Application of Humanized Zebrafish Model in the Suppression of SARS-CoV-2 Spike Protein Induced Pathology by Tri-Herbal Medicine Coronil via Cytokine Modulation
Abstract
1. Introduction
2. Results
2.1. Ultra-High-Performance Liquid Chromatography (UHPLC) Analysis of Coronil Detected the Presence of Bio-Active Metabolites
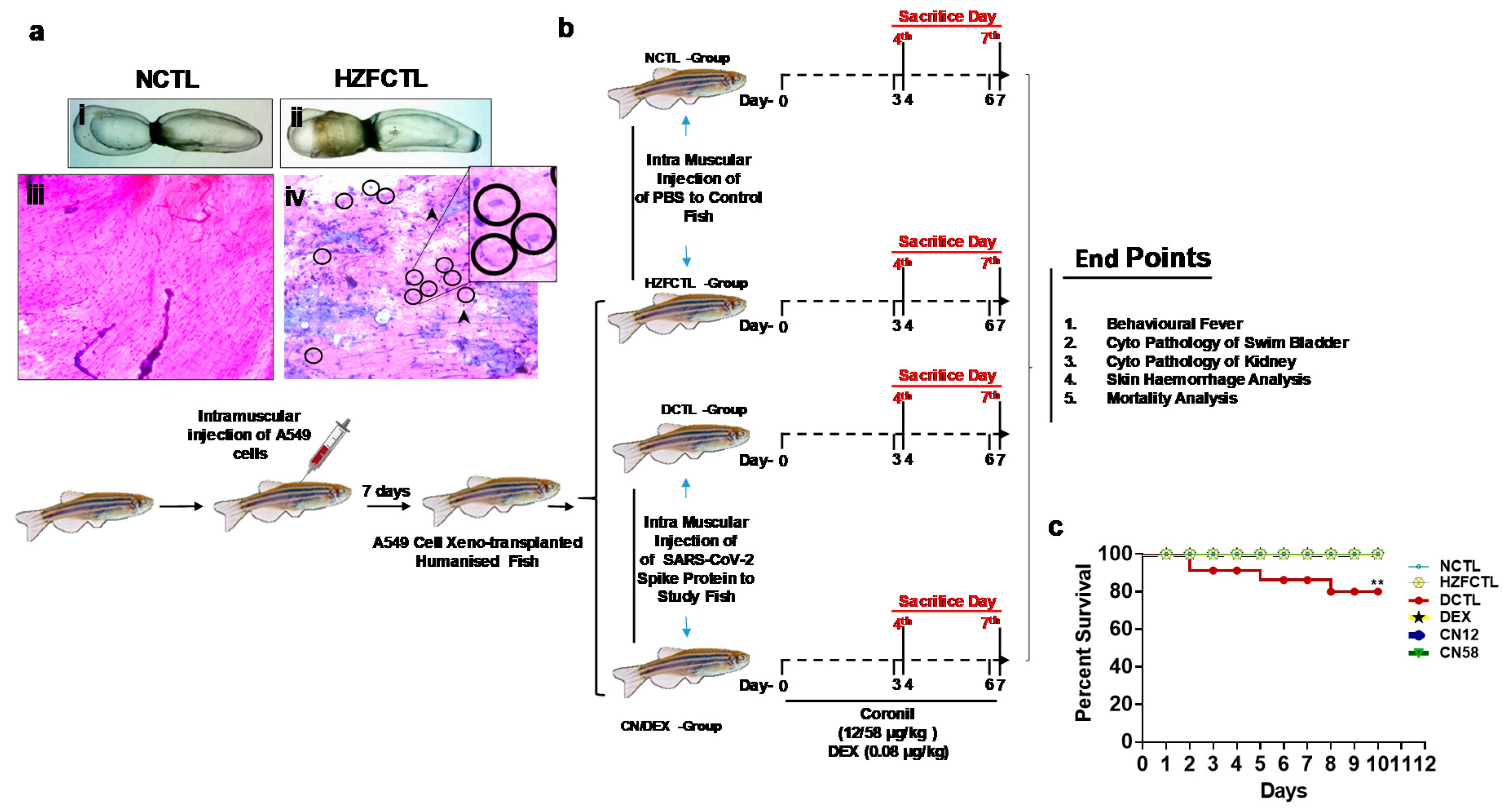
2.2. Generation of Humanized Zebrafish (HZF) Model and Induction of SARS-CoV-2 Spike Protein Stimulated Pathology
2.3. Study Design for Translational Dosing
2.4. Coronil Inhibits SARS-CoV-2 Spike Protein Induced Mortality of Zebrafish
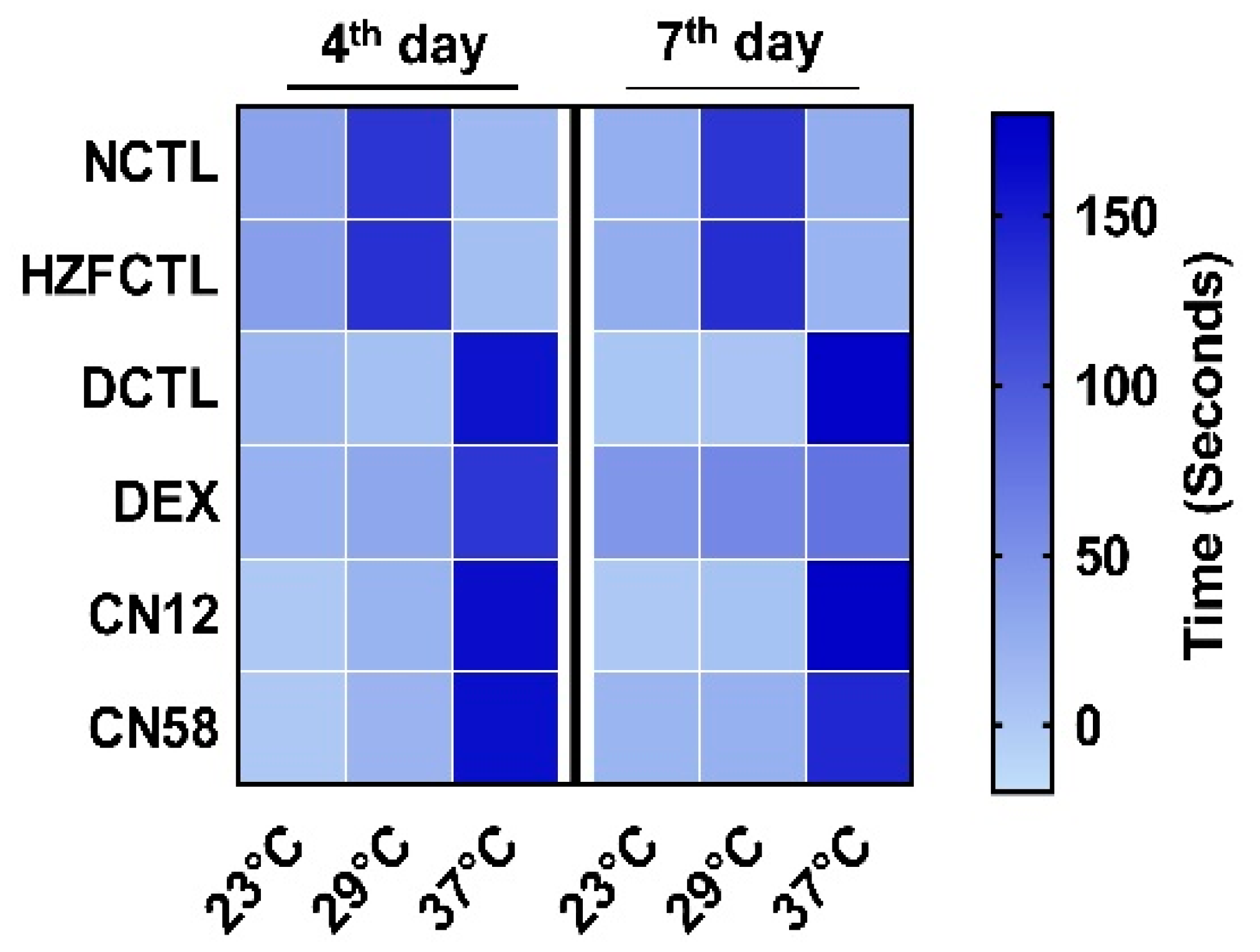
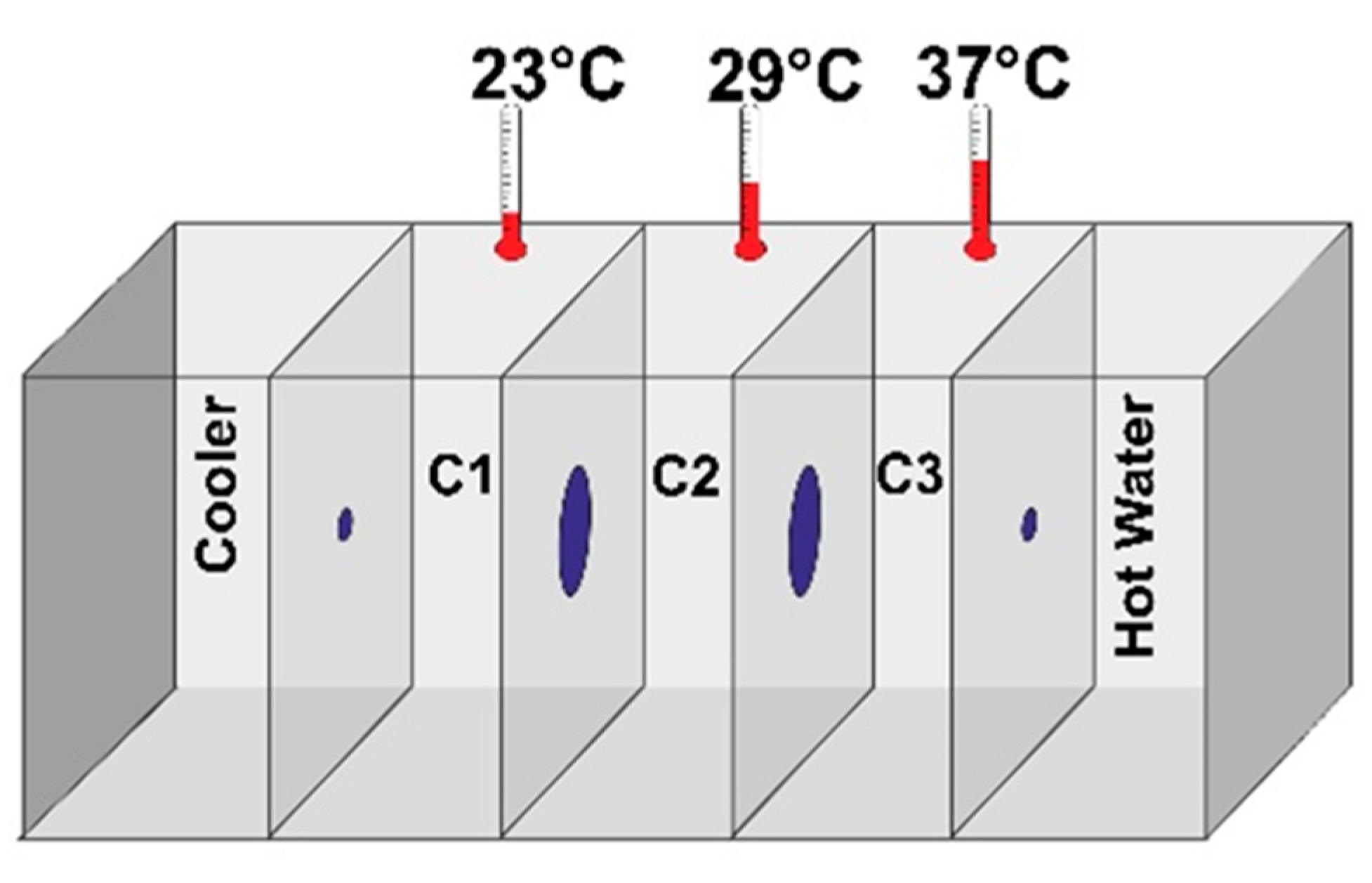
2.5. Coronil Reduces SARS-CoV-2 Spike Protein Induced Behavioral Fever
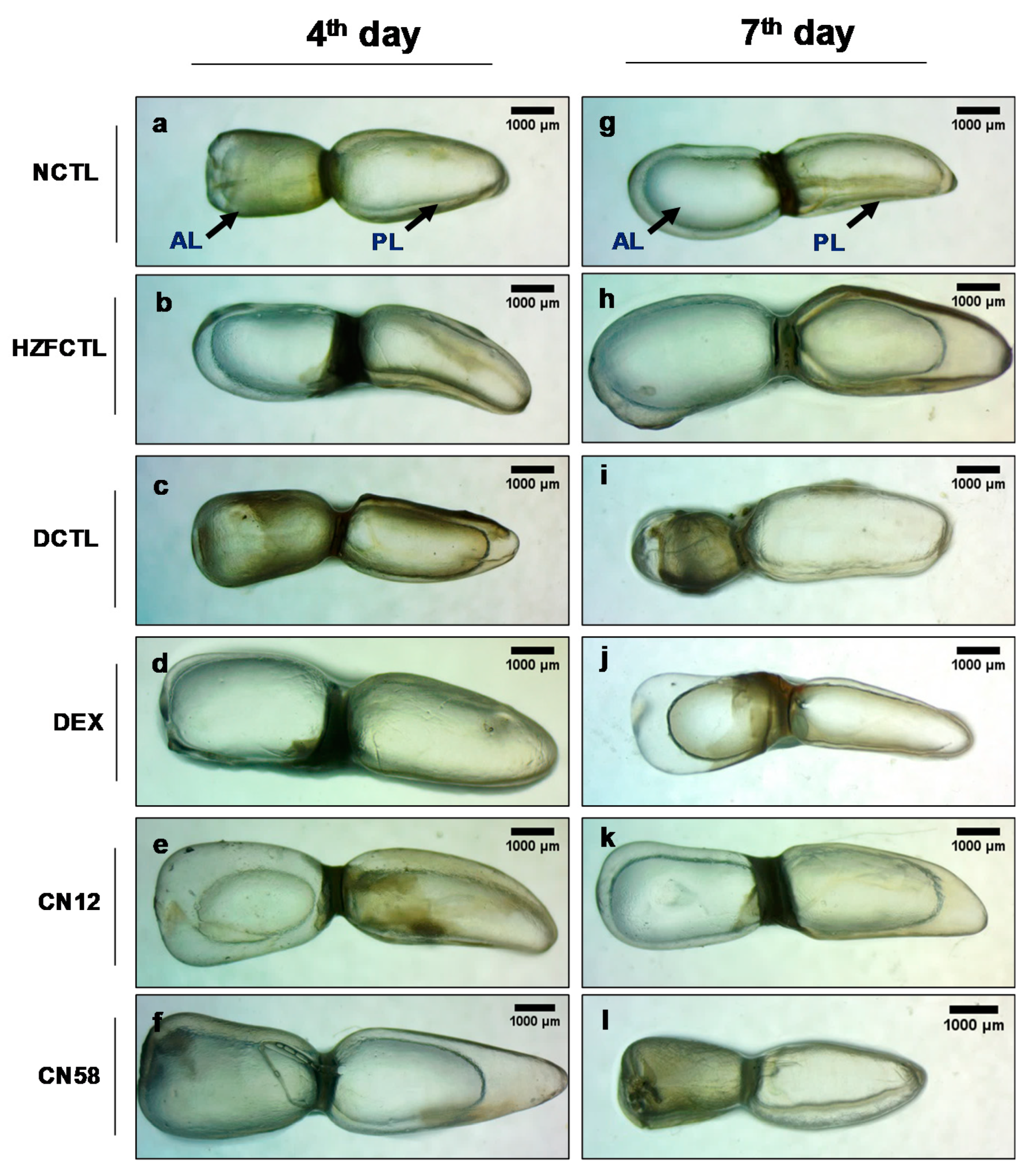
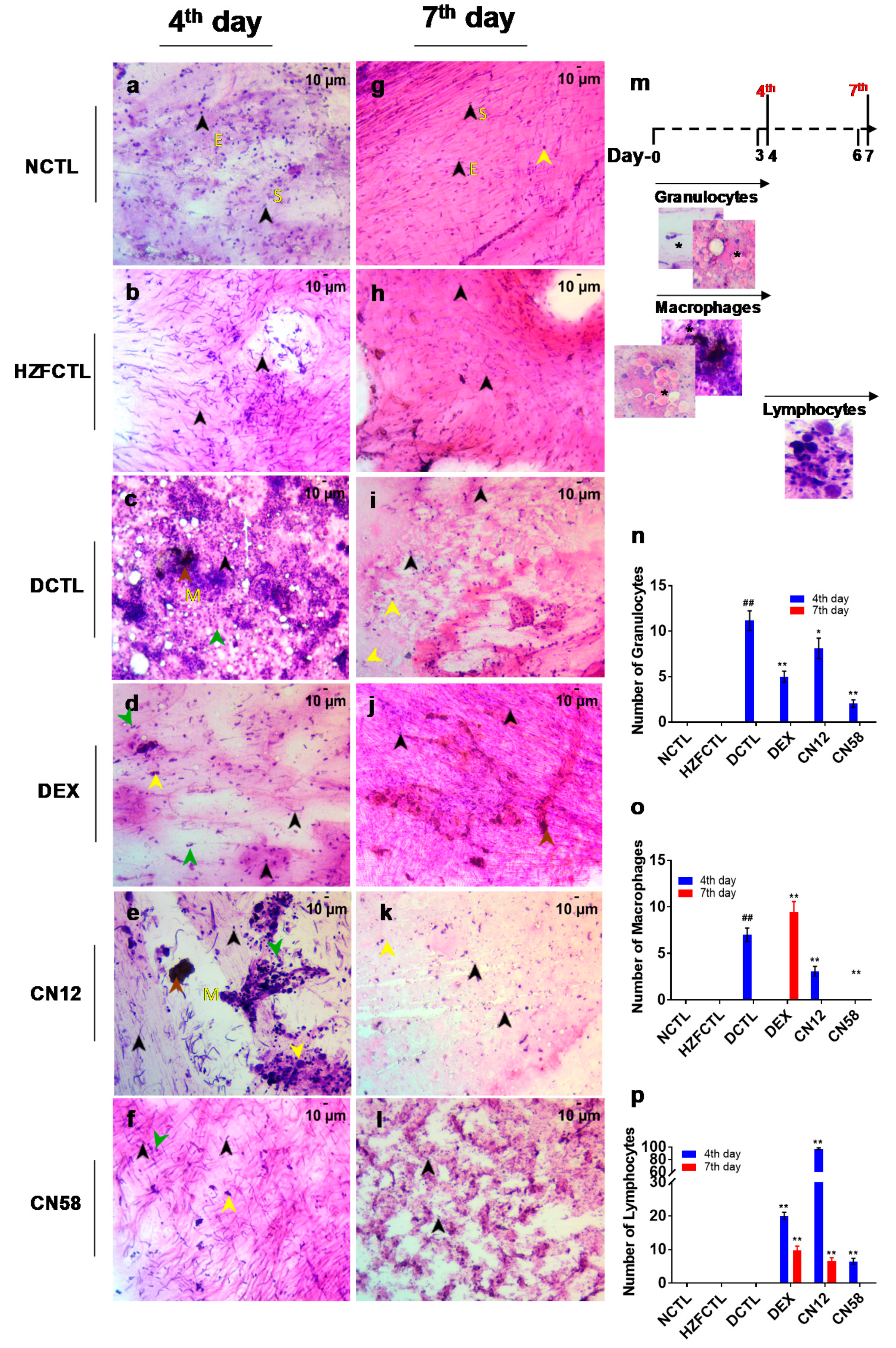
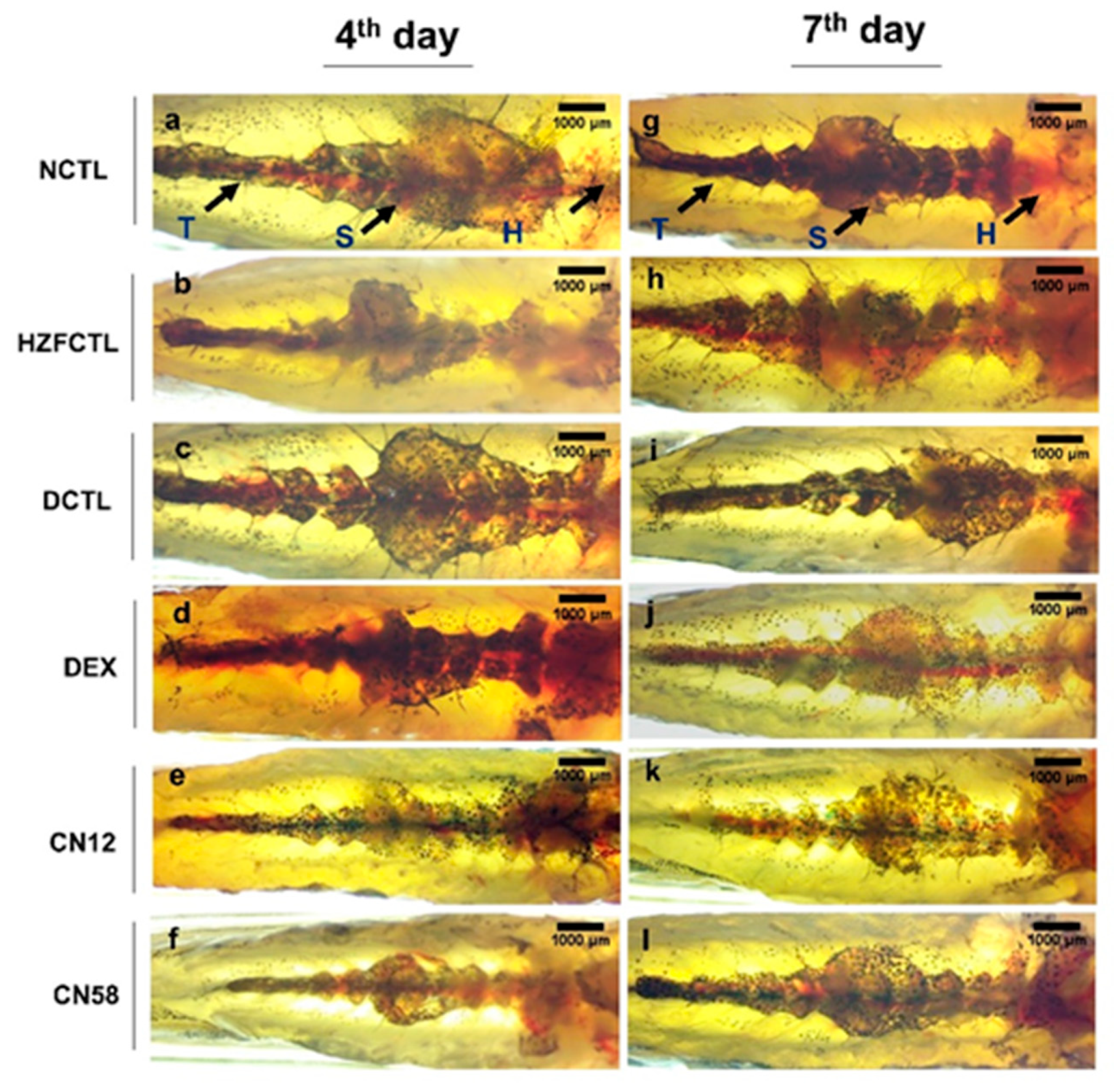
2.6. Swim Bladder Analysis Identified the Protective Response of Coronil
2.7. Coronil Attenuates SARS-CoV-2 Spike Protein Induced Inflammation in Swim Bladder
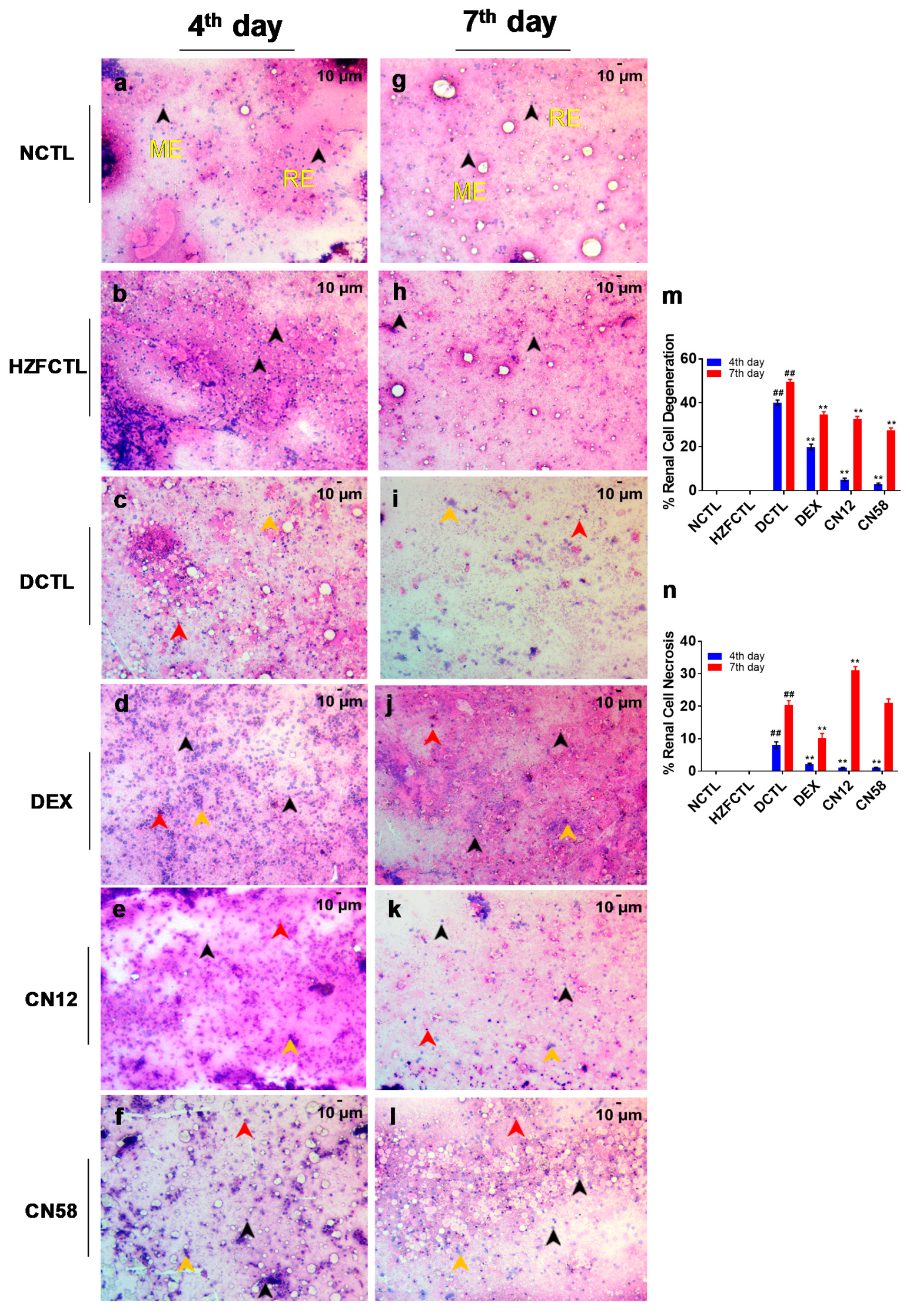
2.8. Coronil Inhibits SARS-CoV-2 Spike Protein Induced Renal Cell Necrosis
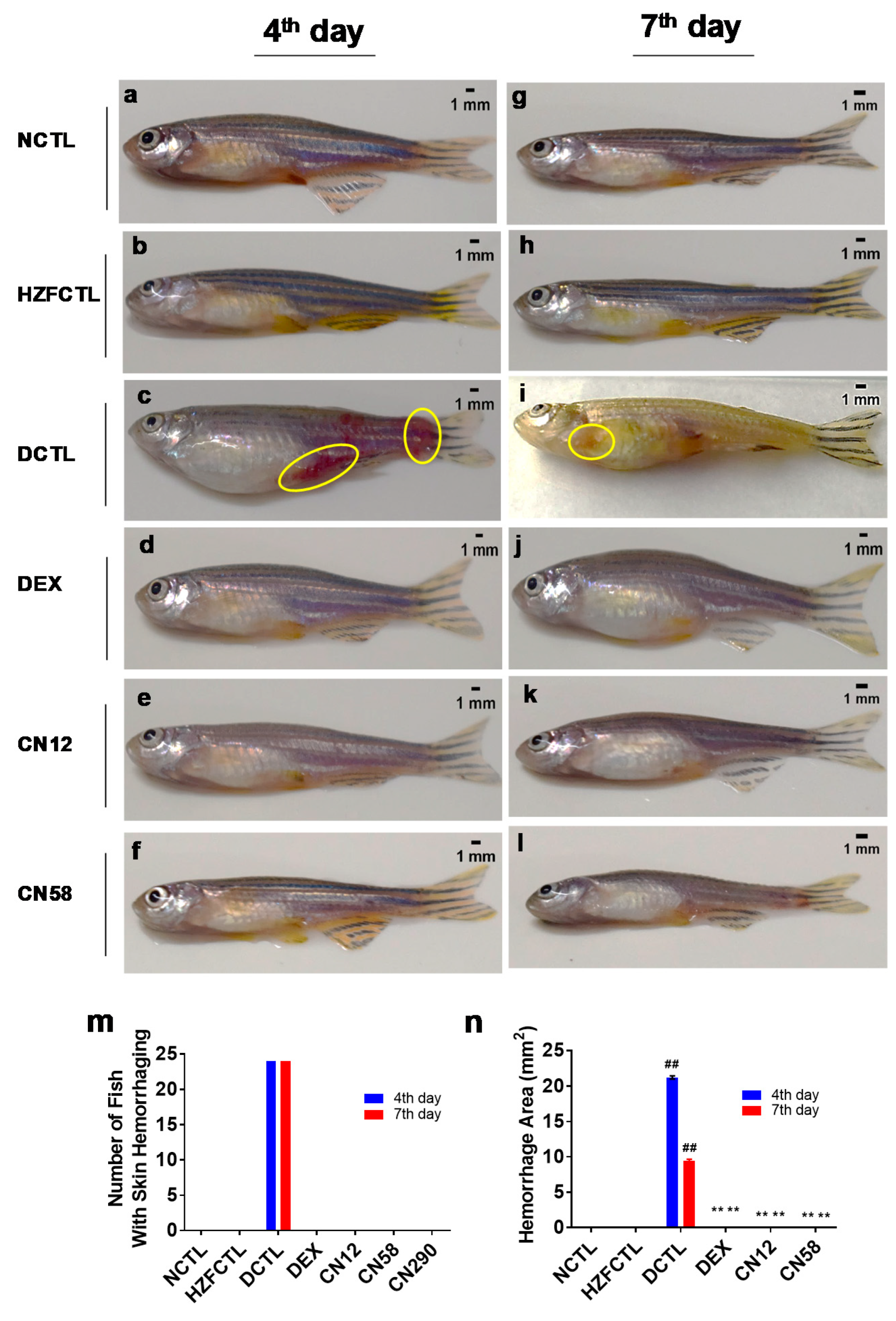
2.9. Coronil Attenuates SARS-CoV-2 Spike Protein Induced Hemorrhage
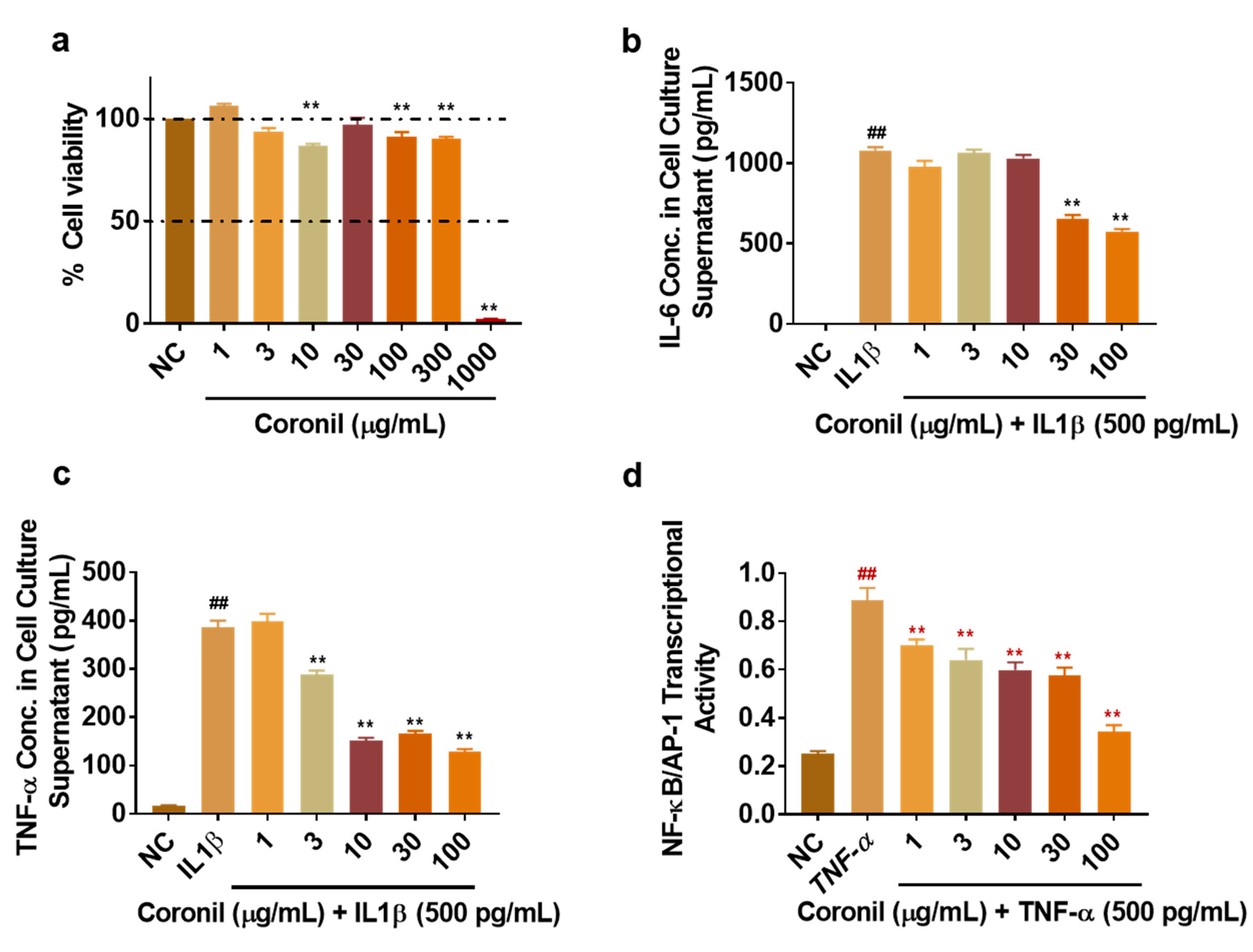
2.10. Coronil Inhibits IL-1β Induced Secretion of IL-6 and TNF-α in Human Alveolar Epithelial Cells and Attenuates Transcriptional Activation of NF-κB/AP-1 Pathway
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Preparation of Coronil Sample for UHPLC
4.3. Zebrafish Care and Maintenance
4.4. Preparation of Zebrafish Test Feed and Dosing
4.5. Xeno-Transplantation of A549 Cells into Humanized Zebrafish Using Intramuscular Injection
4.6. Induction of Pathology Milieu of SARS-CoV-2 Infection in Zebrafish
4.7. Survival Tests
4.8. Behavioral Fever Assessment
4.9. Examination for Presence of Skin Hemorrhage
4.10. Harvesting Swim Bladder and Kidney for Anatomical Observations
4.11. Cytology of Swim Bladder and Kidney
4.12. In Vitro Cell-Biology Assays
4.12.1. Cell Culture
4.12.2. A549 Cell Viability Assay
4.12.3. In Vitro Anti-Inflammatory Activity of Coronil and Quantification of IL-6 and TNF-α Cytokines
4.12.4. Secreted Embryonic Alkaline Phosphatase (SEAP) Based NF-κB/AP-1 Reporter Assay
