A Review on Lead-Free Hybrid Halide Perovskites as Light Absorbers for Photovoltaic Applications Based on Their Structural, Optical, and Morphological Properties
Abstract
1. Introduction
1.1. Pb Content of Perovskite and Crystalline Silicon Solar Cells
1.2. Composition Engineering
1.3. Bandgap Engineering
1.4. Morphology Engineering
2. Single Perovskite Absorbers (ABX3)
2.1. D ABX3 Metal Halide Perovskites and Perovskite-Related Absorbers with Diverse Dimensionalities
2.2. Sn-Based 3D Perovskite Absorbers (ASnX3)
2.3. Ge-Based 3D Perovskite Absorbers (AGeX3)
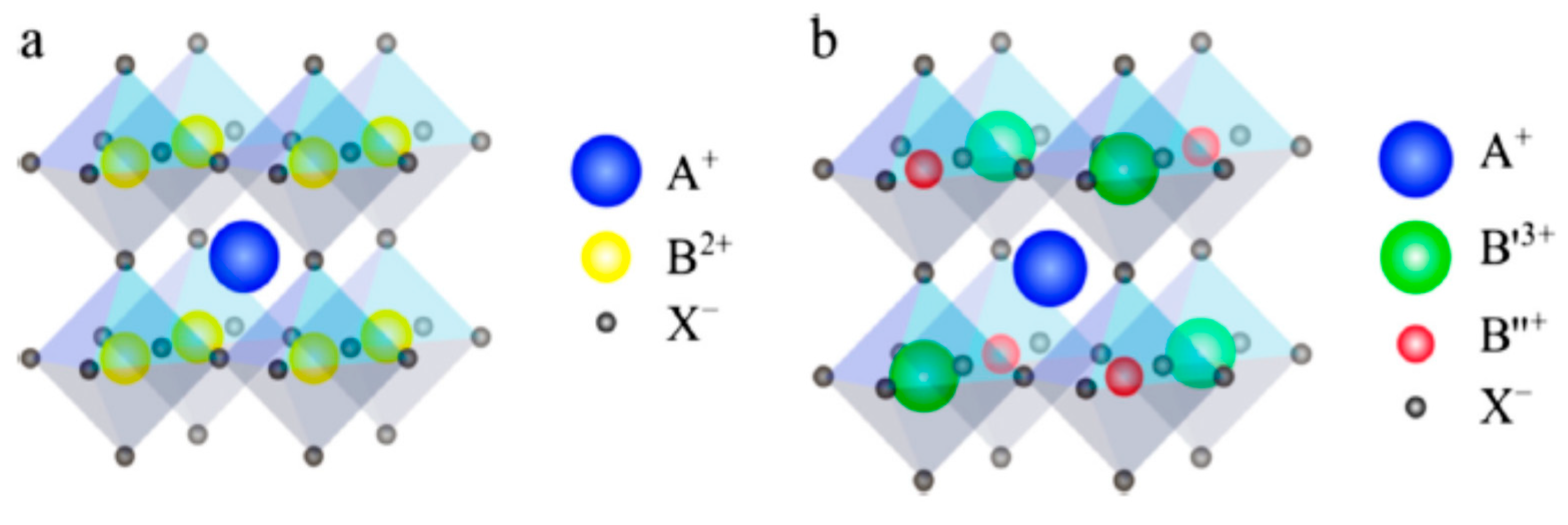
3. Double Perovskite Absorbers (A2BB′X6)
Ordered-Vacancy Double Perovskite Absorbers (A2BX6)
4. Two-Dimensional (2D) Perovskite Absorbers
4.1. Cu-Based 2D Perovskite Absorbers
4.2. Fe-Based 2D Perovskite Absorbers
4.3. Pd-Based 2D Perovskite Absorbers
4.4. Mn-Based 2D Perovskite Absorbers
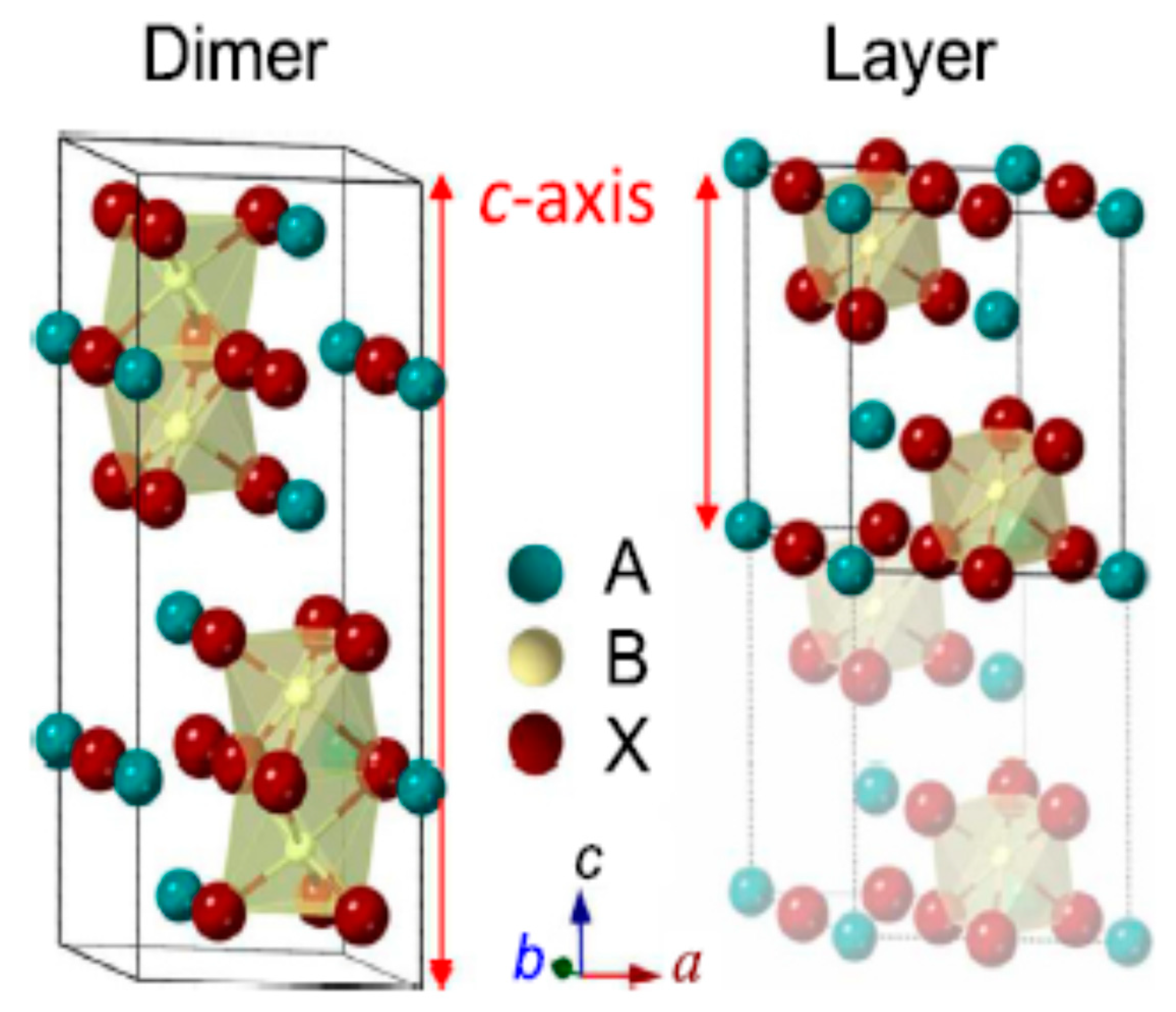
5. Perovskite-Like Halide Absorbers (A3B2X9)
5.1. Sb-Based Perovskite-Like Halides
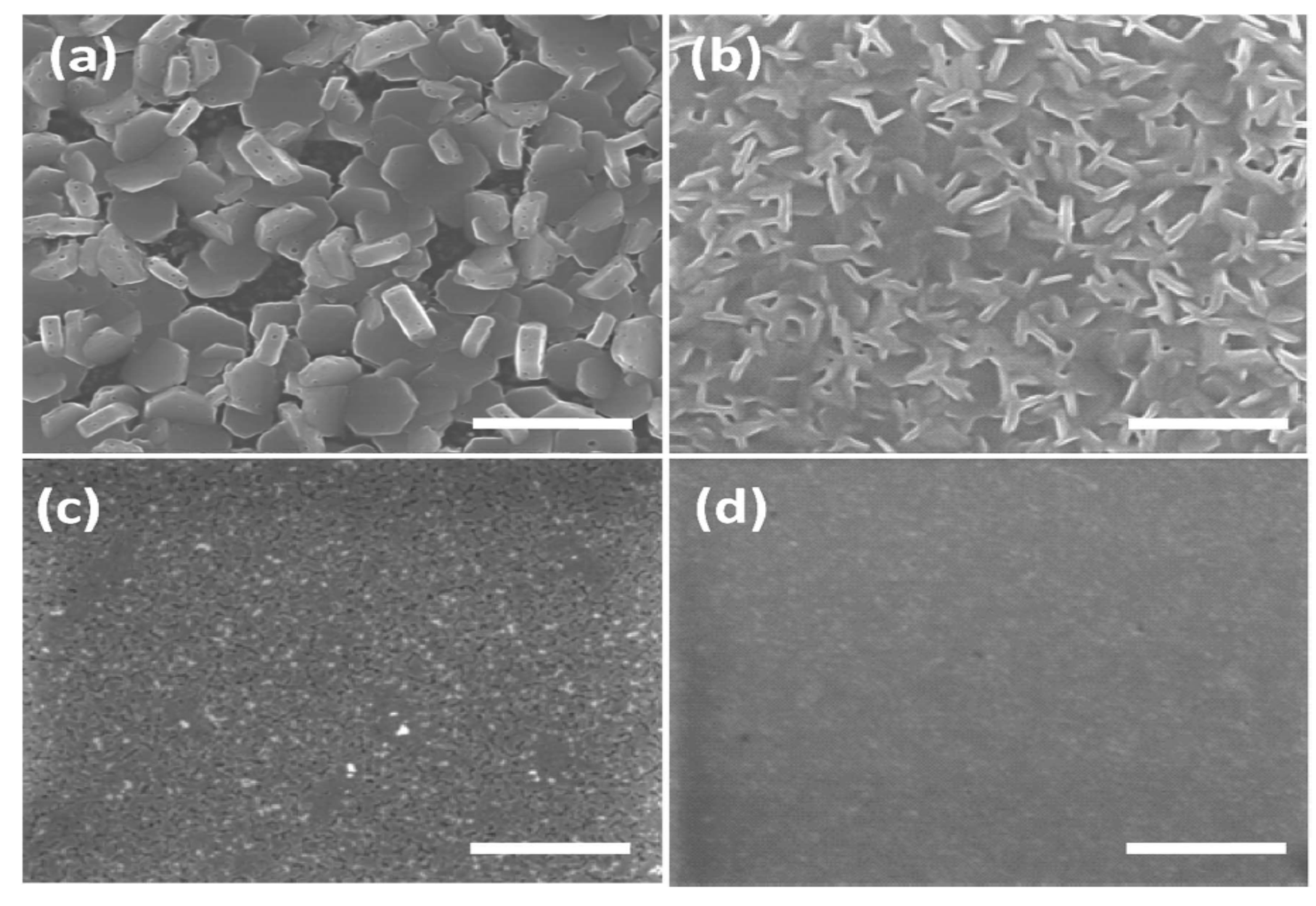
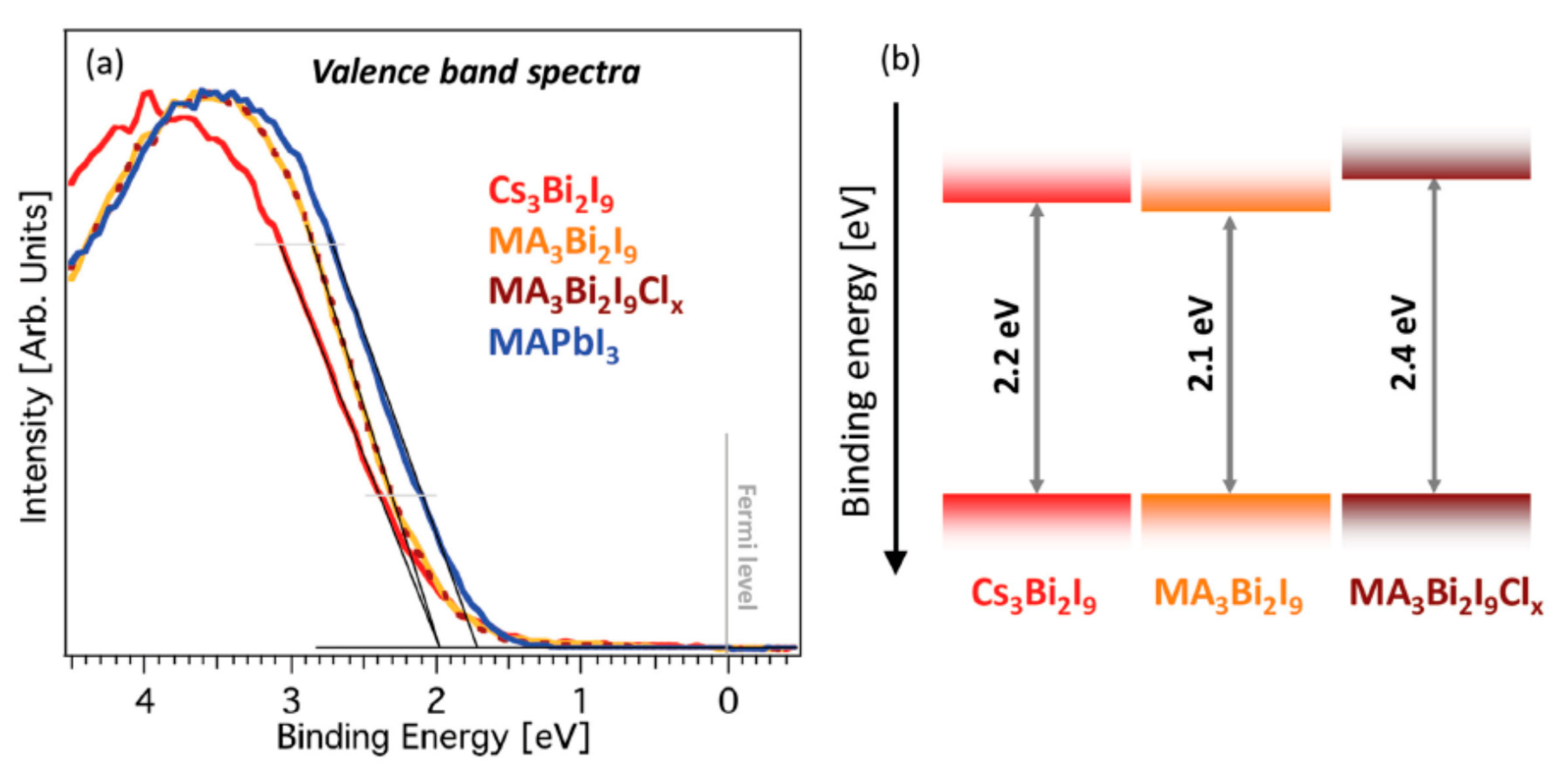
5.2. Bi-Based Perovskite-Like Halides
6. Conclusions and Prospects
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 0-D | zero-dimensional |
| 1-D | one-dimensional |
| 2-D | two-dimensional |
| 3-D | three-dimensional |
| Eg | Bandgap |
| FA | Formamidinium |
| FF | fill factor |
| FTO | fluorine-doped tin oxide |
| ITO | indium tin oxide |
| Jsc | Short-circuit current |
| (LiTFSI) | bis(trifluoromethane)sulfonimide lithium |
| MA | methylammonium |
