Evaluation of Anthocyanin Content, Antioxidant Potential and Antimicrobial Activity of Black, Purple and Blue Colored Wheat Flour and Wheat-Grass Juice against Common Human Pathogens
Abstract
1. Introduction
2. Results
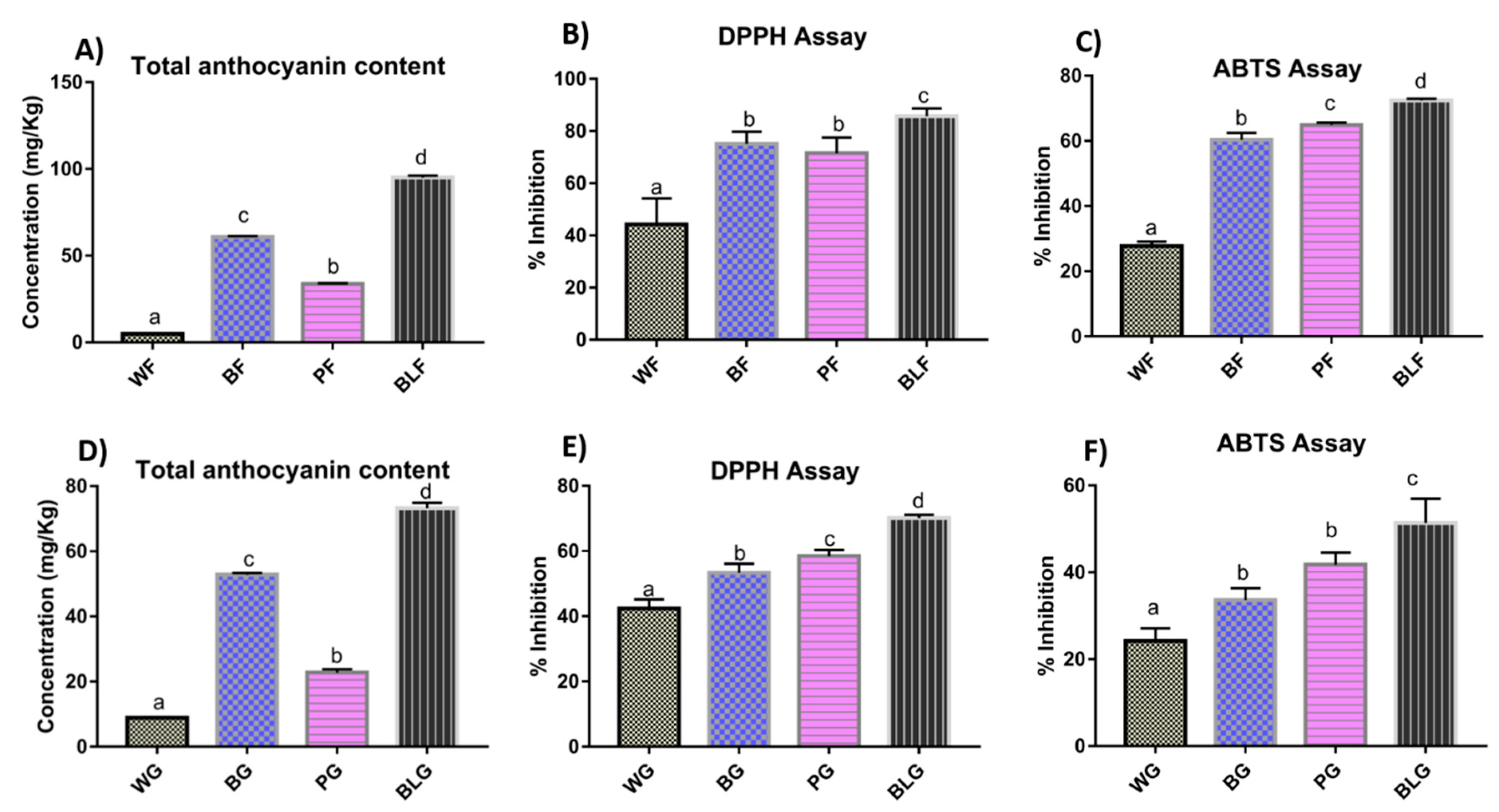
2.1. Total Anthocyanin Content in Wheat Flour and Wheat-Grass Juice
2.2. Antioxidant Potential of Wheat Flour and Wheat-Grass Juice
2.2.1. DPPH Assay
2.2.2. ABTS Assay
2.3. Determination of Anthocyanins by UPLC
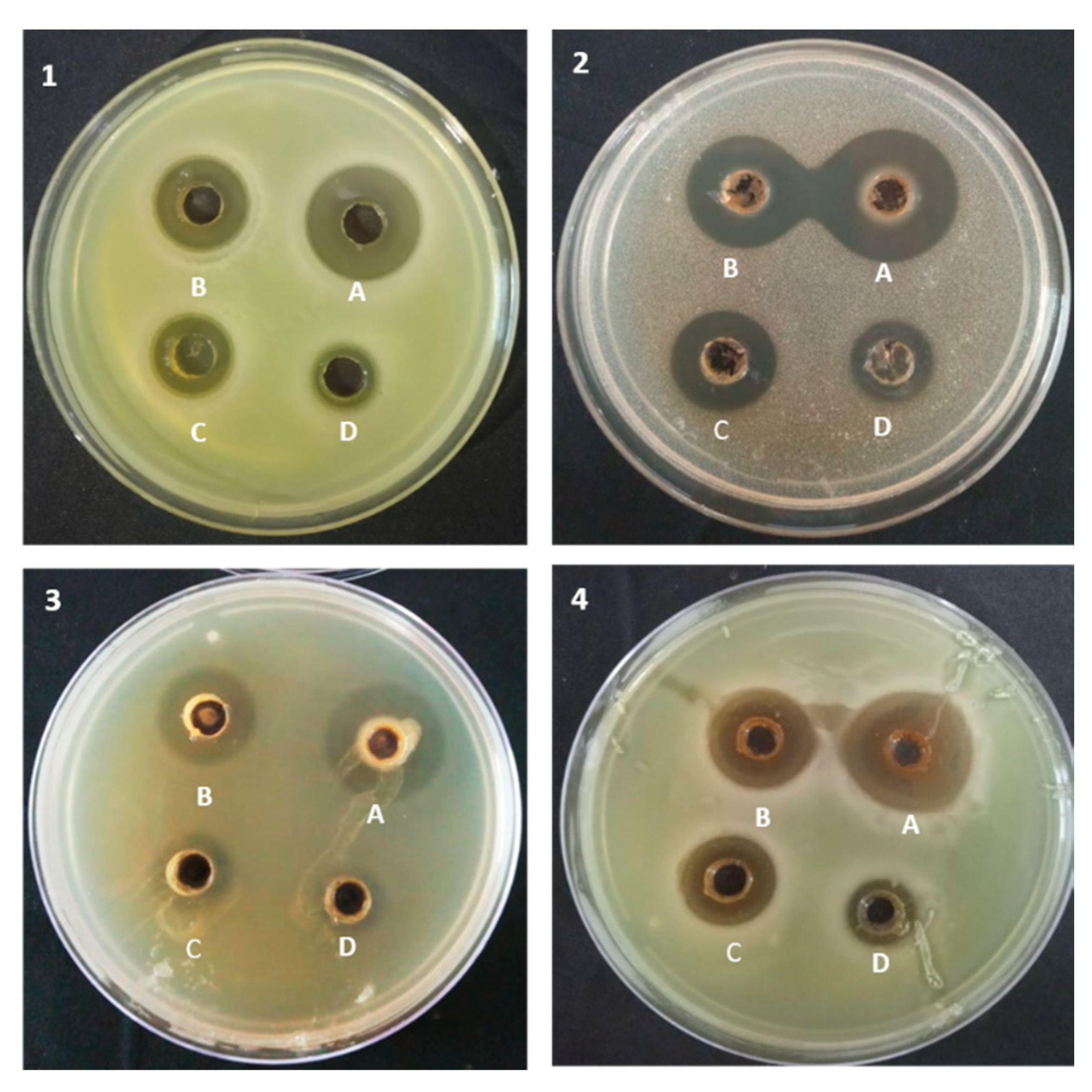
2.4. Antimicrobial Activity of Colored Wheat Anthocyanins Against Microbial Strains Using Agar-Overlay Method
2.5. Minimum Inhibitory Concentration (MIC) and Minimum Microbicidal Concentration (MMC) of Colored Wheat Anthocyanin Extracts Against Human Pathogens
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Preparation of Wheat Flour
4.3. Preparation of Lyophilized Colored Wheat-Grass Juice
4.4. Microbial Cultures
4.5. Total Anthocyanin Content (TAC) in Wheat Flour and Wheat-Grass
4.6. Anthocyanin Determination by Ultra Performance Liquid Chromatography (UPLC)
4.6.1. Anthocyanin Standards
4.6.2. UPLC Analysis
4.7. Antioxidant Potential of Colored Wheat Flour and Wheat-Grass Juice
4.7.1. DPPH Radical Scavenging Assay
4.7.2. ABTS Radical Scavenging Assay
4.8. Antimicrobial Activity of Colored Wheat Anthocyanins Against Microbial Strains Using Agar-Overlay Method
4.9. MIC and MMC of Colored Wheat Anthocyanin Extracts Against Human Pathogens
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Braun, H.J.; Atlin, G.; Payne, T. Multi-location testing as a tool to identify plant response to global climate change. In Climate Change and Crop Production; Reynolds, P.M., Ed.; International Maize and Wheat Improvement Center; CABI: Mexico City, Mexico, 2010; Volume 13, pp. 115–138. [Google Scholar]
- Shewry, P.R.; Hey, S.J. The contribution of wheat to human diet and health. Food Energy Secur. 2015, 4, 178–202. [Google Scholar] [CrossRef]
- Roy, S.; Banerjee, S.; Paul, U.; Zaman, S.; Roy, M.; Mitra, A. Comparative Analysis of Antimicrobial Activity of Different Aged Wheat Grass Grown in the Green House of Techno India University, West Bengal. Int. J. Environ. Health Eng. 2018, 2, 138–143. [Google Scholar]
- Hänninen, O.; Kaartinen, K.; Rauma, A.-L.; Nenonen, M.; Törrönen, R.; Häkkinen, A.S.; Adlercreutz, H.; Laakso, J. Antioxidants in vegan diet and rheumatic disorders. Toxicology 2000, 155, 45–53. [Google Scholar] [CrossRef]
- Walters, R. The Alternative Cancer Therapy Book; Avery Publishing Group: New York, NY, USA, 1992; pp. 299–308. [Google Scholar]
- Calzuola, I.; Marsili, V.; Gianfranceschi, G.L. Synthesis of antioxidants in wheat sprouts. J. Agric Food Chem. 2004, 52, 5201–5206. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Sofic, E.; Prior, R.L. Antioxidant Capacity of Tea and Common Vegetables. J. Agric. Food Chem. 1996, 44, 3426–3431. [Google Scholar] [CrossRef]
- Kulkarni, S.D.; Acharya, R.; Nair, A.G.C.; Rajurkar, N.S.; Reddy, A.V.R. Determination of elemental concentration profiles in tender wheatgrass (Triticum aestivum L.) using instrumental neutron activation analysis. Food Chem. 2006, 95, 699–707. [Google Scholar] [CrossRef]