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wu, F.; Zhao, S.; Yu, B.; Chen, Y.-M.; Wang, W.; Song, Z.-G.; Hu, Y.; Tao, Z.-W.; Tian, J.-H.; Pei, Y.-Y.; et al. A new coronavirus associated with human respiratory disease in China. Nature 2020, 579, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Dong, E.; Du, H.; Gardner, L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect. Dis. 2020, 20, 533–534. [Google Scholar] [CrossRef]
- Fehr, A.R.; Perlman, S. Coronaviruses: An overview of their replication and pathogenesis. Methods Mol. Biol. 2015, 1282, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Peiris, J.S.; Yuen, K.Y.; Osterhaus, A.D.; Stöhr, K. The severe acute respiratory syndrome. N. Engl. J. Med. 2003, 349, 2431–2441. [Google Scholar] [CrossRef] [PubMed]
- Zaki, A.M.; Van Boheemen, S.; Bestebroer, T.M.; Osterhaus, A.D.; Fouchier, R.A. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 2012, 367, 1814–1820. [Google Scholar] [CrossRef] [PubMed]
- Gorbalenya, A.E.; Baker, S.C.; Baric, R.S.; de Groot, R.J.; Drosten, C.; Gulyaeva, A.A.; Haagmans, B.L.; Lauber, C.; Leontovich, A.M.; Neuman, B.W.; et al. The species severe acute respiratory syndrome-related coronavirus: Classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020, 5, 536–544. [Google Scholar]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Li, W.; Moore, M.J.; Vasilieva, N.; Sui, J.; Wong, S.K.; Berne, M.A.; Somasundaran, M.; Sullivan, J.L.; Luzuriaga, K.; Greenough, T.C.; et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nat. Cell Biol. 2003, 426, 450–454. [Google Scholar] [CrossRef]
- Lan, J.; Ge, J.; Yu, J.; Shan, S.; Zhou, H.; Fan, S.; Zhang, Q.; Shi, X.; Wang, Q.; Zhang, L.; et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nat. Cell Biol. 2020, 581, 215–220. [Google Scholar] [CrossRef]
- Hamming, I.; Timens, W.; Bulthuis, M.L.C.; Lely, A.T.; Navis, G.J.; Van Goor, H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004, 203, 631–637. [Google Scholar] [CrossRef]
- Walls, A.C.; Park, Y.-J.; Tortorici, M.A.; Wall, A.; McGuire, A.T.; Veesler, D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell 2020, 181, 281–292. [Google Scholar] [CrossRef]
