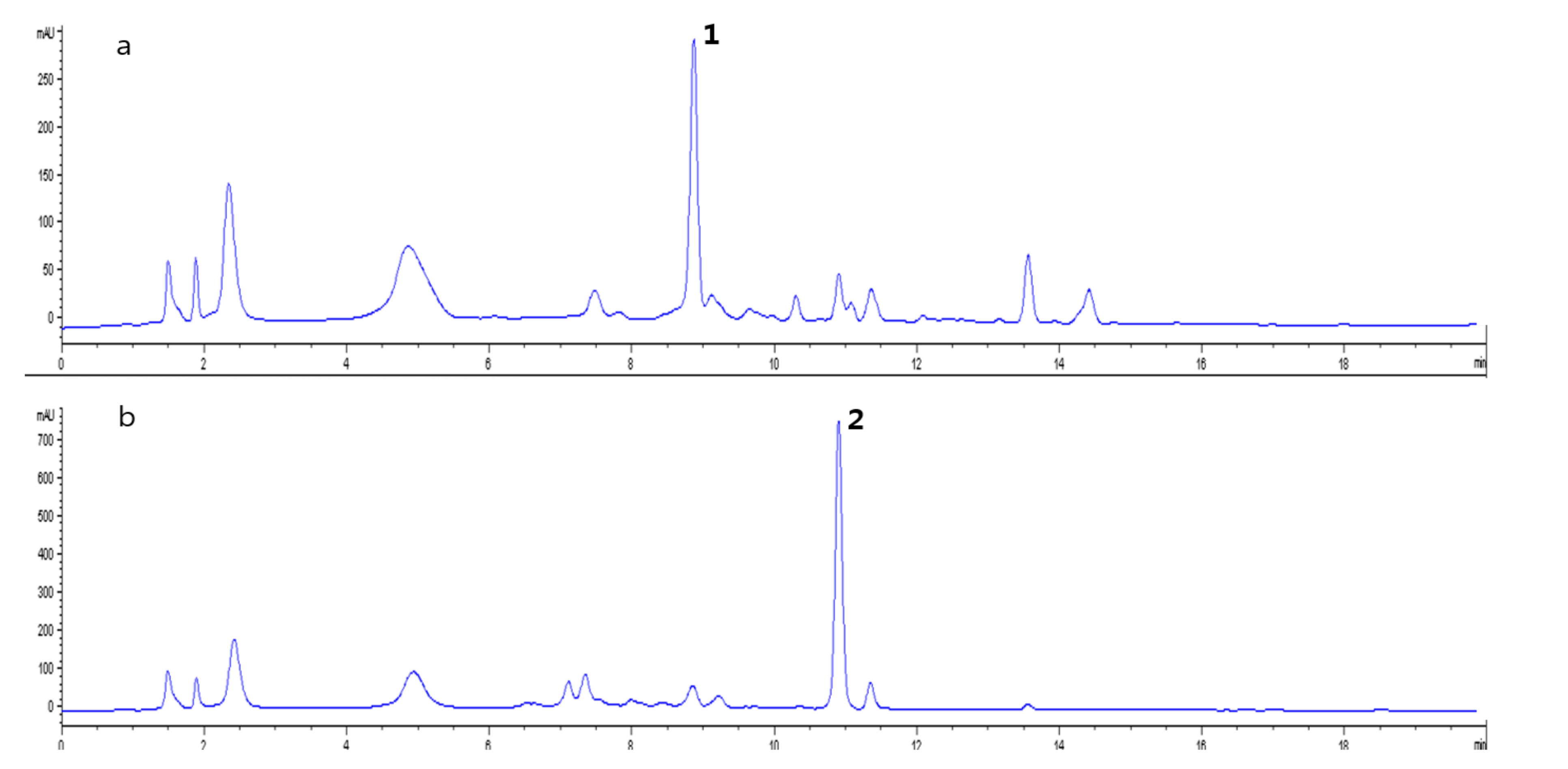
2.4. Identification of Compounds with Different Contents in L. chinense and L. barbarum
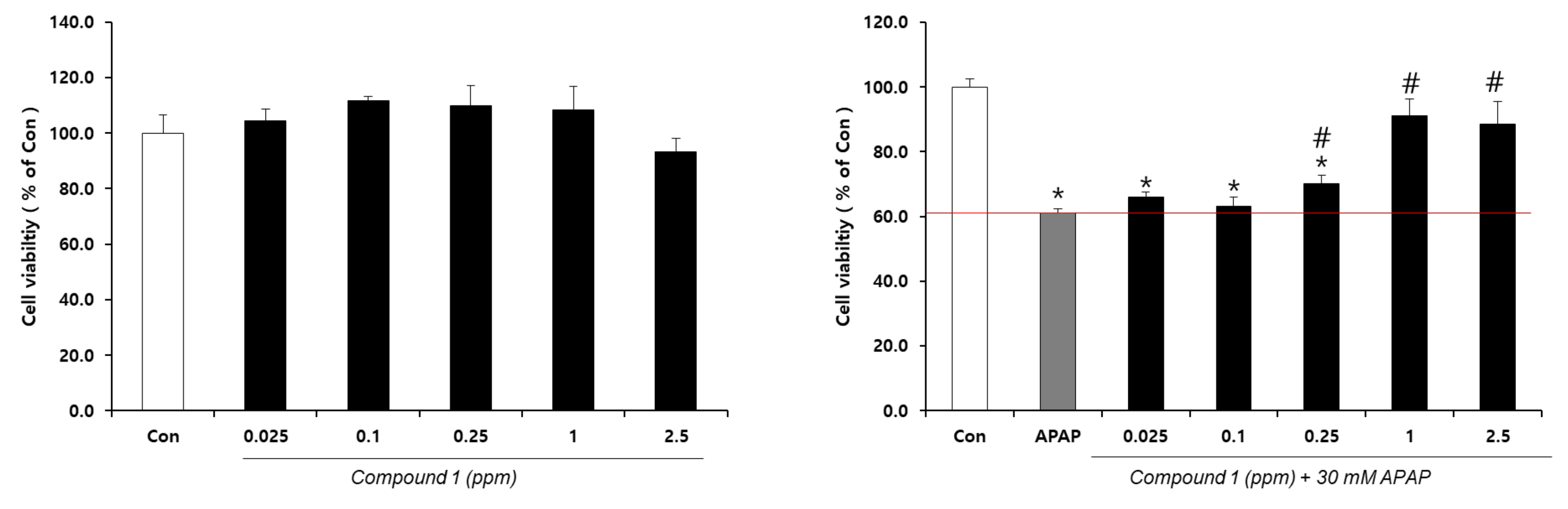
2.5. Hepatoprotective Effect of 4-(2-formyl-5-(hydroxymethyl)-1H-pyrrol-1-yl)Butanoic Acid Isolated from L. chinense
3. Materials and Methods
3.1. Plant Material
3.2. Chemical and Reagents
3.3. Extraction, Fractionation, and Isolation of Compounds
3.4. Hepatoprotection Assay
3.5. Gas Chromatography-Mass Spectrometry
3.6. Sample Preparation for Amino Acids Profiling Analysis
3.7. Sample Preparation for Phenolic Acids Profiling Analysis
3.8. Star Symbol Plotting
3.9. HPLC Analysis
3.10. NMR Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kim, Y.; Choi, H.; Shin, J.; Jo, A.; Lee, K.E.; Cho, S.S.; Hwang, Y.P.; Choi, C. Molecular discrimination of Cynanchum wilfordii and Cynanchum auriculatum by InDel markers of chloroplast DNA. Molecules 2018, 23, 1337. [Google Scholar] [CrossRef]
- Han, E.H.; Lee, S.J.; Kim, M.B.; Shin, Y.W.; Kim, Y.H.; Lee, S.W. Molecular marker analysis of Cynanchum wilfordii and C. auriculatum using the simple ARMS-PCR method with mismatched primers. Plant Biotechnol. Rep. 2017, 11, 127–133. [Google Scholar] [CrossRef]
- Guo, M.; Ren, L.; Pang, X. Inspecting the true identity of herbal materials from Cynanchum using ITS2 barcode. Front. Plant Sci. 2017, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Ma, C.; Li, Y.; Leung, K.S.Y.; Jiang, Z.H.; Zhao, Z. Quantification