1. Introduction
Magnetic nanoparticles are a new trend in various scientific fields, such as drug delivery, DNA/RNA purification, improved magnetic resonance imaging (MRI), immobilization, food industry, medical diagnostics, cell harvesting, bioremediation, and others [
1,
2,
3,
4]. They are a class of particles consisting of a magnetic and a functional chemical component with a diameter varying from 1 to 100 nm and can be controlled by magnetic fields displaying superparamagnetism.
Haematococcus pluvialis (
H. pluvialis) is a microalgae strain rich in astaxanthin as well as lipids (20–25% per dry weight with 10% of it being polyunsaturated fatty acids), proteins (29–45% per dry weight), and carotenoids (2–5% per dry weight). Its ability to grow even in extreme conditions makes it a strategic tool for human diet and animal feeding and can be used even for anticancer and anti-inflammatory purposes [
5]. Skin, heart and eye health, and photoprotection are also fields where
H. pluvialis is an important implement [
6].
Improved cultivation conditions that enhance the production of the above-mentioned high-value-added products as well as better harvesting methods are needed to fulfil the increased demands of
H. pluvialis usage. Applications of nanomaterials in microalgae cultivation and harvesting are widely used for enhancing biomass and lipid production, reducing production costs, more efficient collection and purification, as well as the refinement process, giving the ability to reuse nanomaterials [
7]. The use of magnetic nanoparticles, naked or surface functionalized iron oxide (Fe
3O
4) particles, has been reported in algal biomass harvesting as well as in biomedical applications [
3,
8,
9,
10]. Moreover, the generation of magnetically modified by the insertion of magnetic nanoparticles or with newly acquired genetic properties by the insertion of macromolecules bound to magnetic nanoparticles [
11] into the microalgae protoplasts can provide new insights into microalgae biotechnological applications.
The microalgae cell wall is constituted by rigid components embedded in a polymeric matrix constituted by 70% cellulose as well as glycoproteins, pectin, and algaenan, which enhance the stiffness of their cell walls [
12]. High-pressure homogenization following acidic and thermal pre-treatment, single enzymatic digestion with cellulase, hemicellulase, and pectinase, or a mix of them as well as combinatory methods of enzymatic and mechanical treatment (sonication and microwaves) are used for microalgae protoplast formation [
13,
14,
15].
Protoplasts can be used as a convenient system to improve the delivery of nanocomposites or foreign DNA/RNA into microalgae. Nowadays, protoplast fusion of different species or organisms is a common technique for genome combination and development of microorganisms with desired properties as well as in biotechnological applications [
14,
16,
17,
18,
19]. The extent of cell wall thickening, temperature and duration of the enzymatic incubation, pH, agitation, as well as the nature of osmotic solution are key factors for protoplast formation [
13].
Protoplast isolation is also a complex task, especially when enzymatic digestion is used, which is a stress-induced procedure due to peroxisome production as well as degradation products that enhance cell lysis [
20]. If necessary, flow cytometry sorting or high-gradient sucrose centrifugation is used for the separation of protoplasts from intact cells [
21]. After protoplast isolation, the regeneration of the cell wall is of vital importance in order to fully exploit the genetic or biotechnological features that have been modified in the parental cells. Medium with a non-metabolizable sugar alcohol like mannitol or sorbitol, and with auxins and cytokinins, may provide protection against osmotic pressure and support mitosis and daughter cell formation [
13]. Studies on
Chlorophyta species after physical wounding demonstrate the recreation of a cell membrane on the surface of protoplasts 12 h after the wounding, while Golgi bodies with numerous vesicles at the peripheral region of the rebuilding cell at 24 h after the wounding began to develop [
22].
H. pluvialis, a freshwater species of
Chlorophyta of interest in many biotechnological applications, has not been studied in detail so far. Its cell wall has a different composition compared to other microalgae species [
14]. In this work, a comparative study of the efficiency of enzymatic (cellulase) and mechanical (glass bead vortexing) treatments of
H. pluvialis microalgae species targeting protoplasts formation and wall reconstruction, which is based on modified media enriched with different carbon and amino acids sources, was undertaken. Using scanning electron microscopy, the regeneration of the cell wall was analyzed; however, the survival rates were not investigated. Subsequently, iron oxide magnetic nanoparticles were used to test the efficiency of transformation via electroporation. Our findings provide important basic information on how to easily prepare and recover high-quantity
