Activity and Diversity of Microorganisms in Root Zone of Plant Species Spontaneously Inhabiting Smelter Waste Piles
Abstract
1. Introduction
2. Results and Discussion
2.1. Soil Chemical Characteristics
2.2. Plant Chemical Composition
2.3. Soil Enzyme Activities
2.4. Abundance of Cultivable Microorganisms
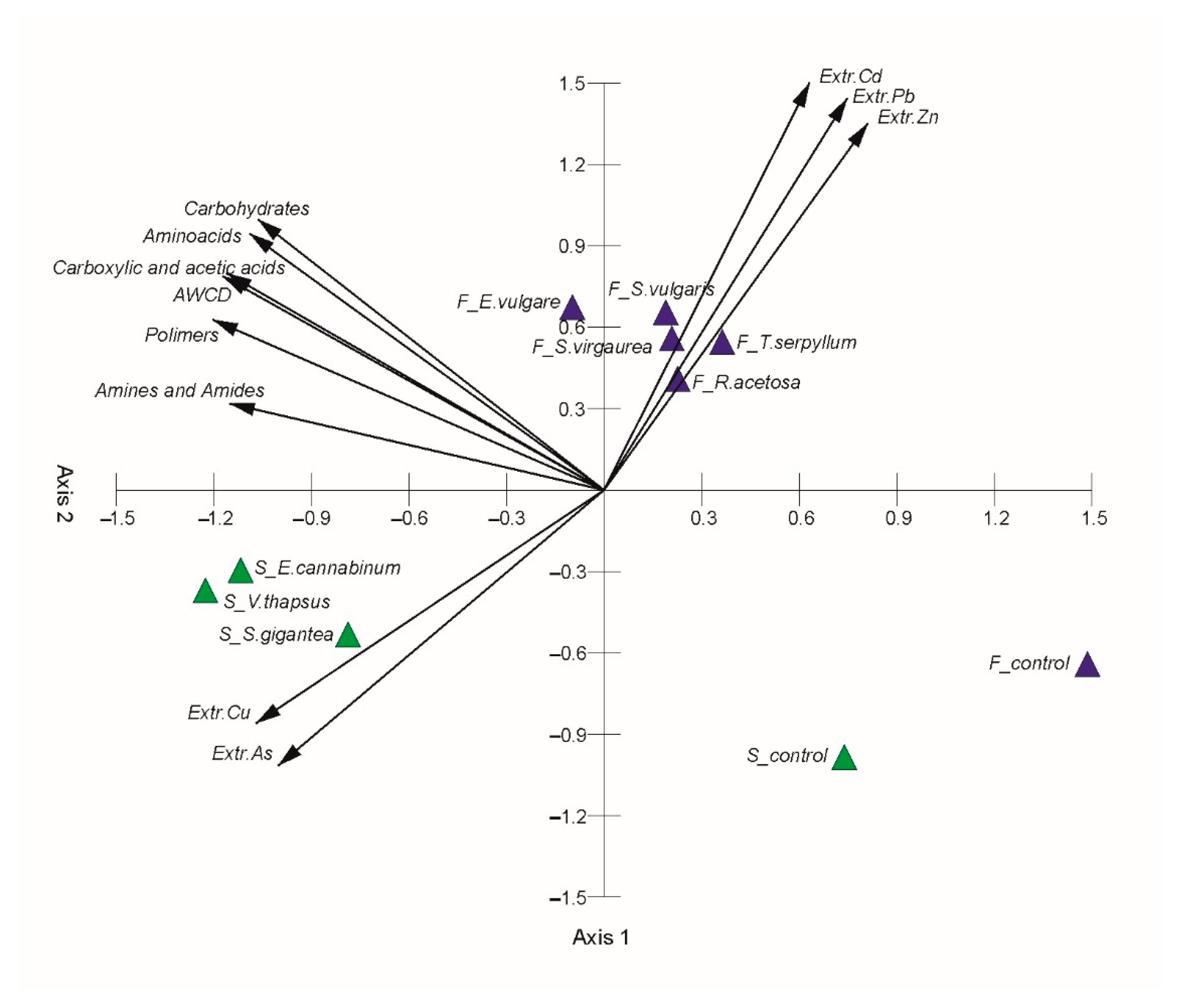
2.5. Effects of Soil Chemistry on Abundance and Activity of Microorganisms
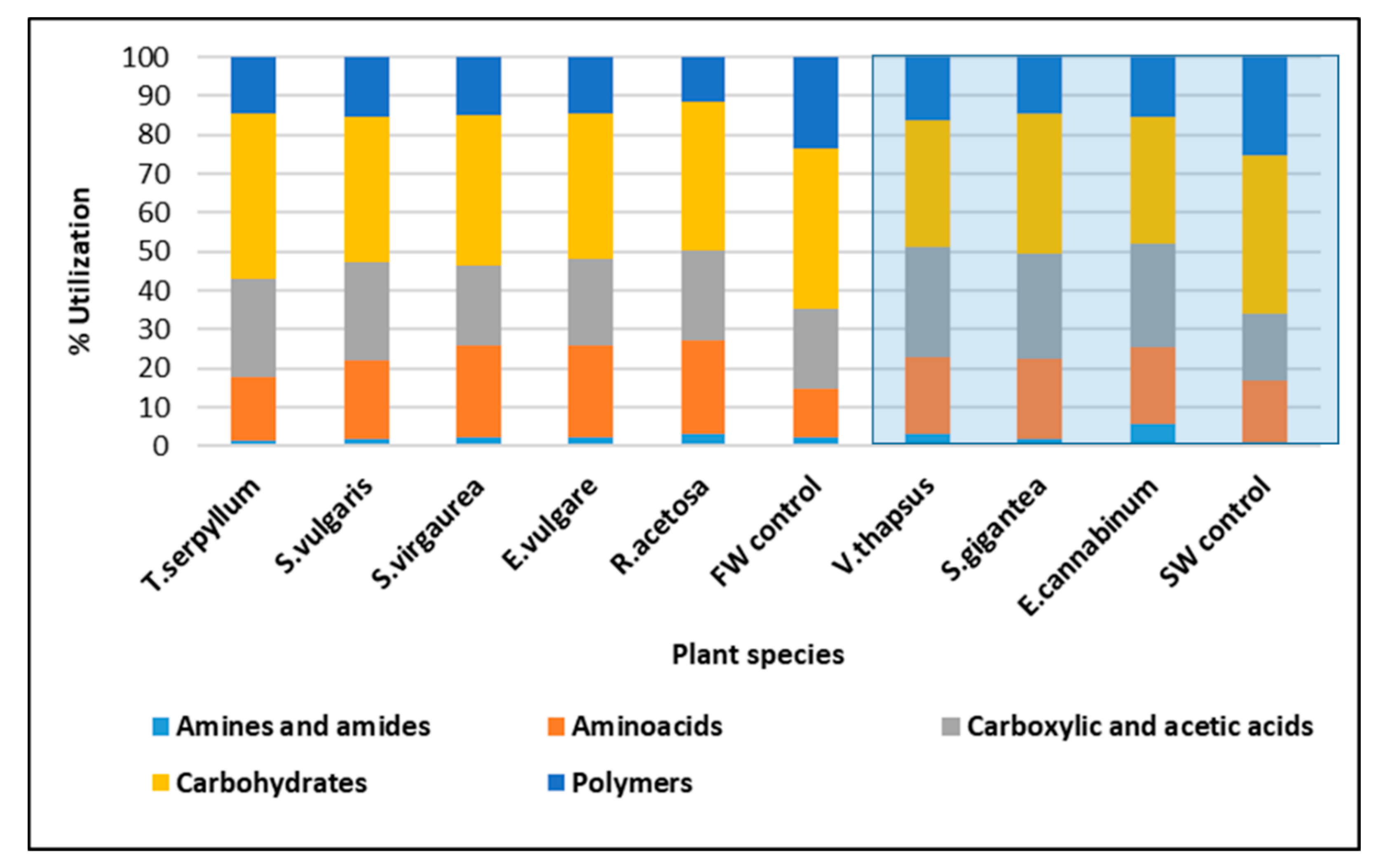
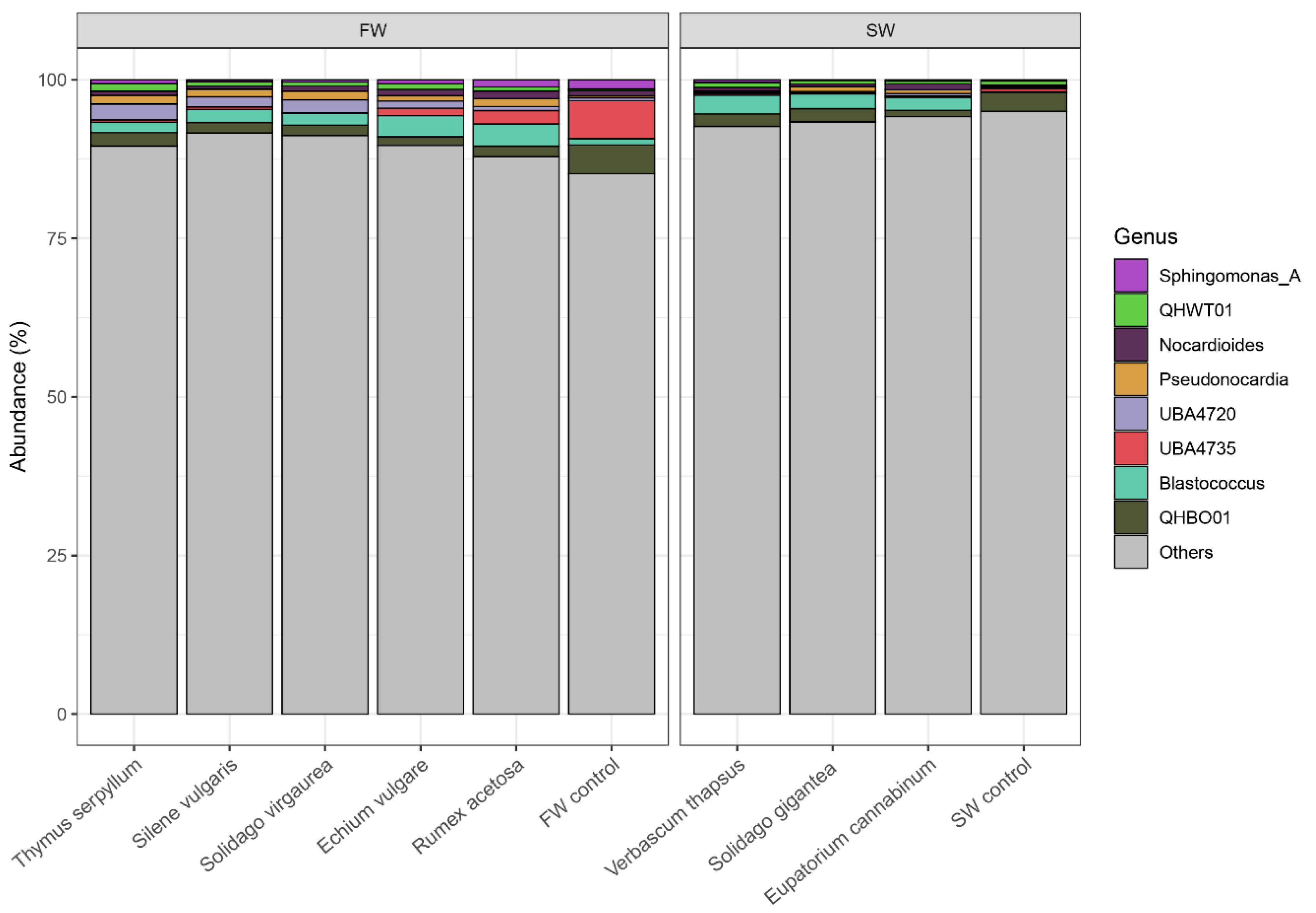
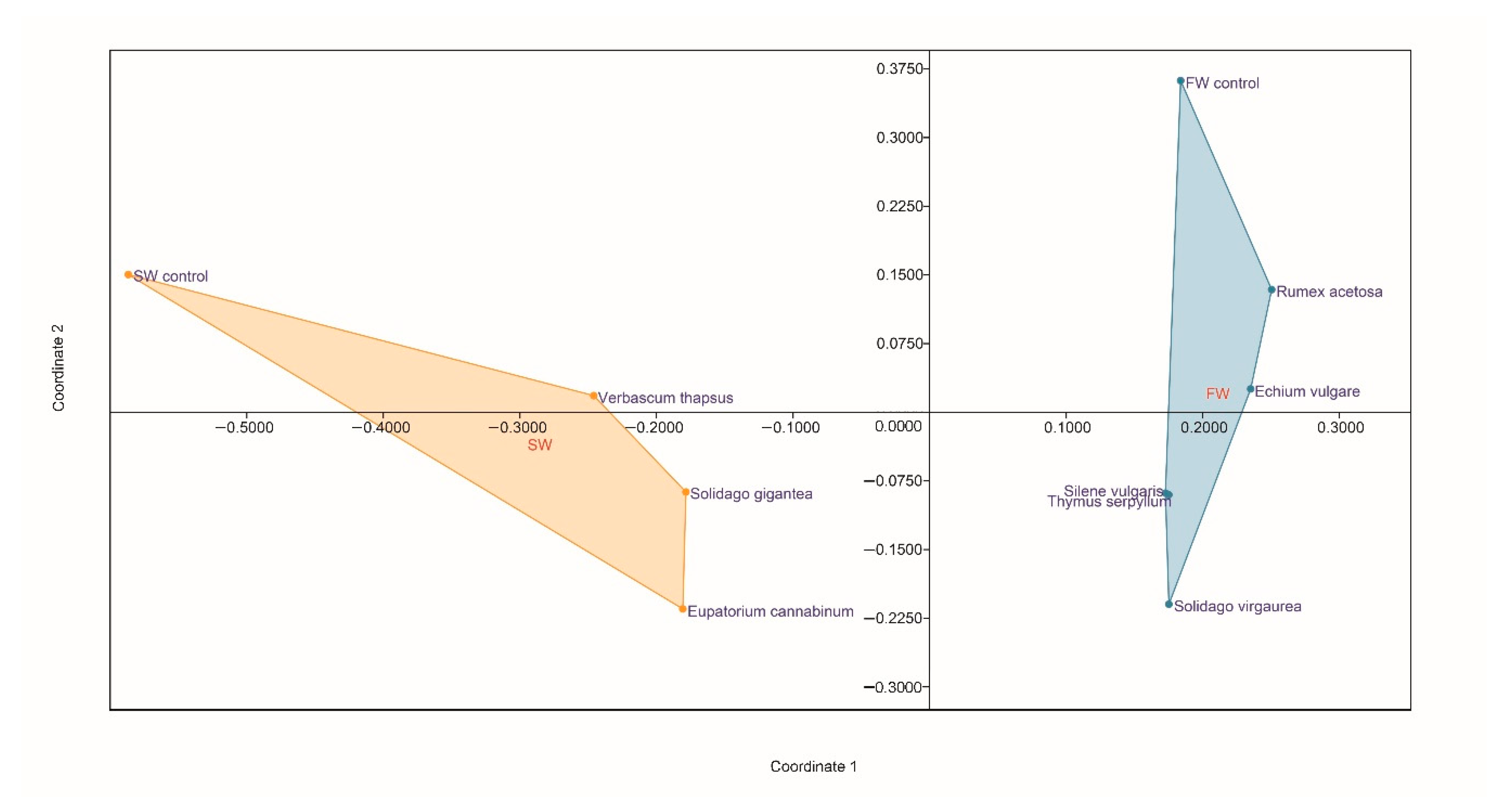
2.6. Plant Driven Changes in Functional and Genetic Diversity of Microorganisms
3. Materials and Methods
3.1. Description of the Experimental Site
3.2. Waste Pile Top Layer and Plant Sampling
3.3. Microbiological Soil Analysis
3.4. Chemical Soil Analysis and Plant Analysis
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dulias, R. The Impact of Mining on the Landscape. A Study of the Upper Silesian Coal Basin in Poland. Environmental Science and Engineering; Springer: Cham, Switzerland, 2016; p. 209. [Google Scholar]
- Panagos, P.; Van Liedekerke, M.; Yigini, Y.; Montanarella, L. Contaminated Sites in Europe: Review of the Current Situation Based on Data Collected through a European Network. J. Environ. Public Health 2013. [Google Scholar] [CrossRef] [PubMed]
- Stuczynski, T.; Siebielec, G.; Daniels, W.L.; Mccarty, G.; Chaney, R.L. Biological aspects of metal waste reclamation with biosolids. J. Environ. Qual. 2007, 36, 1154–1162. [Google Scholar] [CrossRef] [PubMed]
- Siebielec, S.; Siebielec, G.; Stuczyński, T.; Sugier, P.; Grzęda, E.; Grządziel, J. Long term insight into biodiversity of a smelter wasteland reclaimed with biosolids and by-product lime. Sci. Total Environ. 2018, 636, 1048–1057. [Google Scholar] [CrossRef] [PubMed]
- Wójcik, M.; Sugier, P.; Siebielec, G. Metal accumulation strategies in plants spontaneously inhabiting Zn-Pb waste deposits. Sci. Total Environ. 2014, 487, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Siebielec, G.; Smreczak, B.; Klimkowicz-Pawlas, A.; Maliszewska-Kordybach, B.; Terelak, H.; Koza, P.; Łysiak, M.; Gałazka, R.; Pecio, M.; Suszek, B.; et al. Monitoring of Soil Chemical Quality of Agricultural Land in Poland in 2010–2012; Biblioteka Monitoringu Środowiska: Warszawa, Poland, 2012. (In Polish) [Google Scholar]
- Sindhu, S.S.; Parmar, P.; Phour, M.; Sehrawat, A. Potassium-Solubilizing Microorganisms (KSMs) and Its Effect on Plant Growth Improvement. In Potassium Solubilizing Microorganisms for Sustainable Agriculture; Springer Science and Business Media LLC: Berlin, Germany, 2016; pp. 171–185. [Google Scholar]
- Bruemmer, G.W.; Gerth, J.; Tiller, K.G. Reaction kinetics of the adsorption and desorption of nickel, zinc and cadmium by goethite. I. Adsorption and diffusion of metals. Eur. J. Soil Sci. 1988, 39, 37–52. [Google Scholar] [CrossRef]
