Long-Term Stability Analysis of 3D and 2D/3D Hybrid Perovskite Solar Cells Using Electrochemical Impedance Spectroscopy
(This article belongs to the Section Electrochemistry)
Abstract
1. Introduction
2. Results
3. Experimental
3.1. Materials and Device Fabrication
3.2. Characterization of the PSCs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Christians, J.A.; Miranda Herrera, P.A.; Kamat, P.V. Transformation of the excited state and photovoltaic efficiency of CH3NH3PbI3 perovskite upon controlled exposure to humidified air. J. Am. Chem. Soc. 2015, 137, 1530–1538. [Google Scholar] [CrossRef] [PubMed]
- Leguy, A.M.A.; Hu, Y.; Campoy-Quiles, M.; Alonso, M.I.; Weber, O.J.; Azarhoosh, P.; van Schilfgaarde, M.; Weller, M.T.; Bein, T.; Nelson, J.; et al. Reversible hydration of CH3NH3PbI3 in films, single crystals, and solar cells. Chem. Mater. 2015, 27, 3397–3407. [Google Scholar] [CrossRef]
- Jiang, P.; Xiong, Y.; Xu, M.; Mei, A.; Sheng, Y.; Hong, L.; Jones, T.W.; Wilson, G.J.; Xiong, S.; Li, D.; et al. The influence of the work function of hybrid carbon electrodes on printable mesoscopic perovskite solar cells. J. Phys. Chem. C 2018, 122, 16481–16487. [Google Scholar] [CrossRef]
- Parida, B.; Ryu, J.; Yoon, S.; Lee, S.; Seo, Y.; Cho, J.S.; Kang, D.-W. Two-step growth of CsPbI3−xBrx films employing dynamic CsBr treatment: Toward all-inorganic perovskite photovoltaics with enhanced stability. J. Mater. Chem. A 2019, 7, 18488–18498. [Google Scholar] [CrossRef]
- Abbas, M.S.; Hussain, S.; Zhang, J.; Wang, B.; Yang, C.; Wang, Z.; Wei, Z.; Ahmad, R. Orientationally engineered 2D/3D perovskite for high efficiency solar cells. Sustain. Energy Fuels 2020, 4, 324–330. [Google Scholar] [CrossRef]
- Kang, Y.-J.; Kwon, S.-N.; Cho, S.-P.; Seo, Y.-H.; Choi, M.-J.; Kim, S.-S.; Na, S.-I. Antisolvent additive engineering containing dual-function additive for triple-cation p–i–n perovskite solar cells with over 20% PCE. ACS Energy Lett. 2020, 5, 2535–2545. [Google Scholar] [CrossRef]
