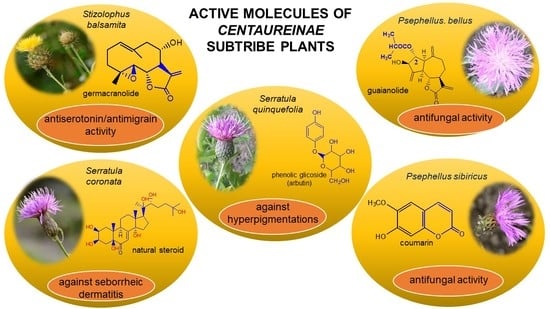
Phytotherapy Perspectives for Treating Fungal Infections, Migraine, Sebhorreic Dermatitis and Hyperpigmentations with the Plants of the Centaureinae Subtribe (Asteraceae)
Abstract
1. Introduction
2. Selected Centaureinae (Asteraceae) Plant Materials in Phytotherapy
2.1. Phytotherapy Possibilities for Treating Fungal Infections
- Very Susceptible, with the diameter of inhibition zone 20 mm or over 20 mm
- Susceptible: with the diameter of inhibition zone between 10 mm and 19 mm
- Moderately susceptible: with the diameter of inhibition zone between 1 mm and 9 mm
- Resistant: no inhibition zone
2.2. Possibilities for Phytotherapy in Serotonin Inhibition
2.3. Phytotherapy’s Possibilities in Treating Seborrheic Dermatitis
2.4. Phytotherapy’s Possibilities in Treating Skin Discoloration
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bruno, M.; Bancheva, S.; Roselli, S.; Maggio, A. sesquiterpenoidsin subtribe Centaureinae (Cass) Dumort (tribe Cardueae, Asteraceae): Distribution, 13C NMR spectra data and biological properties. Phytochemistry 2013, 95, 19–93. [Google Scholar] [CrossRef] [PubMed]
- Lyß, G.; Glasl, S.; Jurenitsch, J.; Pahl, H.L.; Merfort, I. A sesquiterpene and sesquiterpene lactones from Achillea millefolium group possess anti-inflammatory properties but do not inhibit the transcription factor NF-κ B. Pharm. Pharmacol. Lett. 2000, 10, 13–15. [Google Scholar]
- Matsuda, K.; Kagerura, T.; Toguchida, I.; Ueda, H.; Morikawa, T.; Yoshikawa, M. Inhibitory Effects of Sesquiterpenes from Bay-lef on Nitric Oxide Production in Lipopolisaccharide-actived Macrophages: Structure Requirement and Role of Heat-shock Protein Induction. Life Sci. 2000, 66, 2151–2157. [Google Scholar] [CrossRef]
- Nawrot, J.; Budzianowski, J.; Nowak, G. Phytochemical profiles of leaves of Stizolophus balsamita and Psephellus sibiricus and their chemotaxonomic implications. Phytochemistry 2019, 159, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Malinowska, M.; Bielawska, K. Metabolism and antioxidant properties of coumarins. Bromat. Chem. Toksykol. 2013, 3, 393–403. [Google Scholar]
- European Medicines Agency. 2015EMA/HMPC/680374/2013 Committee on Herbal Medicinal Products (HMPC) European Union Herbal Monograph on Hieracium pilosella L.; herba cum radice. 2015. Available online: https://www.ema.europa.eu/en/documents/herbal-monograph/final-european-union-herbal-monograph-hieracium-pilosella-l-herba-cum-radice_en.pdf (accessed on 9 November 2020).