- Chomchan, R.; Siripongvutikorn, A.P.D.S.; Puttarak, D.P.; Rattanapon, M.R. Investigation of Phytochemical Constituents, Phenolic Profiles and Antioxidant Activities of Ricegrass Juice compared to Wheatgrass Juice. Funct. Food Health Dis. 2016, 6, 822. [Google Scholar] [CrossRef]
- Capparelli, R.; Amoroso, M.G.; Palumbo, D.; Iannaccone, M.; Faleri, C.; Cresti, M. Two plant puroindolines colocalize in wheat seed and in vitro synergistically fight against pathogens. Plant Mol. Biol. 2005, 58, 857–867. [Google Scholar] [CrossRef]
- Wakeham, P. The medicinal and pharmacological screening of wheatgrass juice (Triticum aestivum L.): An investigation into chlorophyll content and antimicrobial activity. Plymouth Stud. Sci. 2013, 6, 20–30. [Google Scholar]
- Choi, E.J.; Ka, E.H.; Jo, C.Y.; Jo, S.H.; Apostolidis, E.; Lee, M.S.; Jang, H.D.; Kwon, Y.I. Comparison of the antimicrobial and antioxidant activities of selected wheat varieties. Food Sci. Biotechnol. 2014, 23, 791–797. [Google Scholar] [CrossRef]
- Kim, M.H.; Jo, S.H.; Ha, K.S.; Song, J.H.; Jang, H.D.; Kwon, Y.I. Antimicrobial activities of 1, 4-benzoquinones and wheat germ extract. J. Microbiol. Biotechnol. 2010, 20, 1204–1209. [Google Scholar] [CrossRef] [PubMed]
- Sushma, B.; Murali, R.; Shamala, A.; Yalamalli, M.; Kashyap, B. Antibacterial Activity of Herbal Extracts against Oral Bacteria: An Invitro Study. IOSR-JDMS 2020, 19, 22–29. [Google Scholar]
- Sundaresan, A.; Selvi, A.; Manonmani, H.K. The anti-microbial properties of Triticum aestivum (wheat grass) extract. Int. J. Biotechnol. Wellness Ind. 2015, 4, 84–91. [Google Scholar] [CrossRef]
- Ashok, S.A. Phytochemical and Pharmacological Screening of Wheatgrass Juice (Triticum aestivum L.). Int. J. Pharm. Sci. Rev. Res. 2011, 9, 159–164. [Google Scholar]
- Sytar, O.; Bośko, P.; Živčák, M.; Brestic, M.; Smetanska, I. Bioactive phytochemicals and antioxidant properties of the grains and sprouts of colored wheat genotypes. Molecules 2018, 23, 2282. [Google Scholar] [CrossRef] [PubMed]
- Calderaro, A.; Barreca, D.; Bellocco, E.; Smeriglio, A.; Trombetta, D.; Lagana, G. Colored phytonutrients: Role and applications in the functional foods of anthocyanins. In Phytonutrients in Food, 1st ed.; Nabavi, S.M., Suntar, I., Barreca, D., Khan, H., Eds.; Woodhead Publishing: Cambridge, UK, 2019; pp. 177–195. [Google Scholar]
- Garg, M.; Kumar, A.; Mundey, J.K.; Saini, M.K.; Chawla, M.; Sharma, N.K.; Kaur, N.; Kumar, R.; Sharma, S.; Singh, S.P.; et al. Transfer of grain colors to elite wheat cultivars and their characterization. J. Cereal Sci. 2016, 71, 138–144. [Google Scholar] [CrossRef]
- Wang, C.J.; Wang, J.M.; Lin, W.L.; Chu, C.Y.; Chou, F.P.; Tseng, T.H. Protective effect of Hibiscus anthocyanins against tert-butyl hydroperoxide-induced hepatic toxicity in rats. Food Chem. Toxicol. 2000, 38, 411–416. [Google Scholar] [CrossRef]
- Burdulis, D.; Sarkinas, A.; Jasutiené, I.; Stackevicené, E.; Nikolajevas, L.; Janulis, V. Comparative study of anthocyanin composition, antimicrobial and antioxidant activity in bilberry (Vaccinium myrtillus L.) and blueberry (Vaccinium corymbosum L.) fruits. Acta Pol. Pharm. 2009, 66, 399–408. [Google Scholar]