H. pluvialis protoplasts for biotechnological applications.
3. Discussion
Biotechnological or genetic transformation of microalgae cells could be succeeded through a suitable cell wall perturbation, which permits the insertion of DNA/RNA or nanoparticles and simultaneously supports the normal regeneration and growth of microalgae. Microalgae cells, like bacteria, present a rigid cell wall that surrounds the plasma membrane. Besides structural properties, the cell wall is a formidable barrier that does not allow the insertion of macromolecules into the cytoplasm, even by forcing them with methodologies like electroporation. Indeed, we tried to transform microalgae cells with magnetic nanoparticles, but the rate of magnetic cells was nearly zero. Therefore, we opted to transform protoplast. Protoplasts obtained from terrestrial or aquatic photosynthetic organisms find a variety of applications both in basic and applied research. They can be prepared from multicellular plant tissues or simply by removing the cell wall from unicellular photosynthetic microorganisms, such as microalgae. Recent studies have verified that protoplasts derived from various tissues have crucial differences and properties, especially those that are genetically or biotechnologically transformed [
16]. Depending on the final application, the degree of cell wall removal might vary. Indeed, for cell fusion, perfectly “naked” protoplasts are required since the presence of any rigid cell walls may hamper the process, reducing its efficiency and yield. Fused microalgae (
Ochromonas danica and
Haematococcus pluvialis) protoplasts via PEG treatment resulted in enhanced fatty acid production, while
Chlorella kessleri and rat insulinoma cell line fusion created insulin-producing cells [
25,
26]. On the other hand, when protoplasts are intended to be used for genetic manipulation, e.g., transfection with plasmid, siRNA, CRISPR, and others, removal of the entire cell wall might not be mandatory since the intracellular delivery of such macromolecules can be forced with appropriate methodologies. Other important parameters to be considered in protoplasts’ preparation are the time needed for the recovery (cell wall reconstruction), incubation temperature, means of transfection, number of protoplasts cells required (biomass), and cost of protoplast preparation. These parameters are crucial especially when protoplasts are used in plant/algae industrial applications where the economic viability and sustainability of the entire production process is carefully evaluated.
In this work, we reported how to weaken the
H. pluvialis cell wall, through single-enzyme digestion and mechanical processes as well as their regeneration, in order to produce large quantities of transformable protoplasts, for use in several fields of blue-biotechnology. All experiments were performed with relatively high volumes, 20–100 mL, which can be easily scaled up, and cost minimization related to the enzymes and energy utilized. The
H. pluvialis cell wall is constituted by 19% of carbohydrates and 75% of proteins in the exponential phase of growth, while at the stationary phase of growth, 70% of carbohydrates (66% hexoses and mannose), 3% cellulose, and 6% proteins, as well as a remaining 3% of acetolysis-resistant material, are crucial components of the cell wall [
14]. Cells of this strain can be categorized into flagellates and resting cells. Several enzymes have been reported to be used in protoplasts formation from microalgae, such as proteinase K, a mixture of cellulase, hemicellulase, pectinase, sulfatase, tyrosinase, and others, with good results [