- Martins, L.; Hellwig, F.K. Systematic position of the genera Serratula and Klasea within Centaureinae (Cardueae, Asteraceae) inferred from ETS and ITS sequence data and new combinations in Klasea. Taxon 2005, 54, 632–638. [Google Scholar] [CrossRef]
- Báthori, M.; Pongrácz, Z. Phytoecdysteroids—From isolation to their effects on humans. Curr. Med. Chem. 2005, 12, 153–172. [Google Scholar] [CrossRef]
- Dinan, L. The Karlson lecture Phytoecdysteroids: What use are they? Arch. Insect. Biochem. Physiol. 2009, 72, 126–141. [Google Scholar] [CrossRef]
- Hajar, T.; Leshem, Y.A.; Hanifin, J.M.; Nedorost, S.T.; Lio, P.A.; Paller, A.S.; Block, J.; Simpson, E.L. (The National Eczema Association Task Force). A systematic review of topical corticosteroid withdrawal (“steroid addiction”) in patients with atopic dermatitis and other dermatoses. J. Am. Acad. Dermatol. 2015, 72, 541–549. [Google Scholar] [CrossRef]
- Garcia de Arriba, S.; Naser, B.; Nolte, K.U. Risk assessment of free hydroquinone derived from Arctostaphylos uva-ursi folium herbal preparations. Int. J. Toxicol. 2013, 32, 442–453. [Google Scholar] [CrossRef]
- Nowak, G.; Drozdz, B.; Holub, M. Sesquiterpene lactones of the Cardueae, subtribe Centaureinae. In Compositae: Systematics. Proceedings of the International Compositae Conference; Hind, D.J.N., Beentje, H.J., Eds.; Royal Botanic Gardens, Kew: Richmond, UK, 1994; Volume 1, pp. 219–227. [Google Scholar]
- Jain, P.K.; Joshi, H. Coumarin: Chemical and pharmacological profile. J. Appl. Pharm. Sci. 2012, 2, 236–240. [Google Scholar]
- Morąg, M.; Nowak, G.; Michalak, M. The leaves of Serratula quinquefolia M.B. as a new arbutin source. Post. Fitoter. 2013, 1, 17–21. [Google Scholar]
- Budesinsky, M.; Nowak, G.; Rychlewska, U.; Hodgson, D.J.; Saman, D.; Daniewski, W.M.; Holub, M. Structure of sesquiterpene lactones of some species of subtribe Centaureinae. Collect. Czech. Chem. Commun. 1994, 59, 1175–1201. [Google Scholar] [CrossRef]
- Daniewski, W.M.; Nowak, G. Further sesquiterpene lactones of Centaurea bella. Phytochemistry 1993, 32, 204–205. [Google Scholar] [CrossRef]
- Nycz, E.J.; Małecki, G.J.; Morag, M.; Nowak, G.; Ponikiewski, L.; Switlicka, A.; Kusz, J. Arbutin: Isolation, X-ray structure and computional studies. J. Mol. Struct. 2010, 980, 13–17. [Google Scholar] [CrossRef]
- El-Dahmy, S.; Bohlmann, F.; Sarg, T.M.; Ateya, A.; Farrag, N. New guaianolides from Centaurea aegyptica. Planta Med. 1985, 51, 176–177. [Google Scholar] [CrossRef]
- Bohlmann, F.; Ziesche, J. Naturally occurring terpene derivatives. Part 253. New guaianolides and acetyleniccompounds from Ptilostemon species. Phytochemistry 1980, 19, 692–696. [Google Scholar] [CrossRef]
- Gonzalez, A.G.; Bermejo, J.; Massanet, G.M. Aportacion al estudio quimiotaxonomico del genero Centaurea. Rev. Lat. Quim. 1977, 8, 176–181. [Google Scholar]
- González, A.G.; Bermejo, J.L.J.; Bretón, J.L.; Massanet, G.M.; Triana, J. Chlorohyssopifolin C, D, E and vahlenin, four new sesquiterpene lactones from Centaurea hyssopifolia. Phytochemistry 1974, 13, 1193–1197. [Google Scholar] [CrossRef]
- Nowak, G.; Drozdz, B.; Holub, M.; Budesinsky, M.; Saman, D. Sesquiterpene lactones. XXXI. New guaianolides in Centaurea bella Trautv. and Centaurea adjarica Alb. Acta Soc. Bot. Pol. 1986, 55, 227–231. [Google Scholar] [CrossRef][Green Version]