- Cisowska, A.; Wojnicz, D.; Hendrich, A.B. Anthocyanins as antimicrobial agents of natural plant origin. Nat. Prod. Commun. 2011, 6, 149–156. [Google Scholar] [CrossRef]
- Lacombe, A.; Wu, V.C.; Tyler, S.; Edwards, K. Antimicrobial action of the American cranberry constituents; phenolics, anthocyanins, and organic acids, against Escherichia coli O157: H7. Int. J. Food Microbiol. 2010, 139, 102–107. [Google Scholar] [CrossRef]
- Demirdöven, A.; Karabıyıklı, S.; Tokatlı, K.; Oncül, N. Inhibitory effects of red cabbage and sour cherry pomace anthocyanin extracts on food borne pathogens and their antioxidant properties. Food Sci. Technol. 2015, 63, 8–13. [Google Scholar] [CrossRef]
- Molina, A.K.; Vega, E.N.; Pereira, C.; Dias, M.I.; Heleno, S.A.; Rodrigues, P.; Fernandes, I.P.; Barreiro, M.F.; Kostić, M.; Soković, M.; et al. Promising antioxidant and antimicrobial food colourants from Lonicera caerulea L. var. kamtschatica. Antioxidants 2019, 8, 394. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aal, S.M.; Young, J.C.; Rabalski, I. Anthocyanin composition in black, blue, pink, purple, and red cereal grains. J. Agric. Food Chem. 2006, 54, 4696–4704. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Muller, D.; Richling, E.; Wink, M. Anthocyanin-rich purple wheat prolongs the life span of Caenorhabditis elegans probably by activating the DAF-16/FOXO transcription factor. J. Agric. Food Chem. 2013, 61, 3047–3053. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Chunduri, V.; Kumar, A.; Kumar, R.; Khare, P.; Kondepudi, K.K.; Bishnoi, M.; Garg, M. Anthocyanin bio-fortified colored wheat: Nutritional and functional characterization. PLoS ONE 2018, 13, e0194367. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Khare, P.; Kumar, A.; Chunduri, V.; Kumar, A.; Kapoor, P.; Mangal, P.; Kondepudi, K.K.; Bishnoi, M.; Garg, M. Anthocyanin-biofortified Colored Wheat Prevents High Fat Diet-induced Alterations in Mice: Nutrigenomics Studies. Mol. Nutr. Food Res. 2020, 64, 1900999. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aal, E.S.M.; Hucl, P.; Rabalski, I. Compositional and antioxidant properties of anthocyanin-rich products prepared from purple wheat. Food Chem. 2018, 254, 13–19. [Google Scholar] [CrossRef]
- Abdel-Aal, E.S.M.; Hucl, P. Composition and stability of anthocyanins in blue-grained wheat. J. Agric. Food Chem. 2003, 51, 2174–2180. [Google Scholar] [CrossRef]
- Liu, Q.; Qiu, Y.; Beta, T. Comparison of antioxidant activities of different colored wheat grains and analysis of phenolic compounds. J. Agric. Food Chem. 2010, 58, 9235–9241. [Google Scholar] [CrossRef]
- Li, Y.; Ma, D.; Sun, D.; Wang, C.; Zhang, J.; Xie, Y.; Guo, T. Total phenolic, flavonoid content, and antioxidant activity of flour, noodles, and steamed bread made from different colored wheat grains by three milling methods. Crop J. 2015, 3, 328–334. [Google Scholar] [CrossRef]
