Effect of Potato Pulp Pectic Polysaccharide on the Stability of Acidified Milk Drinks
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Extraction of Potato Pulp Pectic Polysaccharide
2.3. Protein, Uronic Acid Content and Monosaccharide Composition
2.4. Molecular Weight Determination
2.5. Degree of Esterification Measurement
2.6. Preparation of AMDs
2.7. Zeta Potential
2.8. Particle Size
2.9. Rheology
2.10. Serum Separation
2.11. Confocal Laser Scanning Microscopy
2.12. Statistical Analysis
3. Results and Discussion
3.1. Compositional Characterization of PPP, HMP and LMP
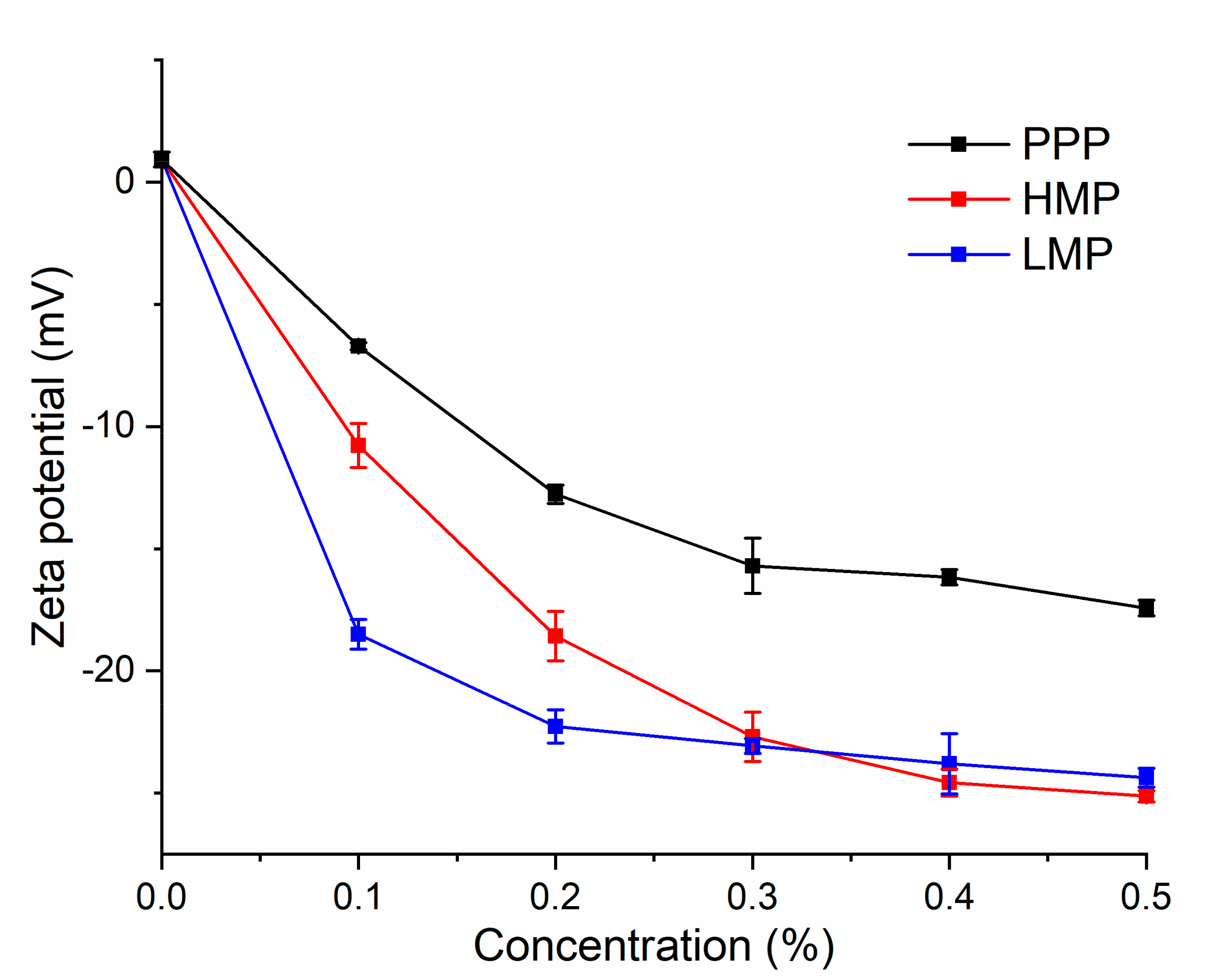
3.2. Zeta Potential
3.3. Particle Size
3.4. Rheological Analysis
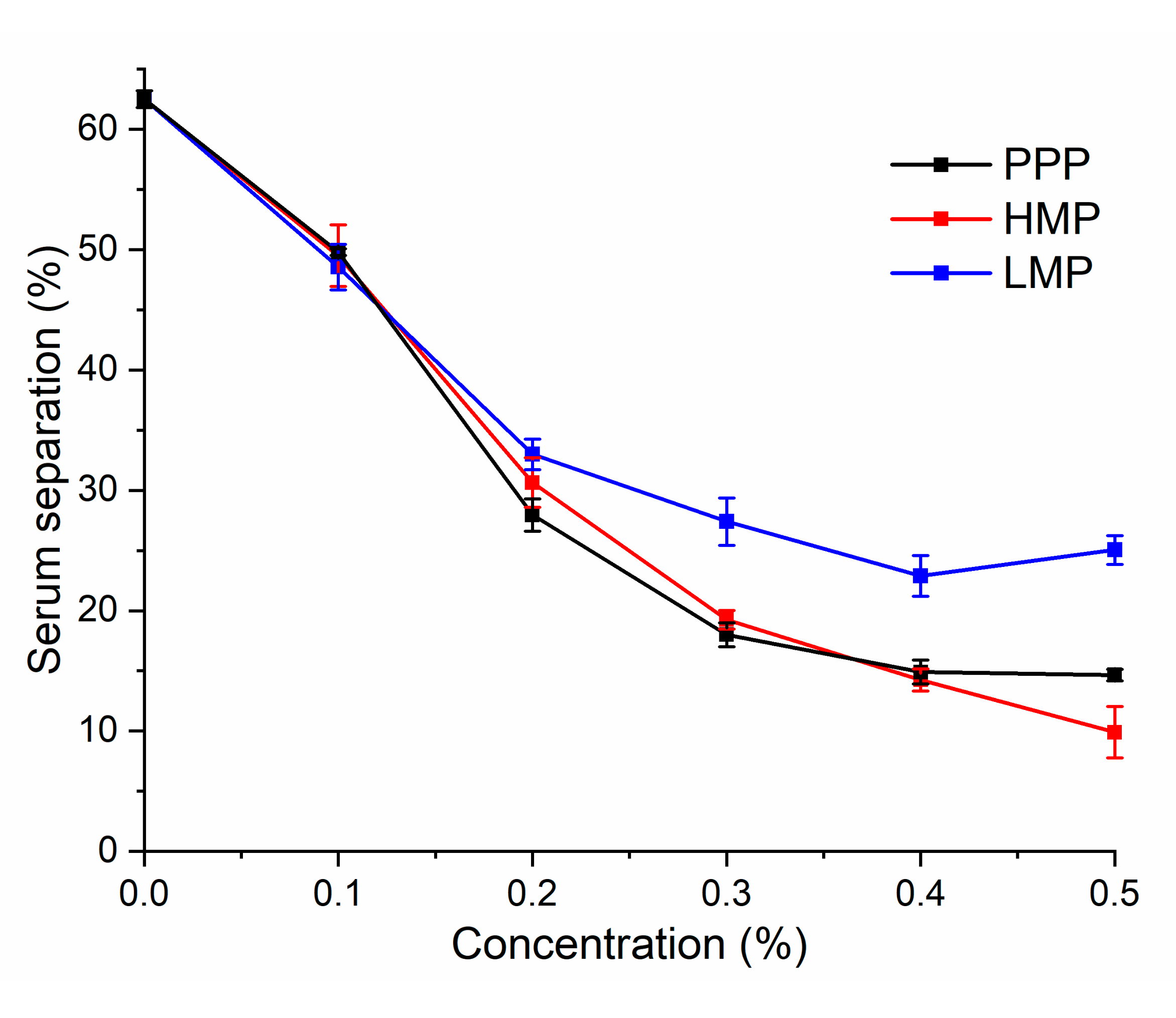
3.5. Serum Separation
3.6. Confocal Laser Scanning Microscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- O’Neill, M.A.; Ishii, T.; Albersheim, P.; Darvill, A.G. Rhamnogalacturonan II: Structure and function of a borate cross-linked cell wall pectic polysaccharide. Annu. Rev. Plant Biol. 2004, 55, 109–139. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, J.; Li, J.; Yan, L.; Li, S.; Ye, X.; Liu, D.; Ding, T.; Linhardt, R.J.; Orfila, C.; et al. Extraction and characterization of RG-I enriched pectic polysaccharides from mandarin citrus peel. Food Hydrocoll. 2018, 79, 579–586. [Google Scholar] [CrossRef]
- Wu, D.; Zheng, J.; Mao, G.; Hu, W.; Ye, X.; Linhardt, R.J.; Chen, S. Rethinking the impact of RG-I mainly from fruits and vegetables on dietary health. Crit. Rev. Food Sci. 2019, 60, 2938–2960. [Google Scholar] [CrossRef] [PubMed]
- May, C.D. Industrial pectins: Sources, production and applications. Carbohyd. Polym. 1990, 12, 79–99. [Google Scholar] [CrossRef]
- Jensen, S.; Rolin, C.; Ipsen, R. Stabilisation of acidified skimmed milk with HM pectin. Food Hydrocoll. 2010, 24, 291–299. [Google Scholar] [CrossRef]
- Yang, J.; Mu, T.; Ma, M. Extraction, structure, and emulsifying properties of pectin from potato pulp. Food Chem. 2018, 244, 197–205. [Google Scholar] [CrossRef]