- Estratova, R.J.; Rybalko, K.S.; Rzasade, R.Y. Acroptilin—A new sesquiterpene lactone from Acroptilon repens. Khim. Prir. Soed. 1967, 4, 284–286. [Google Scholar]
- Samek, F.C.; Holub, M.; Drozdz, B.; Jomni, G.; Corbella, A.; Gariboldi, P. Sesquiterpene lactones of Cynara scolymus L. species. Tetrahedrom Lett. 1971, 50, 4775–4778. [Google Scholar] [CrossRef]
- Nowak, G.; Drozdz, B.; Holub, M.; Lagodzinska, A. Sesquiterpene XXXIII. Guaianolides in the subgenus Psephellus (Cass.) Schmalh. genus Centaurea L. Acta Soc. Bot. Pol. 1986, 55, 629–637. [Google Scholar] [CrossRef][Green Version]
- Stevens, K.I. Sesquiterpene lactones from Centaurea repens. Phytochemistry 1982, 21, 1093–1098. [Google Scholar] [CrossRef]
- Jakupovic, J.; Jia, Y.; Pathak, V.P.; King, R.M. Bisabolone derivatives and sesquiterpene lactones from Centaurea species. Planta Med. 1986, 52, 399–401. [Google Scholar] [CrossRef]
- Bohlmann, F.; Singh, P.; King, R.M.; Robinson, H.E. New guaianolides from Pseudostifftia kingii. Phytochemistry 1982, 21, 1171–1172. [Google Scholar] [CrossRef]
- Dean, F.M. Naturally occurring coumarins. Chem. Org. Nat. 1952, 9, 225–291. [Google Scholar]
- Ma, C.H.; Ke, W.; Sun, Z.L.; Peng, J.Y.; Li, Z.H.; Zhou, X. Large scale isolation and purification of scoparone from Herba Artemisiae scopariae by high speed counter-current chromatography. Chromatographia 2006, 64, 81–87. [Google Scholar] [CrossRef]
- Tsukamoto, H.; Hisada, S.; Nishibe, S.; Roux, D.G.; Rourke, J.P. Phenolic glucosides from Olea europea subsp. africana. Phytochemistry 1984, 23, 2839–2841. [Google Scholar] [CrossRef]
- Huitink, G.M. Substituted Coumarins as Metallofluorochromic Indicators. Retrospective Theses and Dissertations, Iowa State University, Ames, IA, USA, 1967. Paper 3942. [Google Scholar]
- Hewlett, M.J.; Begley, M.J.; Groenewegen, W.A.; Heptinstall, S.; Knight, D.W.; May, J.; Salan, U.; Toplis, D. Sesquiterpene lactones from feverfew, Tanacetum parthenium: Isolation, structural revision, activity against human blood platelet function and implications for migraine therapy. J. Chem. Soc. Perkin Trans. 1996, 16, 1979–1986. [Google Scholar] [CrossRef]
- Rybalko, K.S.; Mukametzhanov, M.N.; Sheinchenko, V.I.; Konovalova, O.A. Sesquiterpene lactones of Stizolophus balsamita. Khim. Prir. Soedin. 1976, 12, 467–468. [Google Scholar]
- Nowak, G.; Drozdz, B.; Budesinsky, M.; Holub, M. Sesquiterpene lactones. XXXVII. Germacranolides in the genus Stizolophus Cass. Acta Soc. Bot. Pol. 1989, 58, 247–251. [Google Scholar] [CrossRef]
- Mukametzhanov, M.N.; Sheinchenko, V.I.; Bankowskii, A.I.; Rybalko, K.S. A sesquiterpene lactone from Stizolophus balsamita. Khim.Prir. Soedin. 1971, 7, 405–406. [Google Scholar]
- Tyson, R.L.; Chang, C.J.; Mc Laughlin, J.L.; Aynehchi, Y.; Cassady, J.M. 9-hydroxyparthenolide a novel antitumor sesquiterpene lactone from Anvilea garcini (Burm.) DC. Experientia 1981, 37, 441–442. [Google Scholar] [CrossRef]
- Oksuz, S.; Ayyidiz, H. Sesqiuterpene lactones from Stizolophus coronopifolia. Phytochemistry 1986, 25, 535–537. [Google Scholar] [CrossRef]