- Pasqualone, A.; Bianco, A.M.; Paradiso, V.M.; Summo, C.; Gambacorta, G.; Caponio, F.; Blanco, A. Production and characterization of functional biscuits obtained from purple wheat. Food Chem. 2015, 180, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Beta, T. Flour and bread from black-, purple-, and blue-colored wheats. In Flour and Breads and Their Fortification in Health and Disease Prevention, 2nd ed.; Victor Preedy, V., Watson, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 59–67. [Google Scholar]
- Kumari, A.; Sharma, S.; Sharma, N.; Chunduri, V.; Kapoor, P.; Goel, A.; Garg, M. Influence of biofortified colored wheats (purple, blue, black) on physicochemical, antioxidant and sensory characteristics of chapatti (Indian flatbread). Molecules 2020, 25, 5071. [Google Scholar] [CrossRef] [PubMed]
- Havrlentová, M.; Pšenáková, I.; Žofajová, A.; Rückschloss; Kraic, J. Anthocyanins in wheat seed—A mini review. Nova Biotechnol. Chim. 2014, 13, 1–12. [Google Scholar] [CrossRef]
- Francavilla, A.; Joye, I.J. Anthocyanins in Whole Grain Cereals and Their Potential Effect on Health. Nutrients 2020, 12, 2922. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Oroudjev, E.; Wilson, L.; Ayoub, G. Delphinidin and cyanidin exhibit antiproliferative and apoptotic effects in MCF7 human breast cancer cells. Integr. Cancer Sci. Ther. 2015, 2, 82–86. [Google Scholar]
- Pereira-Caro, G.; Watanabe, S.; Crozier, A.; Fujimura, T.; Yokota, T.; Ashihara, H. Phytochemical profile of a Japanese black–purple rice. Food Chem. 2013, 141, 2821–2827. [Google Scholar] [CrossRef]
- Hao, J.; Zhu, H.; Zhang, Z.; Yang, S.; Li, H. Identification of anthocyanins in black rice (Oryza sativa L.) by UPLC/QTOF-MS and their in vitro and in vivo anti-oxidant activities. J Cereal Sci. 2015, 64, 92–99. [Google Scholar] [CrossRef]
- Abdel-Aal, E.S.M.; Abou-Arab, A.A.; Gamel, T.H.; Hucl, P.J.; Young, C.; Rabalski, I. Fractionation of blue wheat anthocyanin compounds and their contribution to antioxidant properties. J. Agric. Food Chem. 2008, 56, 11171–11177. [Google Scholar] [CrossRef]
- Tyl, C.E.; Bunzel, M. Antioxidant activity-guided fractionation of blue wheat (UC66049 Triticum aestivum L.). J. Agric. Food Chem. 2012, 60, 731–739. [Google Scholar] [CrossRef]
- Ficco, D.B.; De Simone, V.; Colecchia, S.A.; Pecorella, I.; Platani, C.; Nigro, F.; Finocchiaro, F.; Papa, R.; De Vita, P. Genetic variability in anthocyanin composition and nutritional properties of blue, purple, and red bread (Triticum aestivum L.) and durum (Triticum turgidum L. ssp. turgidum convar. durum) wheats. J. Agric. Food Chem. 2014, 62, 8686–8695. [Google Scholar] [CrossRef]
- Žilić, S.; Dodig, D.; Vančetović, J.; Grčić, N.; Perić, V.; Titan, P.; Maksimović, V. Composition of anthocyanins in colored grains and the relationship of their non-acylated and acylated derivatives. Pol. J. Food Nutr. Sci. 2019, 69, 137–146. [Google Scholar] [CrossRef]
- Nankar, A.N.; Dungan, B.; Paz, N.; Sudasinghe, N.; Schaub, T.; Holguin, F.O.; Pratt, R.C. Quantitative and qualitative evaluation of kernel anthocyanins from south-western United States blue corn. J. Sci. Food Agric. 2016, 96, 4542–4552. [Google Scholar] [CrossRef] [PubMed]
