Recovery of a Bacteriocin-Like Inhibitory Substance from Lactobacillus bulgaricus FTDC 1211 Using Polyethylene-Glycol Impregnated Amberlite XAD-4 Resins System
Abstract
1. Introduction
2. Results
2.1. Antimicrobial of BLIS from L. bulgaricus FTDC 1211
2.2. Purification of BLIS from L. bulgaricus FTDC 1211 Using AIRS
2.2.1. Effect of Molecular Weight and Concentration of PEG
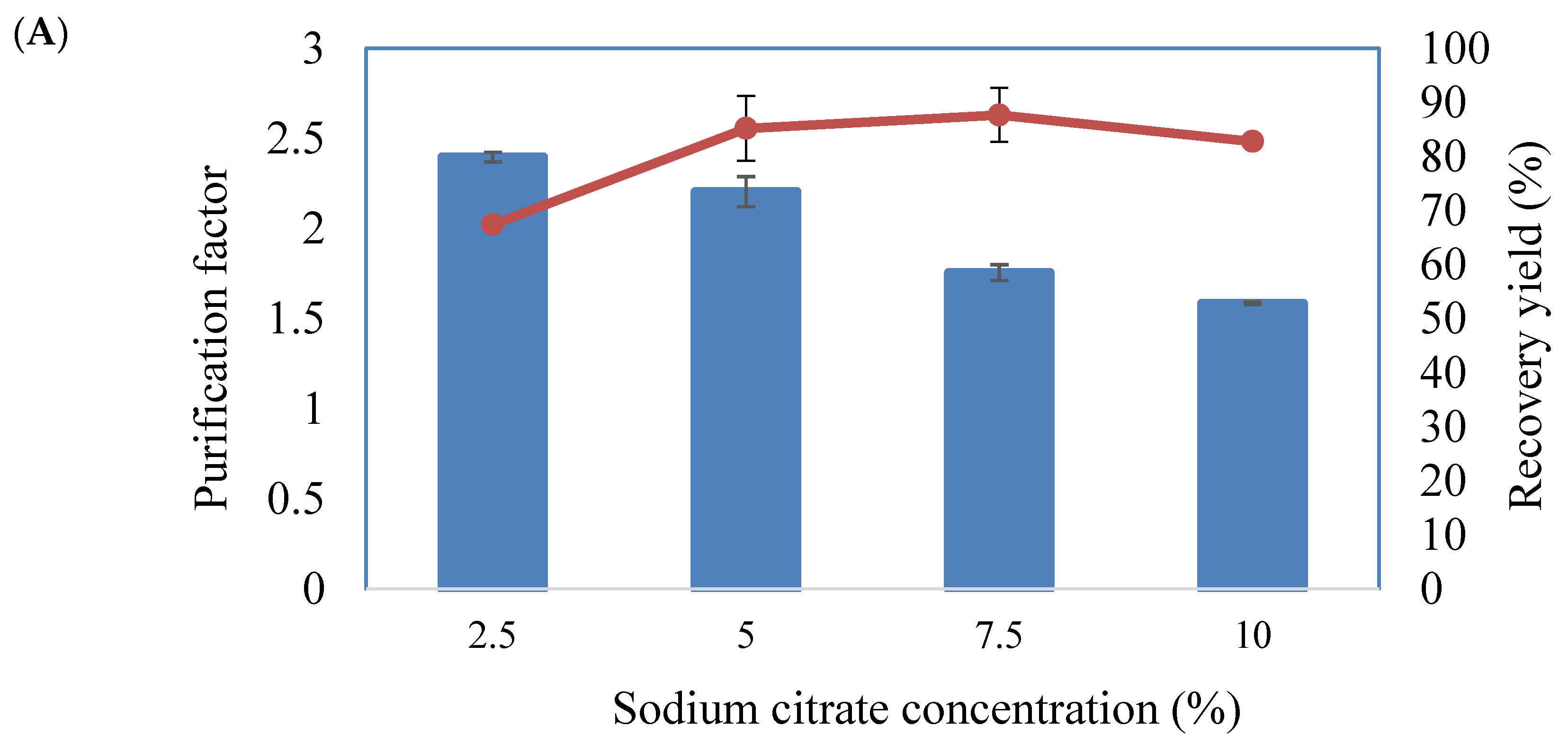
2.2.2. Effect of Sodium Citrate Concentration
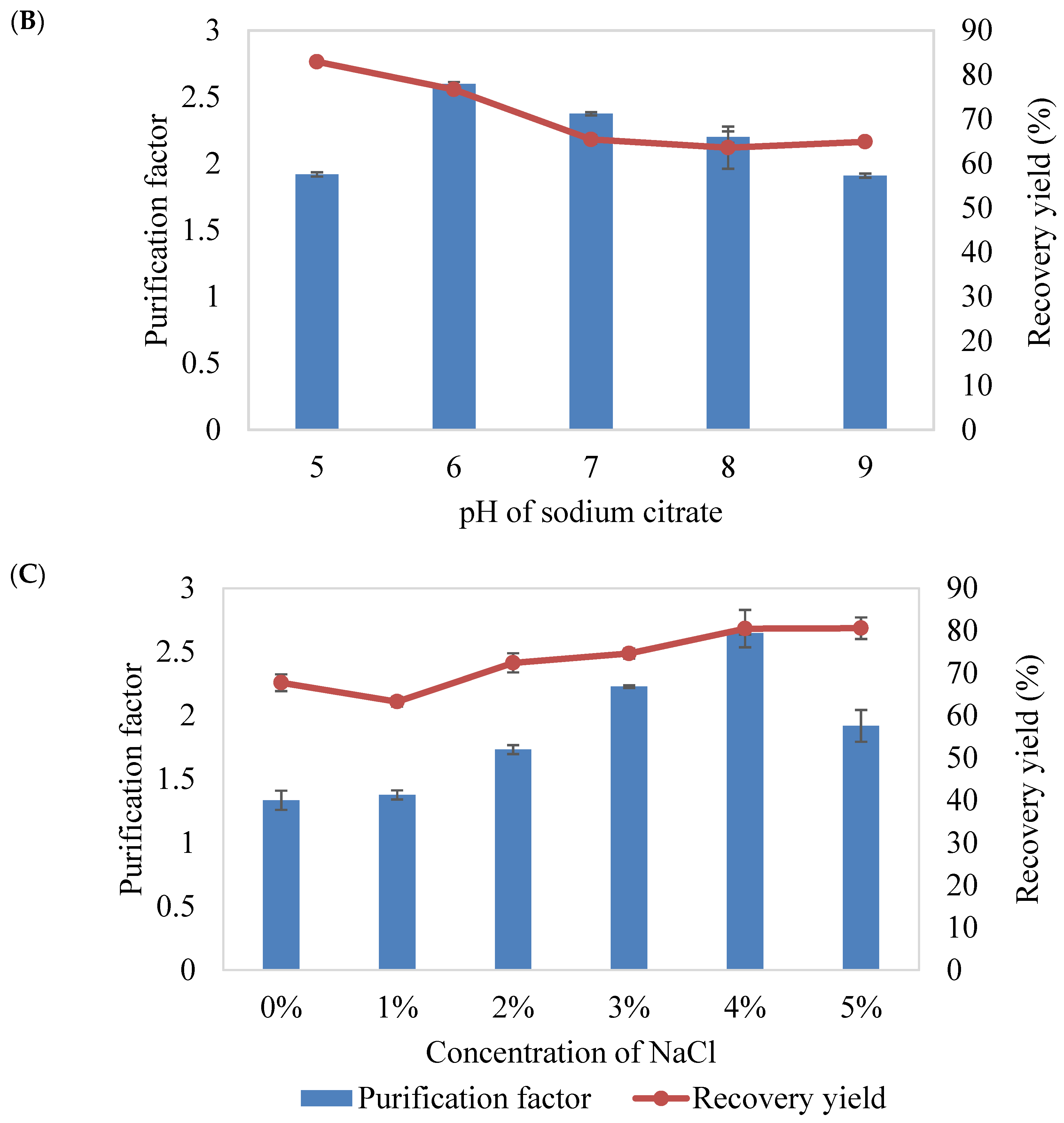
2.2.3. Effect of pH of Sodium Citrate
2.2.4. Effect of Different Concentration of NaCl
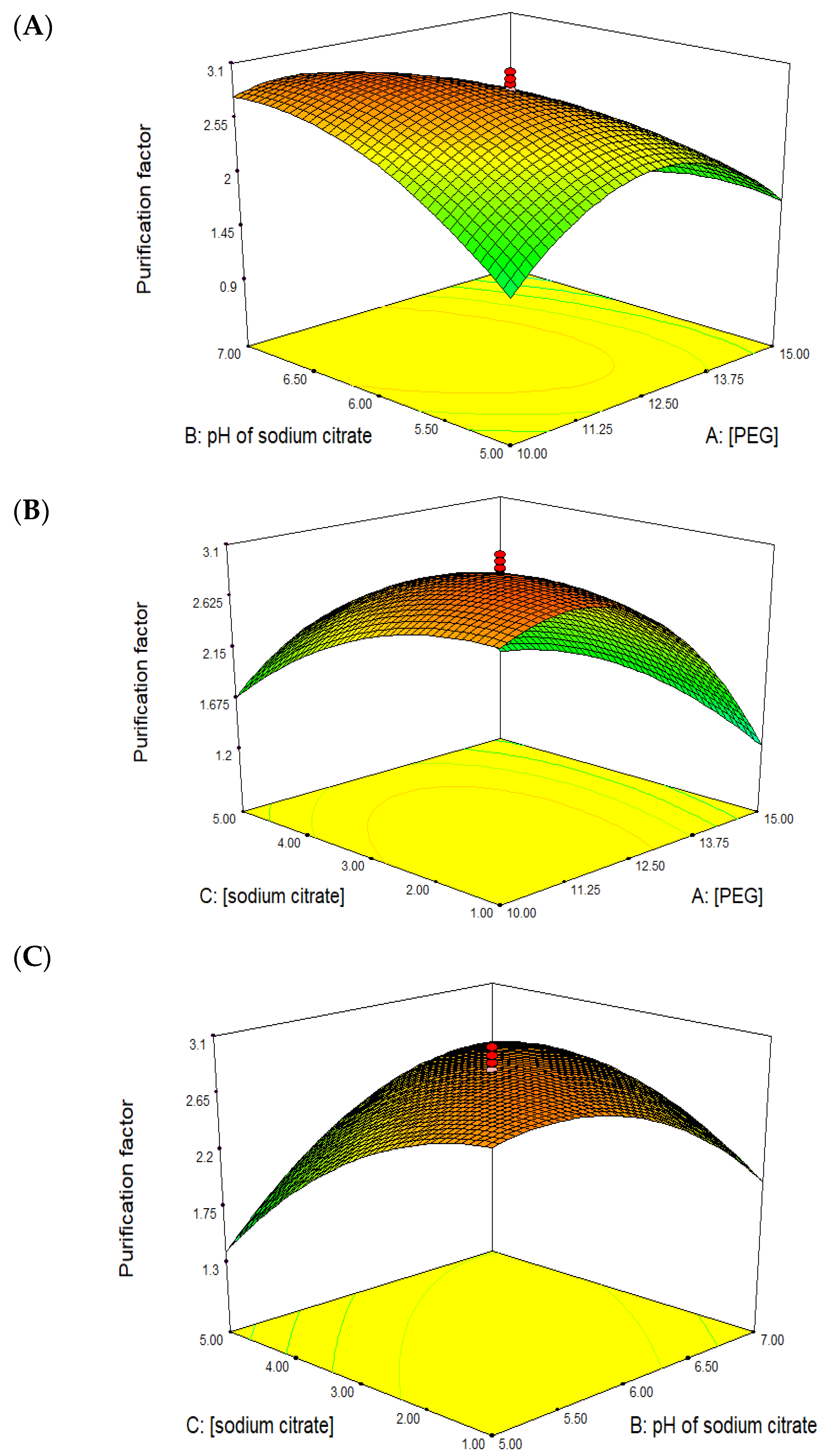
2.3. Optimization of Purification Parameters Using Response Surface Methodology
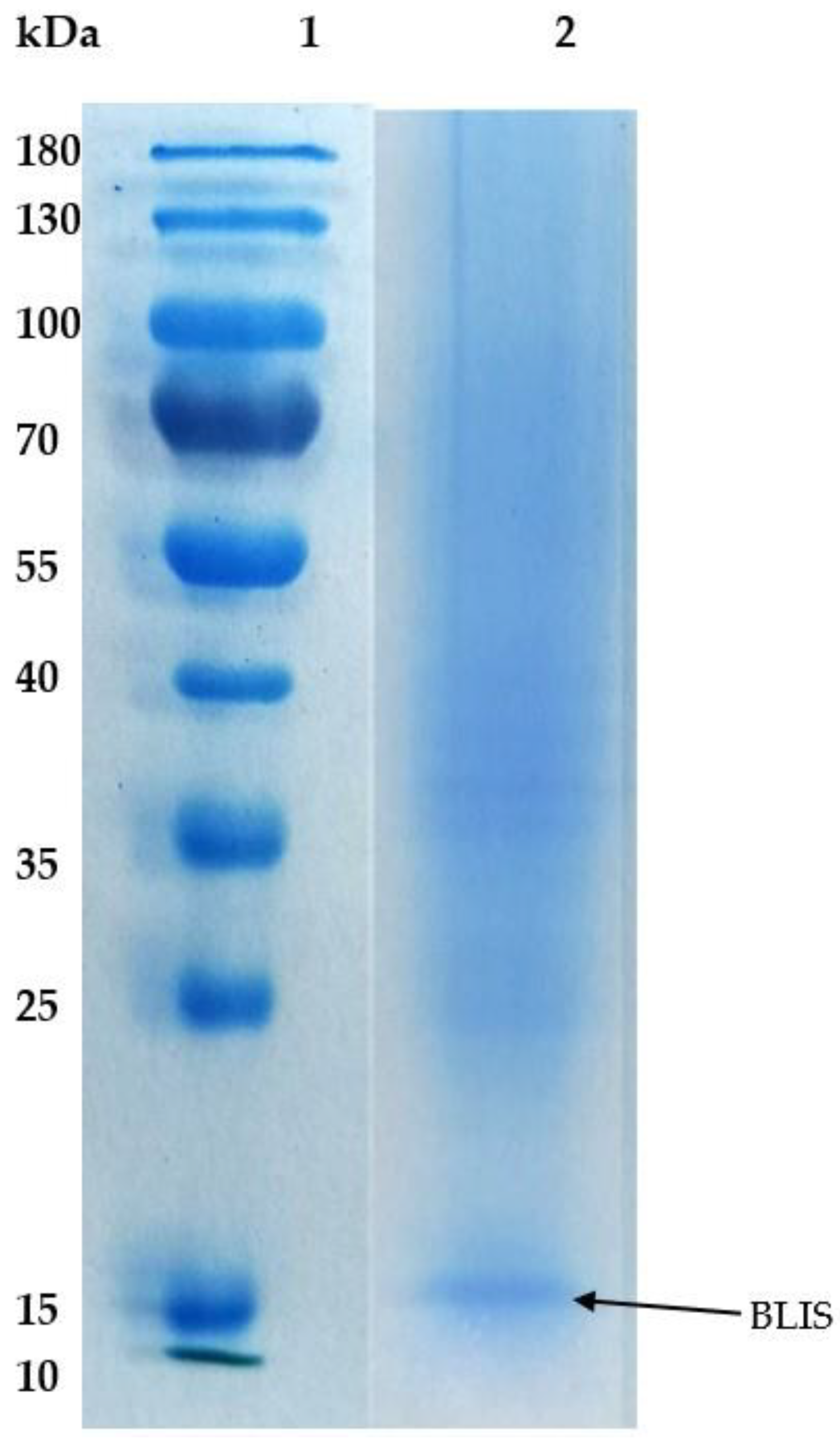
2.4. Purity of BLIS on SDS-PAGE
3. Discussion
4. Materials and Methods
4.1. Supplies
4.2. Bacteriocin-Producing LAB and BLIS Preparation
4.3. Purification of BLIS
4.4. Antimicrobial Activity Assay
4.5. Total Protein Content Determination
4.6. Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis
4.7. Determination of Purification Factor and Recovery Yield
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Morell, E.A.; Balkin, D.M. Methicillin-resistant Staphylococcus aureus: A pervasive pathogen highlights the need for new antimicrobial development. Yale J. Biol. Med. 2010, 83, 223–233. [Google Scholar]