| PC71BM | [6,6]-phenyl C71butyric acid methyl-ester |
| PCBM | [6,6]-phenyl-C61-butyric acid methyl ester |
| PC61BM | [6,6]-phenyl-C61-butyric acid methyl ester |
| PCE | power conversion efficiency |
| PEDOT: PSS | poly(3,4-ethylenedioxythiophene) poly(styrenesulfonate) |
| PSCs | perovskite solar cells |
| PL | Photoluminescence |
| PV | Photovoltaics |
| SEM | Scanning electron microscope |
| spiro-OMeTAD | 2,20,7,70-tetrakis-(N,N-di-p-methoxyphenylamine)9,90-spirobifluorene |
| TGA | Thermogravimetric analysis |
| Voc | open circuit voltage |
| XRD | X-ray diffraction |
| UV-vis | ultraviolet-visible absorption |
References
- Mutalikdesai, A.; Ramasesha, S.K. Emerging solar technologies: Perovskite solar cell. Resonance 2017, 22, 1061–1083. [Google Scholar] [CrossRef]
- Heo, J.H.; Han, H.J.; Kim, D.; Ahn, T.K.; Im, S.H. Hysteresis-less inverted CH 3 NH 3 PbI 3 planar perovskite hybrid solar cells with 18.1% power conversion efficiency. Energy Environ. Sci. 2015, 8, 1602–1608. [Google Scholar] [CrossRef]
- Snaith, H.J.; Abate, A.; Ball, J.M.; Eperon, G.E.; Leijtens, T.; Noel, N.K.; Stranks, S.D.; Wang, J.T.-W.; Wojciechowski, K.; Zhang, W. Anomalous hysteresis in perovskite solar cells. J. Phys. Chem. Lett. 2014, 5, 1511–1515. [Google Scholar] [CrossRef] [PubMed]
- Park, N.-G. Perovskite solar cells: An emerging photovoltaic technology. Mater. Today 2015, 18, 65–72. [Google Scholar] [CrossRef]
- NREL Solar Cell Efficiency Chart. Available online: https://www.nrel.gov/pv/cell-efficiency.html (accessed on 17 September 2020).