- Camelo-Méndez, G.A.; Agama-Acevedo, E.; Sanchez-Rivera, M.M.; Bello-Pérez, L.A. Effect on in vitro starch digestibility of Mexican blue maize anthocyanins. Food Chem. 2016, 211, 281–284. [Google Scholar]
- Lao, F.; Giusti, M.M. Quantification of purple corn (Zea mays L.) anthocyanins using spectrophotometric and HPLC approaches: Method comparison and correlation. Food Anal. Methods. 2016, 9, 1367–1380. [Google Scholar] [CrossRef]
- Hosseinian, F.S.; Li, W.; Beta, T. Measurement of anthocyanins and other phytochemicals in purple wheat. Food Chem. 2008, 109, 916–924. [Google Scholar] [CrossRef]
- Amini, A.M.; Muzs, K.; Spencer, J.P.; Yaqoob, P. Pelargonidin-3-O-glucoside and its metabolites have modest anti-inflammatory effects in human whole blood cultures. Nutr. Res. 2017, 46, 88–95. [Google Scholar] [CrossRef]
- Cherian, S.; Kumar, R.V.; Augusti, K.T.; Kidwai, J.R. Antidiabetic effect of a glycoside of pelargonidin isolated from the bark of Ficus bengalensis Linn. Indian J. Biochem. Biophys. 1992, 29, 380–382. [Google Scholar]
- Saha, S.; Islam, Z.; Islam, S.; Hossain, S.; Islam, S.M. Evaluation of antimicrobial activity of wheat (Triticum aestivum L.) against four bacterial strains. SKUAST J. Res. 2018, 20, 58–62. [Google Scholar]
- Molist, F.; Hermes, R.G.; De Segura, A.G.; Martín-Orúe, S.M.; Gasa, J.; Manzanilla, E.G.; Pérez, J.F. Effect and interaction between wheat bran and zinc oxide on productive performance and intestinal health in post-weaning piglets. Br. J. Nutr. 2011, 105, 1592–1600. [Google Scholar] [CrossRef]
- Rajpurohit, L.; Mehta, N.; Ankola, A.V.; Gadiyar, A. Evaluation of the anti-microbial activity of various concentration of wheat grass (Triticum aestivum) extract against Gram-positive bacteria: An in vitro study. J. Dent. Res. Rev. 2015, 2, 70. [Google Scholar] [CrossRef]
- Das, A.; Raychaudhuri, U.; Chakraborty, R. Antimicrobial effect of edible plant extract on the growth of some foodborne bacteria including pathogens. Nutrafoods 2012, 11, 99–104. [Google Scholar] [CrossRef]
- Deshwal, V.K. Deepshikha. Antimicrobial investigation of wheat grass (Triticum aestivum L.) against Escherichia coli. Eur. J. Mol. Biol. Biochem. 2018, 5, 9–12. [Google Scholar]
- Joshi, B.; Sah, G.P.; Basnet, B.B.; Bhatt, M.R.; Sharma, D.; Subedi, K.; Pandey, J.; Malla, R. Phytochemical extraction and antimicrobial properties of different medicinal plants: Ocimum sanctum (Tulsi), Eugenia caryophyllata (Clove), Achyranthes bidentata (Datiwan) and Azadirachta indica (Neem). J. Microbiol. Antimicrob. 2011, 3, 1–7. [Google Scholar]
- Sharma, A.K.; Gangwar, M.; Tilak, R.; Nath, G.; Sinha, A.S.K.; Tripathi, Y.B.; Kumar, D. Comparative in vitro antimicrobial and phytochemical evaluation of methanolic extract of root, stem and leaf of Jatropha curcas Linn. Phcog. J. 2012, 4, 34–40. [Google Scholar] [CrossRef]
- Naz, S.; Siddiqi, R.; Ahmad, S.; Rasool, S.A.; Sayeed, S.A. Antibacterial activity directed isolation of compounds from Punica granatum. J. Food Sci. 2007, 72, M341–M345. [Google Scholar] [CrossRef] [PubMed]