- Chaney, R.L. Zinc Phytotoxicity. In Zinc in Soils and Plants; Springer Science and Business Media LLC: Berlin, Germany, 1993; pp. 135–150. [Google Scholar]
- Chaney, R.L.; Li, Y.-M.; Brown, S.L.; Homer, F.A.; Malik, M.; Angle, J.S.; Baker, A.J.M.; Reeves, R.D.; Chin, M. Improving Metal Hyperaccumulator Wild Plants to Develop Commercial Phytoextraction Systems: Approaches and Progress. In Phytoremediation of Contaminated Soil and Water; Informa UK Limited: London, UK, 2020; pp. 129–158. [Google Scholar]
- Nebeská, D.; Pidlisnyuk, V.; Stefanovska, T.; Trögl, J.; Shapoval, P.; Popelka, J.; Černý, J.; Medkow, A.; Kvak, V.; Malinská, H. Impact of plant growth regulators and soil properties on Miscanthus x giganteus biomass parameters and uptake of metals in military soils. Rev. Environ. Health 2019, 34, 283–291. [Google Scholar] [CrossRef]
- Sarret, G.; Harada, E.; Choi, Y.-E.; Isaure, M.-P.; Geoffroy, N.; Fakra, S.; Marcus, M.A.; Birschwilks, M.; Clemens, S.; Manceau, A. Trichomes of tobacco excrete zinc as zinc-substituted calcium carbonate and other zinc-containing compounds. Plant Physiol. 2006, 141, 1021–1034. [Google Scholar] [CrossRef]
- Eshanmugam, V.; Tsednee, M.; Yeh, K.-C. Zinc tolerance induced by iron 1 reveals the importance of glutathione in the cross-homeostasis between zinc and iron in Arabidopsis thaliana. Plant J. 2012, 69, 1006–1017. [Google Scholar] [CrossRef]
- Stefanowicz, A.M.; Stanek, M.; Woch, M.W.; Kapusta, P. The accumulation of elements in plants growing spontaneously on small heaps left by the historical Zn-Pb ore mining. Environ. Sci. Pollut. Res. 2016, 23, 6524–6534. [Google Scholar] [CrossRef]
- Clemens, S. Lead in Plants. In Encyclopedia of Metalloproteins; Springer Science and Business Media LLC: Berlin, Germany, 2013; pp. 1179–1183. [Google Scholar]
- Massenssini, A.; Bonduki, V.H.A.; Melo, C.A.D.; Totola, M.R.; Ferreira, F.A.; Costa, M.D. Relative importance of soil physico-chemical characteristics and plant species identity to the determination of soil microbial community structure. Appl. Soil Ecol. 2015, 91, 8–15. [Google Scholar] [CrossRef]
- Xie, Y.; Fan, J.; Zhu, W.; Amombo, E.; Lou, Y.; Chen, L.; Fu, J. Effect of heavy metals pollution on soil microbial diversity and bermudagrass genetic variation. Front. Plant Sci. 2016, 7, 755. [Google Scholar] [CrossRef] [PubMed]
- Chodak, M.; Gołębiewski, M.; Morawska-Płoskonka, J.; Kuduk, K.; Niklińska, M. Diversity of microorganisms from forest soils differently polluted with heavy metals. Appl. Soil Ecol. 2013, 64, 7–14. [Google Scholar] [CrossRef]