of zeaxanthin dipalmitate and total carotenoids in Lycium fruits (Fructus Lycii). Plant Foods Hum. Nutr. 2005, 60, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Chen, Y.; Chen, G. Far infrared-assisted extraction followed by capillary electrophoresis for the determination of bioactive constituents in the leaves of Lycium barbarum Linn. J. Chromatogr. A 2010, 1217, 4511–4516. [Google Scholar] [CrossRef]
- Kim, K.S.; Shim, S.H.; Jeong, G.H.; Cheong, C.S.; Ko, K.H.; Park, J.H.; Huh, H.; Lee, B.J.; Kim, B.-K. Anti-diabetic activity of constituents of Lycii Fructus. J. Appl. Pharmacol. 1998, 6, 378–382. [Google Scholar]
- Kim, S.Y.; Lee, E.J.; Kim, H.P.; Kim, Y.C.; Moon, A.; Kim, Y.C. A Novel cerebroside from Lycii Fructus preserves the hepatic glutathione redox system in primary cultures of rat hepatocytes. Biol. Pharm. Bull. 1999, 22, 873–875. [Google Scholar] [CrossRef]
- Chin, Y.W.; Lim, S.W.; Kim, S.H.; Shin, D.Y.; Suh, Y.G.; Kim, Y.B.; Kim, Y.C.; Kim, J. Hepatoprotective pyrrole derivatives of Lycium chinense fruits. Bioorganic Med. Chem. Lett. 2003, 13, 79–81. [Google Scholar] [CrossRef]
- Lee, H.W.; Kim, Y.H.; Kim, Y.H.; Lee, G.H.; Lee, M.Y. Discrimination of Lycium chinense and Lycium barbarum by taste pattern and betaine analysis. Int. J. Clin. Exp. Med. 2014, 7, 2053–2059. [Google Scholar]