12,
27,
28,
29,
30,
31,
32,
33,
34]. We sought to investigate the possibility of using a single-enzyme digestion with cellulase for the minimum digestion time in order to minimize cost and reduce the time of
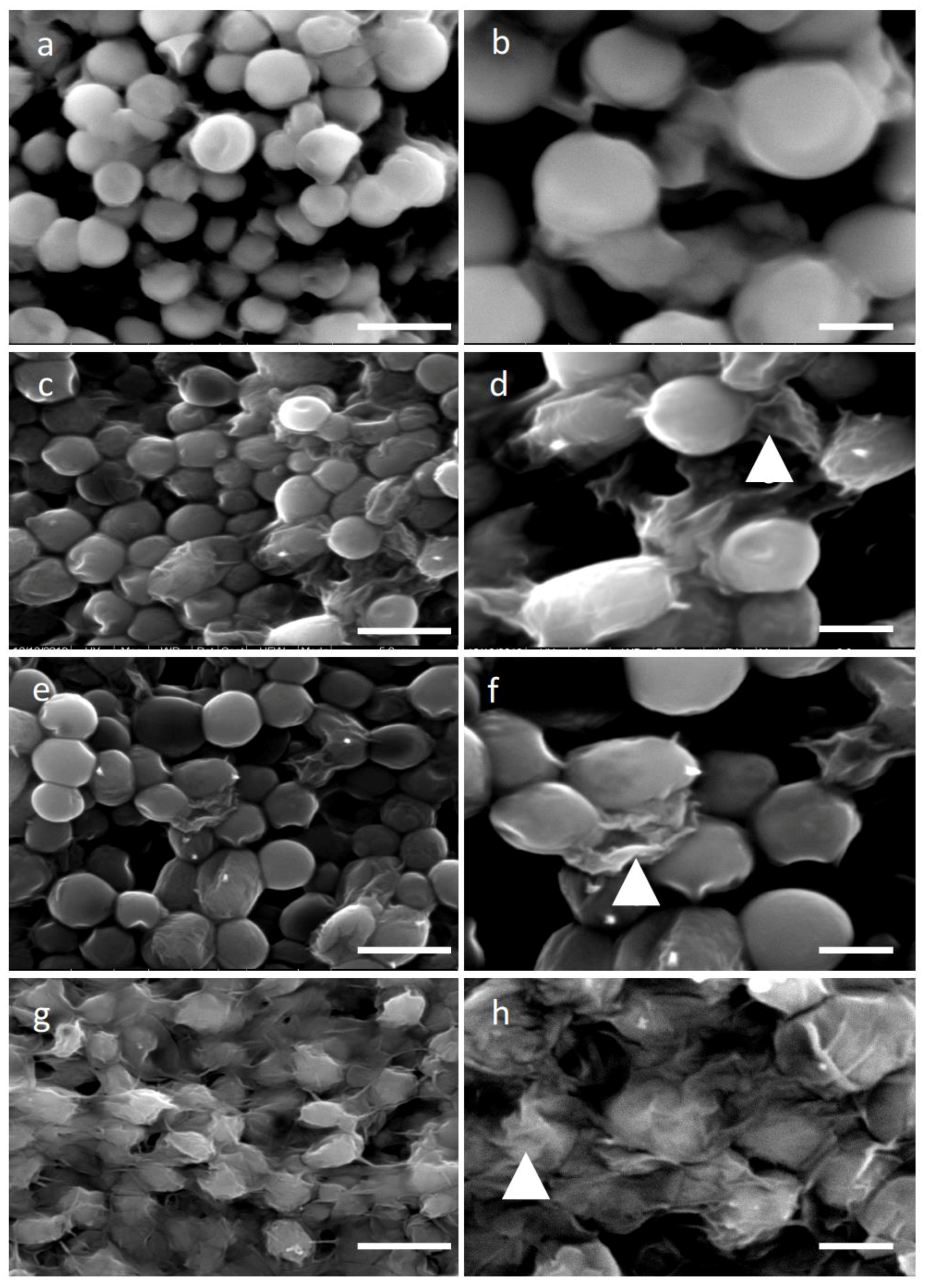
H. pluvialis protoplast production. In our study, the usage of cellulase, which catabolizes cellulose as a linear polymer of D-glucose with β-1,4 linkages, decomposed the cell wall almost to the same efficiency at different treatment periods between 4 and 16 h as it was shown by the SEM images (
Figure 1a–f). To identify the minimum digestion time using only cellulase as the hydrolytic enzyme at a concentration of 2% (
w/
v), we performed a series of digestions with incubation of 4, 8, 12, and 16 h. After each incubation, the entity of cell wall damage was studied by SEM, as well as the transfectability by introducing, via electroporation, magnetic nanoparticles with a diameter of 100 nm. Analyzing the SEM images, we did not observe significant differences between 4 and 16 h of digestion time, as shown in
Figure 1c,d,e,f, though a good transfection outcome was obtained with cells digested for at least 8 h.
We also noticed that morphologically, the digestion time did not widely affect the cell shape (
Figure 1). The fact that after 16 h, digestion protoplasts presented almost the same appearance could be due to the low percentage of cellulose in the cell wall. Indeed, as mentioned above, cellulose represents only 3% of the cell wall, but it plays an important role in the cell wall structure since cellulose microfibrils offer support for sugar-based scaffolds, such as hemicellulose, proteins, and other macromolecules. Thus, even though it is not visible, a long hydrolysis of cellulose microfibrils may have a significant effect on the permeability of the cell wall to macromolecules as well as certain nanomaterials. In addition, it has to be considered that different protoplast yield variations can be due to physiological and biochemical conditions and even to be related to the culture ageing [
27] while different enzyme batches may affect the yield between the same species.
A second parameter we tried to optimize was the incubation temperature. In the literature, incubation temperatures of 20–25 °C have been reported as the optimal temperatures for species like
Gracilaria and
Ulva, while an increased temperature may decrease their protoplast formation productivity [
30,
33]. Regarding our experiments with
H. pluvialis, an incubation temperature of 30 °C resulted in optimal cell wall digestion, which confirms the similar performance obtained in
Chlorella species [
35].
Mechanical treatment through glass bead vortexing resulted in a higher efficiency of cell wall disruption of
H. pluvialis cells treated, as this was verified by the SEM images (
Figure 1g,h). Microalgae cells mixed with glass beads and vortexed for 30 s, while a prolonged vortexing time resulted in irreversible damage, which caused cell death. In
Chlamydomonas species, 15 s demonstrates a better recovery than 60 s of glass bead vortexing [
36]. Studies revealed that the pretreatment of the cells upon acidic or thermal conditions may reduce the energy need for mechanical rupture of microalgae cells [
15], while allantoin pretreatment for
Porphyra cells enhanced the protoplasts formation [
37]. The method we proposed to produce protoplasts seems to better in terms of cost and time, since there is no need for sharp control of the incubation temperature, while the glass beads can be reused multiple times after proper cleaning.