- Fazekaš, J.; Fazekašová, D.; Adamišin, P.; Huličová, P.; Benková, E. Functional diversity of microorganisms in metal- and alkali-contaminated soils of Central and North-eastern Slovakia. Soil Water Res. 2019, 14, 32–39. [Google Scholar] [CrossRef]
- Muhlbachova, G.; Sagova-Mareckova, M.; Omelka, M.; Szakova, J.; Tlustos, P. The influence of soil organic carbon on interactions between microbial parameters and metal concentrations at a long-term contaminated site. Sci. Total Environ. 2015, 502, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Klimek, B.; Sitarz, A.; Choczyński, M.; Niklińska, M. The effects of heavy metals and total petroleum hydrocarbons on soil bacterial activity and functional diversity in the Upper Silesia Industrial Region (Poland). Water Air Soil Pollut. 2016, 227, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-S.; Min, H.-G.; Kim, J.-G. Integrating amendment and liquid fertilizer for aided-phytostabilization and its impacts on soil microbiological properties in arsenic-contaminated soil. Appl. Sci. 2020, 10, 3985. [Google Scholar] [CrossRef]
- Vogeler, I.; Vachey, A.; Deurer, M.; Bolan, N. Impact of plants on the microbial activity in soils with high and low levels of copper. Eur. J. Soil Biol. 2008, 44, 92–100. [Google Scholar] [CrossRef]
- Ho, A.; Di Lonardo, D.P.; Bodelier, P.L.E. Revisiting life strategy concepts in environmental microbial ecology. FEMS Microbiol. Ecol. 2017, 93, 1–14. [Google Scholar] [CrossRef]
- Lorenz, N.; Hintemann, T.; Kramarewa, T.; Katayama, A.; Yasuta, T.; Marschner, P.; Kandeler, E. Response of microbial activity and microbial community composition in soils to long-term arsenic and cadmium exposure. Soil Biol. Biochem. 2006, 38, 1430–1437. [Google Scholar] [CrossRef]
- Pereira, S.; Castro, P.M. Diversity and characterization of culturable bacterial endophytes from Zea mays and their potential as plant growth-promoting agents in metal-degraded soils. Environ. Sci. Pollut. Res. 2014, 21, 14110–14123. [Google Scholar] [CrossRef]
- Siebielec, G.; Stuczynski, T.; Korzeniowska-Pucułek, R. Metal bioavailability in long term contaminated soils of Tarnowskie Gory area. Pol. J. Environ. Stud. 2006, 15, 121–129. [Google Scholar]
- Garland, J.L.; Mills, A.L. Classification and characterization of heterotrophic microbial mommunities on the basis of patterns of community-level sole-carbon-source utilization. Appl. Environ. Microbiol. 1991, 57, 2351–2359. [Google Scholar] [CrossRef] [PubMed]
- Garland, J.L. Analytical approaches to the characterization of samples of microbial communities using patterns of potential C source utilization. Soil Biol. Biochem. 1996, 28, 213–221. [Google Scholar] [CrossRef]
- Hayat, S.; Ali, B.; Ahmad, A. Salicylic Acid: Biosynthesis, Metabolism and Physiological Role in Plants. In Salicylic Acid: A Plant Hormone; Springer Science and Business Media LLC: Berlin, Germany, 2007; pp. 1–14. [Google Scholar]