- Kuba, K.; Imai, Y.; Penninger, J.M. Angiotensin-converting enzyme 2 in lung diseases. Curr. Opin. Pharmacol. 2006, 6, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Tay, M.Z.; Poh, C.M.; Rénia, L.; Macary, P.A.; Ng, L.F.P. The trinity of COVID-19: Immunity, inflammation and intervention. Nat. Rev. Immunol. 2020, 20, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Siu, K.-L.; Chan, C.-P.; Kok, K.-H.; Woo, P.C.-Y.; Jin, D.-Y. Suppression of innate antiviral response by severe acute respiratory syndrome coronavirus M protein is mediated through the first transmembrane domain. Cell. Mol. Immunol. 2014, 11, 141–149. [Google Scholar] [CrossRef]
- Ruan, Q.; Yang, K.; Wang, W.; Jiang, L.; Song, J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensiv. Care Med. 2020, 46, 846–848. [Google Scholar] [CrossRef] [PubMed]
- Takayama, K. In Vitro and Animal Models for SARS-CoV-2 research. Trends Pharmacol. Sci. 2020, 41, 513–517. [Google Scholar] [CrossRef]
- Galindo-Villegas, J. The zebrafish disease and drug screening model: A strong ally against Covid-19. Front. Pharmacol. 2020, 11, 680. [Google Scholar] [CrossRef]
- Levraud, J.-P.; Palha, N.; Langevin, C.; Boudinot, P. Through the looking glass: Witnessing host–virus interplay in zebrafish. Trends Microbiol. 2014, 22, 490–497. [Google Scholar] [CrossRef]
- Balkrishna, A.; Solleti, S.K.; Verma, S.; Varshney, A. Validation of a novel zebrafish model of dengue virus (DENV-3) pathology using the pentaherbal medicine Denguenil Vati. Biomolecules 2020, 10, 971. [Google Scholar] [CrossRef]
- Taylor, K.L.; Grant, N.J.; Temperley, N.D.; Patton, E.E. Small molecule screening in zebrafish: An in vivo approach to identifying new chemical tools and drug leads. Cell Commun. Signal. 2010, 8, 11. [Google Scholar] [CrossRef]
- Varela, M.; Figueras, A.; Novoa, B. Modelling viral infections using zebrafish: Innate immune response and antiviral research. Antivir. Res. 2017, 139, 59–68. [Google Scholar] [CrossRef]
- Sepahi, A.; Kraus, A.; Casadei, E.; Johnston, C.A.; Galindo-Villegas, J.; Kelly, C.; García-Moreno, D.; Muñoz, P.; Mulero, V.; Huertas, M.; et al. Olfactory sensory neurons mediate ultrarapid antiviral immune responses in a TrkA-dependent manner. Proc. Natl. Acad. Sci. USA 2019, 116, 12428–12436. [Google Scholar] [CrossRef]
- Gomes, M.C.; Mostowy, S. The case for modeling human infection in zebrafish. Trends Microbiol. 2020, 28, 10–18. [Google Scholar] [CrossRef]