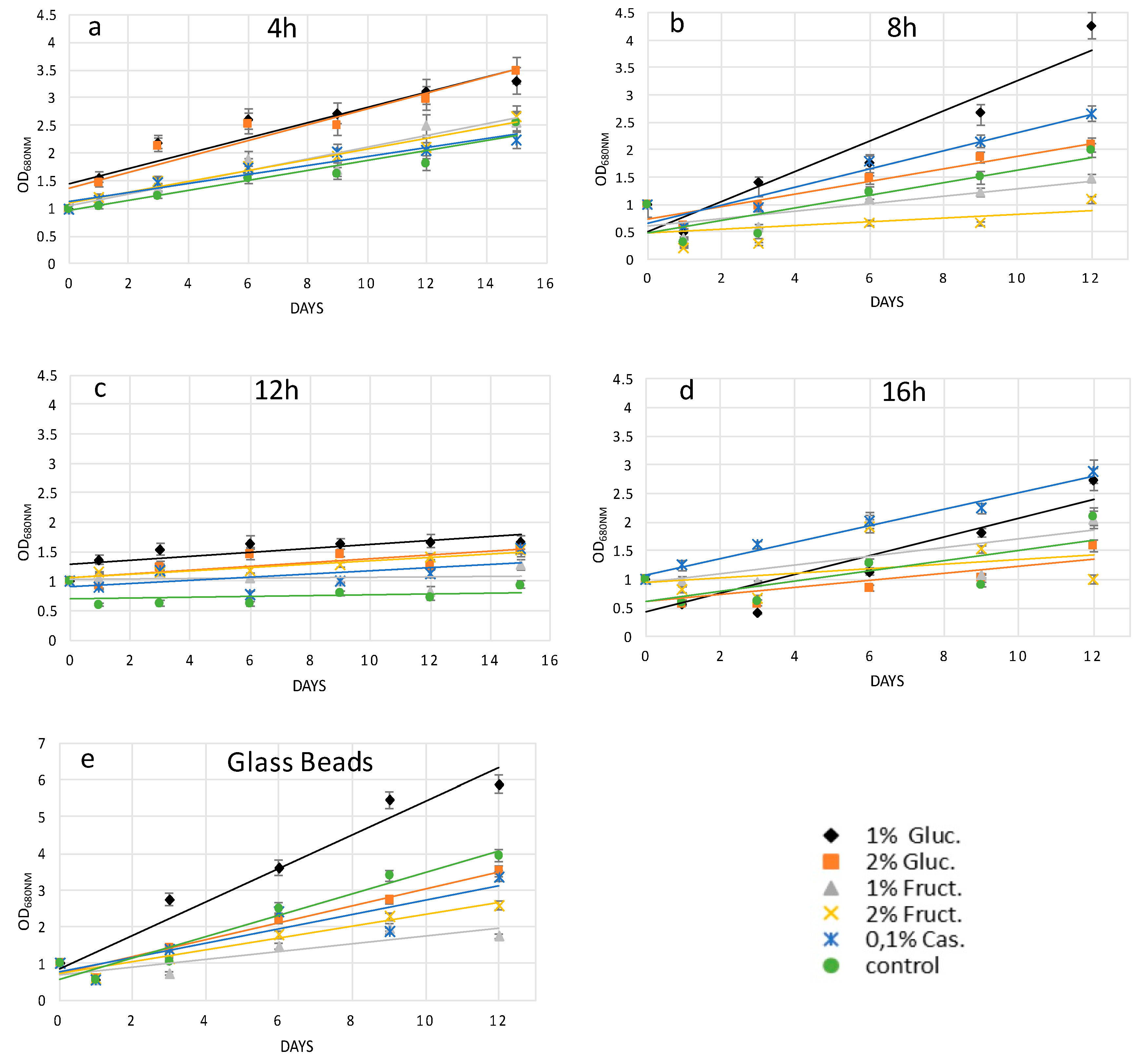
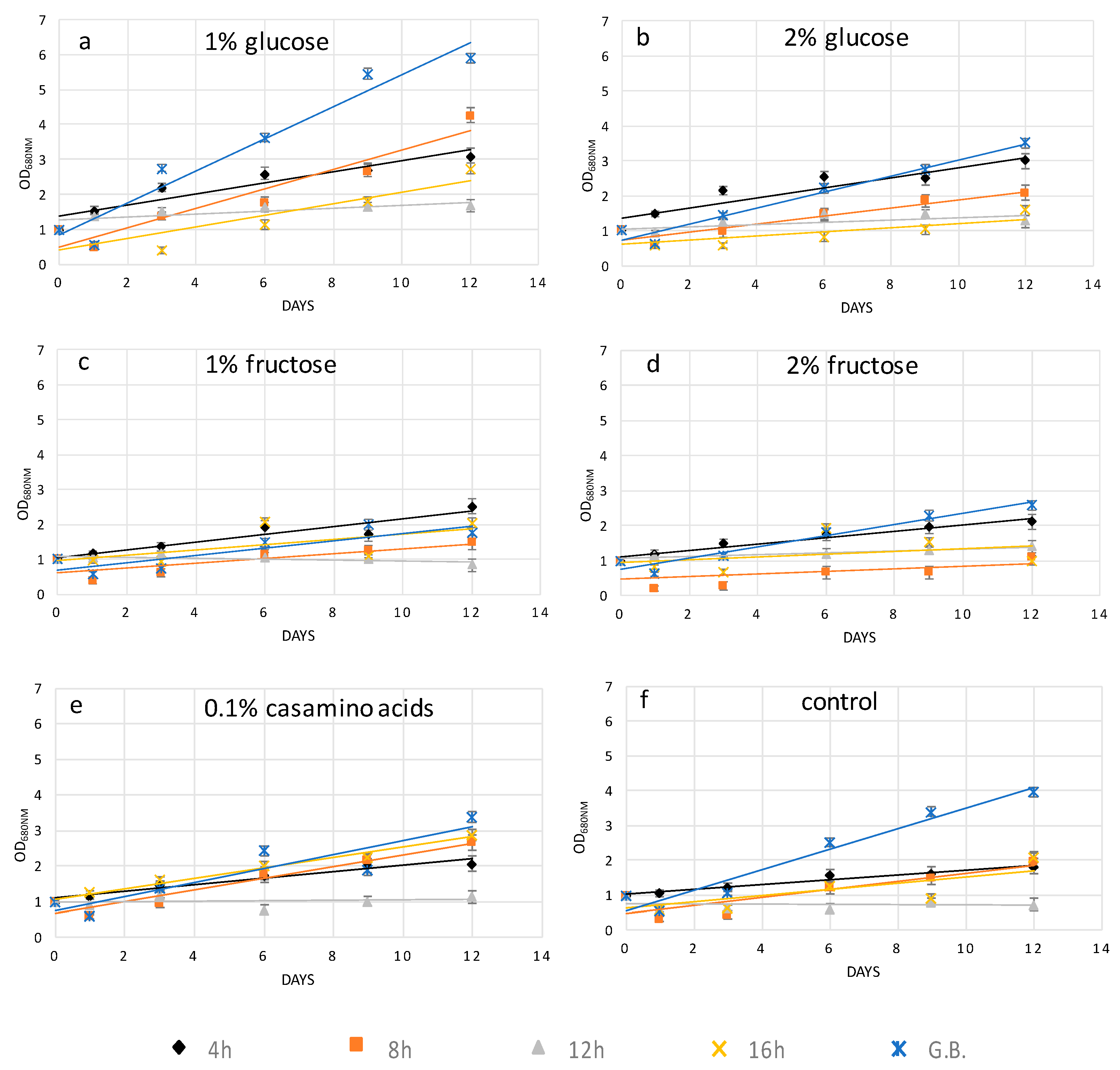
Independently of the protoplast preparation method used, the cells undergo stressing conditions, which may lead to their death if not properly recovered. Cell wall regeneration is an important step in order to have fully exploitable protoplasts cells able to be properly grown. In this work, in order to facilitate cell wall reconstruction, the growth medium was supplemented with an organic source of carbon or nitrogen. Indeed, it was noticed that protoplast cells, especially during the initial days after their preparation, had a reduced photosynthetic ability and most probably were not able to produce an adequate quantity of glucose necessary for all biological processes. It was also observed that high light and agitation time negatively affected the protoplast culture viability (data not shown). Glucose at 1%
w/
v was the crucial compound, which enhanced the recovery trend of
H. pluvialis protoplasts upon the mechanical process and enzymatic digestion after 4, 8, and 12 h of treatment, while casamino acids at 0.1%
w/
v better supported protoplast renewal after 16 h of cellulase treatment, as depicted in
Figure 2a–e. The different key compounds used for protoplast recovery imply the activation of different metabolic pathways, which are related to the degree of cell wall degradation. For instance, glucose in comparison to fructose as the initial compound of glycolysis may lead to enhanced production of cellulose. In conclusion, glucose as the key metabolite for glycolysis enhanced the recovery trends of
H. pluvialis protoplasts after the short-term cellulase treatment and glass bead agitation, while amino acids more efficiently promoted protoplast regeneration after the enzymatic treatment.