- Abbasiliasi, S.; Tan, J.S.; Ibrahim, T.A.T.; Ramanan, R.N.; Vakhshiteh, F.; Mustafa, S.; Ling, T.C.; Rahim, R.A.; Ariff, A.B. Isolation of Pediococcus acidilactici Kp10 with ability to secrete bacteriocin-like inhibitory substance from milk products for applications in food industry. BMC Microbiol. 2012, 12, 260. [Google Scholar] [CrossRef] [PubMed]
- Hor, Y.Y.; Liong, M.T. Use of extracellular extracts of lactic acid bacteria and bifidobacteria for the inhibition of dermatological pathogen Staphylococcus aureus. Dermatol. Sin. 2014, 32, 141–147. [Google Scholar] [CrossRef]
- Abbasiliasi, S.; Tan, J.S.; Ibrahim, T.A.T.; Kadkhodaei, S.; Ng, H.S.; Vakhshiteh, F.; Ajdari, Z.; Mustafa, S.; Ling, T.C.; Rahim, R.A. Primary recovery of a bacteriocin-like inhibitory substance derived from Pediococcus acidilactici Kp10 by an aqueous two-phase system. Food Chem. 2014, 151, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yang, Y.; Yang, H.; Bu, Y.; Yi, H.; Zhang, L.; Han, X.; Ai, L. Purification and partial characterization of Bacteriocin Lac-B23, a novel Bacteriocin production by Lactobacillus plantarum J23, isolated from Chinese traditional fermented milk. Front. Microbiol. 2018, 9, 2165. [Google Scholar] [CrossRef]
- Yamato, M.; Ozaki, K.; Ota, F. Partial purification and characterization of the bacteriocin produced by Lactobacillus acidophilus YIT 0154. Microbiol. Res. 2003, 158, 169–172. [Google Scholar] [CrossRef]