- Imai, S.; Murta, S.; Koreeda, M. Structure of ajugasterone C, a phytoecdysone with an 11-hydroxy-group. J. Chem. Soc. D. 1969, 10, 546–547. [Google Scholar] [CrossRef]
- Jizba, J.; Herout, V.; Sorm, F. Polypodine B—A novel ecdysones-like substance from plant material. Tedrahedrom Lett. 1967, 18, 1689–1691. [Google Scholar] [CrossRef]
- Hocks, P.; Wiechert, R. 20-hydroxyecdysone isoliert aus insekten. Tetrahedron Lett. 1996, 26, 2089–2993. [Google Scholar]
- Ghosh, P.N.; Fisher, M.C.; Bates, K.A. Diagnosing Emerging Fungal Threats: A One Health Perspective. Front. Genet. 2018, 54. [Google Scholar] [CrossRef]
- Harsha, M.V.; Venkatachalam, S.; Pooja, M.; Paranjothy, M. Emerging fungal pathogens: A major threat to human life. Int. J. Pharm. Sci. Res. 2017, 8, 1923–1934. [Google Scholar]
- Rosseeuv, D. Achilles foot screening project: Preliminary results of patients screened by dermatologists. J. Eur. Acad. Derm. Venerol. 1999, 12, 6–9. [Google Scholar] [CrossRef]
- Badiee, P.; Hashenmizadeh, Z. Opportunistic invasive fungal infections: Diagnosis & clinical management. Indian J. Med. Res. 2014, 139, 195–204. [Google Scholar] [PubMed]
- Segal, E.; Elad, D. Special Issue: Treatments for Fungal Infections. J. Fungi 2018, 4, 135. [Google Scholar] [CrossRef] [PubMed]
- Barrero, A.F.; Oltra, J.E.; Alvarez, M.; Raslan, D.S.; Saude, D.A.; Aksira, M. New sources and antifungal activity of sesquiterpene lactone. Fitoterapia 2000, 71, 60–64. [Google Scholar] [CrossRef]
- Montagner, C.; de Souze, S.M.; Crospo, C.; Monache, F.D.; Smânia, E.F.; Smânia, A. Antifungal activity of coumarins. Z. Nat. C. J. Biosci. 2008, 63, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Iranshahi, M.; Askari, M.; Sahebkar, A.; Adjipavlou-Litina, D. Evaluation of antioxidant, anti-inflammatory and lipoxygenase inhibitory activities of the prenylated coumarin umbelliprenin. Daru J. Pharm. Sci. 2009, 17, 99–103. [Google Scholar]
- Kamińska, B. Antifungal Activity of Selected Compounds and Extracts of Some Species Centaurea L. Genus. Ph.D. Thesis, Department of Medicinal and Cosmetic Natural Products, Poznan University of Medical Sciences, Poznań, Poland, 2017. [Google Scholar]
- Deen, M.; Christensen, C.E.; Hougaard, A.; Hansen, H.D.; Knudsen, G.M.; Ashina, M. Serotonergic mechanisms in the migraine brain—A systematic review. Cephalalgia 2017, 37, 251–264. [Google Scholar] [CrossRef]
- Bogrdorff, P.; Tangelder, G.J. Migraine: Possible Role of Shear-Induced Platelet Aggregation with Serotonin Release. Headache 2012, 52, 1298–1318. [Google Scholar]
- Materazzi, S.; Benemei, S.; Fusi, C.; Gualdani, R.; De Siena, G.; Vastani, N.; Anderson, D.A.; Trevisan, G.; Moncelli, M.R.; Wei, X.; et al. Parthenolide inhibits nociception and neurogenic vasodilatation in the trigeminovascular system by targetting TRPA1 chanel. Pain 2013, 154, 2750–2758. [Google Scholar] [CrossRef]
- European Scientific Cooperative on Phytotherapy (ESCOP). ESCOP Monographs: The Scientific Foundation for Herbal Medicinal Products, 2nd ed.; Thieme: New York, NY, USA, 2003; pp. 492–498. [Google Scholar]
- Tassorelli, C.; Greco, R.; Morazzoni, P.; Riva, A.; Sandrini, G.; Nappi, G. Parthenolide is the component of Tanacetum parthenium that inhibits nitroglycerin-induced Fos activation: Studies in an animal model of migraine. Cephalalgia 2005, 25, 612–621. [Google Scholar] [CrossRef]