- Shen, W.; Pu, J.; Sun, J.; Tan, B.; Wang, W.; Wang, L.; Cheng, J.; Zuo, Y. Zebrafish xenograft model of human lung cancer for studying the function of LINC00152 in cell proliferation and invasion. Cancer Cell Int. 2020, 20, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.; Cho, J.Y.; Pham, A.; Ramsdell, J.; Broide, D.H. Adiponectin and functional adiponectin receptor 1 are expressed by airway epithelial cells in chronic obstructive pulmonary disease. J. Immunol. 2008, 182, 684–691. [Google Scholar] [CrossRef]
- Carterson, A.J.; Zu Bentrup, K.H.; Ott, C.M.; Clarke, M.S.; Pierson, D.L.; Vanderburg, C.R.; Buchanan, K.L.; Nickerson, C.A.; Schurr, M.J. A549 lung epithelial cells grown as three-dimensional aggregates: Alternative tissue culture model for pseudomonas aeruginosa pathogenesis. Infect. Immun. 2005, 73, 1129–1140. [Google Scholar] [CrossRef]
- The RECOVERY Collaborative Group; Horby, P.; Lim, W.S.; Emberson, J.R.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A.; et al. Dexamethasone in hospitalized patients with COVID-19—Preliminary report. N. Engl. J. Med. 2020. [Google Scholar] [CrossRef]
- Ren, J.-L.; Zhang, A.-H.; Wang, X.-J. Traditional Chinese medicine for COVID-19 treatment. Pharmacol. Res. 2020, 155, 104743. [Google Scholar] [CrossRef] [PubMed]
- Runfeng, L.; Yunlong, H.; Jicheng, H.; Weiqi, P.; Qinhai, M.; Yongxia, S.; Chufang, L.; Jin, Z.; Zhenhua, J.; Haiming, J.; et al. Lianhuaqingwen exerts anti-viral and anti-inflammatory activity against novel coronavirus (SARS-CoV-2). Pharmacol. Res. 2020, 156, 104761. [Google Scholar] [CrossRef]
- Rastogi, S.; Pandey, D.N.; Singh, R.H. COVID-19 pandemic: A pragmatic plan for ayurveda intervention. J. Ayurveda Integr. Med. 2020. [Google Scholar] [CrossRef]
- Sharma, U.; Bala, M.; Kumar, N.; Singh, B.; Munshi, R.K.; Bhalerao, S. Immunomodulatory active compounds from Tinospora cordifolia. J. Ethnopharmacol. 2012, 141, 918–926. [Google Scholar] [CrossRef] [PubMed]
- Uddin, Q.; Samiulla, L.; Singh, V.K.; Jamil, S.S. Phytochemical and pharmacological profile of Withania somnifera dunal: A review. J. Appl. Pharm. Sci. 2012, 2, 170–175. [Google Scholar]
- Cohen, M.M. Tulsi—Ocimum sanctum: A herb for all reasons. J. Ayurveda Integr. Med. 2014, 5, 251–259. [Google Scholar] [CrossRef]