- Abriouel, H.; Valdivia, E.; Martınez-Bueno, M.; Maqueda, M.; Gálvez, A. A simple method for semi-preparative-scale production and recovery of enterocin AS-48 derived from Enterococcus faecalis subsp. liquefaciens A-48-32. J. Microbiol. Methods 2003, 55, 599–605. [Google Scholar] [CrossRef]
- Tan, J.S.; Abbasiliasi, S.; Ariff, A.B.; Ng, H.S.; Bakar, M.H.A.; Chow, Y.H. Extractive purification of recombinant thermostable lipase from fermentation broth of Escherichia coli using an aqueous polyethylene glycol impregnated resin system. 3 Biotech 2018, 8, 288. [Google Scholar] [CrossRef]
- Abdul Aziz, N.F.H.; Abbasiliasi, S.; Ariff, A.B.; Ng, H.S.; Lan, J.C.-W.; Ahmad, R.; Tan, J.S. Optimization of recovery of esterase from Serratia marcescens using combination of the solvent impregnated resin and aqueous two-phase extraction techniques. Sep. Sci. Technol. 2018, 53, 2952–2960. [Google Scholar] [CrossRef]
- Da Costa, R.J.; Voloski, F.L.; Mondadori, R.G.; Duval, E.H.; Fiorentini, Â.M. Preservation of meat products with bacteriocins produced by lactic acid bacteria isolated from meat. J. Food Qual. 2019, 2019, 4726510. [Google Scholar] [CrossRef]
- Ketnawa, S.; Rungraeng, N.; Rawdkuen, S. Phase partitioning for enzyme separation: An overview and recent applications. Int. Food Res. J. 2017, 24, 1–24. [Google Scholar]