- Meng, H.; Shao, Z.; Wang, L.; Li, Z.; Liu, R.; Fan, Y.; Cui, G.; Pang, S. Chemical composition and phase evolution in DMAI-derived inorganic perovskite solar cells. ACS Energy Lett. 2019, 5, 263–270. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, Y.; Wang, Z.; Liu, Y.; Zhao, Z.; Xu, G.; Han, T.-H.; Lee, J.-W.; Chen, C.; Bao, D.; et al. Hermetic seal for perovskite solar cells: An improved plasma enhanced atomic layer deposition encapsulation. Nano Energy 2020, 69. [Google Scholar] [CrossRef]
- Targhi, F.F.; Jalili, Y.S.; Kanjouri, F. MAPbI3 and FAPbI3 perovskites as solar cells: Case study on structural, electrical and optical properties. Results Phys. 2018, 10, 616–627. [Google Scholar] [CrossRef]
- Ubaid, F.; Radwan, A.B.; Naeem, N.; Shakoor, R.; Ahmad, Z.; Montemor, M.; Kahraman, R.; Abdullah, A.M.; Soliman, A. Multifunctional self-healing polymeric nanocomposite coatings for corrosion inhibition of steel. Surf. Coat. Technol. 2019, 372, 121–133. [Google Scholar] [CrossRef]
- Saliba, M.; Matsui, T.; Seo, J.Y.; Domanski, K.; Correa-Baena, J.P.; Nazeeruddin, M.K.; Zakeeruddin, S.M.; Tress, W.; Abate, A.; Hagfeldt, A.; et al. Cesium-containing triple cation perovskite solar cells: Improved stability, reproducibility and high efficiency. Energy Environ. Sci 2016, 9, 1989–1997. [Google Scholar] [CrossRef]
- Jeon, N.J.; Noh, J.H.; Yang, W.S.; Kim, Y.C.; Ryu, S.; Seo, J.; Seok, S.I. Compositional engineering of perovskite materials for high-performance solar cells. Nature 2015, 517, 476–480. [Google Scholar] [CrossRef] [PubMed]
- Pellet, N.; Gao, P.; Gregori, G.; Yang, T.Y.; Nazeeruddin, M.K.; Maier, J.; Gratzel, M. Mixed-organic-cation perovskite photovoltaics for enhanced solar-light harvesting. Angew. Chem. Int. Ed. Engl. 2014, 53, 3151–3157. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yang, M.; Park, J.-S.; Wei, S.-H.; Berry, J.J.; Zhu, K. Stabilizing perovskite structures by tuning tolerance factor: Formation of formamidinium and cesium lead Iodide solid-state alloys. Chem. Mater. 2015, 28, 284–292. [Google Scholar] [CrossRef]