- Leja, M.; Kamińska, I.; Kramer, M.; Maksylewicz-Kaul, A.; Kammerer, D.; Carle, R.; Baranski, R. The content of phenolic compounds and radical scavenging activity varies with carrot origin and root color. Plant Foods Hum. Nutr. 2013, 68, 163–170. [Google Scholar] [CrossRef]
- Saleem, M.Q.; Akhtar, S.; Imran, M.; Riaz, M.; Rauf, A.; Mubarak, M.S.; Bawazeer, S.; Bawazeer, S.S.; Hassanien, M.F. Antibacterial and anticancer characteristics of black carrot (Daucus Carota) extracts. J. Med. Spice Plants 2018, 22, 40–44. [Google Scholar]
- Kim, S.H.; Woo, H.J.; Lee, M.H.; Park, M.; Nagendran, T.; Rhee, K.J.; Lee, D.; Jin, Y.B.; Choi, S.W.; Seo, W.D.; et al. Antimicrobial effects of black rice extract on Helicobacter pylori infection in Mongolian gerbil. J. Cereal Sci. 2019, 85, 1–5. [Google Scholar] [CrossRef]
- Viswanath, V.; Urooj, A.; Malleshi, N.G. Evaluation of antioxidant and antimicrobial properties of finger millet polyphenols (Eleusine coracana). Food Chem. 2009, 114, 340–346. [Google Scholar] [CrossRef]
- Walsh, S.E.; Maillard, J.Y.; Russell, A.D.; Catrenich, C.E.; Charbonneau, D.L.; Bartolo, R.G. Activity and mechanisms of action of selected biocidal agents on Gram-positive and-negative bacteria. J. Appl. Microbiol. 2003, 94, 240–247. [Google Scholar] [CrossRef]
- Garg, M. NABIMG-11-Black (BW/2* PBW621) (IC0620916; INGR17003), a Wheat (Triticum aestivum) Germplasm with Black grain colour;(purple pericarp+ blue aleuron). Indian J. Plant Genet. Res. 2018, 31, 334–335. [Google Scholar]
- Garg, M. NABIMG-10-Purple (BW/2* PBW621) (IC0620915; INGR17002), a Wheat (Triticum aestivum) Germplasm with purple grain (pericarp) Color. Indian J. Plant. Genet. Res. 2018, 31, 333–334. [Google Scholar]
- Garg, M. NABIMG-9-Blue; BW/2*/PBW621 (IC0620914; INGR17001), a Wheat (Triticum aestivum) Germplasm with blue grain (aleurone) Color. Indian J. Plant. Genet. Res. 2018, 31, 332–333. [Google Scholar]
- Boeing, J.S.; Barizão, E.O.; e Silva, B.C.; Montanher, P.F.; de Cinque Almeida, V.; Visentainer, J.V. Evaluation of solvent effect on the extraction of phenolic compounds and antioxidant capacities from the berries: Application of principal component analysis. Chem. Cent. J. 2014, 8, 48. [Google Scholar] [CrossRef] [PubMed]
- Young, J.C.; Abdel-Aal, E.M. Anthocyanidins. In Analysis of Bioactive Components in Small Grain Cereals; Shewry, P.R., Ward, J.L., Eds.; AACC International Press: St. Paul, MN, USA, 2010; pp. 141–165. [Google Scholar]
- Yu, L.; Beta, T. Identification and Antioxidant Properties of Phenolic Compounds during Production of Bread from Purple Wheat Grains. Molecules 2015, 20, 15525–15549. [Google Scholar] [CrossRef] [PubMed]
- Foldes, T.; Banhegyi, I.; Herpai, Z.; Varga, L.; Szigeti, J. Isolation of Bacillus strains from the rhizosphere of cereals and in vitro screening for antagonism against phytopathogenic, food-borne pathogenic and spoilage micro-organisms. J. Appl. Microbiol. 2000, 89, 840–846. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, M.; Soran, H.; Beyatli, Y. Antimicrobial activities of some Bacillus spp. strains isolated from the soil. Microbiol. Res. 2006, 161, 127–131. [Google Scholar] [CrossRef]