An important parameter that has to be considered in the protoplast preparation from microalgae is the osmotic fluctuation, since they live in salty or fresh water, where electrolytes, ions, and other molecules present affect the osmotic pressure in the inner cell compartment. Therefore, by removing the protective cell wall, microalgae could undergo damage since water can spontaneously enter the cell body and inflate it until its explosion. To prevent such a phenomenon, during protoplast preparation and in all downstream applications, sorbitol and mannitol are added in the medium. These two sugar-based molecules increase the osmotic pressure of media by equilibrating the pressure between the internal cell compartment and the external environment. The optimized concentration of mannitol is 0.6 M, and it is used in all media during digestion, electroporation, and recovery along the medium specific for H. pluvialis.
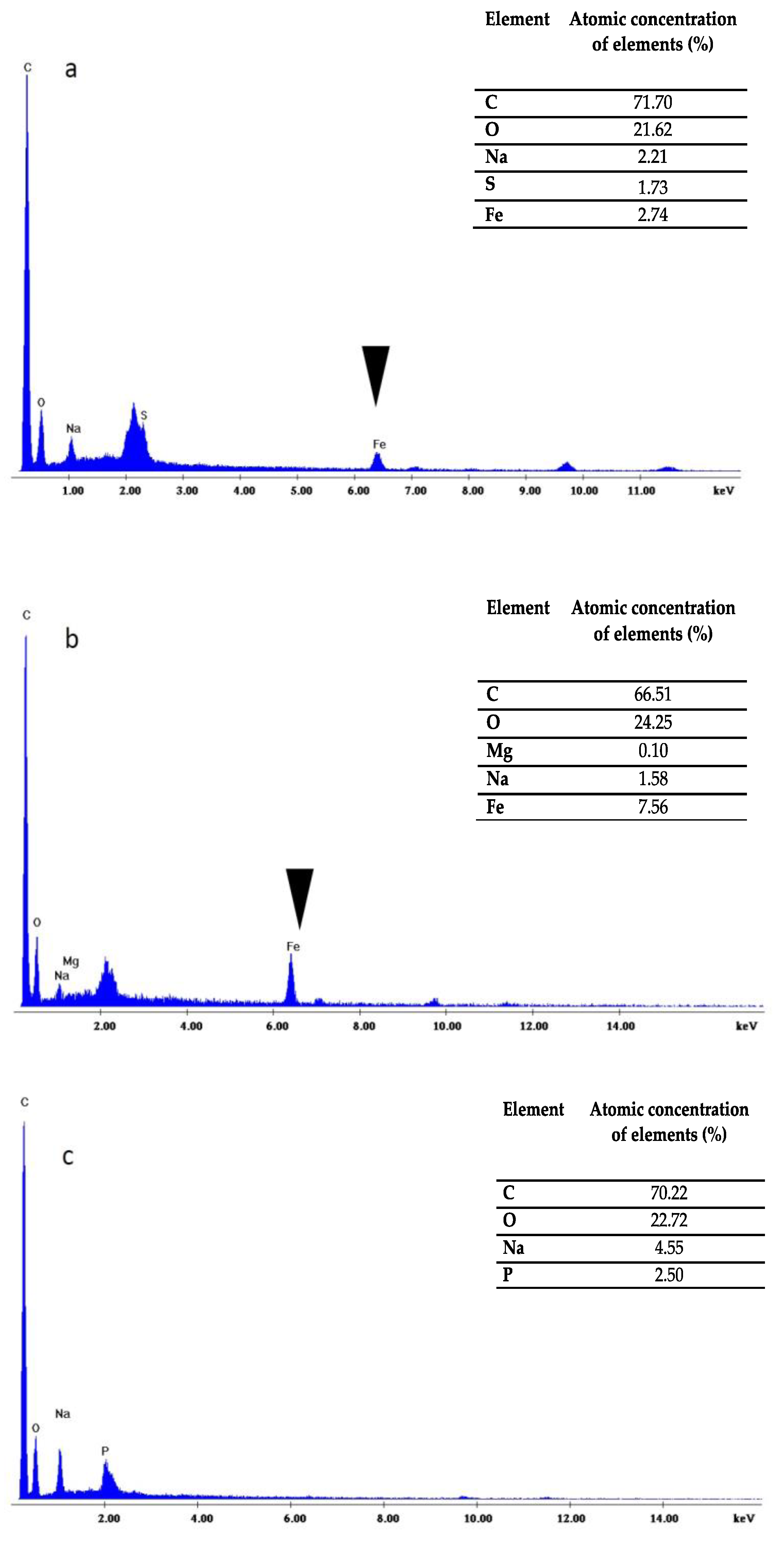
Finally, the quality of the protoplasts prepared with both methods was tested via their transformation with magnetic nanoparticles. The presence of the magnetic particles inside microalgae cells was easily verified by means of magnetism and by the Prussian blue test. Our results showed that with enzymatic digestion and glass bead pre-treatment, the transformation efficiency with magnetic particles was around 20% and 30%, respectively. It is worth noting that when Prussian blue staining was negative (no blue color overlapped with green), the attraction of the cells with permanent magnets was not possible, suggesting that only when nanoparticles were internalized the cells were magnetic. The transformation yield after cellulase treatment was lower compared to plant protoplasts transformed with iron nanoparticles due to the double-enzymatic effect of cellulose and macerozyme R10 compared to our single-enzymatic treatment [
38], while similar results to ours were demonstrated in aminoclay transformation with vortexing of
Chlamydomonas reinhardtii [
39].