- Borse, S.; Saggam, S.; Bhat, V.; Walia, S.; Sagar, S.; Chavan-Gautam, P.; Tillu, G. Ayurveda botanicals in COVID-19 management: An in silico-multitarget approach. Research Square 2020. Available online: https://www.researchsquare.com/article/rs-30361/v1 (accessed on 24 October 2020). [CrossRef]
- Coperchini, F.; Chiovato, L.; Croce, L.; Magri, F.; Rotondi, M. The cytokine storm in COVID-19: An overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev. 2020, 53, 25–32. [Google Scholar] [CrossRef]
- Barnes, P.J. Transcription factors in airway diseases. Lab. Investig. 2006, 86, 867–872. [Google Scholar] [CrossRef]
- National Institutes of Health. Available online: https://www.covid19treatmentguidelines.nih.gov/antiviral-therapy/ (accessed on 24 October 2020).
- Chibber, P.; Haq, S.A.; Ahmed, I.; Andrabi, N.I.; Singh, G. Advances in the possible treatment of COVID-19: A review. Eur. J. Pharmacol. 2020, 883, 173372. [Google Scholar] [CrossRef]
- Bhatia, M.; Zemans, R.L.; Jeyaseelan, S. Role of Chemokines in the Pathogenesis of Acute Lung Injury. Am. J. Respir. Cell Mol. Biol. 2012, 46, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Menter, T.; Haslbauer, J.D.; Nienhold, R.; Savic, S.; Hopfer, H.; Deigendesch, N.; Frank, S.; Turek, D.; Willi, N.; Pargger, H.; et al. Post-mortem examination of COVID19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings of lungs and other organs suggesting vascular dysfunction. Histopathology 2020, 77, 198–209. [Google Scholar] [CrossRef]
- Benedetti, C.; Waldman, M.; Zaza, G.; Riella, L.V.; Cravedi, P. COVID-19 and the kidneys: An update. Front. Med. 2020, 7, 423. [Google Scholar] [CrossRef]
- Batlle, D.; Soler, M.J.; Sparks, M.A.; Hiremath, S.; South, A.M.; Welling, P.A.; Swaminathan, S. Acute kidney injury in COVID-19: Emerging evidence of a distinct pathophysiology. J. Am. Soc. Nephrol. 2020, 31, 1380–1383. [Google Scholar] [CrossRef]
- Yang, L.; Liu, S.; Liu, J.; Zhang, Z.; Wan, X.; Huang, B.; Chen, Y.; Zhang, Y. COVID-19: Immunopathogenesis and immunotherapeutics. Signal Transduct. Target. Ther. 2020, 5, 1–8. [Google Scholar] [CrossRef]
- Haick, A.K.; Rzepka, J.P.; Brandon, E.; Balemba, O.B.; Miura, T.A. Neutrophils are needed for an effective immune response against pulmonary rat coronavirus infection, but also contribute to pathology. J. Gen. Virol. 2014, 95, 578–590. [Google Scholar] [CrossRef]