- Dempsey, D.A.; Vlot, A.C.; Wildermuth, M.C.; Klessig, D.F. Salicylic Acid Biosynthesis and Metabolism. Arab. Book 2011, 9, e0156. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, J.-J.; Banerjee, S.; Zhou, N.; Zhao, Z.; Zhang, K.; Hu, M.; Tian, C. Biogeographical distribution of bacterial communities in saline agricultural soil. Geoderma 2020, 361, 114095. [Google Scholar] [CrossRef]
- Castro, J.F.; Nouioui, I.; Sangal, V.; Choi, S.; Yang, S.-J.; Kim, B.-Y.; Trujillo, M.E.; Riesco, R.; Montero-Calasanz, M.D.C.; Rahmani, T.P.D.; et al. Blastococcus atacamensis sp. nov., a novel strain adapted to life in the Yungay core region of the Atacama Desert. Int. J. Syst. Evol. Microbiol. 2018, 68, 2712–2721. [Google Scholar] [CrossRef]
- Touceda-González, M.; Kidd, P.S.; Smalla, K.; Prieto-Fernández, A. Bacterial communities in the rhizosphere of different populations of the Ni-hyperaccumulator Alyssum serpyllifolium and the metal-excluder Dactylis glomerata growing in ultramafic soils. Plant Soil 2018, 431, 317–332. [Google Scholar] [CrossRef]
- Gtari, M.; Essoussi, I.; Maaoui, R.; Sghaier, H.; Boujmil, R.; Gury, J.; Pujic, P.; Brusetti, L.; Chouaia, B.; Crotti, E.; et al. Contrasted resistance of stone-dwelling Geodermatophilaceae species to stresses known to give rise to reactive oxygen species. FEMS Microbiol. Ecol. 2012, 80, 566–577. [Google Scholar] [CrossRef]
- Dastager, S.G.; Lee, J.-C.; Ju, Y.-J.; Park, D.-J.; Kim, C.-J. Nocardioides halotolerans sp. nov., isolated from soil on Bigeum Island, Korea. Syst. Appl. Microbiol. 2008, 31, 24–29. [Google Scholar] [CrossRef]
- Yoon, J.; Cao, X.; Zhou, Q.; Ma, L.Q. Accumulation of Pb, Cu, and Zn in native plants growing on a contaminated Florida site. Sci. Total Environ. 2006, 368, 456–464. [Google Scholar] [CrossRef]
- Essel, E.; Xie, J.; Deng, C.; Peng, Z.; Wang, J.; Shen, J.; Xie, J.; Coulter, J.A.; Li, L. Bacterial and fungal diversity in rhizosphere and bulk soil under different long-term tillage and cereal/legume rotation. Soil Tillage Res. 2019, 194, 104302. [Google Scholar] [CrossRef]
- Apinya, T.; Sombatsompop, N.; Prapagdee, B. Selection of a Pseudonocardia sp. RM423 that accelerates the biodegradation of poly(lactic) acid in submerged cultures and in soil microcosms. Int. Biodeterior. Biodegrad. 2015, 99, 23–30. [Google Scholar] [CrossRef]
- Pascual, J.; Blanco, S.; Ramos, J.-L.; Van Dillewijn, P. Responses of bulk and rhizosphere soil microbial communities to thermoclimatic changes in a Mediterranean ecosystem. Soil Biol. Biochem. 2018, 118, 130–144. [Google Scholar] [CrossRef]
- Gorski, T.; Zaliwski, A. Agro-climate model of Poland. Pam. Pul. 2002, 130, 251–260. (In Polish) [Google Scholar]