- Fishman, M.L.; Chau, H.K.; Cooke, P.H.; Yadav, M.P.; Hotchkiss, A.T. Physico-chemical characterization of alkaline soluble polysaccharides from sugar beet pulp. Food Hydrocoll. 2009, 23, 1554–1562. [Google Scholar] [CrossRef]
- Marić, M.; Grassino, A.N.; Zhu, Z.; Barba, F.J.; Brnčić, M.; Rimac Brnčić, S. An overview of the traditional and innovative approaches for pectin extraction from plant food wastes and by-products: Ultrasound-, microwaves-, and enzyme-assisted extraction. Trends Food Sci. Technol. 2018, 76, 28–37. [Google Scholar] [CrossRef]
- Khodaei, N.; Karboune, S. Extraction and structural characterisation of rhamnogalacturonan I-type pectic polysaccharides from potato cell wall. Food Chem. 2013, 139, 617–623. [Google Scholar] [CrossRef]
- Karboune, S.; Khodaei, N. Structures, isolation and health-promoting properties of pectic polysaccharides from cell wall-rich food by-products: A source of functional ingredients. Curr. Opin. Food Sci. 2016, 8, 50–55. [Google Scholar] [CrossRef]
- Michalak, M.; Thomassen, L.V.; Roytio, H.; Ouwehand, A.C.; Meyer, A.S.; Mikkelsen, J.D. Expression and characterization of an endo-1,4-β-galactanase from Emericella nidulans in Pichia pastoris for enzymatic design of potentially prebiotic oligosaccharides from potato galactans. Enzym. Microb. Tech. 2012, 50, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, F.; Liu, X.; St Ange, K.; Zhang, A.; Li, Q.; Linhardt, R.J. Isolation of a lectin binding rhamnogalacturonan-I containing pectic polysaccharide from pumpkin. Carbohyd. Polym. 2017, 163, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Thakur, B.R.; Singh, R.K.; Handa, A.K. Chemistry and Uses of Pectin—A Review. Crit. Rev. Food Sci. 1997, 37, 47–73. [Google Scholar] [CrossRef] [PubMed]
- Khodaei, N.; Karboune, S.; Orsat, V. Microwave-assisted alkaline extraction of galactan-rich rhamnogalacturonan I from potato cell wall by-product. Food Chem. 2016, 190, 495–505. [Google Scholar] [CrossRef]
- McMahon, D.J.; Oommen, B.S. Supramolecular structure of the casein micelle. J. Dairy Sci. 2008, 91, 1709–1721. [Google Scholar] [CrossRef]