- Wang, L.; Wang, K.; Zou, B. Pressure-induced structural and optical properties of organometal halide perovskite-based formamidinium lead bromide. J. Phys. Chem. Lett. 2016, 7, 2556–2562. [Google Scholar] [CrossRef] [PubMed]
- Saliba, M.; Matsui, T.; Seo, J.-Y.; Domanski, K.; Correa-Baena, J.-P.; Nazeeruddin, M.K.; Zakeeruddin, S.M.; Tress, W.; Abate, A.; Hagfeldt, A. Cesium-containing triple cation perovskite solar cells: Improved stability, reproducibility and high efficiency. Energy Environ. Sci. 2016, 9, 1989–1997. [Google Scholar] [CrossRef]
- Wang, F.; Bai, S.; Tress, W.; Hagfeldt, A.; Gao, F. Defects engineering for high-performance perovskite solar cells. npj Flex. Electron. 2018, 2, 1–14. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, L.; Zhang, Y.; Gao, H.; Yan, H. Large-area perovskite solar cells—A review of recent progress and issues. RSC Adv. 2018, 8, 10489–10508. [Google Scholar] [CrossRef]
- Gao, P.; Grätzel, M.; Nazeeruddin, M.K. Organohalide lead perovskites for photovoltaic applications. Energy Environ. Sci. 2014, 7, 2448–2463. [Google Scholar] [CrossRef]
- Guo, X.; Burda, C. Coordination engineering toward high performance organic–inorganic hybrid perovskites. Coord. Chem. Rev. 2016, 320–321, 53–65. [Google Scholar] [CrossRef]
- Loi, M.A.; Hummelen, J.C. Hybrid solar cells: Perovskites under the sun. Nat. Mater. 2013, 12, 1087–1089. [Google Scholar]
- Hussain, I.; Tran, H.P.; Jaksik, J.; Moore, J.; Islam, N.; Uddin, M.J. Functional materials, device architecture, and flexibility of perovskite solar cell. Emergent Mater. 2018, 1, 133–154. [Google Scholar] [CrossRef]
- Ahmed, M.I.; Habib, A.; Javaid, S.S. Perovskite solar cells: Potentials, challenges, and opportunities. Int. J. Photoenergy 2015, 2015, 1–13. [Google Scholar] [CrossRef]
- Tanaka, H.; Oku, T.; Ueoka, N. Structural stabilities of organic–inorganic perovskite crystals. Jpn. J. Appl. Phys. 2018, 57, 08RE12. [Google Scholar] [CrossRef]
- Suzuki, A.; Oku, T. Effects of transition metals incorporated into perovskite crystals on the electronic structures and magnetic properties by first-principles calculation. Heliyon 2018, 4, e00755. [Google Scholar] [CrossRef] [PubMed]
- Kieslich, G.; Sun, S.; Cheetham, A.K. An extended tolerance factor approach for organic–inorganic perovskites. Chem. Sci. 2015, 6, 3430–3433. [Google Scholar] [CrossRef]
- Huang, T.J.; Thiang, Z.X.; Yin, X.; Tang, C.; Qi, G.; Gong, H. (CH3NH3) 2PdCl4: A compound with two-dimensional organic–inorganic layered perovskite structure. Chem. Eur. J. 2016, 22, 2146–2152. [Google Scholar] [CrossRef]
- Sun, P.-P.; Li, Q.-S.; Yang, L.-N.; Li, Z.-S. Theoretical insights into a potential lead-free hybrid perovskite: Substituting Pb2+ with Ge2+. Nanoscale 2016, 8, 1503–1512. [Google Scholar] [CrossRef]
- Li, Z.; Yang, M.; Park, J.-S.; Wei, S.-H.; Berry, J.J.; Zhu, K. Stabilizing perovskite structures by tuning tolerance factor: Formation of formamidinium and cesium lead iodide solid-state alloys. Chem. Mater. 2016, 28, 284–292. [Google Scholar] [CrossRef]
- Travis, W.; Glover, E.N.K.; Bronstein, H.; Scanlon, D.O.; Palgrave, R.G. On the application of the tolerance factor to inorganic and hybrid halide perovskites: A revised system. Chem. Sci. 2016, 7, 4548–4556. [Google Scholar] [CrossRef]
- Maughan, A.E.; Ganose, A.M.; Almaker, M.A.; Scanlon, D.O.; Neilson, J.R. Tolerance factor and cooperative tilting effects in vacancy-ordered double perovskite halides. Chem. Mater. 2018, 30, 3909–3919. [Google Scholar] [CrossRef]
- Bartel, C.J.; Sutton, C.; Goldsmith, B.R.; Ouyang, R.; Musgrave, C.B.; Ghiringhelli, L.M.; Scheffler, M. New tolerance factor to predict the stability of perovskite oxides and halides. Sci. Adv. 2019, 5, eaav0693. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Jing, L.; Yuan, Y.; Du, S.; Yao, Q.; Zhang, J.; Ding, J.; Zhou, T. Centimeter-size square 2D layered Pb-free hybrid perovskite single crystal (CH3NH3)2 MnCl4 for red photoluminescence. CrystEngComm 2019, 21, 4085–4091. [Google Scholar] [CrossRef]
- Yi, Z.; Ladi, N.H.; Shai, X.; Li, H.; Shen, Y.; Wang, M. Will organic–inorganic hybrid halide lead perovskites be eliminated from optoelectronic applications? Nanoscale Adv. 2019, 1, 1276–1289. [Google Scholar] [CrossRef]
- Kong, D.; Cheng, D.; Wang, X.; Zhang, K.; Wang, H.; Liu, K.; Li, H.; Sheng, X.; Yin, L. Solution processed lead-free cesium titanium halide perovskites and their structural, thermal and optical characteristics. J. Mater. Chem. C 2020, 8, 1591–1597. [Google Scholar] [CrossRef]
- Akbulatov, A.F.; Tsarev, S.A.; Elshobaki, M.; Luchkin, S.Y.; Zhidkov, I.S.; Kurmaev, E.Z.; Aldoshin, S.M.; Stevenson, K.J.; Troshin, P.A. Comparative Intrinsic Thermal and Photochemical Stability of Sn (II) Complex Halides as Next-Generation Materials for Lead-Free Perovskite Solar Cells. J. Phys. Chem. C 2019, 123, 26862–26869. [Google Scholar] [CrossRef]
- Liu, X.; Yan, K.; Tan, D.; Liang, X.; Zhang, H.; Huang, W. Solvent engineering improves efficiency of lead-free tin-based hybrid perovskite solar cells beyond 9%. ACS Energy Lett. 2018, 3, 2701–2707. [Google Scholar] [CrossRef]
- Kim, H.-S.; Hagfeldt, A.; Park, N.-G. Morphological and compositional progress in halide perovskite solar cells. Chem. Commun. 2019, 55, 1192–1200. [Google Scholar] [CrossRef]
- Adjokatse, S.; Fang, H.-H.; Loi, M.A. Broadly tunable metal halide perovskites for solid-state light-emission applications. Mater. Today 2017, 20, 413–424. [Google Scholar] [CrossRef]