- Tsui, P.T.; Kwok, M.L.; Yuen, H.; Lai, S.T. Severe Acute Respiratory Syndrome: Clinical Outcome and Prognostic Correlates. Emerg. Infect. Dis. 2003, 9, 1064–1069. [Google Scholar] [CrossRef]
- Nagata, N.; Iwata, N.; Hasegawa, H.; Fukushi, S.; Harashima, A.; Sato, Y.; Saijo, M.; Taguchi, F.; Morikawa, S.; Sata, T. Mouse-passaged severe acute respiratory syndrome-associated coronavirus leads to lethal pulmonary edema and diffuse alveolar damage in adult but not young mice. Am. J. Pathol. 2008, 172, 1625–1637. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Cassatella, M.A.; Costantini, C.; Jaillon, S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat. Rev. Immunol. 2011, 11, 519–531. [Google Scholar] [CrossRef]
- Zhao, Q.; Meng, M.; Kumar, R.; Wu, Y.; Huang, J.; Deng, Y.; Weng, Z.; Yang, L. Lymphopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: A systemic review and meta-analysis. Int. J. Infect. Dis. 2020, 96, 131–135. [Google Scholar] [CrossRef]
- Wagner, J.; Dupont, A.; Larson, S.; Cash, B.; Farooq, A. Absolute lymphocyte count is a prognostic marker in COVID-19: A retrospective cohort review. Int. J. Lab. Hematol. 2020, 13288. [Google Scholar] [CrossRef] [PubMed]
- Walls, A.C.; Xiong, X.; Park, Y.-J.; Tortorici, M.A.; Snijder, J.; Quispe, J.; Cameroni, E.; Gopal, R.; Dai, M.; Lanzavecchia, A.; et al. Unexpected receptor functional mimicry elucidates activation of coronavirus fusion. Cell 2019, 176, 1026–1039. [Google Scholar] [CrossRef]
- Conti, P.; Ronconi, G.; Caraffa, A.; Gallenga, C.; Ross, R.; Frydas, I.; Kritas, S. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by COVID-19: Anti-inflammatory strategies. J. Biol. Regul. Homeost. Agents 2020, 34, 327–331. [Google Scholar]
- Zhang, H.; Penninger, J.M.; Li, Y.; Zhong, N.; Slutsky, A.S. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: Molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020, 46, 586–590. [Google Scholar] [CrossRef]
- Moon, C. Fighting COVID-19 exhausts T cells. Nat. Rev. Immunol. 2020, 20, 277. [Google Scholar] [CrossRef]
- Tao, L.; Lowe, A.; Wang, G.; Dozmorov, I.; Chang, T.; Yan, N.; A Reese, T. Metabolic control of viral infection through PPAR-α regulation of STING signaling. bioRxiv. 2019, p. 731208. Available online: https://www.biorxiv.org/content/10.1101/731208v3.full (accessed on 24 October 2020).