- Zhao, B.T.; Jeong, S.Y.; Hwangbo, K.; Moon, D.C.; Seo, E.K.; Lee, D.; Lee, J.H.; Min, B.S.; Ma, E.S.; Son, J.K.; et al. Quantitative analysis of betaine in Lycii Fructus by HILIC-ELSD. Arch. Pharm. Res. 2013, 36, 1231–1237. [Google Scholar] [CrossRef]
- Kang, P.; Liu, Y.; Zhu, H.; Zhang, J.; Shi, H.; Li, S.; Pi, D.; Leng, W.; Wang, X.; Wu, H.; et al. The effect of dietary asparagine supplementation on energy metabolism in liver of weaning pigs when challenged with lipopolysaccharide. Asian Australas. J. Anim. Sci. 2018, 31, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.C.; Jiang, Z.M.; Li, D.M. Glutamine: A precursor of glutathione and its effect on liver. World J. Gastroenterol. 1999, 5, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Obayashi, Y.; Arisaka, H.; Yoshida, S.; Mori, M.; Takahashi, M. Proline protects liver from d-galactosamine hepatitis by activating the IL-6/STAT3 survival signaling pathway. Amino Acids 2012, 43, 2371–2380. [Google Scholar] [CrossRef] [PubMed]
- Rukkumani, R.; Aruna, K.; Varma, P.S.; Menon, V.P. Hepatoprotective role of ferulic acid: A dose-dependent study. J. Med. Food 2004, 7, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Itoh, A.; Isoda, K.; Kondoh, M.; Kawase, M.; Kobayashi, M.; Tamesada, M.; Yagi, K. Hepatoprotective effect of syringic acid and vanillic acid on concanavalin A-induced liver injury. Biol. Pharm. Bull. 2009, 32, 1215–1219. [Google Scholar] [CrossRef]
- Lee, S., II; An, S.M.; Mun, G.I.; Lee, S.J.; Park, K.M.; Park, S.H.; Boo, Y.C. Protective effect Sasa quelpaertensis and p-courmaric acid on ethanol-induced hepatotoxicity in mice. J. Appl. Biol. Chem. 2008, 51, 148–154. [Google Scholar] [CrossRef]