- Li, J.; Cao, H.-L.; Jiao, W.-B.; Wang, Q.; Wei, M.; Cantone, I.; Lü, J.; Abate, A. Biological impact of lead from halide perovskites reveals the risk of introducing a safe threshold. Nat. Commun. 2020, 11, 310. [Google Scholar] [CrossRef]
- Urbina, A. The balance between efficiency, stability and environmental impacts in perovskite solar cells: A review. J. Phys. Energy 2020, 2, 022001. [Google Scholar] [CrossRef]
- Li, X.; Zhang, F.; He, H.; Berry, J.J.; Zhu, K.; Xu, T. On-device lead sequestration for perovskite solar cells. Nature 2020, 578, 555–558. [Google Scholar] [CrossRef] [PubMed]
- Eberstein, M.; Falk-Windisch, H.; Peschel, M.; Schilm, J.; Seuthe, T.; Wenzel, M.; Kretzschmar, C.; Partsch, U. Sintering and contact formation of glass containing silver pastes. Energy Procedia 2012, 27, 522–530. [Google Scholar] [CrossRef]
- Sun, X.; Yao, S.; Xing, J.; Zhang, J.; Yang, Y.; Li, H.; Tong, H.; Yuan, X. Mechanism of silver/glass interaction in the metallization of crystalline silicon solar cells. Mater. Res. Express 2020, 7, 016315. [Google Scholar] [CrossRef]
- Zhou, J.; Gan, W.; Li, Y.; Huang, B.; Yang, C. Immobilization of silver nanoparticles onto the surface of Pb-based glass frit by a one-pot procedure involving polyol process and its application in conductive thick films. J. Mater. Sci. Mater. Electron. 2015, 26, 234–241. [Google Scholar] [CrossRef]
- Zhao, Z.; Gu, F.; Li, Y.; Sun, W.; Ye, S.; Rao, H.; Liu, Z.; Bian, Z.; Huang, C. Mixed-Organic-Cation Tin Iodide for Lead-Free Perovskite Solar Cells with an Efficiency of 8.12%. Adv. Sci. 2017, 4, 1700204. [Google Scholar] [CrossRef]
- Chen, Q.; De Marco, N.; Yang, Y.M.; Song, T.-B.; Chen, C.-C.; Zhao, H.; Hong, Z.; Zhou, H.; Yang, Y. Under the spotlight: The organic–inorganic hybrid halide perovskite for optoelectronic applications. Nano Today 2015, 10, 355–396. [Google Scholar] [CrossRef]
- Roccanova, R.; Ming, W.; Whiteside, V.R.; McGuire, M.A.; Sellers, I.R.; Du, M.-H.; Saparov, B. Synthesis, crystal and electronic structures, and optical properties of (CH3NH3) 2CdX4 (X = Cl, Br, I). Inorg. Chem. 2017, 56, 13878–13888. [Google Scholar] [CrossRef]
- Li, G.; Zou, X.; Cheng, J.; Chen, D.; Yao, Y.; Chang, C.; Yu, X.; Zhou, Z.; Wang, J.; Liu, B. Impact of perovskite composition on film formation quality and photophysical properties for flexible perovskite solar cells. Molecules 2020, 25, 732. [Google Scholar] [CrossRef]
- Bin Mohd Yusoff, A.R.; Nazeeruddin, M.K. Organohalide lead perovskites for photovoltaic applications. J. Phys. Chem. Lett. 2016, 7, 851–866. [Google Scholar] [CrossRef]
- Reshi, H.A.; Zargar, R.A. Perovskite Solar Cells: The Challenging Issues for Stable Power Conversion Efficiency. Recent Dev. Optoelectron. Devices 2018, 117. [Google Scholar] [CrossRef]
- Vidyasagar, C.C.; Muñoz Flores, B.M.; Jiménez Pérez, V.M. Recent Advances in Synthesis and Properties of Hybrid Halide Perovskites for Photovoltaics. Nano-Micro Lett. 2018, 10, 68. [Google Scholar] [CrossRef] [PubMed]
- Calvo, M.E. Materials chemistry approaches to the control of the optical features of perovskite solar cells. J. Mater. Chem. A 2017, 5, 20561–20578. [Google Scholar] [CrossRef]
- Bhunia, H.; Chatterjee, S.; Pal, A.J. Band Edges of Hybrid Halide Perovskites under the Influence of Mixed-Cation Approach: A Scanning Tunneling Spectroscopic Insight. ACS Appl. Energy Mater. 2018, 1, 4351–4358. [Google Scholar] [CrossRef]
- Walsh, A. Principles of chemical bonding and band gap engineering in hybrid organic–inorganic halide perovskites. J. Phys. Chem. C 2015, 119, 5755–5760. [Google Scholar] [CrossRef]
- Chen, K.; Schünemann, S.; Song, S.; Tüysüz, H. Structural effects on optoelectronic properties of halide perovskites. Chem. Soc. Rev. 2018, 47, 7045–7077. [Google Scholar] [CrossRef]
- Dualeh, A.; Tétreault, N.; Moehl, T.; Gao, P.; Nazeeruddin, M.K.; Grätzel, M. Effect of annealing temperature on film morphology of organic–inorganic hybrid pervoskite solid-state solar cells. Adv. Funct. Mater. 2014, 24, 3250–3258. [Google Scholar] [CrossRef]
- Li, W.; Fan, J.; Li, J.; Mai, Y.; Wang, L. Controllable grain morphology of perovskite absorber film by molecular self-assembly toward efficient solar cell exceeding 17%. J. Am. Chem. Soc. 2015, 137, 10399–10405. [Google Scholar] [CrossRef]
- Sanders, S.; Stümmler, D.; Pfeiffer, P.; Ackermann, N.; Schimkat, F.; Simkus, G.; Heuken, M.; Baumann, P.K.; Vescan, A.; Kalisch, H. Morphology Control of Organic–Inorganic Bismuth-Based Perovskites for Solar Cell Application. Phys. Status Solidi 2018, 215, 1800409. [Google Scholar] [CrossRef]
- Stoumpos, C.C.; Kanatzidis, M.G. Halide Perovskites: Poor Man’s high-performance semiconductors. Adv. Mater. 2016, 28, 5778–5793. [Google Scholar] [CrossRef]
- Yang, L.; Barrows, A.T.; Lidzey, D.G.; Wang, T. Recent progress and challenges of organometal halide perovskite solar cells. Reports Prog. Phys. 2016, 79, 026501. [Google Scholar] [CrossRef] [PubMed]
- Green, M.A.; Ho-Baillie, A.; Snaith, H.J. The emergence of perovskite solar cells. Nat. Photonics 2014, 8, 506–514. [Google Scholar] [CrossRef]
- Hao, F.; Stoumpos, C.C.; Cao, D.H.; Chang, R.P.H.; Kanatzidis, M.G. Lead-free solid-state organic–inorganic halide perovskite solar cells. Nat. Photonics 2014, 8, 489–494. [Google Scholar] [CrossRef]