In conclusion, our study demonstrated a simplified methodology to obtain good-quality H. pluvialis protoplasts. Glucose should be added to the recovery medium as the key metabolite for enhancement of the recovery trends of the protoplasts after short-term cellulase treatment or glass bead agitation, while amino acids more efficiently promote protoplast regeneration after the enzymatic process. Osmotic stabilizers are necessary in all steps, from pretreatment to recovery. By following our study, it will be possible to easily prepare a large quantity of H. pluvialis protoplasts biomass, transform them with proper molecules in order to acquire magnetic properties, and recover them after 4–5 days of growth with conditional media.
4. Materials and Methods
4.1. Organism and Growth Conditions
The
H. pluvialis strain (CCAP 34/6) was purchased from Culture Collection of Algae and Protozoa (Dunbeg, UK). The strain was grown in medium with the following composition (per liter): Ca(NO
3)
2, 0.15 g; KNO
3, 0.10 g; β-glycerophosphoric acid disodium salt pentahydrate, 0.05 g; MgSO
4·7H
2O, 0.04 g; Tris-aminomethane, 0.50 g; thiamine, 0.01 mg; PIV metal solution, 3.00 mL; biotin 0.10 μg; and vitamin B
12, 0.10 μg. One liter of PIV metal solution contained Na
2EDTA, 1.0 g; FeCl
3·6H
2O, 0.196 g; MnCl
2·4H
2O, 36.0 mg; ZnSO
4·7H
2O, 22.0 mg; CoCl
2·6H
2O, 4.0 mg; and Na
2MoO
4·2H
2O, 2.5 mg [
40]. The pH was adjusted to 7.5 and the temperature was controlled at 23 °C. Cell cultures of
H. pluvialis were grown in 250-mL Erlenmeyer flasks containing a culture volume of 100 mL at 150 rpm supplemented with NaHCO
3 at a concentration of 1 g/L. The re-culture concentration was adapted to an optical density of 0.1 at 680
nm (OD
680 nm). Periodic purity assessment was performed by microscopic examination. The photobioreactor was illuminated at 60 μmol photon m
−2 s
−1 24/7 white LED lamps at the cell culture surface.
H. pluvialis growth was monitored by measuring the OD at 680 nm with a UV-vis spectrophotometer (Shimadzu, Kyoto, Japan) for up to 15 days. All experiments were conducted in duplicate while samples were analyzed in triplicate.
4.2. Cell Wall Disruption by Enzymatic Lyses
H. pluvialis cells were grown in a 500-mL flask for 6–8 days and harvested by centrifugation at 1000× g for 5 min. Then, the cell pellet was suspended in 25 mM phosphate buffer (pH 7.0) containing 0.6 M D-mannitol and the polysaccharide-degrading enzyme cellulase (2%). The incubation temperature was chosen based on the literature as well as our preliminary data, where different incubation temperatures (25–37 °C) were examined and 30 °C was found to give the best results based on our morphological observations via SEM. Each reaction mixture was incubated at 30 °C for 4, 8, 12, and 16 h. Afterwards, the cells were centrifuged at 1000× g for 3 min, the pellet was re-suspended in 0.6 M D-mannitol solution, and was washed twice with sugar solution. The protoplast layer was transferred into 5 mL of buffer (pH 7.0) containing 0.6 M D-mannitol solution for further analysis. Generally, cells were grown up to an optical density of 0.8–1.0 at 680 nm. Then, from this cultivation, 100 mL of cells were withdrawn, washed, and further processed. The digestion was performed in a final volume of 30 mL. After digestion, enzymes and digestion debris were removed by 2 washing steps and cells resuspended in 20 mL of medium for electroporation. All solutions used for digesting, washing, and electroporation contained 0.6 M D-mannitol to equilibrate the osmotic pressure.