- Illesca, P.; Valenzuela, R.; Espinosa, A.; Echeverría, F.; Soto-Alarcon, S.; Ortiz, M.; Videla, L.A. Hydroxytyrosol supplementation ameliorates the metabolic disturbances in white adipose tissue from mice fed a high-fat diet through recovery of transcription factors Nrf2, SREBP-1c, PPAR-γ and NF-κB. Biomed. Pharmacother. 2019, 109, 2472–2481. [Google Scholar] [CrossRef]
- Hernández-Rodas, M.C.; Valenzuela, R.; Echeverría, F.; Cervera, M.R.; Espinosa, A.; Illesca, P.; Muñoz, P.; Corbari, A.; Romero, N.; Gonzalez-Mañan, D.; et al. Supplementation with docosahexaenoic acid and extra virgin olive oil prevents liver steatosis induced by a high-fat diet in mice through PPAR-α and Nrf2 upregulation with concomitant SREBP-1c and NF-kB downregulation. Mol. Nutr. Food Res. 2017, 61, 1700479. [Google Scholar] [CrossRef]
- Brocker, C.; Thompson, D.; Matsumoto, A.; Nebert, D.W.; Vasiliou, V. Evolutionary divergence and functions of the human interleukin (IL) gene family. Hum. Genom. 2010, 5, 30–55. [Google Scholar] [CrossRef]
- A Hunter, C.; A Jones, S. IL-6 as a keystone cytokine in health and disease. Nat. Immunol. 2015, 16, 448–457. [Google Scholar] [CrossRef]
- Bethea, J.R.; Gillespie, G.Y.; Benveniste, E.N. Interleukin-1β induction of TNF-α gene expression: Involvement of protein kinase C. J. Cell. Physiol. 1992, 152, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.-S.; Cho, D.-H.; Kim, K.-S.; Kim, K.-H.; Park, J.; Kim, Y.; Jung, J.H.; Kim, K.-I.; Jung, H.; Jang, H.-J. Anti-inflammatory effects of embelin in A549 cells and human asthmatic airway epithelial tissues. Immunopharmacol. Immunotoxicol. 2018, 40, 83–90. [Google Scholar] [CrossRef]
- Carswell, E.A.; Old, L.J.; Kassel, R.L.; Green, S.; Fiore, N.; Williamson, B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc. Natl. Acad. Sci. USA 1975, 72, 3666–3670. [Google Scholar] [CrossRef]
- Haga, S.; Yamamoto, N.; Nakai-Murakami, C.; Osawa, Y.; Tokunaga, K.; Sata, T.; Sasazuki, T.; Ishizaka, Y. Modulation of TNF-α-converting enzyme by the spike protein of SARS-CoV and ACE2 induces TNF-α production and facilitates viral entry. Proc. Natl. Acad. Sci. USA 2008, 105, 7809–7814. [Google Scholar] [CrossRef]
- Panchabhai, T.S.; Kulkarni, U.P.; Rege, N.N. Validation of therapeutic claims of Tinospora cordifolia: A review. Phytotherapy Res. 2008, 22, 425–441. [Google Scholar] [CrossRef] [PubMed]
- Ernst, E. Major herbs of Ayurveda. Focus Altern. Complement. Ther. 2010, 8, 98. [Google Scholar] [CrossRef]
- Vyas, V.K.; Bhandari, P.; Patidar, R. A comprehensive review on Withania somnifera dunal. J. Nat. Remedies 2011, 11, 1–13. [Google Scholar] [CrossRef]
- Pattanayak, P.; Behera, P.; Das, D.; Panda, S.K. Ocimum sanctum Linn. A reservoir plant for therapeutic applications: An overview. Pharmacogn. Rev. 2010, 4, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Krupanidhi, S.; Peele, K.A.; Venkateswarulu, T.; Ayyagari, V.S.; Bobby, N.; Babu, D.J.; Narayana, A.V.; Aishwarya, G. Screening of phytochemical compounds of Tinospora cordifolia for their inhibitory activity on SARS-CoV-2: An in silico study. J. Biomol. Struct. Dyn. 2020, 1–5. [Google Scholar] [CrossRef]
- Balkrishna, A.; Subarna, P.; Varshney, A. Tinocordiside from Tinospora cordifolia (Giloy) may curb SARS-CoV-2 contagion by disrupting the electrostatic interactions between host ACE2 and viral S-protein receptor binding domain. Comb. Chem. High Throughput Screen. 2020, in press. [Google Scholar]
- Chowdhury, P. In silico investigation of phytoconstituents from Indian medicinal herb ‘Tinospora cordifolia (giloy)’ against SARS-CoV-2 (COVID-19) by molecular dynamics approach. J. Biomol. Struct. Dyn. 2020, 1–18. [Google Scholar] [CrossRef]
- Sharma, P.; Parmar, J.; Verma, P.; Goyal, P.K. Radiation-induced testicular injury and its amelioration by Tinospora cordifolia (an indian medicinal plant) extract. Evidence-Based Complement. Altern. Med. 2011, 2011, 1–9. [Google Scholar] [CrossRef]
- Van Kiem, P.; Van Minh, C.; Dat, N.T.; Van Kinh, L.; Hang, D.T.; Nam, N.H.; Cuong, N.X.; Huong, H.T.; Van Lau, T. Aporphine alkaloids, clerodane diterpenes, and other constituents from Tinospora cordifolia. Fitoterapia 2010, 81, 485–489. [Google Scholar] [CrossRef]
- Lee, B.-H.; Chathuranga, K.; Uddin, B.; Weeratunga, P.; Kim, M.S.; Cho, W.-K.; Kim, H.I.; Ma, J.Y.; Lee, J.-S. Coptidis Rhizoma extract inhibits replication of respiratory syncytial virus in vitro and in vivo by inducing antiviral state. J. Microbiol. 2017, 55, 488–498. [Google Scholar] [CrossRef]
- Balkrishna, A.; Pokhrel, S.; Singh, J.; Varshney, A. Withanone from Withania somnifera may inhibit novel coronavirus (COVID-19) entry by disrupting interactions between viral S-protein receptor binding domain and host ACE2 receptor. Research Square 2020. Available online: https://www.researchsquare.com/article/rs-17806/v1 (accessed on 24 October 2020). [CrossRef]
- Chikhale, R.V.; Gurav, S.S.; Patil, R.B.; Sinha, S.K.; Prasad, S.K.; Shakya, A.; Shrivastava, S.K.; Gurav, N.S.; Prasad, R.S. SARS-CoV-2 host entry and replication inhibitors from Indian ginseng: An in-silico approach. J. Biomol. Struct. Dyn. 2020, 1–12. [Google Scholar] [CrossRef]
- Kumar, V.; Dhanjal, J.K.; Bhargava, P.; Kaul, A.; Wang, J.; Zhang, H.; Kaul, S.C.; Wadhwa, R.; Sundar, D. Withanone and withaferin-A are predicted to interact with transmembrane protease serine 2 (TMPRSS2) and block entry of SARS-CoV-2 into cells. J. Biomol. Struct. Dyn. 2020, 1–13. [Google Scholar] [CrossRef]