- Tsai, C.; Mohanta, N.; Wang, C.; Lin, Y.; Yang, Y.; Wang, C.; Hung, C.; Diau, E.W. Formation of Stable Tin Perovskites Co-crystallized with Three Halides for Carbon-Based Mesoscopic Lead-Free Perovskite Solar Cells. Angew. Chemie 2017, 129, 14007–14011. [Google Scholar] [CrossRef]
- Tsarev, S.; Boldyreva, A.G.; Luchkin, S.Y.; Elshobaki, M.; Afanasov, M.I.; Stevenson, K.J.; Troshin, P.A. Hydrazinium-assisted stabilisation of methylammonium tin iodide for lead-free perovskite solar cells. J. Mater. Chem. A 2018, 6, 21389–21395. [Google Scholar] [CrossRef]
- Koh, T.M.; Krishnamoorthy, T.; Yantara, N.; Shi, C.; Leong, W.L.; Boix, P.P.; Grimsdale, A.C.; Mhaisalkar, S.G.; Mathews, N. Formamidinium tin-based perovskite with low E g for photovoltaic applications. J. Mater. Chem. A 2015, 3, 14996–15000. [Google Scholar] [CrossRef]
- Ke, W.; Stoumpos, C.C.; Zhu, M.; Mao, L.; Spanopoulos, I.; Liu, J.; Kontsevoi, O.Y.; Chen, M.; Sarma, D.; Zhang, Y. Enhanced photovoltaic performance and stability with a new type of hollow 3D perovskite {en} FASnI3. Sci. Adv. 2017, 3, e1701293. [Google Scholar] [CrossRef]
- Kayesh, M.E.; Chowdhury, T.H.; Matsuishi, K.; Kaneko, R.; Kazaoui, S.; Lee, J.-J.; Noda, T.; Islam, A. Enhanced photovoltaic performance of FASnI3-based perovskite solar cells with hydrazinium chloride coadditive. ACS Energy Lett. 2018, 3, 1584–1589. [Google Scholar] [CrossRef]
- Gao, W.; Ran, C.; Li, J.; Dong, H.; Jiao, B.; Zhang, L.; Lan, X.; Hou, X.; Wu, Z. Robust stability of efficient lead-free formamidinium tin iodide perovskite solar cells realized by structural regulation. J. Phys. Chem. Lett. 2018, 9, 6999–7006. [Google Scholar] [CrossRef]
- Shao, S.; Liu, J.; Portale, G.; Fang, H.; Blake, G.R.; ten Brink, G.H.; Koster, L.J.A.; Loi, M.A. Highly reproducible Sn-based hybrid perovskite solar cells with 9% efficiency. Adv. Energy Mater. 2018, 8, 1702019. [Google Scholar] [CrossRef]
- Kumar, M.H.; Dharani, S.; Leong, W.L.; Boix, P.P.; Prabhakar, R.R.; Baikie, T.; Shi, C.; Ding, H.; Ramesh, R.; Asta, M. Lead-free halide perovskite solar cells with high photocurrents realized through vacancy modulation. Adv. Mater. 2014, 26, 7122–7127. [Google Scholar] [CrossRef] [PubMed]
- Sabba, D.; Mulmudi, H.K.; Prabhakar, R.R.; Krishnamoorthy, T.; Baikie, T.; Boix, P.P.; Mhaisalkar, S.; Mathews, N. Impact of anionic Br–substitution on open circuit voltage in lead free perovskite (CsSnI3−xBrx) solar cells. J. Phys. Chem. C 2015, 119, 1763–1767. [Google Scholar] [CrossRef]
- Zhang, T.; Li, H.; Ban, H.; Sun, Q.; Shen, Y.; Wang, M. Efficient CsSnI3-based inorganic perovskite solar cells based on a mesoscopic metal oxide framework via incorporating a donor element. J. Mater. Chem. 2020, 8, 4118–4124. [Google Scholar] [CrossRef]
- Gupta, S.; Bendikov, T.; Hodes, G.; Cahen, D. CsSnBr3, a lead-free halide perovskite for long-term solar cell application: Insights on SnF2 addition. ACS Energy Lett. 2016, 1, 1028–1033. [Google Scholar] [CrossRef]
- Krishnamoorthy, T.; Ding, H.; Yan, C.; Leong, W.L.; Baikie, T.; Zhang, Z.; Sherburne, M.; Li, S.; Asta, M.; Mathews, N. Lead-free germanium iodide perovskite materials for photovoltaic applications. J. Mater. Chem. A 2015, 3, 23829–23832. [Google Scholar] [CrossRef]
- Kopacic, I.; Friesenbichler, B.; Hoefler, S.F.; Kunert, B.; Plank, H.; Rath, T.; Trimmel, G. Enhanced performance of germanium halide perovskite solar cells through compositional engineering. ACS Appl. Energy Mater. 2018, 1, 343–347. [Google Scholar] [CrossRef]
- Eperon, G.E.; Stranks, S.D.; Menelaou, C.; Johnston, M.B.; Herz, L.M.; Snaith, H.J. Formamidinium lead trihalide: A broadly tunable perovskite for efficient planar heterojunction solar cells. Energy Environ. Sci. 2014, 7, 982. [Google Scholar] [CrossRef]
- Schötz, K.; Askar, A.M.; Köhler, A.; Shankar, K.; Panzer, F. Investigating the Tetragonal-to-Orthorhombic Phase Transition of Methylammonium Lead Iodide Single Crystals by Detailed Photoluminescence Analysis. Adv. Opt. Mater. 2020, 8, 2000455. [Google Scholar] [CrossRef]
- Feng, J.; Xiao, B. Crystal Structures, Optical Properties, and Effective Mass Tensors of CH3 NH3PbX3 (X = I and Br) Phases Predicted from HSE06. J. Phys. Chem. Lett. 2014, 5, 1278–1282. [Google Scholar] [CrossRef]
- Zhou, C.; Lin, H.; He, Q.; Xu, L.; Worku, M.; Chaaban, M.; Lee, S.; Shi, X.; Du, M.-H.; Ma, B. Low dimensional metal halide perovskites and hybrids. Mater. Sci. Eng. R Rep. 2019, 137, 38–65. [Google Scholar] [CrossRef]
- Gai, C.; Wang, J.; Wang, Y.; Li, J. The Low-Dimensional Three-Dimensional Tin Halide Perovskite: Film Characterization and Device Performance. Energies 2020, 13, 2. [Google Scholar] [CrossRef]
- Noel, N.K.; Stranks, S.D.; Abate, A.; Wehrenfennig, C.; Guarnera, S.; Haghighirad, A.-A.; Sadhanala, A.; Eperon, G.E.; Pathak, S.K.; Johnston, M.B. Lead-free organic–inorganic tin halide perovskites for photovoltaic applications. Energy Environ. Sci. 2014, 7, 3061–3068. [Google Scholar] [CrossRef]
- Singh, P.K.; Singh, R.; Singh, V.; Bhattacharya, B.; Khan, Z.H. New class of lead free perovskite material for low-cost solar cell application. Mater. Res. Bull. 2018, 97, 572–577. [Google Scholar]
- Zhang, M.; Lyu, M.; Yun, J.-H.; Noori, M.; Zhou, X.; Cooling, N.A.; Wang, Q.; Yu, H.; Dastoor, P.C.; Wang, L. Low-temperature processed solar cells with formamidinium tin halide perovskite/fullerene heterojunctions. Nano Res. 2016, 9, 1570–1577. [Google Scholar] [CrossRef]