- Da Silva Sabo, S.; Lopes, A.M.; de Carvalho Santos-Ebinuma, V.; de Oliveira Rangel-Yagui, C.; de Souza Oliveira, R.P. Bacteriocin partitioning from a clarified fermentation broth of Lactobacillus plantarum ST16Pa in aqueous two-phase systems with sodium sulfate and choline-based salts as additives. Process Biochem. 2018, 66, 212–221. [Google Scholar] [CrossRef]
- Nainegali, B.; Iyyaswami, R.; Belur, P. Aqueous two-phase extraction of anthocyanin from fruits of garcinia indica. Int. J. Earth Sci. Eng. 2017, 10, 688–692. [Google Scholar]
- Asenjo, J.A.; Andrews, B.A. Aqueous two-phase systems for protein separation: A perspective. J. Chromatogr. A 2011, 1218, 8826–8835. [Google Scholar] [CrossRef]
- Marcos, J.; Fonseca, L.; Ramalho, M.; Cabral, J. Variation of penicillin acylase partition coefficient with phase volume ratio in poly (ethylene glycol)–sodium citrate aqueous two-phase systems. J. Chromatogr. B 1998, 711, 295–299. [Google Scholar] [CrossRef]
- Chaiwut, P.; Pintathong, P.; Rawdkuen, S. Extraction and three-phase partitioning behavior of proteases from papaya peels. Process Biochem. 2010, 45, 1172–1175. [Google Scholar] [CrossRef]
- Nour, I.; Fattouh, F.; El-Adawi, H. Antibacterial bioactivity of selected lactic acid bacterial strains against some human pathogenic bacteria. Int. J. Pharmacol. 2015, 11, 440–447. [Google Scholar]