- Singh, R.; Sandhu, S.; Yadav, H.; Lee, J.J. Stable triple-cation (Cs(+)-MA(+)-FA(+)) perovskite powder formation under ambient conditions for hysteresis-free high-efficiency solar cells. ACS Appl. Mater. Interfaces 2019, 11, 29941–29949. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, Z.; Noma, T.; Paek, S.; Cho, K.T.; Taguchi, D.; Iwamoto, M.; Manaka, T.; Nazeeruddin, M.K.; Touati, F.; Al-Muhtaseb, S.A. Stability in 3D and 2D/3D hybrid perovskite solar cells studied by EFISHG and IS techniques under light and heat soaking. Org. Electron. 2019, 66, 7–12. [Google Scholar] [CrossRef]
- Cho, K.T.; Grancini, G.; Lee, Y.; Oveisi, E.; Ryu, J.; Almora, O.; Tschumi, M.; Schouwink, P.A.; Seo, G.; Heo, S.; et al. Selective growth of layered perovskites for stable and efficient photovoltaics. Energy Environ. Sci. 2018, 11, 952–959. [Google Scholar] [CrossRef]
- Choi, H.S.; Kim, H.S. 3D/2D bilayerd perovskite solar cells with an enhanced stability and performance. Materials 2020, 13, 3868. [Google Scholar] [CrossRef]
- Grancini, G.; Roldan-Carmona, C.; Zimmermann, I.; Mosconi, E.; Lee, X.; Martineau, D.; Narbey, S.; Oswald, F.; De Angelis, F.; Graetzel, M.; et al. One-Year stable perovskite solar cells by 2D/3D interface engineering. Nat. Commun. 2017, 8, 15684. [Google Scholar] [CrossRef]
- Krishna, A.; Gottis, S.; Nazeeruddin, M.K.; Sauvage, F. Mixed dimensional 2D/3D hybrid perovskite absorbers: The future of perovskite solar cells? Adv. Funct. Mater. 2019, 29. [Google Scholar] [CrossRef]
- Yao, D.; Zhang, C.; Zhang, S.; Yang, Y.; Du, A.; Waclawik, E.; Yu, X.; Wilson, G.J.; Wang, H. 2D-3D mixed organic-inorganic perovskite layers for solar cells with enhanced efficiency and stability induced by n-propylammonium iodide additives. ACS Appl. Mater. Interfaces 2019, 11, 29753–29764. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Lin, Q.; Chmiel, F.P.; Sakai, N.; Herz, L.M.; Snaith, H.J. Efficient ambient-air-stable solar cells with 2D–3D heterostructured butylammonium-caesium-formamidinium lead halide perovskites. Nat. Energy 2017, 2. [Google Scholar] [CrossRef]