- Lesiak, K.; Koprowska, K.; Zalesna, I.; Nejc, D.; Duchler, M.; Czyź, M. Parthenolide, a sesquiterpene lactone from the medical herb feverfew, shows anti-cancer activity against human melanoma cells in vitro. Melanoma Res. 2010, 20, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Wyrebska, A.; Gach, K.; Janecka, A. Combined effect of parthenolide and various anti-cancer drugs or anticancer candidate substances on malignant cells in vitro and in vivo. Mini Rev. Med. Chem. 2014, 14, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Won, J.K.; Ong, C.N.; Shi, X.; Shen, H.M. Chemopreventive activity of parthenolide against UVB-induced skin cancer and its mechanisms. Carcinogenesis 2004, 25, 1449–1458. [Google Scholar] [CrossRef] [PubMed]
- Napierała, M.; Nawrot, J.; Gornowicz-Porowska, J.; Florek, E.; Moroch, A.; Adamski, Z.; Kroma, A.; Miechowicz, I.; Nowak, G. Separation and HPLC characterisation natural steroids and a standardised extract from the Serratula coronata herb with antiseborrheic dermatitis activity. Int. J. Environ. Res. Public Health 2020, 17, 6453. [Google Scholar] [CrossRef]
- Heptinstall, S.; Groenwegen, W.A.; Spangenberg, P.; Loesche, W. Extracts Feverfew may inhibit behavior via naturalisation of sulphydryl groups. J. Pharm. Pharm. 1987, 39, 459–465. [Google Scholar] [CrossRef]
- Wyganowska-Swiatkowska, M.; Nohawica, M.; Grocholewicz, K.; Nowak, G. Influence of herbal medicines on HMBG1 release, SARS-CoV-2 viral attachment, acute respiratory failure and sepsis. A literature review. Int. J. Mol. Sci. 2020, 21, 4639. [Google Scholar] [CrossRef]
- Hadas, E.; Derda, M.; Nawrot, J.; Nowak, G.; Thiem, B. Evaluation of the amoebicidal activities of Centaurea bella, Centaurea daghestanica, Rhaponticum pulchrum and Tanacetum vulgarae against pathogenic Acathamoeba spp. Acta Pol. Pharm. 2017, 74, 1827–1832. [Google Scholar]
- Picman, A.K. Biological activities of sesquiterpene lactones. Biochem. Syst. Ecol. 1986, 14, 255–281. [Google Scholar] [CrossRef]
- Dinan, L. Phytoecdysteroids biological aspects. Phytochemistry 2001, 57, 325–339. [Google Scholar] [CrossRef]
- Clark, G.W.; Pope, S.M.; Jaboori, K.A. Diagnosis and treatment of seborrheic dermatitis. Am. Fam. Physician 2015, 91, 185–190. [Google Scholar]
- Dinan, L.; Lafont, R. Effects and applications of arthropod steroid hormones (ecdysteroids) in mammals. J. Endocrinol. 2006, 191, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.S.; Savjani, J.K. Systematic review of plant steroids as potential anti-inflammatory agents: Current status and future perspectives. J. Phytopharm. 2015, 4, 121–125. [Google Scholar]
- Meybeck, A.; Bonté, F. Ecdysteroid-containing liposomes for wound healing and skin regeneration. Chem. Abstr. 1990, 114, 30138. [Google Scholar]
- Ali, S.; Khan, F.I.; Mohammad, T.; Lan, D.; Hassan, M.I.; Wang, Y. Identification and Evaluation of Inhibitors of Lipase from Malassezia restricta using Virtual High-Throughput Screening and Molecular Dynamics Studies. Int. J. Mol. Sci. 2019, 20, 884. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Han, J.; Zhang, M. 20-Hydroxyecdysone enhances Immulectin-1ediated immune response against entomogenous fungus in Locusta migratoria. Pest Manag. Sci. 2020, 76, 304–313. [Google Scholar] [CrossRef]