- Kim, H.J.; Kim, J.H.; Son, J.H.; Seo, H.J.; Park, S.J.; Paek, N.S.; Kim, S.K. Characterization of bacteriocin produced by Lactobacillus bulgaricus. J. Microbiol. Biotechnol. 2004, 14, 503–508. [Google Scholar]
- Balogu, T.; Yunusa, A.; Aliyu, H. Antimicrobial efficiency of purified and characterized bacteriocins produced by Lactobacillus bulgaricusY34 and LactococcuslactisN22 isolated from fermented milk products. Int. J. Adv. Res. 2013, 1, 63–70. [Google Scholar]
- Ng, Z.J.; Zarin, M.A.; Lee, C.K.; Phapugrangkul, P.; Tan, J.S. Isolation and characterization of Enterococcus faecium DSM 20477 with ability to secrete antimicrobial substance for the inhibition of oral pathogen Streptococcus mutans UKMCC 1019. Arch. Oral Biol. 2020, 110, 104617. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]

| PEG Molecular Weight g/mol | Concentration % (w/w) | Purification Factor | Recovery Yield % |
|---|---|---|---|
| 2000 | 10 | 0 | 0 |
| 20 | 1.15 | 81.79 | |
| 30 | 1.15 | 84.89 | |
| 40 | 1.20 | 84.25 | |
| 4000 | 10 | 1.64 | 82.63 |
| 20 | 1.40 | 86.68 | |
| 30 | 1.10 | 65.50 | |
| 40 | 0.77 | 47.83 | |
| 6000 | 10 | 1.16 | 55.54 |
| 20 | 1.10 | 78.16 | |
| 30 | 1.06 | 84.46 | |
| 40 | 1.00 | 86.13 | |
| 8000 | 10 | 1.30 | 80.41 |
| 20 | 0.82 | 69.10 | |
| 30 | 0.71 | 72.38 | |
| 40 | 0.62 | 62.38 |