- An, S.M.; Lee, S.I.; Choi, S.W.; Moon, S.W.; Boo, Y.C. p-Coumaric acid, a constituent of Sasa quelpaertensis Nakai, inhibits cellular melanogenesis stimulated by α-melanocyte stimulating hormone. Br. J. Dermatol. 2008, 159, 292–299. [Google Scholar] [CrossRef]
- Park, S.H.; Kim, D.S.; Park, S.H.; Shin, J.W.; Youn, S.W.; Park, K.C. Inhibitory effect of p-coumaric acid by Rhodiola sachalinensis on melanin synthesis in B16F10 cells. Pharmazie 2008, 63, 290–295. [Google Scholar] [CrossRef]
- An, S.M.; Koh, J.S.; Boo, Y.C. p-coumaric acid not only inhibits human tyrosinase activity in vitro but also melanogenesis in cells exposed to UVB. Phyther. Res. 2010, 24, 1175–1180. [Google Scholar] [CrossRef]
- Kim, M.; Lee, E.J.; Lim, K.-M. Ibuprofen increases the hepatotoxicity of ethanol through potentiating oxidative stress. Biomol. Ther. 2020. [Google Scholar] [CrossRef]
- Paik, M.-J.; Moon, S.-M.; Kim, K.-R.; Choi, S.; Ahn, Y.-H.; Lee, G. Target metabolic profiling analysis of free amino acids in plasma as EOC/TBDMS derivatives by GC-SIM-MS. Biomed. Chromatogr. 2008, 22, 339–342. [Google Scholar] [CrossRef] [PubMed]
- Paik, M.J.; Kim, K.R. Sequential ethoxycarbonylation, methoximation and tert- butyldimethylsilylation for simultaneous determination of amino acids and carboxylic acids by dual-column gas chromatography. J. Chromatogr. A 2004, 1034, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Paik, M.J.; Lee, H.J.; Kim, K.R. Simultaneous retention index analysis of urinary amino acids and carboxylic acids for graphic recognition of abnormal state. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2005, 821, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Seo, C.; Hwang, Y.H.; Lee, H.S.; Kim, Y.; Shin, T.H.; Lee, G.; Son, Y.J.; Kim, H.; Yee, S.T.; Park, A.K.; et al. Metabolomic study for monitoring of biomarkers in mouse plasma with asthma by gas chromatography–mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2017, 1063, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Seo, C.; Na, M.; Jang, J.; Park, M.; Choi, B.; Lee, S.; Paik, M.J. Monitoring of altered amino acid metabolic pattern in rat urine following intraperitoneal injection with γ-hydroxybutyric acid. Metabolomics 2018, 14, 111. [Google Scholar] [CrossRef] [PubMed]
- Sooklert, K.; Wongjarupong, A.; Cherdchom, S.; Wongjarupong, N.; Jindatip, D.; Phungnoi, Y.; Rojanathanes, R.; Sereemaspun, A. Molecular and morphological evidence of hepatotoxicity after silver nanoparticle exposure: A systematic review, in silico, and ultrastructure investigation. Toxicol. Res. 2019, 35, 257–270. [Google Scholar]

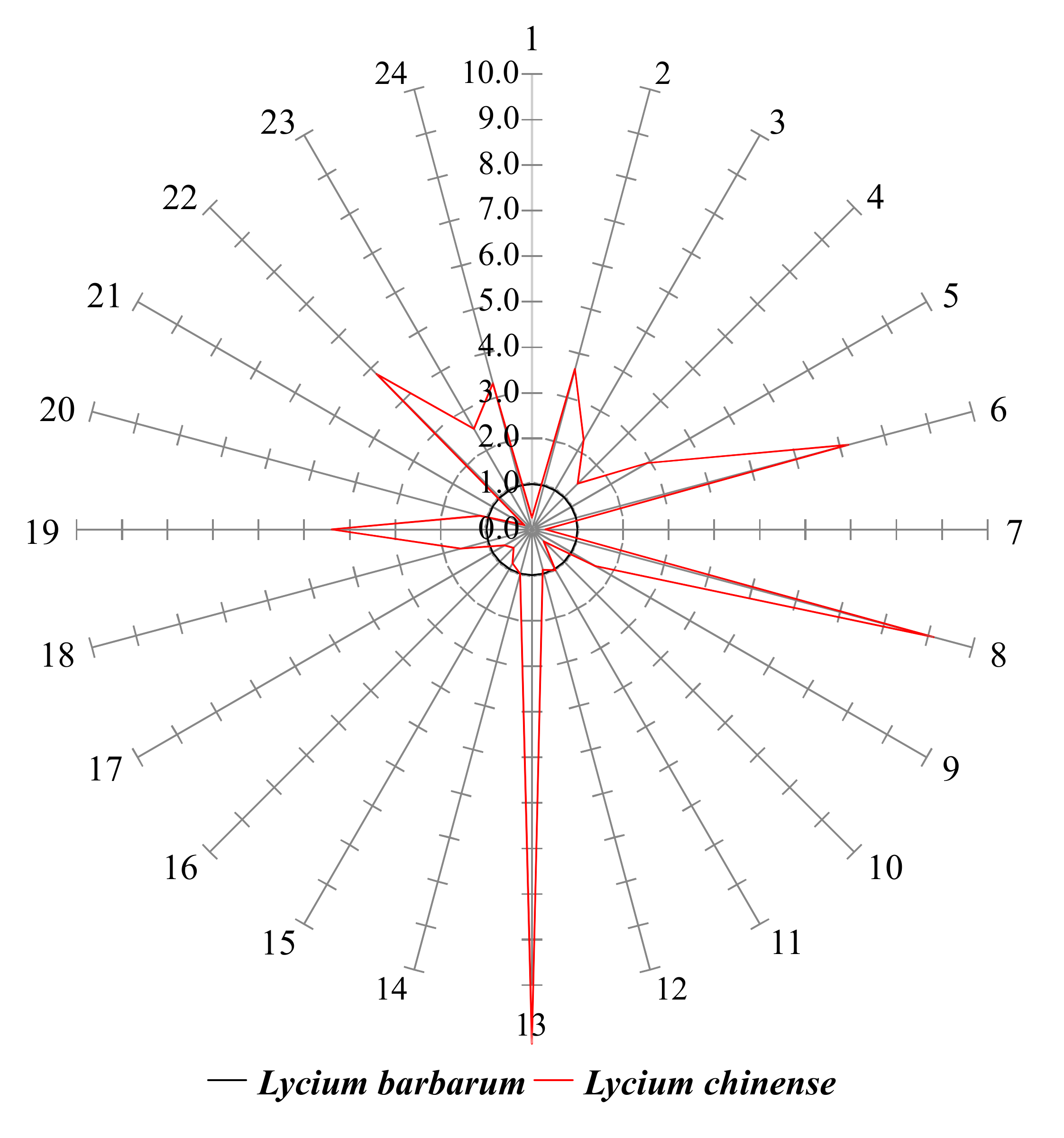
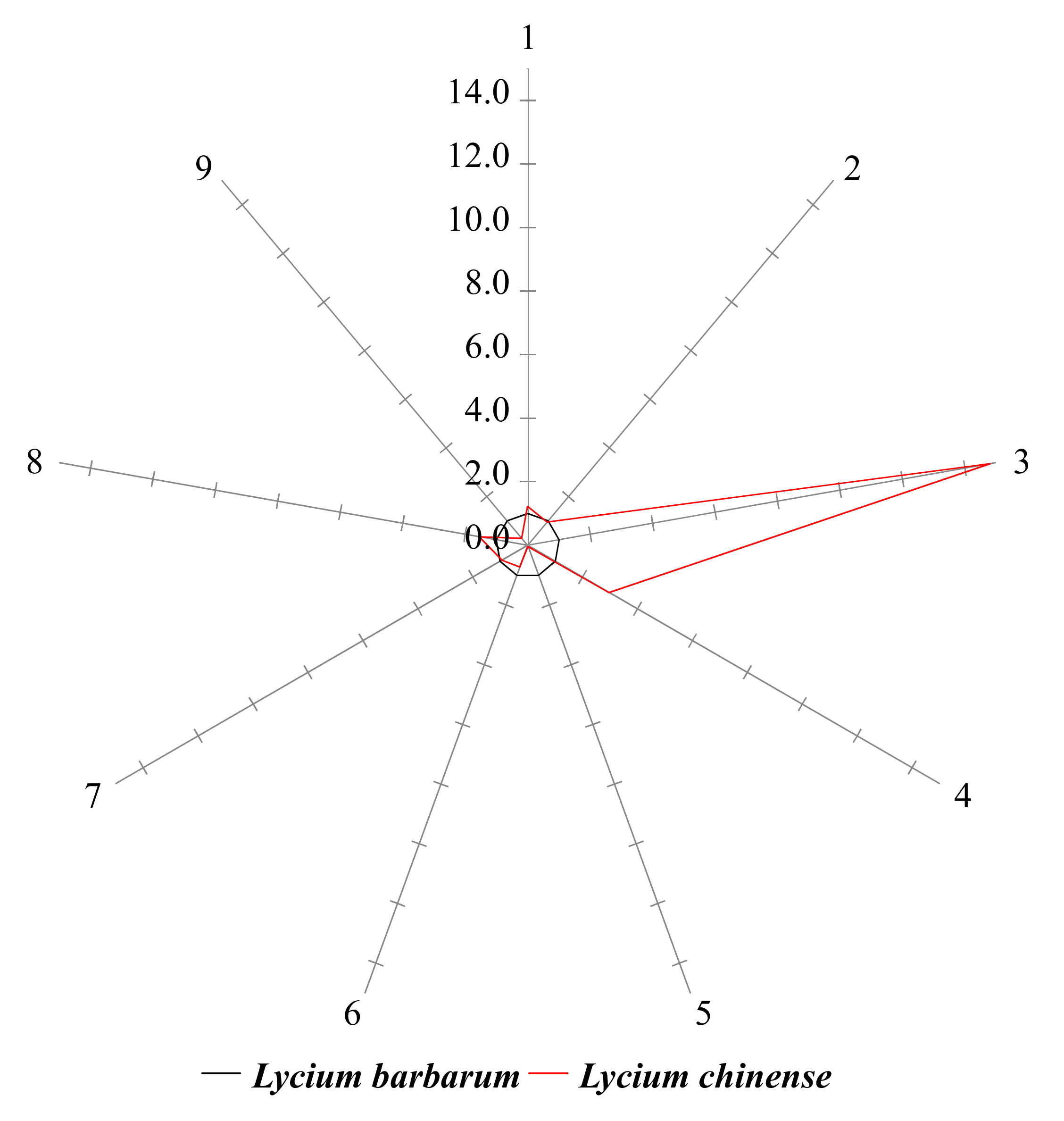
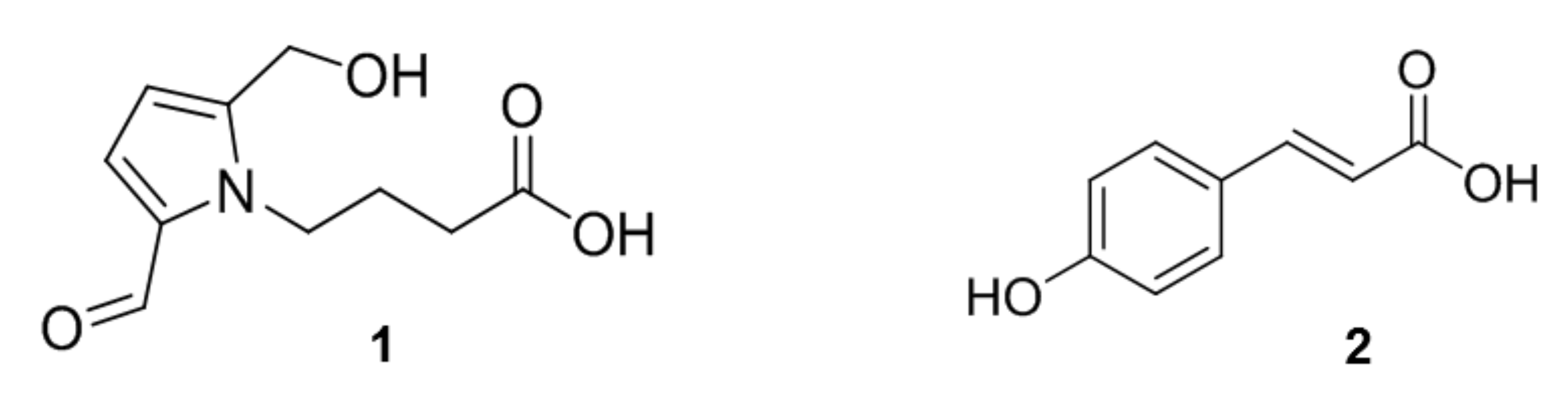
| No. | Amino Acid | Retention Time | Selected Ions 1 (m/z) | Amount (ng/mg, Mean ± SD) | Normalized Value 2 | |||