- Tabatabai, M.A. Soil Enzymes. In Methods of Soil Analysis. Part 2. Microbiological and Biochemical Properties; Weaver, R.W., Angle, S., Bottomley, P., Bezdicek, D., Smith, S., Tabatabai, A., Wollum, A., Eds.; SSSA Book Series 5; SSSA: Madison, WI, USA, 1994; pp. 775–826. [Google Scholar]
- Casida, L.E.; Klein, D.A.; Santoro, T. Soil dehydrogenase activity. Soil Sci. 1964, 98, 371–376. [Google Scholar] [CrossRef]
- Tabatabai, M.A.; Bremner, J.M. Use of p-nitrophenylphosphate for assay of soil phosphatase activity. Soil Biol. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Wallace, R.H.; Lochhead, A.G. qualitative studies of soil microorganisms: IX. Amino acid requirements of rhizosphere bacteria. Can. J. Res. 1950, 28, 1–6. [Google Scholar] [CrossRef]
- Rodina, A. Mikrobiologiczne Metody Badania Wód; Wydawnictwo PWRiL: Warszawa, Poland, 1968. (In Polish) [Google Scholar]
- Fenglerowa, W. Simple method for counting Azotobacter in soil samples. Acta Microbiol. Pol. 1965, 14, 203–206. [Google Scholar]
- Martin, J.P. Use of acid, rose bengal, and streptomycin in the plate method for estimating soil fungi. Soil Sci. 1950, 69, 215–232. [Google Scholar] [CrossRef]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glöckner, F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Parks, D.H.; Chuvochina, M.; Chaumeil, P.A.; Rinke, C.; Mussig, A.J.; Hugenholtz, P. Selection of representative genomes for 24,706 bacterial and archaeal species clusters provide a complete genome-based taxonomy. BioRxiv 2019, 771964. [Google Scholar] [CrossRef]
- Chaumeil, P.-A.; Mussig, A.J.; Hugenholtz, P.; Parks, D.H. GTDB-Tk: A toolkit to classify genomes with the Genome Taxonomy Database. Bioinformatics 2019, 36, 1925–1927. [Google Scholar] [CrossRef] [PubMed]
- Murali, A.; Bhargava, A.; Wright, E.S. IDTAXA: A novel approach for accurate taxonomic classification of microbiome sequences. Microbiome 2018, 6, 1–14. [Google Scholar] [CrossRef] [PubMed]
- McMurdie, P.J.; Holmes, S.P. phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. VSEARCH: A versatile open source tool for metagenomics. PeerJ 2016, 4, e2584. [Google Scholar] [CrossRef]
- Větrovský, T.; Baldrian, P.; Morais, D.C. SEED 2: A user-friendly platform for amplicon high-throughput sequencing data analyses. Bioinformatics 2018, 34, 2292–2294. [Google Scholar] [CrossRef]
- Abdi, H.; Williams, L. Principal components analysis. Wiley Interdiscip. Rev. Comput. Stat. 2010, 2, 439–459. [Google Scholar] [CrossRef]
- Kovach, W.L. MVSP—A Multivariate Statistical Package for Windows Ver. 3.1; Kovach Computing Service: Pentraeth, UK, 1999. [Google Scholar]
- Kot, M.; Daniel, W.A. The relative contribution of human cytochrome P450 isoforms to the four caffeine oxidation pathways: An in vitro comparative study with cDNA-expressed P450s including CYP2C isoforms. Biochem. Pharmacol. 2008, 76, 543–551. [Google Scholar] [CrossRef]