| PEG Concentration | pH of Sodium Citrate | Concentration of Sodium Citrate | Purification Factor | Recovery Yield (%) | ||
|---|---|---|---|---|---|---|
| Actual | Predicted | Actual | Predicted | |||
| 10.00 | 5.00 | 1.00 | 2.46 | 2.33 | 86.32 | 91.87 |
| 15.00 | 5.00 | 1.00 | 1.79 | 1.80 | 88.02 | 82.62 |
| 10.00 | 7.00 | 1.00 | 2.44 | 2.48 | 86.58 | 95.34 |
| 10.00 | 5.00 | 5.00 | 0.31 | 0.23 | 88.44 | 35.94 |
| 15.00 | 5.00 | 5.00 | 1.17 | 1.05 | 78.54 | 94.72 |
| 10.00 | 7.00 | 5.00 | 2.53 | 2.44 | 83.36 | 49.01 |
| 15.00 | 7.00 | 5.00 | 1.22 | 1.27 | 83.87 | 42.70 |
| 8.30 | 6.00 | 3.00 | 1.33 | 1.46 | 91.24 | 49.16 |
| 12.50 | 4.32 | 3.00 | 1.52 | 1.69 | 92.34 | 95.26 |
| 12.50 | 7.68 | 3.00 | 2.03 | 2.00 | 83.87 | 73.35 |
| 12.50 | 6.00 | -0.36 | 2.29 | 2.31 | 84.38 | 91.07 |
| 12.50 | 6.00 | 6.36 | 1.54 | 1.65 | 80.57 | 89.08 |
| 12.50 | 6.00 | 3.00 | 3.00 | 2.84 | 82.35 | 82.21 |
| 12.50 | 6.00 | 3.00 | 2.87 | 2.84 | 82.60 | 82.21 |
| 12.50 | 6.00 | 3.00 | 2.83 | 2.84 | 85.65 | 82.21 |
| 12.50 | 6.00 | 3.00 | 2.50 | 2.84 | 83.87 | 82.21 |
| 12.50 | 6.00 | 3.00 | 2.88 | 2.84 | 83.7 | 82.21 |
| 12.50 | 6.00 | 3.00 | 2.94 | 2.84 | 81.58 | 82.21 |
| Predicted optimum conditions | ||||||
| 11.44 | 6.06 | 1.98 | 3.25 ± 0.03 | 2.98 | 82.69 ± 0.06 | 84.28 |
| Source | Sum of Squares | Degree of Freedom | Mean Square | F Value | p-Value Prob > F |
|---|---|---|---|---|---|
| (a) Purification Factor | |||||