|---|---|---|---|---|---|---|---|---|
| L. barbarum | L. chinense | |||||||
| 1 | Alanine | 5.17 | 190 | 218 | 260 | 2187.6 ± 67.8 | 565.2 ± 13.6 | 0.26 |
| 2 | Glycine | 5.40 | 176 | 204 | 246 | 36.5 ± 1.1 | 132.9 ± 1.0 | 3.64 |
| 3 | α-Aminobutyric acid | 6.06 | 130 | 204 | 232 | 12.9 ± 0.3 | 29.2 ± 0.6 | 2.27 |
| 4 | Valine | 6.51 | 144 | 218 | 246 | 290.3 ± 10.4 | 412.8 ± 15.2 | 1.42 |
| 5 | Leucine | 7.37 | 158 | 232 | 260 | 234.1 ± 8.0 | 683.4 ± 7.2 | 2.92 |
| 6 | Isoleucine | 7.62 | 158 | 232 | 260 | 55.7 ± 1.5 | 400.7 ± 5.7 | 7.19 |
| 7 | Proline | 8.09 | 142 | 216 | 244 | 9798.5 ± 242.4 | 2931.3 ± 106.0 | 0.30 |
| 8 | γ-Aminobutyric acid | 8.12 | 186 | 201 | 346 | 121.4 ± 4.2 | 1108.0 ± 31.3 | 9.13 |
| 9 | Pipecolic acid | 8.81 | 156 | 230 | 258 | 61.0 ± 1.7 | 97.6 ± 5.0 | 1.60 |
| 10 | Pyroglutamic acid | 10.25 | 272 | 300 | 342 | 653.7 ± 20.6 | 240.0 ± 8.7 | 0.37 |
| 11 | Serine | 11.03 | 216 | 302 | 348 | 514.0 ± 59.5 | 530.8 ± 42.9 | 1.03 |
| 12 | Threonine | 11.12 | 230 | 248 | 261 | 159.6 ± 33.2 | 146.2 ± 17.7 | 0.92 |
| 13 | Phenylalanine | 12.17 | 195 | 205 | 294 | 39.1 ± 1.7 | 440.7 ± 17.9 | 11.28 |
| 14 | Cysteine | 12.58 | 216 | 276 | 322 | 157.8 ± 0.5 | 158.7 ± 0.3 | 1.01 |
| 15 | Aspartic acid | 12.81 | 287 | 330 | 287 | 330 | 1079.9 ± 24.0 | 0.85 |
| 16 | N-Methyl-DL-aspartic acid | 13.01 | 244 | 287 | 390 | 40.5 ± 1.7 | 23.1 ± 0.5 | 0.57 |
| 17 | Homocysteine | 13.85 | 234 | 290 | 336 | 81.2 ± 7.6 | 56.5 ± 0.3 | 0.70 |