- Dermar, M.; Dumas, M.; Bonte, F. Effect of ecdysterone on the differentiation of normal keratinocytes in vitro. Eur. J. Derm. 1994, 4, 558–569. [Google Scholar]
- Perez-Bernal, A.; Munoz-Perez, M.A.; Camacho, F. Management of facial hyperpigmentation. Am. J. Clin. Derm. 2007, 6, 195–202. [Google Scholar]
- Qian, W.; Liu, W.; Zhu, D. Natural skin-whitening compounds for the treatment of melanogenesis (Review). Exp. Med. 2020, 20, 173–185. [Google Scholar] [CrossRef]
- Sarkar, R.; Pooja Arora, P.; Garg, K.V.J. Cosmeceuticals for Hyperpigmentation: What is Available? Cutan. Aesthet. Surg. 2013, 6, 4–11. [Google Scholar] [CrossRef]
- Balkrishnan, R.; Kelly, A.P.; Mc Michael, A.; Torok, H. Improved quality of life with effective treatment of facial melasma: The pigment trial. J. Drugs Derm. 2004, 3, 377–381. [Google Scholar]
- Morag, M.; Nawrot, J.; Siatkowski, I.; Adamski, Z.; Fedorowicz, T.; Dawid-Pać, R.; Urbańska, M.; Nowak, G. A double-blind, placebo-controlled randomized trial of Serratulae quinquefoliae folium, a new source of β-arbutin, in selected skin hyperpigmentations. J. Cosmet. Derm. 2015, 14, 185–190. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds—cebellin L, cebellin A, cebellin B, acroptilin, scoparone, scopoletin, umbelliferone, izospiciformin, stizolin, stizolicin, aiugasterone C, polypodine B, 20-hydroxyecdysone, β-arbutin are available. |

| Source | Compound | Structure | Properties/Uses |
|---|---|---|---|
| Psephellus bellus herb | Cebellin L (1) Budesinsky et al. (1994) [15] | 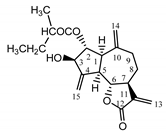 | anti-inflammatory/antifungal |
| Cebellin O (2) Daniewski and Nowak (1993) [16] | 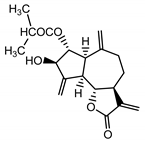 | anti-inflammatory/antifungal | |
| Cebellin K (3) Budesinsky et al. (1994) [15] | 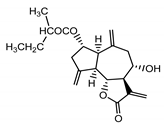 | anti-inflammatory/antifungal | |
| Cebellin N (4) Daniewski and Nowak (1993) [16] | 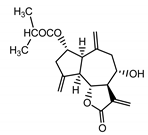 | anti-inflammatory/antifungal | |
| 19-deoxychloro-janerin (5) El-Dahmy et al. (1985) [18] | 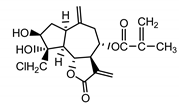 | anti-inflammatory/antifungal (in extract) | |
| 17,18-epoxy-19-deoxy-chlorojanerin (6) Budesinsky et al. (1994) [16] | 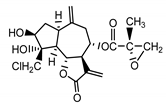 | anti-inflammatory/antifungal (in extract) | |
| Cebellin M (7) Budesinsky et al. (1994) [16] | 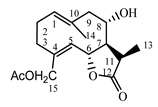 | anti-inflammatory/antifungal (in extract) | |
| 8-desacylo-8α-(2′-methyl-acryloxy)-subluteolide (8) Bohlmann and Ziesche (1980) [19] | 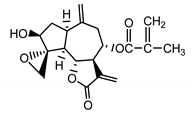 | anti-inflammatory/antifungal (in extract) | |
| Repin (9) Gonzales et al. (1977) [20] | 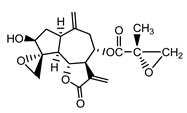 | anti-inflammatory/antifungal (in extract) | |
| Centaurepensin (10) Gonzales et al. (1974) [21] | 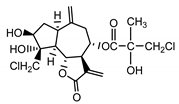 | anti-inflammatory/antifungal (in extract) | |
| Cebellin A (11) Nowak et al. (1986a) [22] | 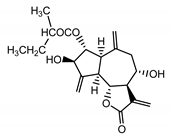 | anti-inflammatory/antifungal | |