- Zou, Y.; Cui, Y.; Wang, H.Y.; Cai, Q.; Mu, C.; Zhang, J.P. Highly efficient and stable 2D-3D perovskite solar cells fabricated by interfacial modification. Nanotechnology 2019, 30, 275202. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Zhao, Y.; Zhang, X.; Yang, X.; Chen, Y.; Chu, Z.; Ye, Q.; Li, X.; Yin, Z.; You, J. Surface passivation of perovskite film for efficient solar cells. Nat. Photonics 2019, 13, 460–466. [Google Scholar] [CrossRef]
- Zhou, L.; Lin, Z.; Ning, Z.; Li, T.; Guo, X.; Ma, J.; Su, J.; Zhang, C.; Zhang, J.; Liu, S.; et al. Highly efficient and stable planar perovskite solar cells with modulated diffusion passivation toward high power conversion efficiency and ultrahigh fill factor. Sol. RRL 2019, 3. [Google Scholar] [CrossRef]
- Niu, T.; Lu, J.; Jia, X.; Xu, Z.; Tang, M.-C.; Barrit, D.; Yuan, N.; Ding, J.; Zhang, X.; Fan, Y.J.N.l. Interfacial engineering at the 2D/3D heterojunction for high-performance perovskite solar cells. 2019, 19, 7181–7190. [Google Scholar] [CrossRef]
- Wang, Z.; Fang, J.; Mi, Y.; Zhu, X.; Ren, H.; Liu, X.; Yan, Y. Enhanced performance of perovskite solar cells by ultraviolet-ozone treatment of mesoporous TiO2. Appl. Surf. Sci. 2018, 436, 596–602. [Google Scholar] [CrossRef]
- Huang, X.; Bi, W.; Jia, P.; Tang, Y.; Lou, Z.; Hu, Y.; Cui, Q.; Hou, Y.; Teng, F. Enhanced efficiency and light stability of planar perovskite solar cells by diethylammonium bromide induced large-grain 2D/3D hybrid film. Org. Electron. 2019, 67, 101–108. [Google Scholar] [CrossRef]
- Ahmed, Z.; Mishra, A.; Abdulrahim, S.; Taguchi, D.; Paek, S.; Aziz, F.; Iwamoto, M.; Manaka, T.; Nazeeruddin, K.; Touati, F.; et al. The consequence of aging at Au/HTM/Perovskite interface in triple cation 3D and 2D/3D hybrid perovskite solar cell. Sci. Rep. 2021, in press. [Google Scholar]
- Rizvi, S.M.H.; Mantri, P.; Mazhari, B. Traps signature in steady state current-voltage characteristics of organic diode. J. Appl. Phys. 2014, 115, 244502. [Google Scholar] [CrossRef]