- Kumar, V.; Dhanjal, J.K.; Kaul, S.C.; Wadhwa, R.; Sundar, D. Withanone and caffeic acid phenethyl ester are predicted to interact with main protease (Mpro) of SARS-CoV-2 and inhibit its activity. J. Biomol. Struct. Dyn. 2020, 1–27. [Google Scholar] [CrossRef]
- Tripathi, M.K.; Singh, P.; Sharma, S.; Singh, T.P.; Ethayathulla, A.S.; Kaur, P. Identification of bioactive molecule from Withania somnifera (Ashwagandha) as SARS-CoV-2 main protease inhibitor. J. Biomol. Struct. Dyn. 2020, 1–14. [Google Scholar] [CrossRef]
- Tohmé, M.J.; Giménez, M.G.; Peralta, A.; Colombo, M.I.; Delgui, L.R. Ursolic acid: A novel antiviral compound inhibiting rotavirus infection in vitro. Int. J. Antimicrob. Agents 2019, 54, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Yao, D.; Li, H.; Gou, Y.; Zhang, H.; Vlessidis, A.G.; Zhou, H.; Evmiridis, N.P.; Liu, Z. Betulinic acid-mediated inhibitory effect on hepatitis B virus by suppression of manganese superoxide dismutase expression. FEBS J. 2009, 276, 2599–2614. [Google Scholar] [CrossRef]
- Kumar, A.; Choudhir, G.; Shukla, S.K.; Sharma, M.; Tyagi, P.; Bhushan, A.; Rathore, M. Identification of phytochemical inhibitors against main protease of COVID-19 using molecular modeling approaches. J. Biomol. Struct. Dyn. 2020, 1–21. [Google Scholar] [CrossRef]
- Liu, J.-X.; Zhang, Y.; Hu, Q.-P.; Li, J.-Q.; Liu, Y.-T.; Wu, Q.-G.; Wu, J.; Lai, X.-P.; Zhang, Z.-D.; Li, X.; et al. Anti-inflammatory effects of rosmarinic acid-4-O-β-D-glucoside in reducing acute lung injury in mice infected with influenza virus. Antivir. Res. 2017, 144, 34–43. [Google Scholar] [CrossRef]
- Ducharme, N.A.; Reif, D.M.; Gustafsson, J.-A.; Bondesson, M. Comparison of toxicity values across zebrafish early life stages and mammalian studies: Implications for chemical testing. Reprod. Toxicol. 2015, 55, 3–10. [Google Scholar] [CrossRef]
- Shin, J.; Seol, I.; Son, C. Interpretation of animal dose and human equivalent dose for drug development. J. Korean Orient. Med. 2010, 31, 1–7. [Google Scholar]
- Boltaña, S.; Reynaldo, V.; Roher, N.; Vargas, R.; Huerta, M.; Huntingford, F.A.; Goetz, F.W.; Moore, J.; Garcia-Valtanen, P.; Estepa, A.; et al. Behavioural fever is a synergic signal amplifying the innate immune response. Proc. R. Soc. B Boil. Sci. 2013, 280, 20131381. [Google Scholar] [CrossRef]
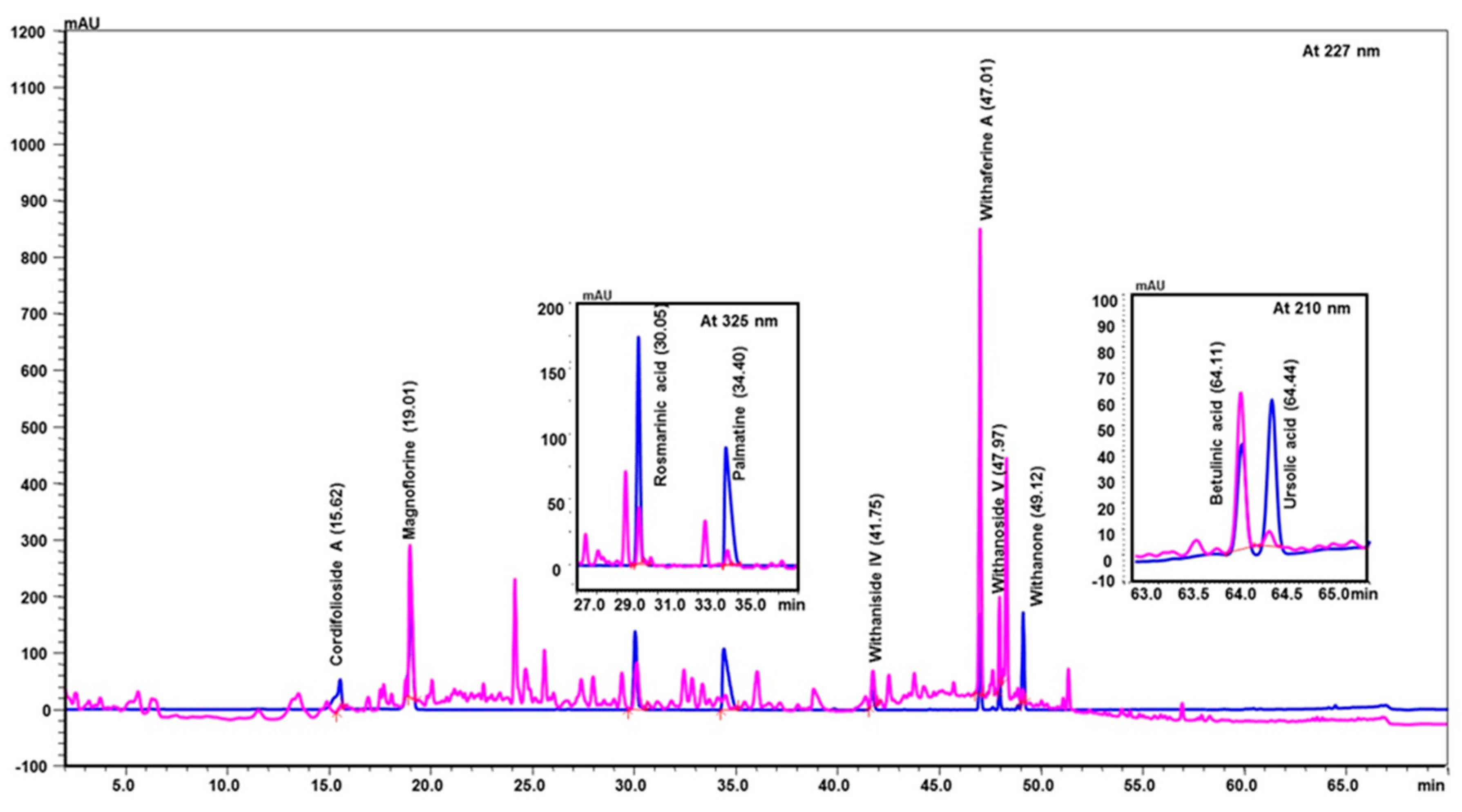
| Peak No. | Name of Phyto-Metabolites | Structure (as per PubChem Database) | Retention Time (min.) | Quantity (µg/mg) | Plant Species of Origin |
|---|---|---|---|---|---|
| 1 | Cordifolioside A | 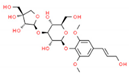 | 15.64 | 0.080 | Tinospora cordifolia |
| 2 | Magnoflorine | 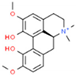 | 19.03 | 1.041 | Tinospora cordifolia |
| 3 | Rosmarinic acid | 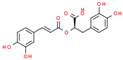 | 30.05 | 0.233 | Ocimum sanctum |
| 4 | Palmatine | 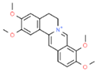 | 34.40 | 0.071 | Tinospora cordifolia |
| 5 | Withanoside IV | 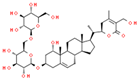 | 41.66 | 1.870 | Withania somnifera |
| 6 | Withaferine A | 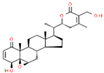 | 46.98 | 4.891 | Withania somnifera |
| 7 | Withanoside V | 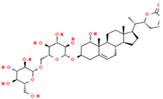 | 47.94 | 2.072 | Withania somnifera |
| 8 | Withanone | 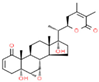 | 49.08 | 0.113 | Withania somnifera |
| 9 | Betulinic acid | 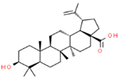 | 64.11 | 1.270 | Ocimum sanctum |
| 10 | Ursolic acid | 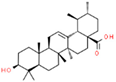 | 64.44 | 0.082 | Ocimum sanctum |
| Study Groups | Time (in Seconds) Spent in the Temperature Gradient Chamber | |||||
|---|---|---|---|---|---|---|
| 4th Day | 7th Day | |||||
| 23 °C | 29 °C | 37 °C | 23 °C | 29 °C | 37 °C | |
| NCTL | 35.58 ± 3.0 | 129.63 ± 1.4 | 14.92 ± 2.7 | 24.13 ± 0.7 | 130.00 ± 0.8 | 25.88 ± 0.7 |
| HZFCTL | 37.79 ± 1.5 | 134.00 ± 1.5 | 8.21 ± 2.0 | 25.88 ± 1.3 | 135.75 ± 1.5 | 18.38 ± 1.3 |
| DCTL | 15.96 ± 1.6 | 7.04 ± 1.3 | 157.08 ± 1.6 | 2.38 ± 1.1 | 4.75 ± 1.3 | 172.88 ± 1.3 |
| DEX | 21.13 ± 2.3 | 30.46 ± 2.3 | 128.29 ± 1.9 | 45.54 ± 1.6 | 57.54 ± 2.0 | 76.92 ± 2.1 |
| CN12 | 0 | 19.08 ± 2.1 | 160.92 ± 2.1 | 0 | 5.04 ± 1.7 | 174.96 ± 1.7 |
| CN58 | 0 | 19.92 ± 2.1 | 160.08 ± 2.1 | 17.54 ± 1.7 | 21.50 ± 1.8 | 140.96 ± 2.0 |
Sample Availability: Samples of the compounds are available from the authors. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balkrishna, A.; Solleti, S.K.; Verma, S.; Varshney, A. Application of Humanized Zebrafish Model in the Suppression of SARS-CoV-2 Spike Protein Induced Pathology by Tri-Herbal Medicine Coronil via Cytokine Modulation. Molecules 2020, 25, 5091. https://doi.org/10.3390/molecules25215091
Balkrishna A, Solleti SK, Verma S, Varshney A. Application of Humanized Zebrafish Model in the Suppression of SARS-CoV-2 Spike Protein Induced Pathology by Tri-Herbal Medicine Coronil via Cytokine Modulation. Molecules. 2020; 25(21):5091. https://doi.org/10.3390/molecules25215091
Chicago/Turabian StyleBalkrishna, Acharya, Siva Kumar Solleti, Sudeep Verma, and Anurag Varshney. 2020. "Application of Humanized Zebrafish Model in the Suppression of SARS-CoV-2 Spike Protein Induced Pathology by Tri-Herbal Medicine Coronil via Cytokine Modulation" Molecules 25, no. 21: 5091. https://doi.org/10.3390/molecules25215091
APA StyleBalkrishna, A., Solleti, S. K., Verma, S., & Varshney, A. (2020). Application of Humanized Zebrafish Model in the Suppression of SARS-CoV-2 Spike Protein Induced Pathology by Tri-Herbal Medicine Coronil via Cytokine Modulation. Molecules, 25(21), 5091. https://doi.org/10.3390/molecules25215091