- Chung, I.; Song, J.-H.; Im, J.; Androulakis, J.; Malliakas, C.D.; Li, H.; Freeman, A.J.; Kenney, J.T.; Kanatzidis, M.G. CsSnI3: Semiconductor or metal? High electrical conductivity and strong near-infrared photoluminescence from a single material. High hole mobility and phase-transitions. J. Am. Chem. Soc. 2012, 134, 8579–8587. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, J.J.; Ren, Y.; Yu, C.; Shum, K. Schottky solar cells based on CsSnI3 thin-films. Appl. Phys. Lett. 2012, 101, 93901. [Google Scholar] [CrossRef]
- Stoumpos, C.C.; Frazer, L.; Clark, D.J.; Kim, Y.S.; Rhim, S.H.; Freeman, A.J.; Ketterson, J.B.; Jang, J.I.; Kanatzidis, M.G. Hybrid germanium iodide perovskite semiconductors: Active lone pairs, structural distortions, direct and indirect energy gaps, and strong nonlinear optical properties. J. Am. Chem. Soc. 2015, 137, 6804–6819. [Google Scholar] [CrossRef]
- Zhou, C.; Lin, H.; Lee, S.; Chaaban, M.; Ma, B. Organic–inorganic metal halide hybrids beyond perovskites. Mater. Res. Lett. 2018, 6, 552–569. [Google Scholar] [CrossRef]
- Lozhkina, O.A.; Murashkina, A.A.; Elizarov, M.S.; Shilovskikh, V.V.; Zolotarev, A.A.; Kapitonov, Y.V.; Kevorkyants, R.; Emeline, A.V.; Miyasaka, T. Microstructural analysis and optical properties of the halide double perovskite Cs2BiAgBr6 single crystals. Chem. Phys. Lett. 2018, 694, 18–22. [Google Scholar] [CrossRef]
- Gao, W.; Ran, C.; Xi, J.; Jiao, B.; Zhang, W.; Wu, M.; Hou, X.; Wu, Z. High-Quality Cs2AgBiBr6 Double Perovskite Film for Lead-Free Inverted Planar Heterojunction Solar Cells with 2.2% Efficiency. ChemPhysChem 2018, 19, 1696–1700. [Google Scholar] [CrossRef]
- Karmakar, A.; Dodd, M.S.; Agnihotri, S.; Ravera, E.; Michaelis, V.K. Cu (II)-doped Cs2SbAgCl6 double perovskite: A lead-free, low-bandgap material. Chem. Mater. 2018, 30, 8280–8290. [Google Scholar] [CrossRef]
- Lee, B.; Krenselewski, A.; Baik, S.I.; Seidman, D.N.; Chang, R.P.H. Solution processing of air-stable molecular semiconducting iodosalts, Cs2SnI6−xBrx, for potential solar cell applications. Sustain. Energy Fuels 2017, 1, 710–724. [Google Scholar] [CrossRef]
- Chu, L.; Ahmad, W.; Liu, W.; Yang, J.; Zhang, R.; Sun, Y.; Yang, J.; Li, X. Lead-Free Halide Double Perovskite Materials: A New Superstar Toward Green and Stable Optoelectronic Applications. Nano-Micro Lett. 2019, 11, 1–18. [Google Scholar] [CrossRef]
- Volonakis, G.; Filip, M.R.; Haghighirad, A.A.; Sakai, N.; Wenger, B.; Snaith, H.J.; Giustino, F. Lead-free halide double perovskites via heterovalent substitution of noble metals. J. Phys. Chem. Lett. 2016, 7, 1254–1259. [Google Scholar] [CrossRef]
- Li, Z.; Kavanagh, S.; Napari, M.; Palgrave, R.G.; Abdi-Jalebi, M.; Andaji-Garmaroudi, Z.; Davies, D.W.; Laitinen, M.; Julin, J.; Friend, R.H. Bandgap Lowering in Mixed Alloys of Cs2Ag (SbxBi1-x) Br6 Double Perovskite Thin Films. arXiv 2020, arXiv:2007.00388. [Google Scholar]
- Ji, F.; Klarbring, J.; Wang, F.; Ning, W.; Wang, L.; Yin, C.; Figueroa, J.S.M.; Christensen, C.K.; Etter, M.; Ederth, T.; et al. Lead-Free Halide Double Perovskite Cs2AgBiBr6 with Decreased Band Gap. Angew. Chemie 2020, 132, 15303–15306. [Google Scholar] [CrossRef]
- Wei, F.; Deng, Z.; Sun, S.; Hartono, N.T.P.; Seng, H.L.; Buonassisi, T.; Bristowe, P.D.; Cheetham, A.K. Enhanced visible light absorption for lead-free double perovskite Cs2AgSbBr6. Chem. Commun. 2019, 55, 3721–3724. [Google Scholar] [CrossRef]
- Cao, X.; Kang, L.; Guo, S.; Zhang, M.; Lin, Z.; Gao, J. Cs2NaVCl6: A Pb-Free Halide Double Perovskite with Strong Visible and Near-Infrared Light Absorption. ACS Appl. Mater. Interfaces 2019, 11, 38648–38653. [Google Scholar] [CrossRef]
- Dahl, J.C.; Osowiecki, W.T.; Cai, Y.; Swabeck, J.K.; Bekenstein, Y.; Asta, M.; Chan, E.M.; Alivisatos, A.P. Probing the Stability and Band Gaps of Cs2AgInCl6 and Cs2AgSbCl6 Lead-Free Double Perovskite Nanocrystals. Chem. Mater. 2019, 31, 3134–3143. [Google Scholar] [CrossRef]
- Zhou, W.; Han, P.; Zhang, X.; Zheng, D.; Yang, S.; Yang, Y.; Luo, C.; Yang, B.; Hong, F.; Wei, D.; et al. Lead-Free Small-Bandgap Cs2CuSbCl6 Double Perovskite Nanocrystals. J. Phys. Chem. Lett. 2020, 11, 6463–6467. [Google Scholar] [CrossRef]
- Peedikakkandy, L.; Chatterjee, S.; Pal, A.J. Bandgap Engineering and Efficient Conversion of a Ternary Perovskite (Cs3Bi2I9) to a Double Perovskite (Cs2NaBiI6) with the Aid of Alkali Metal Sulfide. J. Phys. Chem. C 2020, 124, 10878–10886. [Google Scholar] [CrossRef]
- Ghosh, B.; Febriansyah, B.; Harikesh, P.C.; Koh, T.M.; Hadke, S.S.; Wong, L.H.; England, J.; Mhaisalkar, S.G.; Mathews, N. Direct Bandgap Mixed-valence Organic-inorganic Gold Perovskite as Visible Light Absorber. Chem. Mater. 2020, 32. [Google Scholar] [CrossRef]
- Slavney, A.H.; Hu, T.; Lindenberg, A.M.; Karunadasa, H.I. A bismuth-halide double perovskite with long carrier recombination lifetime for photovoltaic applications. J. Am. Chem. Soc. 2016, 138, 2138–2141. [Google Scholar] [CrossRef]
- McClure, E.T.; Ball, M.R.; Windl, W.; Woodward, P.M. Cs2AgBiX6 (X= Br, Cl): New visible light absorbing, lead-free halide perovskite semiconductors. Chem. Mater. 2016, 28, 1348–1354. [Google Scholar] [CrossRef]
- Greul, E.; Petrus, M.L.; Binek, A.; Docampo, P.; Bein, T. Highly stable, phase pure Cs2AgBiBr6double perovskite thin films for optoelectronic applications. J. Mater. Chem. A 2017, 5, 19972–19981. [Google Scholar] [CrossRef]