Sample Availability: Samples are not available from the authors. |



| Plant Species | pH | Organic Matter (OM) | Available P (P2O5) | Available K (K2O) | N-NH4 |
|---|---|---|---|---|---|
| % | mg kg−1 | ||||
| T. serpyllum | 8.00 ± 0.08 1,a2 | 14.0 ± 0.3 ab | 17.8 ± 0.3 a | 176 ± 2.9 bc | 8.20 ±0.7 c |
| S. vulgaris | 8.13 ± 0.05 ab | 13.0 ±1.0 a | 19.6 ± 0.1 ab | 194 ± 5.7 c | 7.07 ± 2.1 bc |
| S. virgaurea | 8.13 ± 0.05 ab | 12.7 ±2.3 a | 18.5 ± 0.1 a | 172 ± 1.6 abc | 9.88 ± 0.6 c |
| E. vulgare | 8.23 ± 0.05 abc | 12.1 ± 0.3 a | 18.0 ± 0.1 a | 67 ± 2.1 ab | 3.44 ± 1.4 a |
| R. acetosa | 8.17 ± 0.05 ab | 12.4 ± 0.3 a | 15.1 ± 0.3 a | 50 ± 1.0 a | 4.02 ± 1.5 ab |
| FW control | 8.23 ± 0.05 abc | 11.8 ± 0.7 a | 10.1 ± 0.1 a | 67 ± 1.0 ab | 2.01 ± 0.3 a |
| V. thapsus | 8.23 ± 0.05 abc | 18.1 ± 0.7 b | 31.8 ± 0.6 b | 160 ± 6.6 abc | 2.61 ± 1.6 a |
| S. gigantea | 8.27 ± 0.09 abc | 11.9 ± 3.7 a | 18.0 ± 0.9 a | 206 ± 4.4 c | 2.27 ± 0.3 a |
| E. cannabin. | 8.43 ± 0.09 bc | 16.0 ± 1.1 ab | 22.3 ± 0.3 ab | 111 ± 6.6 abc | 3.86 ± 0.6 ab |
| SW control | 8.53 ± 0.05 c | 16.7 ± 1.5 ab | 9.8 ± 0.5 a | 91 ± 1.0 abc | 1.91 ± 0.1 a |
| Plant Species | Cu | Zn | As | Cd | Pb | Mg | K | Ca | Fe |
|---|---|---|---|---|---|---|---|---|---|
| T. serpyllum | 51 ± 22 1, a2 | 85,501 ± 2950 c | 2091 ± 300 bcd | 418 ± 8 ab | 18,552 ± 1430 ab | 19,722 ± 2160 a | 3077 ± 198 bc | 39,805 ± 4990 a | 255,884 ± 6420 ab |
| S. vulgaris | 35 ± 6 a | 108,838 ± 5160 e | 2517 ± 147 d | 538 ± 25 b | 23,327 ± 1189 ab | 23,959 ± 3600 ab | 3726 ± 317 cd | 47,102 ± 7670 a | 288,467 ± 9700 b |
| S. virgaurea | 49 ± 13 a | 93,037 ± 4700 cd | 2293 ± 146 cd | 468 ± 17 ab | 20,824 ± 846 ab | 20,201 ± 1108 a | 4291 ± 254 d | 41,031 ± 410 a | 273,304 ± 14,560 ab |
| E. vulgare | 34 ± 3 a | 105,111 ± 2440 de | 2562 ± 186 d | 495 ± 21 ab | 22,528 ± 1164 ab | 23,603 ± 2510 ab | 4081 ± 516 cd | 45,033 ± 5130 a | 292,675 ± 5530 b |
| R. acetosa | 30 ± 5 a | 112,211 ± 4330 e | 2505 ± 75 d | 556 ± 20 b | 24,130 b ± 290 | 26,992 ± 2630 abc | 3811 ± 406 cd | 52,559 ± 5470 a | 325,362 ± 16,800 b |
| FW control | 24 ± 2 a | 101,716 ± 2260 de | 2351 ± 364 cd | 514 ± 20 b | 21,286 ± 583 ab | 25,333 ± 1850 ab | 2378 ± 258 ab | 51,918 ± 5530 a | 297,958 ± 5560 b |
| V. thapsus | 2170 ± 360 b | 18,292 ± 2770 a | 1835 d ± 230 abc | 233 ± 127 a | 11,515 ± 2690 a | 31,732 ± 2790 abc | 2389 ± 373 ab | 124,118 ± 14,600 b | 254,603 ± 21,010 ab |
| S. gigantea | 2162 ± 260 b | 18,542 ± 3700 a | 1454 ± 293 ab | 311 ± 121 ab | 12,392 ± 3440 ab | 35,691 ± 4510 bc | 2108 ± 338 ab | 152,248 ± 10,900 b | 245,532 ± 29,680 ab |
| E. cannabinum | 1829 ± 137 b | 25,990 ± 1130 ab | 1284 ± 101 a | 331 ± 107 ab | 11,956 a ± 2110 b | 38,577 ± 4770 c | 1794 ± 153 a | 128,312 ± 15,400 b | 194,861 ± 13,920 a |