| Model | 9.82 | 9 | 1.09 | 32.90 | <0.0001 a |
| A | 0.97 | 1 | 0.97 | 29.18 | 0.0006 |
| B | 0.089 | 1 | 0.089 | 2.69 | 0.1393 |
| C | 0.40 | 1 | 0.40 | 11.97 | 0.0086 |
| AB | 1.30 | 1 | 1.30 | 39.31 | 0.0002 |
| AC | 0.60 | 1 | 0.60 | 18.12 | 0.0028 |
| BC | 1.39 | 1 | 1.39 | 41.85 | 0.0002 |
| A2 | 3.49 | 1 | 3.49 | 105.21 | <0.0001 |
| B2 | 1.70 | 1 | 1.70 | 51.15 | <0.0001 |
| C2 | 1.25 | 1 | 1.25 | 37.78 | 0.0003 |
| Residual | 0.27 | 8 | 0.033 | ||
| Lack of fit | 0.11 | 3 | 0.038 | 1.23 | 0.3904 b |
| Pure error | 0.15 | 5 | 0.031 | ||
| Cor Total | 10.08 | 17 | |||
| (b) Recovery Yield | |||||
| Model | 157.69 | 9 | 17.52 | 2.56 | 0.1001 b |
| A | 5.07 | 1 | 5.07 | 0.74 | 0.4145 |
| B | 9.44 | 1 | 9.44 | 1.38 | 0.2740 |
| C | 26.68 | 1 | 26.68 | 3.90 | 0.0838 |
| AB | 9.65 | 1 | 9.65 | 1.41 | 0.2691 |
| AC | 14.34 | 1 | 14.34 | 2.09 | 0.1859 |
| BC | 0.044 | 1 | 0.044 | 6.485 × 10−3 | 0.9378 |
| A2 | 19.07 | 1 | 19.07 | 2.79 | 0.1336 |
| B2 | 28.60 | 1 | 28.60 | 4.18 | 0.0752 |
| C2 | 4.04 | 1 | 4.04 | 0.59 | 0.4645 |
| Residual | 54.75 | 8 | 6.84 | ||
| Lack of fit | 44.40 | 3 | 14.80 | 7.14 | 0.0295 a |
| Pure error | 10.36 | 5 | 2.07 | ||
| Cor Total | 212.45 | 17 | |||