- Hyun Kim, C.; Yaghmazadeh, O.; Bonnassieux, Y.; Horowitz, G. Modeling the low-voltage regime of organic diodes: Origin of the ideality factor. J. Appl. Phys. 2011, 110. [Google Scholar] [CrossRef]
- Klotz, D.; Tumen-Ulzii, G.; Qin, C.; Matsushima, T.; Adachi, C. Detecting and identifying reversible changes in perovskite solar cells by electrochemical impedance spectroscopy. RSC Adv. 2019, 9, 33436–33445. [Google Scholar] [CrossRef]
- Feng, Y.; Bian, J.; Wang, S.; Zhang, C.; Wang, M.; Shi, Y. Soft interfaces within hybrid perovskite solar cells: Real-time dynamic tracking of interfacial electrical property evolution by EIS. J. Mater. Chem. C 2019, 7, 8294–8302. [Google Scholar] [CrossRef]
- Bag, M.; Renna, L.A.; Jeong, S.P.; Han, X.; Cutting, C.L.; Maroudas, D.; Venkataraman, D. Evidence for reduced charge recombination in carbon nanotube/perovskite-based active layers. Chem. Phys. Lett. 2016, 662, 35–41. [Google Scholar] [CrossRef]
- Pockett, A.; Eperon, G.E.; Peltola, T.; Snaith, H.J.; Walker, A.; Peter, L.M.; Cameron, P.J. Characterization of planar lead halide perovskite solar cells by impedance spectroscopy, open-circuit photovoltage decay, and intensity-modulated photovoltage/photocurrent spectroscopy. J. Phys. Chem. C 2015, 119, 3456–3465. [Google Scholar] [CrossRef]
- Guerrero, A.; Garcia-Belmonte, G.; Mora-Sero, I.; Bisquert, J.; Kang, Y.S.; Jacobsson, T.J.; Correa-Baena, J.-P.; Hagfeldt, A. Properties of contact and bulk impedances in hybrid lead halide perovskite solar cells including inductive loop elements. J. Phys. Chem. C 2016, 120, 8023–8032. [Google Scholar] [CrossRef]
- Zarazua, I.; Sidhik, S.; Lopéz-Luke, T.; Esparza, D.; De la Rosa, E.; Reyes-Gomez, J.; Mora-Sero, I.; Garcia-Belmonte, G. Operating mechanisms of mesoscopic perovskite solar cells through impedance spectroscopy and J–V modeling. J. Phys. Chem. Lett. 2017, 8, 6073–6079. [Google Scholar] [CrossRef]