- Cos, P.; Vlietinck, A.J.; Berghe, D.V.; Maes, L. Anti-infective potential of natural products: How to develop a stronger in vitro ‘proof-of-concept’. J. Ethnopharmacol. 2006, 106, 290–302. [Google Scholar] [CrossRef]

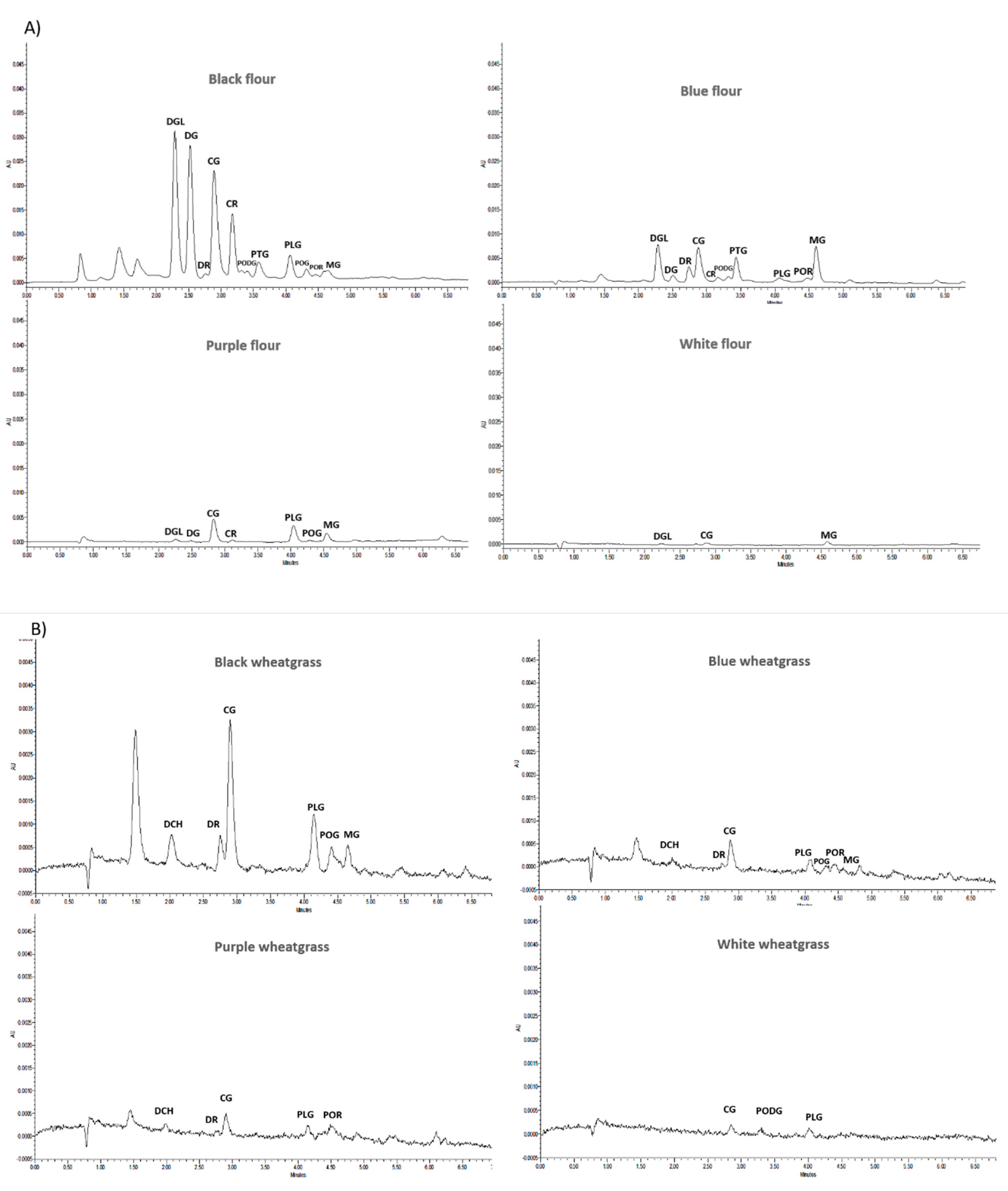

| Wheat Flour | Wheat-Grass | |||||||
|---|---|---|---|---|---|---|---|---|
| Anthocyanins | Black | Purple | Blue | White | Black | Purple | Blue | White |
| DGl | 29.14 ± 0.87 g | 0.40 ± 0.02 b | 4.95 ± 0.04 f | 0.03 ± 0.01 a | - | - | - | - |
| DG | 25.64 ± 0.69 f | 0.08 ± 0.00 a | 0.40 ± 0.01 a | - | - | - | - | - |
| DR | 0.66 ± 0.06 ab | - | 2.08 ± 0.09 c | - | 2.03 ± 0.08 b | 0.21 ± 0.03 b | 2.36 ± 0.05 d | - |
| CG | 20.50 ± 1.06 e | 2.64 ± 0.04 e | 4.50 ± 0.03 e | 0.07 ± 0.01 b | 4.74 ± 0.51 d | 0.23 ± 0.02 b | - | 0.37 ± 0.04 b |
| PODG | 0.23 ± 0.07 a | - | 0.31 ± 0.10 a | - | - | - | - | 0.26 ± 0.04 a |
| PTG | 2.29 ± 0.21 c | - | 3.18 ± 0.30 d | - | - | - | - | - |
| PLG | 2.13 ± 0.05 c | 1.88 ± 0.06 d | 0.39 ± 0.09 a | - | 2.77 ± 0.11 c | 0.41 ± 0.03 d | 0.40 ± 0.03 c | 0.35 ± 0.02 b |
| POG | 1.40 ± 0.09 bc | - | - | - | 1.03 ± 0.15 a | 0.32 ± 0.06 c | 0.14 ± 0.01 a | - |
| POR | 0.97 ± 0.10 ab | - | 0.76 ± 0.26 b | - | - | 0.18 ± 0.03 ab | 0.31 ± 0.03 b | - |
| MG | 2.18 ± 0.17 c | 1.32 ± 0.04 c | 5.50 ± 0.15 g | 0.12 ± 0.01 c | 1.25 ± 0.17 a | - | 0.15 ± 0.01 a | - |
| CR | 11.14 ± 0.25 d | 0.20 ± 0.01 ab | 0.60 ± 0.17 ab | - | - | - | - | - |
| DCH | - | - | - | - | 1.89 ± 0.30 b | 0.14 ± 0.01 a | - | - |
| Wheat Sample | Extract Conc. | S. aureus | P. aeruginosa | E. coli | C. albicans | ||||
|---|---|---|---|---|---|---|---|---|---|
| (mg/mL) | WF 1 | WG 2 | WF 1 | WG 2 | WF 1 | WG 2 | WF 1 | WG 2 | |
| Black | 200 | 2.50 ± 0.05 k | 2.23 ± 0.06 k | 2.73 ± 0.04 g | 2.60 ± 0.10 i | 2.50 ± 0 h | 1.93 ± 0.04 g | 2.57 ± 0.04 g | 2.37 ± 0.04 i |
| 150 | 1.87 ± 0.06 g | 1.77 ± 0.04 i | 2.37 ± 0.06 def | 197 ± 0.12 g | 2.07 ± 0.05 f | 1.60 ± 0.02 ef | 2.03 ± 0.06 e | 2.03 ± 0.06 h | |
| 100 | 1.63 ± 0.05 f | 1.27 ± 0.05 fg | 2.03 ± 0.12 c | 1.46 ± 0.08 f | 1.53 ± 0.06 c | 1.03 ± 0.05 d | 1.83 ± 0.03 d | 1.77 ± 0.03 g | |
| 50 | 1.23 ± 0.04 c | 0.83 ± 0.06 d | 1.63 ± 0.12 b | 1.17 ± 0.04 e | 1.13 ± 0.06 ab | 0.77 ± 0.12 bc | 1.17 ± 0.04 b | 1.17 ± 0.04 de | |
| Purple | 200 | 2.27 ± 0.06 j | 2.20 ± 0.10 k | 2.60 ± 0.10 fg | 2.27 ± 0.07 h | 2.37 ± 0.04 gh | 2.00 ± 0.10 g | 2.40 ± 0.10 f | 1.93 ± 0.12 gh |
| 150 | 2.07 ± 0.05 hi | 1.87 ± 0.06 ij | 2.13 ± 0.12 cd | 1.87 ± 0.05 g | 1.97 ± 0.12 e | 1.80 ± 0.07 fg | 2.03 ± 0.12 e | 1.50 ± 0.03 f | |
| 100 | 1.77 ± 0.07 fg | 1.07 ± 0.05 e | 2.07 ± 0.05 c | 1.03 ± 0.05 de | 1.80 ± 0.10 de | 1.40 ± 0.10 e | 1.67 ± 0.06 c | 1.00 ± 0.10 d | |
| 50 | 1.43 ± 0.12 de | 0.57 ± 0.03 c | 1.57 ± 0.11 b | 0.50 ± 0 b | 1.20 ± 0.10 ab | 0.90 ± 0.04 cd | 1.57 ± 0.06 c | 0.57 ± 0.08 c | |
| Blue | 200 | 1.93 ± 0.06 gh | 1.53 ± 0.05 h | 2.47 ± 0.05 ef | 1.53 ± 0.04 f | 2.30 ± 0.10 g | 1.10 ± 0.11 d | 1.53 ± 0.11 c | 1.53 ± 0.06 f |
| 150 | 1.60 ± 0.10 ef | 1.17 ± 0.06 ef | 2.13 ± 0.05 cd | 0.97 ± 0.06 d | 2.07 ± 0.02 f | 0.87 ± 0.04 cd | 1.07 ± 0.05 b | 1.20 ± 0.10 e | |