- Elzoghby, A.O.; Abo El-Fotoh, W.S.; Elgindy, N.A. Casein-based formulations as promising controlled release drug delivery systems. J. Control. Release 2011, 153, 206–216. [Google Scholar] [CrossRef]
- Horne, D.S. Casein micelle structure: Models and muddles. Curr. Opin. Colloid Interface Sci. 2006, 11, 148–153. [Google Scholar] [CrossRef]
- Tromp, R.H.; de Kruif, C.G.; van Eijk, M.; Rolin, C. On the mechanism of stabilisation of acidified milk drinks by pectin. Food Hydrocoll. 2004, 18, 565–572. [Google Scholar] [CrossRef]
- Tuinier, R.; Rolin, C.; de Kruif, C.G. Electrosorption of pectin onto casein micelles. Biomacromolecules 2002, 3, 632–638. [Google Scholar] [CrossRef]
- Pereyra, R.; Schmidt, K.A.; Wicker, L. Interaction and Stabilization of Acidified Casein Dispersions with Low and High Methoxyl Pectins. J. Agric. Food Chem. 1997, 45, 3448–3451. [Google Scholar] [CrossRef]
- Xia, K.; Zong, P.; Liu, X.; Zhao, J.; Zhang, X. Preparation of methyl alginate and its application in acidified milk drinks. Int. J. Biol. Macromol. 2019, 132, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Leroux, J.; Langendorff, V.; Schick, G.; Vaishnav, V.; Mazoyer, J. Emulsion stabilizing properties of pectin. Food Hydrocoll. 2003, 17, 455–462. [Google Scholar] [CrossRef]
- Peterson, R.B.; Rankin, S.A.; Ikeda, S. Short communication: Stabilization of milk proteins at pH 5.5 using pectic polysaccharides derived from potato tubers. J. Dairy Sci. 2019, 102, 8691–8695. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Li, J.; Zhang, H.; Huang, L.; Chen, P.; Zhou, J. Influence of molecular weight and degree of substitution of carboxymethylcellulose on the stability of acidified milk drinks. Food Hydrocoll. 2009, 23, 1420–1426. [Google Scholar] [CrossRef]
- Filisetti-Cozzi, T.M.C.C.; Carpita, N.C. Measurement of uronic acids without interference from neutral sugars. Anal. Biochem. 1991, 197, 157–162. [Google Scholar] [CrossRef]
- Zhang, Z.; Khan, N.M.; Nunez, K.M.; Chess, E.K.; Szabo, C.M. Complete Monosaccharide Analysis by High-Performance Anion-Exchange Chromatography with Pulsed Amperometric Detection. Anal. Chem. 2012, 84, 4104–4110. [Google Scholar] [CrossRef]