- Romero, B.; del Pozo, G.; Arredondo, B.; Martín-Martín, D.; Hernández-Balaguera, E.; González, M.d.C.L. Characterization of organic and perovskite solar cells by impedance spectroscopy. In Women in Renewable Energy (WiRE); International Society for Optics and Photonics: Bellingham, WA, USA, 2019; p. 110950N. [Google Scholar]
- Gedamu, D.; Asuo, I.M.; Benetti, D.; Basti, M.; Ka, I.; Cloutier, S.G.; Rosei, F.; Nechache, R. Solvent-antisolvent ambient processed large grain size perovskite thin films for high-performance solar cells. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef]
- Del Pozo, G.; Arredondo, B.; Romero, B.; Susanna, G.; Brunetti, F. Degradation of PEIE interlayer in PTB7:[70] PCBM based solar cells characterized by impedance spectroscopy. Sol. Energy 2017, 144, 105–110. [Google Scholar] [CrossRef]
- Chen, P.; Bai, Y.; Wang, S.; Lyu, M.; Yun, J.H.; Wang, L. In situ growth of 2D perovskite capping layer for stable and efficient perovskite solar cells. Adv. Funct. Mater. 2018, 28, 1706923. [Google Scholar] [CrossRef]
- Liu, B.; Long, M.; Cai, M.; Ding, L.; Yang, J. Interfacial charge behavior modulation in 2D/3D perovskite heterostructure for potential high-performance solar cells. Nano Energy 2019, 59, 715–720. [Google Scholar] [CrossRef]
Sample Availability: Data can be provided on the reasonable request to the corresponding author. |
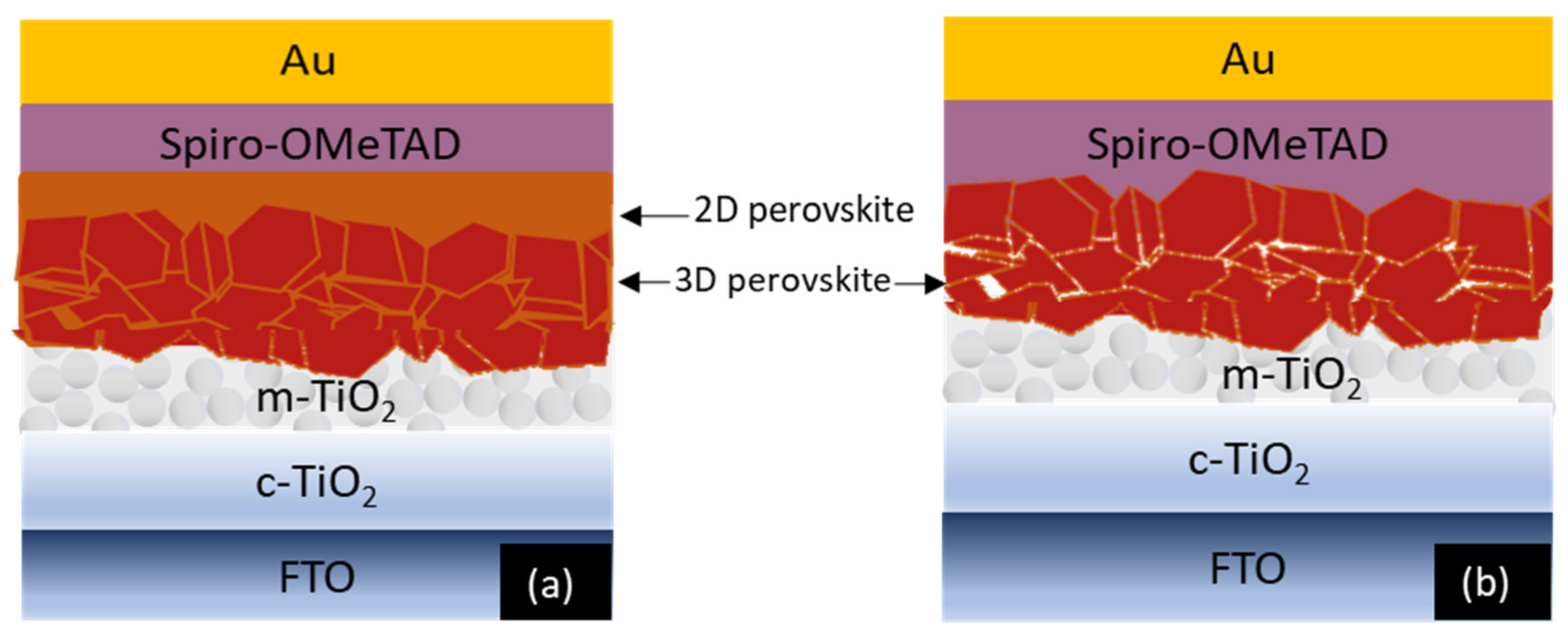

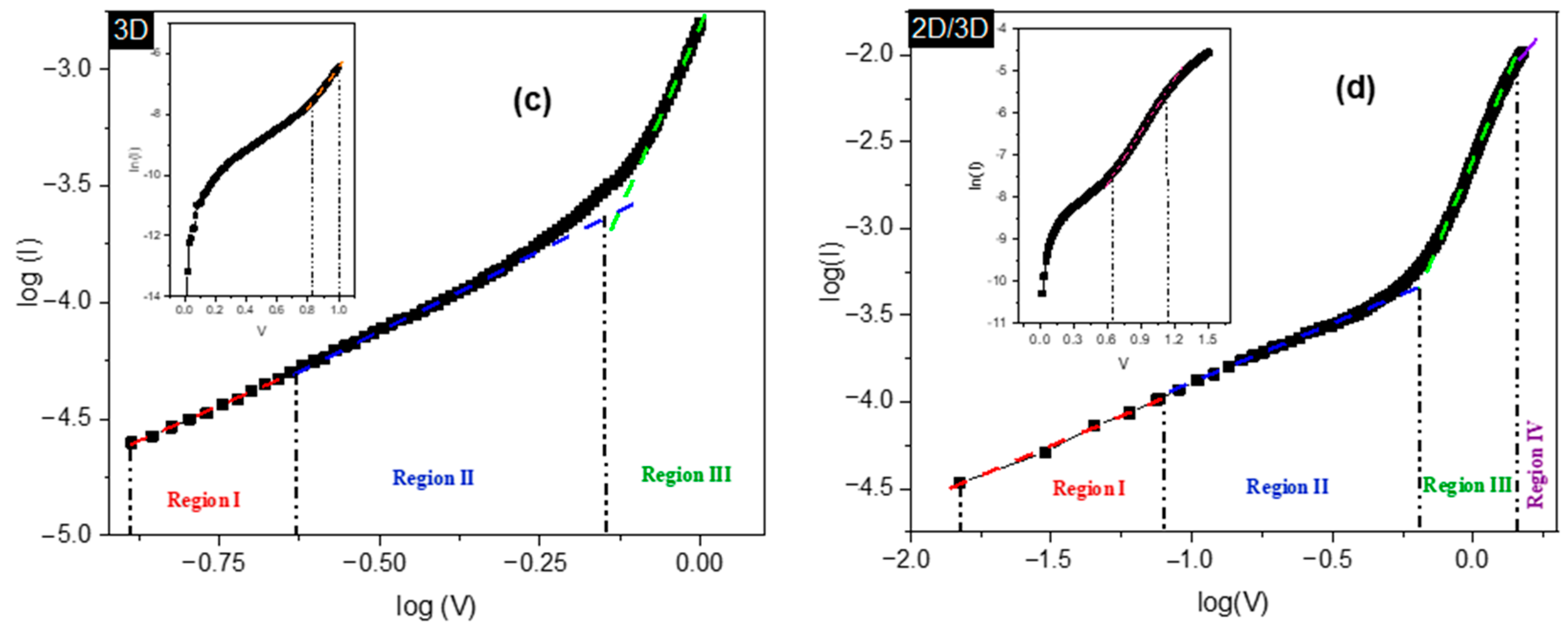

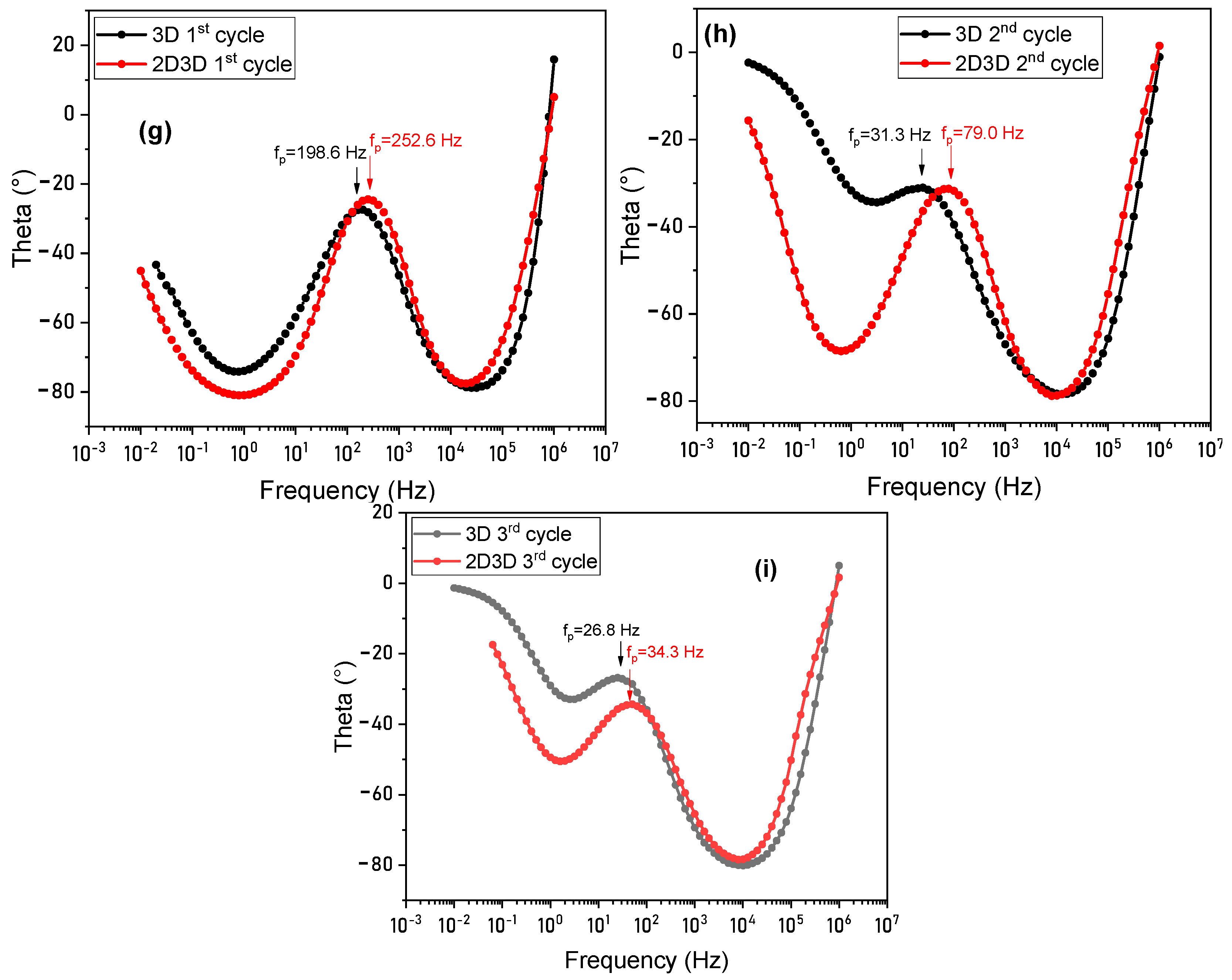
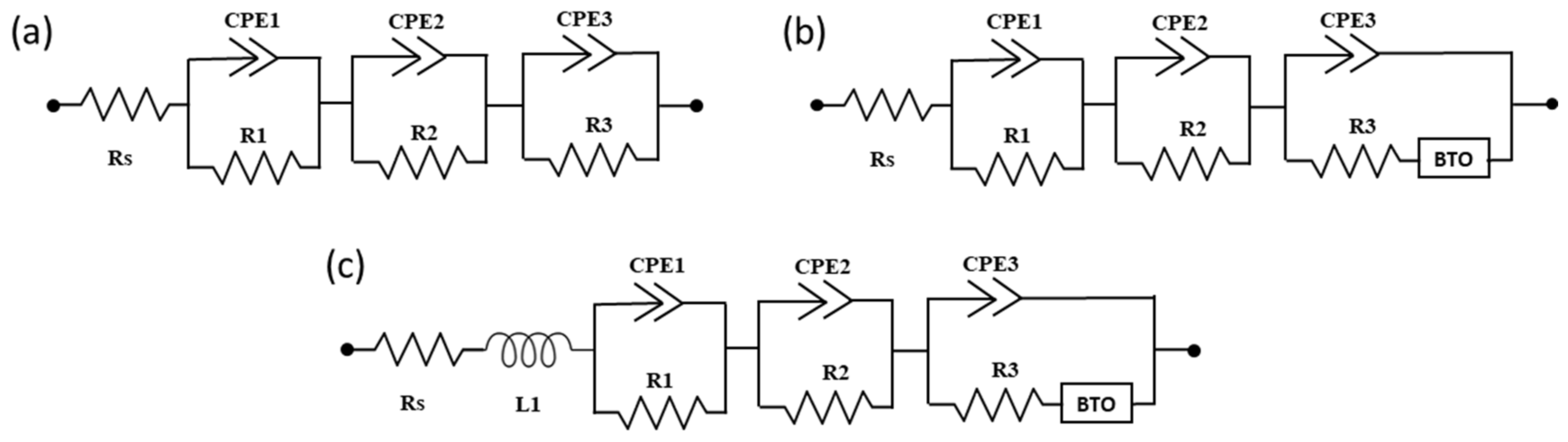
| Sample | Jsc, (mA/cm2) | Voc, (V) | FF | Efficiency (%) | Ideality Factor (n) | |
|---|---|---|---|---|---|---|
| 3D | 1st cycle | 22.96 | 1.09 | 78.17 | 19.44 | 2.03 ± 0.13 |
| 2nd cycle | 8.51 | 0.98 | 57.08 | 4.75 | 5.78 ± 0.040 | |
| 3rd cycle | 6.44 | 0.97 | 43.61 | 2.71 | 6.78 ± 0.091 | |
| 2D/3D | 1st cycle | 23.08 | 1.1 | 79.68 | 20.23 | 2.08 ± 0.22 |
| 2nd cycle | 15.32 | 1.01 | 54.14 | 8.10 | 8.28 ± 0.004 | |
| 3rd cycle | 13.71 | 0.98 | 50.34 | 6.83 | 8.99 ± 0.091 | |
| Sample | Slope (m) | |||