- Li, Y.; Ji, L.; Liu, R.; Zhang, C.; Mak, C.H.; Zou, X.; Shen, H.-H.; Leu, S.-Y.; Hsu, H.-Y. A review on morphology engineering for highly efficient and stable hybrid perovskite solar cells. J. Mater. Chem. A 2018, 6, 12842–12875. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, Q.; Liu, Y.; Luo, W.; Guo, X.; Huang, Z.; Ting, H.; Sun, W.; Zhong, X.; Wei, S. The Dawn of Lead-Free Perovskite Solar Cell: Highly Stable Double Perovskite Cs2AgBiBr6 Film. Adv. Sci. 2018, 5, 1700759. [Google Scholar] [CrossRef]
- Ning, W.; Wang, F.; Wu, B.; Lu, J.; Yan, Z.; Liu, X.; Tao, Y.; Liu, J.; Huang, W.; Fahlman, M. Long Electron–Hole Diffusion Length in High-Quality Lead-Free Double Perovskite Films. Adv. Mater. 2018, 30, 1706246. [Google Scholar] [CrossRef] [PubMed]
- Du, K.; Meng, W.; Wang, X.; Yan, Y.; Mitzi, D.B. Bandgap Engineering of Lead-Free Double Perovskite Cs2AgBiBr6 through Trivalent Metal Alloying. Angew. Chemie Int. Ed. 2017, 56, 8158–8162. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, L.; Wang, M.; Zhong, Y.; Huang, M.; Long, Y.; Zhu, H. Bandgap-tunable double-perovskite thin films by solution processing. Mater. Today 2019, 28, 25–30. [Google Scholar] [CrossRef]
- Volonakis, G.; Haghighirad, A.A.; Milot, R.L.; Sio, W.H.; Filip, M.R.; Wenger, B.; Johnston, M.B.; Herz, L.M.; Snaith, H.J.; Giustino, F. Cs2InAgCl6: A new lead-free halide double perovskite with direct band gap. J. Phys. Chem. Lett. 2017, 8, 772–778. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Xia, Z.; Molokeev, M.S.; Zhang, X.; Peng, D.; Liu, Q. Composition design, optical gap and stability investigations of lead-free halide double perovskite Cs2AgInCl6. J. Mater. Chem. A 2017, 5, 15031–15037. [Google Scholar] [CrossRef]
- Zhang, C.; Gao, L.; Teo, S.; Guo, Z.; Xu, Z.; Zhao, S.; Ma, T. Design of a novel and highly stable lead-free Cs2NaBiI6double perovskite for photovoltaic application. Sustain. Energy Fuels 2018, 2, 2419–2428. [Google Scholar] [CrossRef]
- Han, X.; Liang, J.; Yang, J.; Soni, K.; Fang, Q.; Wang, W.; Zhang, J.; Jia, S.; Martí, A.A.; Zhao, Y.; et al. Lead-Free Double Perovskite Cs2SnX6: Facile Solution Synthesis and Excellent Stability. Small 2019, 15, 1901650. [Google Scholar] [CrossRef]
- Karim, M.M.S.; Ganose, A.M.; Pieters, L.; Winnie Leung, W.W.; Wade, J.; Zhang, L.; Scanlon, D.O.; Palgrave, R.G. Anion Distribution, Structural Distortion, and Symmetry-Driven Optical Band Gap Bowing in Mixed Halide Cs2SnX6Vacancy Ordered Double Perovskites. Chem. Mater. 2019, 31, 9430–9444. [Google Scholar] [CrossRef]
- Schwartz, D.; Murshed, R.; Larson, H.; Usprung, B.; Soltanmohamad, S.; Pandey, R.; Barnard, E.S.; Rockett, A.; Hartmann, T.; Castelli, I.E.; et al. Air Stable, High-Efficiency, Pt-Based Halide Perovskite Solar Cells with Long Carrier Lifetimes. Phys. Status Solidi Rapid Res. Lett. 2020, 14, 2000182. [Google Scholar] [CrossRef]
- Chen, M.; Ju, M.-G.; Carl, A.D.; Zong, Y.; Grimm, R.L.; Gu, J.; Zeng, X.C.; Zhou, Y.; Padture, N.P. Cesium titanium (IV) bromide thin films based stable lead-free perovskite solar cells. Joule 2018, 2, 558–570. [Google Scholar] [CrossRef]
- Maughan, A.E.; Ganose, A.M.; Bordelon, M.M.; Miller, E.M.; Scanlon, D.O.; Neilson, J.R. Defect tolerance to intolerance in the vacancy-ordered double perovskite semiconductors Cs2SnI6 and Cs2TeI6. J. Am. Chem. Soc. 2016, 138, 8453–8464. [Google Scholar] [CrossRef]
- Lee, B.; Stoumpos, C.C.; Zhou, N.; Hao, F.; Malliakas, C.; Yeh, C.-Y.; Marks, T.J.; Kanatzidis, M.G.; Chang, R.P.H. Air-stable molecular semiconducting iodosalts for solar cell applications: Cs2SnI6 as a hole conductor. J. Am. Chem. Soc. 2014, 136, 15379–15385. [Google Scholar] [CrossRef]
- Saparov, B.; Sun, J.-P.; Meng, W.; Xiao, Z.; Duan, H.-S.; Gunawan, O.; Shin, D.; Hill, I.G.; Yan, Y.; Mitzi, D.B. Thin-film deposition and characterization of a Sn-deficient perovskite derivative Cs2SnI6. Chem. Mater. 2016, 28, 2315–2322. [Google Scholar] [CrossRef]
- Sakai, N.; Haghighirad, A.A.; Filip, M.R.; Nayak, P.K.; Nayak, S.; Ramadan, A.; Wang, Z.; Giustino, F.; Snaith, H.J. Solution-processed cesium hexabromopalladate (IV), Cs2PdBr6, for optoelectronic applications. J. Am. Chem. Soc. 2017, 139, 6030–6033. [Google Scholar] [CrossRef]
- Ju, M.-G.; Chen, M.; Zhou, Y.; Garces, H.F.; Dai, J.; Ma, L.; Padture, N.P.; Zeng, X.C. Earth-abundant nontoxic titanium (IV)-based vacancy-ordered double perovskite halides with tunable 1.0 to 1.8 eV bandgaps for photovoltaic applications. ACS Energy Lett. 2018, 3, 297–304. [Google Scholar] [CrossRef]
- Ju, D.; Zheng, X.; Yin, J.; Qiu, Z.; Türedi, B.; Liu, X.; Dang, Y.; Cao, B.; Mohammed, O.F.; Bakr, O.M. Tellurium-Based Double Perovskites A2TeX6 with Tunable Band Gap and Long Carrier Diffusion Length for Optoelectronic Applications. ACS Energy Lett. 2018, 4, 228–234. [Google Scholar] [CrossRef]
- Liu, Y.; Li, F.; Yao, F.; He, Z.; Liu, S.; Xu, L.; Han, X.; Wang, K. Synthesis, Structure and Photoluminescence Properties of 2D Organic–Inorganic Hybrid Perovskites. Appl. Sci. 2019, 9, 5211. [Google Scholar] [CrossRef]
- Cui, X.-P.; Jiang, K.-J.; Huang, J.-H.; Zhang, Q.-Q.; Su, M.-J.; Yang, L.-M.; Song, Y.-L.; Zhou, X.-Q. Cupric bromide hybrid perovskite heterojunction solar cells. Synth. Met. 2015, 209, 247–250. [Google Scholar] [CrossRef]
- Yin, J.; Li, H.; Cortecchia, D.; Soci, C.; Bredas, J.-L. Excitonic and polaronic properties of 2D hybrid organic–inorganic perovskites. ACS Energy Lett. 2017, 2, 417–423. [Google Scholar] [CrossRef]