- Mueller, M.; Cavarkapa, A.; Unger, F.M.; Viernstein, H.; Praznik, W. Prebiotic potential of neutral oligo- and polysaccharides from seed mucilage of Hyptis suaveolens. Food Chem. 2017, 221, 508–514. [Google Scholar] [CrossRef]
- Zhao, R.; Qi, J.; Liu, Q.; Zeng, W.; Yang, X. Fractionation and characterization of soluble soybean polysaccharide esterified of octenyl succinic anhydride and its effect as a stabilizer in acidified milk drinks. Food Hyrdocoll. 2018, 85, 215–221. [Google Scholar] [CrossRef]
- Yuliarti, O.; Mei, K.H.; Kam Xue Ting, Z.; Yi, K.Y. Influence of combination carboxymethylcellulose and pectin on the stability of acidified milk drinks. Food Hydrocoll. 2019, 89, 216–223. [Google Scholar] [CrossRef]
- Øbro, J.; Harholt, J.; Scheller, H.V.; Orfila, C. Rhamnogalacturonan I in Solanum tuberosum tubers contains complex arabinogalactan structures. Phytochemistry 2004, 65, 1429–1438. [Google Scholar] [CrossRef]
- Liu, J.; Nakamura, A.; Corredig, M. Addition of pectin and soy soluble polysaccharide affects the particle size distribution of casein suspensions prepared from acidified skim milk. J. Agric. Food Chem. 2006, 54, 6241–6246. [Google Scholar] [CrossRef] [PubMed]
- Everett, D.W.; McLeod, R.E. Interactions of polysaccharide stabilisers with casein aggregates in stirred skim-milk yoghurt. Int. Dairy J. 2005, 15, 1175–1183. [Google Scholar] [CrossRef]
- Corredig, M.; Sharafbafi, N.; Kristo, E. Polysaccharide-protein interactions in dairy matrices, control and design of structures. Food Hydrocoll. 2011, 25, 1833–1841. [Google Scholar] [CrossRef]
- Lootens, D.; Capel, F.; Durand, D.; Nicolai, T.; Boulenguer, P.; Langendorff, V. Influence of pH, Ca concentration, temperature and amidation on the gelation of low methoxyl pectin. Food Hydrocoll. 2003, 17, 237–244. [Google Scholar] [CrossRef]
- Acero-Lopez, A.; Alexander, M.; Corredig, M. Diffusing wave spectroscopy and rheological studies of rennet-induced gelation of skim milk in the presence of pectin and κ-carrageenan. Int. Dairy J. 2010, 20, 328–335. [Google Scholar] [CrossRef]