Sample Availability: Samples of the compounds are available from the authors. | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdul Aziz, N.F.H.; Abbasiliasi, S.; Ng, Z.J.; Abu Zarin, M.; Oslan, S.N.; Tan, J.S.; Ariff, A.B. Recovery of a Bacteriocin-Like Inhibitory Substance from Lactobacillus bulgaricus FTDC 1211 Using Polyethylene-Glycol Impregnated Amberlite XAD-4 Resins System. Molecules 2020, 25, 5332. https://doi.org/10.3390/molecules25225332
Abdul Aziz NFH, Abbasiliasi S, Ng ZJ, Abu Zarin M, Oslan SN, Tan JS, Ariff AB. Recovery of a Bacteriocin-Like Inhibitory Substance from Lactobacillus bulgaricus FTDC 1211 Using Polyethylene-Glycol Impregnated Amberlite XAD-4 Resins System. Molecules. 2020; 25(22):5332. https://doi.org/10.3390/molecules25225332
Chicago/Turabian StyleAbdul Aziz, Nur Fazrin Husna, Sahar Abbasiliasi, Zhang Jin Ng, Mazni Abu Zarin, Siti Nurbaya Oslan, Joo Shun Tan, and Arbakariya Bin Ariff. 2020. "Recovery of a Bacteriocin-Like Inhibitory Substance from Lactobacillus bulgaricus FTDC 1211 Using Polyethylene-Glycol Impregnated Amberlite XAD-4 Resins System" Molecules 25, no. 22: 5332. https://doi.org/10.3390/molecules25225332
APA StyleAbdul Aziz, N. F. H., Abbasiliasi, S., Ng, Z. J., Abu Zarin, M., Oslan, S. N., Tan, J. S., & Ariff, A. B. (2020). Recovery of a Bacteriocin-Like Inhibitory Substance from Lactobacillus bulgaricus FTDC 1211 Using Polyethylene-Glycol Impregnated Amberlite XAD-4 Resins System. Molecules, 25(22), 5332. https://doi.org/10.3390/molecules25225332