| 18 | Glutamic acid | 14.18 | 288 | 344 | 390 | 346.2 ± 15.3 | 559.5 ± 31.9 | 1.62 |
| 19 | Asparagine | 14.47 | 286 | 329 | 375 | 3460.4 ± 146.9 | 15211.9 ± 1141.6 | 4.40 |
| 20 | α-Aminoadipic acid | 15.36 | 302 | 358 | 404 | 33.6 ± 0.1 | 39.4 ± 0.7 | 1.17 |
| 21 | Glutamine | 15.71 | 287 | 343 | 389 | 7462.7 ± 588.4 | 1468.8 ± 119.1 | 0.20 |
| 22 | Lysine | 15.85 | 156 | 301 | 347 | 185.9 ± 6.1 | 897.2 ± 57.3 | 4.83 |
| 23 | Histidine | 16.35 | 254 | 267 | 356 | 565.5 ± 9.6 | 1441.7 ± 238.2 | 2.55 |
| 24 | Tryptophan | 18.35 | 130 | 244 | 333 | 156.8 ± 3.9 | 520.5 ± 118.1 | 3.32 |
| No. | Phenolic Acid | Retention Time | Selected Ions 1 (m/z) | Amount (ng/mg, Mean ± SD) | Normalized Value 2 | |||
|---|---|---|---|---|---|---|---|---|
| L. barbarum | L. chinense | |||||||
| 1 | Salicylic acid | 4.44 | 73 | 115 | 195 | 1.8 ± 0.1 | 2.3 ± 0.4 | 1.23 |
| 2 | 4-Hydroxybenzoic acid | 5.27 | 73 | 235 | 285 | 8.1 ± 0.3 | 7.8 ± 0.1 | 0.96 |
| 3 | Vanillic acid | 6.02 | 193 | 223 | 267 | 1.8 ± 0.1 | 26.4 ± 0.3 | 14.81 |
| 4 | Syringic acid | 6.74 | 223 | 253 | 297 | 0.3 ± < 0.1 | 0.9 ± < 0.1 | 2.97 |
| 5 | p-Coumaric acid | 7.10 | 73 | 261 | 291 | 178.4 ± 9.3 | 6.8 ± < 0.1 | 0.04 |
| 6 | Protocatechuic acid | 7.47 | 73 | 365 | 395 | 1.0 ± < 0.1 | 0.7 ± < 0.1 | 0.72 |
| 7 | Ferulic acid | 7.86 | 219 | 249 | 293 | 33.6 ± 3.6 | 31.3 ± 0.3 | 0.93 |
| 8 | Gallic acid | 8.88 | 73 | 323 | 439 | 1.2 ± 0.1 | 1.9 ± 0.3 | 1.55 |