|---|---|---|---|---|
| Region I | Region II | Region III | Region IV | |
| 3D | 1.22 ± 0.02 | 1.37 ± 0.01 | 5.56 ± 0.11 | N/A |
| 2D/3D | 1.19 ± 0.026 | 1.16 ± 0.0041 | 3.20 ± 0.007 | 2.91 ± 0.021 |
| 3RC | ||||||||||
| Sample | Time Period | Rs | Ls | HF Region (Charge Transfer) | IF/LF Region (Recombination) | Goodness of Fit | ||||
| (Ω) | (H) | Rct (Ω) | act (10−3) | Yct (10−6) | Rrec (Ω) | arec (10−3) | Yrec (10−6) | |||
| 1st cycle | 8.78 ± 0.22 | - | 3.03 × 103 | 978 | 0.064 | 2.48 × 106 | 844 | 2.91 | 7.35 × 10−3 | |
| 3D | 2nd cycle | 16.0 ± 0.44 | - | 1.37 × 104 | 989 | 0.138 | 1.52 × 105 | 718 | 2.80 | 7.87 × 10−3 |
| 3rd cycle | 17.70 ± 0.31 | - | 1.51 × 104 | 970 | 0.176 | 7.65 × 104 | 801 | 3.44 | 4.24 × 10−3 | |
| 1st cycle | 12.59 ± 0.26 | - | 1.96 × 103 | 977 | 0.073 | 4.79 × 106 | 910 | 2.55 | 4.33 × 10−3 | |
| 2D/3D | 2nd cycle | 28.25 ± 0.45 | - | 1.03 × 104 | 969 | 0.068 | 1.48 × 106 | 859 | 2.15 | 1.65 × 10−3 |
| 3rd cycle | 36.91 ± 0.57 | - | 1.39 × 104 | 955 | 0.317 | 3.72 × 105 | 783 | 2.15 | 1.88 × 103 | |
| 3RC-BTO | ||||||||||
| Sample | Time Period | Rs | Ls | HF Region (Charge Transfer) | IF/LF Region (Recombination) | Goodness of Fit | ||||
| (Ω) | (H) | Rct (Ω) | act (10−3) | Yct (10−6) | Rrec(Ω) | arec (10−3) | Yrec (10−6) | |||
| 1st cycle | 8.27 ± 0.26 | - | 3.20 × 103 | 959 | 0.121 | 2.42 × 106 | 853 | 2.83 | 16.7 × 10−3 | |
| 3D | 2nd cycle | 16.03 ± 0.96 | - | 8.72 × 103 | 963 | 0.130 | 1.02 × 105 | 914 | 3.31 | 1.84 × 10−3 |
| 3rd cycle | 18.12 ± 0.43 | - | 1.42 × 104 | 948 | 0.138 | 7.36 × 104 | 749 | 3.25 | 3.59 × 10−3 | |
| 1st cycle | 12.80 ± 0.26 | - | 2.07 × 103 | 991 | 0.069 | 4.00 × 106 | 921 | 2.48 | 3.54 × 10−3 | |
| 2D/3D | 2nd cycle | 28.5 ± 0.51 | - | 8.27 × 103 | 991 | 0.19 | 1.45 × 106 | 870.5 | 2.16 | 1.31 × 10−3 |
| 3rd cycle | 36.92 ± 0.89 | - | 1.34 × 104 | 977 | 0.25 | 3.78 × 105 | 776 | 2.17 | 1.90 × 10−3 | |
| Ls-3RC-BTO | ||||||||||
| Sample | Period | Rs | Ls | HF Region (Charge Transfer) | IF/LF Region (Recombination) | Goodness of Fit | ||||