4.3. Cell Wall Disruption with Glass Beads
Glass beads (Sigma, St Louis, MO, USA), 1.0 mm in diameter, were washed in concentrated sulfuric acid, then rinsed thoroughly with sterilized water a few times, and baked at 180 °C for 2–3 h. H. pluvialis cells (from the same culture as before) were cultured to the logarithmic phase and harvested by centrifugation at 1000× g for 5 min. Cells were washed three times with 25 mM phosphate buffer (pH 7.0) containing 0.6 M D-mannitol and re-suspended with this medium at a concentration of 2 × 108 cells/mL. A total of 200 mg of dry glass beads and 1 mL of cells (2 × 108 cells/mL) were added to a 1.5-mL Eppendorf tube and agitated at 1500 rpm on a vortex agitator for 30 s. The cells were transferred to sterilized test tubes for further analysis. The experiments were performed with the optimum conditions for 30 s and 200 mg of dry glass beads. Blank controls were transformed without agitation. Every treatment consisted of three independent agitations.
4.4. Morphological Observation with Scanning Electron Microscopy Coupled with an Energy Dispersive X-ray Analyzer System (SEM-EDX)
The H. pluvialis algal cells were sampled for the scanning electron microscopy observations immediately after cell disruption by enzymatic lyses as well as mechanical treatment. The samples of algal cells were fixed in 2.5% (v/v) paraformaldehyde at 4 °C for 1 h, rinsed three times in 0.05 M phosphate buffer (pH 7.4), and successively dehydrated in 20%, 35%, 50%, 75%, 90%, and 100% (v/v) ethanol solution for 5 min. After dehydration, the prepared samples were sputter-coated with Au and examined using a FEI Quanta-200 Scanning Electron Microscope. For validation purposes, magnifications of 5000× and 10,000× were used. The microscope was equipped with an EDX detector in order to obtain a distribution of the elemental composition of cells. The X-ray spectrum of each sample loaded with a given microelement was obtained.
4.5. Nanoparticles
For the experimental investigations, ferrofluid nanoparticles (fluidMAG-lipid from Chemicell GmbH, Berlin, Germany) were used. The nanoparticles consisted of an aqueous dispersion of magnetic iron oxides (diameter of 80–100 nm) and were covered with phosphatidylcholine. The surfactant, phosphatidylcholine, over the iron oxide core, is a coating layer similar to cell membrane. This characteristic gives it an excellent biocompatibility since coated nanoparticles do not cross react with molecules and organelles of the cytosol.
4.6. Protoplast Transformation
The transformation of H. pluvialis protoplasts with magnetic nanoparticles was performed using a homemade continuous flow cuvette device for the electroporation. Pretreated H. pluvialis cells were dissolved in medium containing 0.6 M D-mannitol solution and electroporated with a pulse generator (MicroPulser) from BioRad (USA) using 1 pulse at 3 kV. Nanoparticles and pretreated cells were mixed for 30 min before the electric pulse. A homemade flow-cuvette was used based on 3-D printing of a plastic core where aluminum plaques were glued. The internal volume of the flow cuvette was 1.5 mL with inlet and outlet tubes. A peristaltic pump was used to pump the mixture of protoplast cells and nanoparticles inside the flow cuvette at a rate of ~100 µL per s. The generator was modified with an external electronic circuit in order to deliver a pulse every 15 s.
4.7. Prussian Blue Staining
Cells were fixed with methanol/acetone 7:1 solution for 10 min at room temperature and washed twice with phosphate-buffered saline (Sigma, St Louis, MO, USA). Cells were incubated for 1 h in Prussian blue reagent obtained following the instruction manual of Biopal protocol (BioPhysics Assay Laboratory, Worcester, MA, USA). Stained cells were investigated using the microscope.
4.8. Regeneration of Protoplasts
To regenerate protoplasts, an efficient protocol was established for cell wall regeneration and restoration of the original
H. pluvialis morphology. The
H. pluvialis protoplast suspension was obtained as described above after cell disruption by enzymatic lyses and glass beads. The regeneration medium was supplemented with mannitol for osmotic stabilization of protoplasts [
41], the growth medium, and different concentrations of glucose (1 and 2%
w/
v), fructose (1 and 2%
w/
v), and casamino acids (0.01%
w/
v) to enhance cell wall regeneration and subsequent division. In total, 10 mL of the suspension were placed with 90 mL of regeneration medium. Medium without a supplemented carbon or nitrogen source was used as a control. Protoplasts were incubated at 23 °C, without agitation and with low light intensity 60 μmol photon m
−2 s
−1 for about a week and regenerated protoplasts were observed every 3 days. The culture conditions were described above. The electroporation efficiency was calculated as follows: electroporated cells were grown in the appropriate recovery medium for 4 days, then the OD
680 nm was measured using 1 mL of culture. The same 1 mL was placed in a magnetic rack to attract and retain magnetic cells. After 20 min, the supernatant of the tube was carefully removed and its absorbance (OD
680 nm) was measured again. When both protoplast preparation and electroporation were successful, a pellet of magnetic cells in the tube formed; therefore, the supernatant’s absorbance was lower compared to the initial one. The protoplast and electroporation efficiency was calculated by the rate between the two measurements.