| 9 | Caffeic acid | 9.08 | 73 | 219 | 261 | 2.5 ± 0.3 | 0.7 ± 0.1 | 0.29 |
Sample Availability: Sample of the compound 1 is available from the authors. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryu, M.-J.; Kim, M.; Ji, M.; Lee, C.; Yang, I.; Hong, S.-B.; Chin, J.; Seo, E.K.; Paik, M.-J.; Lim, K.-M.; et al. Discrimination of Lycium chinense and L. barbarum Based on Metabolite Analysis and Hepatoprotective Activity. Molecules 2020, 25, 5835. https://doi.org/10.3390/molecules25245835
Ryu M-J, Kim M, Ji M, Lee C, Yang I, Hong S-B, Chin J, Seo EK, Paik M-J, Lim K-M, et al. Discrimination of Lycium chinense and L. barbarum Based on Metabolite Analysis and Hepatoprotective Activity. Molecules. 2020; 25(24):5835. https://doi.org/10.3390/molecules25245835
Chicago/Turabian StyleRyu, Min-Ji, Minjeong Kim, Moongi Ji, Chaeyoung Lee, Inho Yang, Seong-Bin Hong, Jungwook Chin, Eun Kyoung Seo, Man-Jeong Paik, Kyung-Min Lim, and et al. 2020. "Discrimination of Lycium chinense and L. barbarum Based on Metabolite Analysis and Hepatoprotective Activity" Molecules 25, no. 24: 5835. https://doi.org/10.3390/molecules25245835
APA StyleRyu, M.-J., Kim, M., Ji, M., Lee, C., Yang, I., Hong, S.-B., Chin, J., Seo, E. K., Paik, M.-J., Lim, K.-M., & Nam, S.-J. (2020). Discrimination of Lycium chinense and L. barbarum Based on Metabolite Analysis and Hepatoprotective Activity. Molecules, 25(24), 5835. https://doi.org/10.3390/molecules25245835