| Cebellin B (12) Nowak et al. (1986a) [22] | 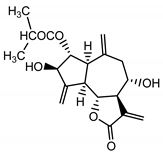 | anti-inflammatory/antifungal | |
| Acroptilin (13) Estratova et al. (1967) [23] | 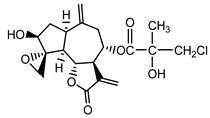 | anti-inflammatory/antifungal (in extract) | |
| Cynaropicrin (14) Samek et al. (1971) [24] | 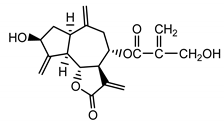 | anti-inflammatory/antifungal (in extract) | |
| Cebellin F (15) Nowak et al. (1986a) [22] | 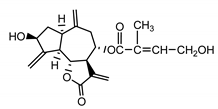 | anti-inflammatory/antifungal (in extract) | |
| 15-deoxyrepin (16) Nowak et al. (1986b) [25] | 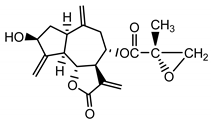 | anti-inflammatory/antifungal (in extract) | |
| Chlorojanerin (17) Stevens (1982) [26] | 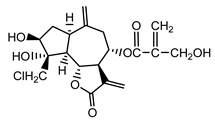 | anti-inflammatory/antifungal (in extract) | |
| 8-desacetyl-centaurepensin-8-O-(4′-hydroxy)-tiglate (18) Stevens (1982) [26] | 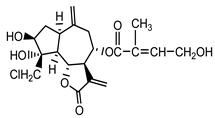 | anti-inflammatory/antifungal (in extract) | |
| Repensolide (19) Jakupovic et al. (1986) [27] | 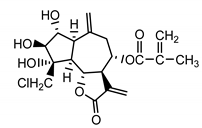 | anti-inflammatory/antifungal (in extract) | |
| Janerin (20) Gonzales et al. (1977) [20] | 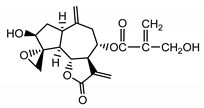 | anti-inflamatory/antifungal (in extract) | |
| 8-4′-tiglinate-8-desacetyl-subluteolide (21) Budesinsky et al. (1994) [16] | 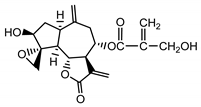 | anti-inflamatory/antifungal (in extract) | |
| Cebellin G (22) Nowak et al. (1986a) [22] | 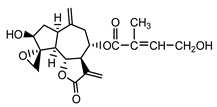 | anti-inflamatory/antifungal (in extract) | |
| Cebellin H (23) Nowak et al. (1986a) [22] | 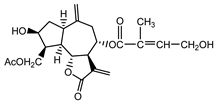 | anti-inflamatory/antifungal (in extract) | |
| Cebellin I (24) Nowak et al. (1986a) [22] | 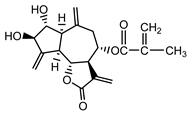 | anti-inflammatory/antifungal (in extract) | |
| Repdiolide (25) Bohlmann et al. (1982) [28] | 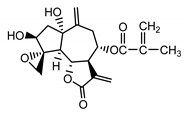 | anti-inflammatory/antifungal (in extract) | |
| Cebellin J (26) Budesinky et al. (1994) [16] | 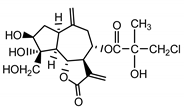 | anti-inflammatory/antifungal (in extract) | |
| Psephellus sibiricus leaf | Coumarin (27) Dean (1952) [29] | 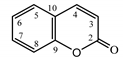 | anti-inflammatory/antifungal (in extract) |
| Scoparone (28) Ma et al. (2006) [30] | 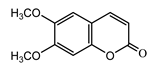 | anti-inflammatory/antifungal (in extract) | |
| Scopoletin (29) Tsukamoto et al. (1984) [31] | 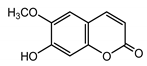 | anti-inflammatory/antifungal | |
| Umbelliferone (30) Hulting (1967) [32] | 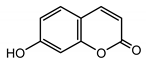 | anti-inflammatory/antifungal (in extract) | |
| Tanacetum parthenium herb | Parthenolide (31) Hevlett et al. (1996) [33] | 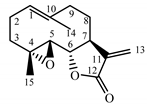 | anti-inflammatory/antimigraine/ Anticancer |
| Stizolophus balsamita leaf | Balsamin (32) Rybalko et al. (1969) [34] | 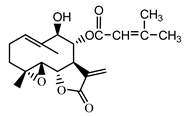 | anti-inflammatory/antiserotonin (in extract) |
| Izospiciformin (33) Nowak et al. (1989) [35] | 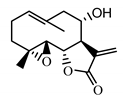 | anti-inflammatory/antiserotonin (in extract) | |
| Stizolin (34) Mukametzhnov et al. (1971) [36] | 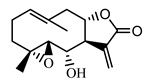 | anti-inflammatory/antiserotonin (in extract) | |