| SW control | 1931 ± 159 b | 32,564 ± 6600 b | 1564 ± 238 abc | 593 ± 236 b | 20,894 a ± 3726 b | 29,627 ± 2760 abc | 2237 ± 130 ab | 126,584 ± 14,560 b | 195,606 ± 12,790 a |
| Plant Species | Zn | Cd | Pb | As | Fe | Ca | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Content | BF | Content | BF | Content | BF | Content | BF | Content | Content | |
| T. serpyllum | 6337 b1 | 0.07 b | 52.7 b | 0.13 b | 1626 b | 0.09 b | 134 b | 0.06 b | 12,231 b | 13,833 a |
| S. vulgaris | 2847 ab | 0.03 bc | 15.2 a | 0.03 d | 506 a | 0.02 d | 56 ab | 0.02 c | 5528 ab | 9009 a |
| S. virgaurea | 800 a | 0.01 c | 5.0 a | 0.01 e | 161 a | 0.01 d | 15 a | 0.01 c | 1488 a | 9471 a |
| E. vulgare | 20,870 c | 0.20 a | 113 c | 0.23 a | 4351 c | 0.19 a | 473 c | 0.18 a | 43,859 c | 40,276 b |
| R. acetosa | 5103 ab | 0.05 b | 22.4 a | 0.04 d | 860 ab | 0.04 c | 96 ab | 0.04 bc | 9160 ab | 18,484 a |
| V. thapsus | 954 a | 0.05 b | 10.4 a | 0.04 d | 470 a | 0.04 c | 41 ab | 0.02 c | 3889 ab | 10,252 a |
| S. gigantea | 236 a | 0.01 c | 4.6 a | 0.01 e | 157 a | 0.01 d | 9 a | 0.01 c | 632 a | 8381 a |
| E. cannabinum | 417 a | 0.02 c | 26.6 ab | 0.08 c | 171 a | 0.01 d | 15 a | 0.01 c | 1419 a | 13,003 a |
| Plant Species | Dehydrogenases | Acidic Phosphatase | Alkaline Phosphatase |
|---|---|---|---|
| µg TPF g−1 DM 24 h−1 | µg PNP g−1 DM h−1 | µg PNP g−1 DM h−1 | |
| T. serpyllum | 24.4 bc | 32.6 c | 73.2 b |
| S. vulgaris | 13.6 abc | 23.1 abc | 40.9 ab |
| S. virgaurea | 18.7 bc | 30.9 bc | 71.9 b |
| E. vulgare | 2.9 a | 19.8 abc | 25.2 a |
| R. acetosa | 3.7 ab | 20.0 abc | 28.2 a |
| FW control | 0.1 a | 15.6 abc | 11.5 a |
| V. thapsus | 0.5 a | 9.0 a | 17.1 a |
| S. gigantea | 5.2 ab | 14.8 ab | 24.2 a |
| E. cannabinum | 1.0 a | 10.6 a | 23.2 a |
| SW control | 0.1 a | 12.8 a | 11.4 a |
| Plant Species | Total Bacteria | Total Fungi | Oligotrophic Bacteria | Copiotrophic Bacteria | Ammonification Bacteria |
|---|---|---|---|---|---|
| 108 | 104 | 107 | 107 | 107 | |
| T. serpyllum | 8.1 ab | 22.4 cd | 45.8 b | 24.9 ab | 0.3 a |
| S. vulgaris | 11.7 b | 45.2 d | 124 c | 54.0 b | 0.4 a |
| S. virgaurea | 4.1 a | 22.1 cd | 31.1 b | 21.2 ab | 0.3 a |
| E. vulgare | 7.1 a | 14.6 bc | 37.1 b | 20.8 ab | 0.4 a |
| R. acetosa | 11.6 b | 15.1 bc | 50.6 b | 46.9 b | 57.9 b |
| FW control | 2.1 a | 0.4 a | 6.4 a | 4.0 a | 8.5 a |
| V. thapsus | 15.5 b | 9.9 b | 44.6 b | 34.5 ab | 41.8 b |
| S. gigantean | 37.2 c | 7.7 b | 221 d | 111 c | 184 c |
| E. cannabinum | 13.4 ab | 13.7 bc | 34.6 b | 22.6 ab | 35.2 b |
| SW control | 3.6 a | 3.0 ab | 13.8 a | 10.5 a | 18.4 ab |
| Microbial Parameter | Independent Variable | Coefficient | Significance Level | Model R2 |
|---|---|---|---|---|