| 100 | 1.30 ± 0.10 cd | 0.73 ± 0.02 d | 1.73 ± 0.12 b | 0.33 ± 0.06 b | 1.27 ± 0.03 b | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.47 ± 0.05 c | |
| 50 | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.97 ± 0.06 a | 0.0 ± 0.0 a | 1.07 ± 0.06 a | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.0 ± 0.0 a | |
| White | 200 | 2.13 ± 0.12 ij | 1.93 ± 0.06 j | 2.23 ± 0.07 cde | 2.17 ± 0.05 h | 2.47 ± 0.07 gh | 1.97 ± 0.12 g | 2.37 ± 0.07 f | 1.07 ± 0.15 de |
| 150 | 1.77 ± 0.06 fg | 1.37 ± 0.04 g | 2.07 ± 0.12 c | 1.83 ± 0.05 g | 1.97 ± 0.11 ef | 1.43 ± 0.05 e | 2.03 ± 0.05 e | 0.20 ± 0.0 b | |
| 100 | 1.30 ± 0.10 cd | 0.87 ± 1.0 d | 1.63 ± 0.12 b | 1.37 ± 0.12 f | 1.67 ± 0.08 cd | 0.90 ± 0.09 d | 1.0 ± 0.10 b | 0.0 ± 0.0 a | |
| 50 | 0.83 ± 0.07 b | 0.27 ± 0.07 b | 0.97 ± 0.15 a | 0.77 ± 0.12 c | 1.53 ± 0.05 c | 0.57 ± 0.05 b | 0.0 ± 0.0 a | 0.0 ± 0.0 a | |
| Wheat Sample | Inhibition | S. aureus | P. aeruginosa | E. coli | C. albicans | ||||
|---|---|---|---|---|---|---|---|---|---|
| WF 1 | WG 2 | WF 1 | WG 2 | WF 1 | WG 2 | WF 1 | WG 2 | ||
| Black | MIC * | 50 | 100 | 50 | 150 | 100 | 100 | 100 | 150 |
| MMC ** | 100 | 200 | 150 | 200 | 200 | - | 200 | - | |
| Purple | MIC * | 50 | 150 | 50 | 150 | 100 | 150 | 150 | 150 |
| MMC ** | 150 | 200 | 150 | - | - | - | - | - | |
| Blue | MIC * | 150 | 200 | 100 | 150 | 150 | 200 | - | - |
| MMC ** | 200 | - | 200 | - | - | - | - | - | |
| White | MIC * | 100 | 150 | 100 | 150 | - | 200 | 200 | - |
| MMC ** | 200 | - | 150 | - | - | - | - | ||
Sample Availability: All the Samples of colored wheat flour are available to the authors at National Agri-Food Biotechnology Institute, India. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, N.; Tiwari, V.; Vats, S.; Kumari, A.; Chunduri, V.; Kaur, S.; Kapoor, P.; Garg, M. Evaluation of Anthocyanin Content, Antioxidant Potential and Antimicrobial Activity of Black, Purple and Blue Colored Wheat Flour and Wheat-Grass Juice against Common Human Pathogens. Molecules 2020, 25, 5785. https://doi.org/10.3390/molecules25245785
Sharma N, Tiwari V, Vats S, Kumari A, Chunduri V, Kaur S, Kapoor P, Garg M. Evaluation of Anthocyanin Content, Antioxidant Potential and Antimicrobial Activity of Black, Purple and Blue Colored Wheat Flour and Wheat-Grass Juice against Common Human Pathogens. Molecules. 2020; 25(24):5785. https://doi.org/10.3390/molecules25245785
Chicago/Turabian StyleSharma, Natasha, Vandita Tiwari, Shreya Vats, Anita Kumari, Venkatesh Chunduri, Satveer Kaur, Payal Kapoor, and Monika Garg. 2020. "Evaluation of Anthocyanin Content, Antioxidant Potential and Antimicrobial Activity of Black, Purple and Blue Colored Wheat Flour and Wheat-Grass Juice against Common Human Pathogens" Molecules 25, no. 24: 5785. https://doi.org/10.3390/molecules25245785
APA StyleSharma, N., Tiwari, V., Vats, S., Kumari, A., Chunduri, V., Kaur, S., Kapoor, P., & Garg, M. (2020). Evaluation of Anthocyanin Content, Antioxidant Potential and Antimicrobial Activity of Black, Purple and Blue Colored Wheat Flour and Wheat-Grass Juice against Common Human Pathogens. Molecules, 25(24), 5785. https://doi.org/10.3390/molecules25245785