Sample Availability: Samples of the pectic polysaccharides and acidified milk drinks are not available from the authors. |



| Property | PPP | HMP | LMP |
|---|---|---|---|
| Protein (%) | 1.31 ± 0.30 a | 1.25 ± 0.34 a | 0.70 ± 0.16 b |
| Uronic acid content (%) | 34.02 ± 4.30 c | 69.18 ± 5.56 a | 60.11 ± 1.92 b |
| DM (%) | 8.32 | 66.29 | 30.14 |
| DA (%) | 11.33 | 3.85 | 1.56 |
| Mw (kDa) | 420.9 | 234.6 | 187.0 |
| Monosaccharide Composition (mol%) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Fuc | Rha | Ara | Gal | Glc | Man | Xyl | GalA | |
| PPP | 1.38 ± 0.02 a | 13.32 ± 0.16 a | 8.49 ± 0.10 b | 36.08 ± 0.60 a | 3.23 ± 0.07 c | 1.33 ± 0.03 a | 1.43 ± 0.04 a | 34.74 ± 0.80 c |
| HMP | 0.74 ± 0.04 c | 6.19 ± 0.71 c | 9.68 ± 1.07 b | 12.95 ± 1.09 b | 5.34 ± 0.46 b | 0.30 ± 0.15 b | 0.43 ± 0.11 b | 64.37 ± 3.11 a |
| LMP | 1.13 ± 0.09 b | 7.76 ± 0.14 b | 20.40 ± 0.34 a | 12.04 ± 0.41 b | 6.47 ± 1.40 a | 0.04 ± 0.04 c | 0.12 ± 0.02 c | 52.03 ± 1.40 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, W.; Yang, W.; Zheng, Y.; Zhang, H.; Fang, H.; Liu, D.; Kong, X.; Chen, S.; Ye, X.; Tian, J. Effect of Potato Pulp Pectic Polysaccharide on the Stability of Acidified Milk Drinks. Molecules 2020, 25, 5632. https://doi.org/10.3390/molecules25235632
Sun W, Yang W, Zheng Y, Zhang H, Fang H, Liu D, Kong X, Chen S, Ye X, Tian J. Effect of Potato Pulp Pectic Polysaccharide on the Stability of Acidified Milk Drinks. Molecules. 2020; 25(23):5632. https://doi.org/10.3390/molecules25235632
Chicago/Turabian StyleSun, Weixuan, Wenhan Yang, Yuxue Zheng, Huiling Zhang, Haitian Fang, Donghong Liu, Xiangli Kong, Shiguo Chen, Xingqian Ye, and Jinhu Tian. 2020. "Effect of Potato Pulp Pectic Polysaccharide on the Stability of Acidified Milk Drinks" Molecules 25, no. 23: 5632. https://doi.org/10.3390/molecules25235632
APA StyleSun, W., Yang, W., Zheng, Y., Zhang, H., Fang, H., Liu, D., Kong, X., Chen, S., Ye, X., & Tian, J. (2020). Effect of Potato Pulp Pectic Polysaccharide on the Stability of Acidified Milk Drinks. Molecules, 25(23), 5632. https://doi.org/10.3390/molecules25235632