| Bacteria | Total Cu Total Fe Extr. As Constant | 0.022 0.000 −153.5 −39.8 | 0.011 | 0.33 |
| Fungi | Avail. K Total Mg Total Ca Constant | 2.14 0.003 −0.0006 −41.2 | <0.001 | 0.52 |
| Oligotrophs | Avail. K Total Cu Total K Total Ca Total Fe Extr. As Constant | 7.93 0.29 −0.07 −0.00 0.00 −2454 −266 | <0.001 | 0.84 |
| Copiotrophs | Avail. K Total Cu Total Fe Extr. As Constant | 3.3 0.089 0.001 −896 −191.7 | <0.001 | 0.61 |
| Ammonification bacteria | Avail. P Total Cu Total Ca Total Fe Extr. As Constant | −51.23 0.34 −0.00 0.00 −1947 −116.9 | <0.001 | 0.85 |
| Acidic phosphatase | OM N-NH4 Total Mg Constant | −0.55 1.63 −0.0005 34.05 | <0.001 | 0.89 |
| Alkaline phosphatase | N-NH4 Total Mg Total Ca Constant | 6.74 −0.002 0.0003 37.07 | <0.001 | 0.88 |
| Dehydrogenase | EC Total Cu Total As Extr. Cd Constant | 0.029 −0.004 −0.014 11.08 22.37 | <0.001 | 0.86 |
| Plant Species | Biodiversity Indices | |||
|---|---|---|---|---|
| AWCD | Shannon Diversity Index (H’) | Evenness Index (J’) | Richness (R) | |
| T. serpyllum | 1.12 d | 3.24 a | 0.95 ab | 30.7 a |
| S. vulgaris | 1.29 cd | 3.27 a | 0.95 ab | 30.7 a |
| S. virgaurea | 1.20 cd | 3.26 a | 0.97 b | 29.0 a |
| E. vulgare | 1.57 abc | 3.23 a | 0.96 ab | 29.3 a |
| R. acetosa | 1.12 d | 3.26 a | 0.96 ab | 30.0 a |
| FW control | 0.16 e | 2.46 b | 0.82 c | 20.3 b |
| V. thapsus | 1.98 a | 3.33 a | 0.97 b | 30.7 a |
| S. gigantea | 1.47 bcd | 3.25 a | 0.95 ab | 31.0 a |
| E. cannabinum | 1.76 ab | 3.39 a | 0.99 a | 30.7 a |
| SW control | 0.32 e | 2.90 a | 0.87 bc | 28.0 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siebielec, S.; Siebielec, G.; Sugier, P.; Woźniak, M.; Grządziel, J.; Gałązka, A.; Stuczyński, T. Activity and Diversity of Microorganisms in Root Zone of Plant Species Spontaneously Inhabiting Smelter Waste Piles. Molecules 2020, 25, 5638. https://doi.org/10.3390/molecules25235638
Siebielec S, Siebielec G, Sugier P, Woźniak M, Grządziel J, Gałązka A, Stuczyński T. Activity and Diversity of Microorganisms in Root Zone of Plant Species Spontaneously Inhabiting Smelter Waste Piles. Molecules. 2020; 25(23):5638. https://doi.org/10.3390/molecules25235638
Chicago/Turabian StyleSiebielec, Sylwia, Grzegorz Siebielec, Piotr Sugier, Małgorzata Woźniak, Jarosław Grządziel, Anna Gałązka, and Tomasz Stuczyński. 2020. "Activity and Diversity of Microorganisms in Root Zone of Plant Species Spontaneously Inhabiting Smelter Waste Piles" Molecules 25, no. 23: 5638. https://doi.org/10.3390/molecules25235638
APA StyleSiebielec, S., Siebielec, G., Sugier, P., Woźniak, M., Grządziel, J., Gałązka, A., & Stuczyński, T. (2020). Activity and Diversity of Microorganisms in Root Zone of Plant Species Spontaneously Inhabiting Smelter Waste Piles. Molecules, 25(23), 5638. https://doi.org/10.3390/molecules25235638