| (Ω) | (H) | Rct (Ω) | act (10−3) | Yct (10−6) | Rrec (Ω) | arec (10−3) | Yrec (10−6) | |||
| 1st cycle | 7.66 ± 0.72 | 1.28 × 10−6 | 3.35 × 103 | 890 | 0.268 | 2.38 × 106 | 821 | 3.9 | 1.08 × 10−3 | |
| 3D | 2nd cycle | 16.0 ± 0.82 | 1.12 × 10−6 | 1.06 × 104 | 874 | 0.192 | 1.37 × 105 | 872 | 4.8 | 4.85 × 10−3 |
| 3rd cycle | 16.94 ± 0.63 | 1.17 × 10−6 | 1.51 × 104 | 953 | 0.324 | 7.56 × 104 | 783 | 3.61 | 2.57 × 10−4 | |
| 1st cycle | 11.98 ± 0.51 | 7.88 × 10−7 | 2.17 × 103 | 963 | 0.086 | 4.06 × 106 | 922 | 2.49 | 1.36 × 10−4 | |
| 2D/3D | 2nd cycle | 27.75 ± 0.62 | 1.38 × 10−6 | 1.05 × 104 | 956 | 0.076 | 1.46 × 106 | 861 | 2.15 | 2.41 × 10−4 |
| 3rd cycle | 36.22 ± 0.87 | 1.31 × 10−6 | 1.30 × 104 | 962 | 0.268 | 3.80 × 105 | 766 | 2.21 | 5.25 × 10−4 | |
| Sample | fp (Hz) | ||
|---|---|---|---|
| 1st Cycle | 2nd Cycle | 3rd Cycle | |
| 3D | 198.6 | 31.3 | 26.8 |
| 2D/3D | 252.6 | 79 | 34.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdulrahim, S.M.; Ahmad, Z.; Bhadra, J.; Al-Thani, N.J. Long-Term Stability Analysis of 3D and 2D/3D Hybrid Perovskite Solar Cells Using Electrochemical Impedance Spectroscopy. Molecules 2020, 25, 5794. https://doi.org/10.3390/molecules25245794
Abdulrahim SM, Ahmad Z, Bhadra J, Al-Thani NJ. Long-Term Stability Analysis of 3D and 2D/3D Hybrid Perovskite Solar Cells Using Electrochemical Impedance Spectroscopy. Molecules. 2020; 25(24):5794. https://doi.org/10.3390/molecules25245794
Chicago/Turabian StyleAbdulrahim, Sumayya M., Zubair Ahmad, Jolly Bhadra, and Noora Jabor Al-Thani. 2020. "Long-Term Stability Analysis of 3D and 2D/3D Hybrid Perovskite Solar Cells Using Electrochemical Impedance Spectroscopy" Molecules 25, no. 24: 5794. https://doi.org/10.3390/molecules25245794
APA StyleAbdulrahim, S. M., Ahmad, Z., Bhadra, J., & Al-Thani, N. J. (2020). Long-Term Stability Analysis of 3D and 2D/3D Hybrid Perovskite Solar Cells Using Electrochemical Impedance Spectroscopy. Molecules, 25(24), 5794. https://doi.org/10.3390/molecules25245794