| 9α-hydroxy-parthenolide (35) Tyson et al. (1981) [37] | 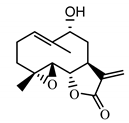 | anti-inflammatory/antiserotonin (in extract) | |
| 8-E-(4′-hydrohy)-senecioyloxy-9α-hydroxyparthe-nolide (36) Oksuz and Ayyildiz (1986) [38] | 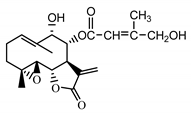 | anti-inflammatory/antiserotonin (in extract) | |
| 11βH,13-dihydro-stizolicin (37) Nawrot et al. (2019) [4] | 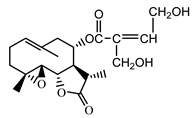 | anti-inflammatory/antiserotonin (in extract) | |
| Stizolicin (38) Mukametzhanov et al. (1971) [36] | 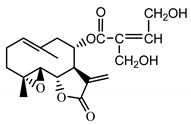 | anti-inflammatory/antiserotonin | |
| Serratula coronata herb | Ajugasterone C (39) Imai et al. (1969) [39] | 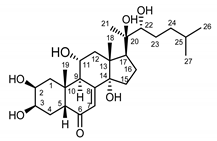 | anti-Malassesia restricta Seborrheic dermatitis (in extract) |
| Polypodine B (40) Jizba et al. (1967) [40] | 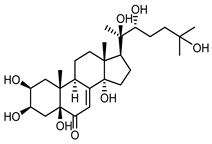 | anti-Malassesia restricta Seborrheic dermatitis (in extract) | |
| 20-hydroxyecdysone (41) Hocks and Wiechert (1996) [41] | 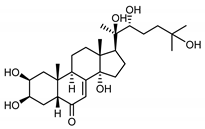 | anti-Malassesia restricta Seborrheic dermatitis (in extract) | |
| Serratula quinquefolia leaf | β-arbutin (42) Nycz et al. (2010) [17] | 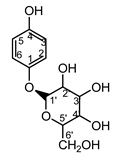 | hyperpigmentations (in extract) |
| Herbal Substance | Candida albicans | Candida famata | Candida glabrata | Candida parapsilosis | Rhodotorula rubra | Trichophyton rubrum | Trichophyton mentagrophytes var. interdigitale | Microsporum canis | Scopulariopsis brevicaulis |
|---|---|---|---|---|---|---|---|---|---|
| Cebellin L | - | S | S | VS | S | S | VS | - | S |
| Cebellins K + N + O | S | - | VS | - | VS | - | VS | VS | MS |
| Cebellin A | M | - | S | - | S | - | S | - | VS |
| Cebellin B | - | S | S | MS | - | S | S | - | MS |
| Acroptilin | - | MS | - | - | - | VS | MS | - | - |
| Scopoletin | - | S | S | MS | - | S | MS | - | MS |
| P. bellus extract | - | S | S | S | - | VS | VS | - | - |
| P. sibiricus extract | - | S | - | S | - | - | S | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nawrot, J.; Gornowicz-Porowska, J.; Nowak, G. Phytotherapy Perspectives for Treating Fungal Infections, Migraine, Sebhorreic Dermatitis and Hyperpigmentations with the Plants of the Centaureinae Subtribe (Asteraceae). Molecules 2020, 25, 5329. https://doi.org/10.3390/molecules25225329
Nawrot J, Gornowicz-Porowska J, Nowak G. Phytotherapy Perspectives for Treating Fungal Infections, Migraine, Sebhorreic Dermatitis and Hyperpigmentations with the Plants of the Centaureinae Subtribe (Asteraceae). Molecules. 2020; 25(22):5329. https://doi.org/10.3390/molecules25225329
Chicago/Turabian StyleNawrot, Joanna, Justyna Gornowicz-Porowska, and Gerard Nowak. 2020. "Phytotherapy Perspectives for Treating Fungal Infections, Migraine, Sebhorreic Dermatitis and Hyperpigmentations with the Plants of the Centaureinae Subtribe (Asteraceae)" Molecules 25, no. 22: 5329. https://doi.org/10.3390/molecules25225329
APA StyleNawrot, J., Gornowicz-Porowska, J., & Nowak, G. (2020). Phytotherapy Perspectives for Treating Fungal Infections, Migraine, Sebhorreic Dermatitis and Hyperpigmentations with the Plants of the Centaureinae Subtribe (Asteraceae). Molecules, 25(22), 5329. https://doi.org/10.3390/molecules25225329