- Lai, H.; Kan, B.; Liu, T.; Zheng, N.; Xie, Z.; Zhou, T.; Wan, X.; Zhang, X.; Liu, Y.; Chen, Y. Two-dimensional Ruddlesden–Popper perovskite with nanorod-like morphology for solar cells with efficiency exceeding 15%. J. Am. Chem. Soc. 2018, 140, 11639–11646. [Google Scholar] [CrossRef]
- Wei, Y.; Audebert, P.; Galmiche, L.; Lauret, J.-S.; Deleporte, E. Photostability of 2D organic-inorganic hybrid perovskites. Materials 2014, 7, 4789–4802. [Google Scholar] [CrossRef]
- Lan, C.; Zhou, Z.; Wei, R.; Ho, J.C. Two-dimensional perovskite materials: From synthesis to energy-related applications. Mater. Today Energy 2019, 11, 61–82. [Google Scholar] [CrossRef]
- Zhou, F.; Abdelwahab, I.; Leng, K.; Loh, K.P.; Ji, W. 2D Perovskites with Giant Excitonic Optical Nonlinearities for High-Performance Sub-Bandgap Photodetection. Adv. Mater. 2019, 31, 1904155. [Google Scholar] [CrossRef]
- Wei, Y.; Chu, H.; Chen, B.; Tian, Y.; Yang, X.; Cai, B.; Zhang, Y.; Zhao, J. Two-dimensional cyclohexane methylamine based perovskites as stable light absorbers for solar cells. Sol. Energy 2020, 201, 13–20. [Google Scholar] [CrossRef]
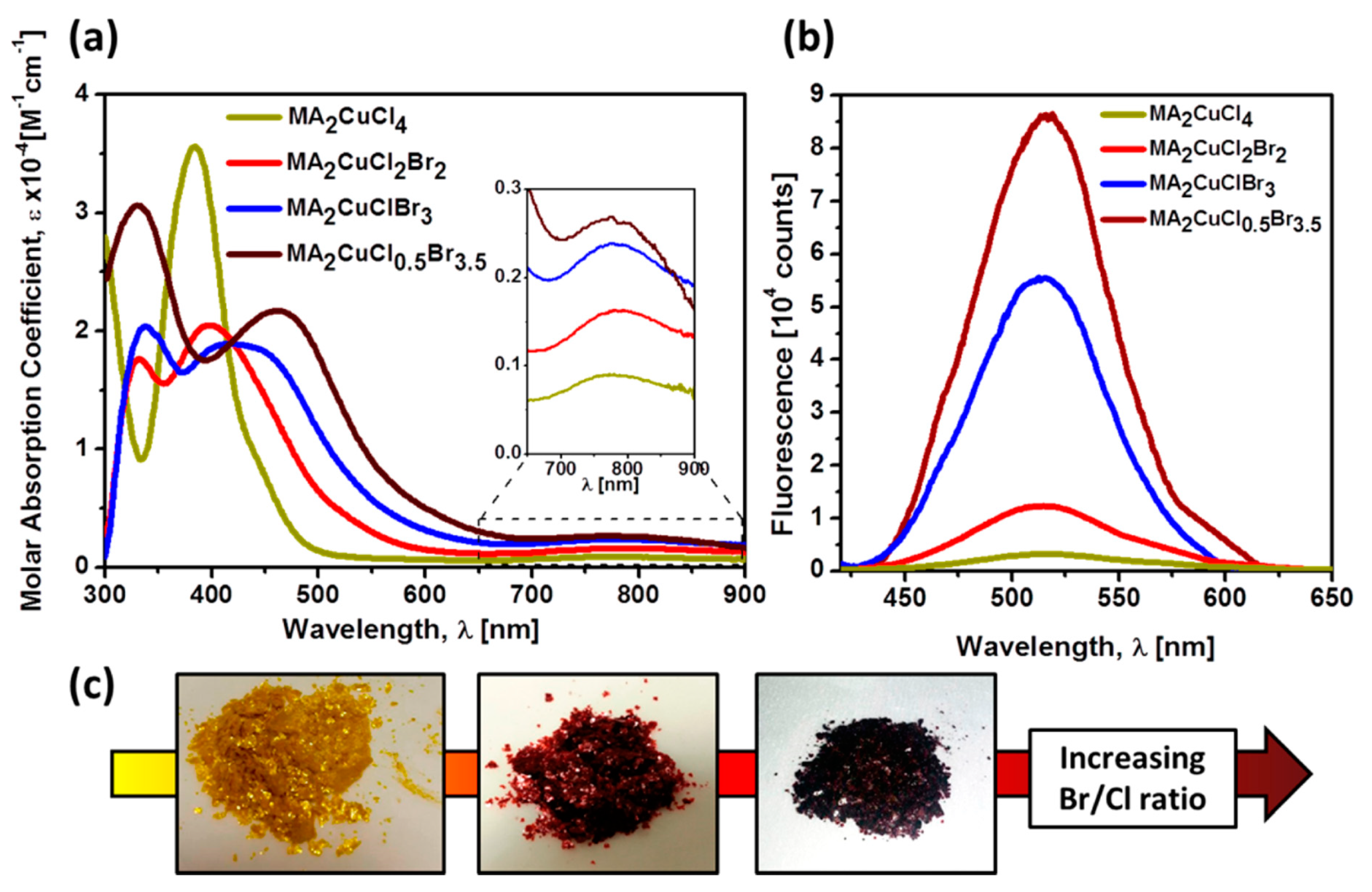
- Cortecchia, D.; Dewi, H.A.; Yin, J.; Bruno, A.; Chen, S.; Baikie, T.; Boix, P.P.; Grätzel, M.; Mhaisalkar, S.; Soci, C. Lead-free MA2CuCl x Br4−x hybrid perovskites. Inorg. Chem. 2016, 55, 1044–1052. [Google Scholar] [CrossRef]
- Yin, J.; Shi, S.; Wei, J.; He, G.; Fan, L.; Guo, J.; Zhang, K.; Xu, W.; Yuan, C.; Wang, Y. Earth-abundant and environment friendly organic–inorganic hybrid tetrachloroferrate salt CH3NH3FeCl4: Structure, adsorption properties and photoelectric behavior. RSC Adv. 2018, 8, 19958–19963. [Google Scholar] [CrossRef]
- Elseman, A.M.; Shalan, A.E.; Sajid, S.; Rashad, M.M.; Hassan, A.M.; Li, M. Copper-substituted lead perovskite materials constructed with different halides for working (CH3NH3) 2CuX4-based perovskite solar cells from experimental and theoretical view. ACS Appl. Mater. Interfaces 2018, 10, 11699–11707. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhong, X.; Hu, Y.; Li, B.; Sheng, Y.; Zhang, Y.; Weng, C.; Feng, M.; Han, H.; Wang, J. Organic–inorganic copper (II)-based material: A low-toxic, highly stable light absorber for photovoltaic application. J. Phys. Chem. Lett. 2017, 8, 1804–1809. [Google Scholar] [CrossRef]
- Hajlaoui, F.; Audebrand, N.; Roisnel, T.; Zouari, N. Structural phase transition, electrical and semiconducting properties in a lead-free 2D hybrid perovskite-like compound: [Cl-(CH2)2-NH3]2[CuCl4]. Appl. Organomet. Chem. 2020, 34, e5293. [Google Scholar] [CrossRef]
- Daub, M.; Stroh, R.; Hillebrecht, H. Synthesis, crystal structure, and optical properties of (CH3NH3) 2CoX4 (X = Cl, Br, I, Cl0.5Br0.5, Cl0.5I0.5, Br0.5I0.5). Zeitschrift für Anorg. und Allg. Chemie 2016, 642, 268–274. [Google Scholar] [CrossRef]
- Zhou, H.; Cui, X.; Yuan, C.; Cui, J.; Shi, S.; He, G.; Wang, Y.; Wei, J.; Pu, X.; Li, W. Band-Gap Tuning of Organic–Inorganic Hybrid Palladium Perovskite Materials for a Near-Infrared Optoelectronics Response. ACS Omega 2018, 3, 13960–13966. [Google Scholar] [CrossRef]
- Nie, Z.; Yin, J.; Zhou, H.; Chai, N.; Chen, B.; Zhang, Y.; Qu, K.; Shen, G.; Ma, H.; Li, Y. Layered and Pb-Free Organic–Inorganic Perovskite Materials for Ultraviolet Photoresponse:(010)-Oriented (CH3NH3) 2MnCl4 Thin Film. ACS Appl. Mater. Interfaces 2016, 8, 28187–28193. [Google Scholar] [CrossRef]
- Singh, T.; Kulkarni, A.; Ikegami, M.; Miyasaka, T. Effect of electron transporting layer on bismuth-based lead-free perovskite (CH3NH3)3Bi2I9 for photovoltaic applications. ACS Appl. Mater. Interfaces 2016, 8, 14542–14547. [Google Scholar] [CrossRef]
- Tang, G.; Xiao, Z.; Hosono, H.; Kamiya, T.; Fang, D.; Hong, J. Layered Halide Double Perovskites Cs3+nM(II)nSb2X9+3n (M = Sn, Ge) for Photovoltaic Applications. J. Phys. Chem. Lett. 2018, 9, 43–48. [Google Scholar] [CrossRef]
- Kim, S.-Y.; Yun, Y.; Shin, S.; Lee, J.H.; Heo, Y.-W.; Lee, S. Wide range tuning of band gap energy of A3B2X9 perovskite-like halides. Scr. Mater. 2019, 166, 107–111. [Google Scholar] [CrossRef]
- Lehner, A.J.; Fabini, D.H.; Evans, H.A.; Hébert, C.-A.; Smock, S.R.; Hu, J.; Wang, H.; Zwanziger, J.W.; Chabinyc, M.L.; Seshadri, R. Crystal and Electronic Structures of Complex Bismuth Iodides A 3Bi2I9 (A = K, Rb, Cs) Related to Perovskite: Aiding the Rational Design of Photovoltaics. Chem. Mater. 2015, 27, 7137–7148. [Google Scholar] [CrossRef]
- Karuppuswamy, P.; Boopathi, K.M.; Mohapatra, A.; Chen, H.-C.; Wong, K.-T.; Wang, P.-C.; Chu, C.-W. Role of a hydrophobic scaffold in controlling the crystallization of methylammonium antimony iodide for efficient lead-free perovskite solar cells. Nano Energy 2018, 45, 330–336. [Google Scholar] [CrossRef]
- Chatterjee, S.; Pal, A.J. Tin (IV) substitution in (CH3NH3) 3Sb2I9: Toward low-band-gap defect-ordered hybrid perovskite solar cells. ACS Appl. Mater. Interfaces 2018, 10, 35194–35205. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Boopathi, K.M.; Mohapatra, A.; Chen, Y.F.; Li, G.; Chu, C.W. Photovoltaic performance of vapor-assisted solution-processed layer polymorph of Cs3Sb2I9. ACS Appl. Mater. Interfaces 2018, 10, 2566–2573. [Google Scholar] [CrossRef]
- Hebig, J.-C.; Kühn, I.; Flohre, J.; Kirchartz, T. Optoelectronic properties of (CH3NH3) 3Sb2I9 thin films for photovoltaic applications. ACS Energy Lett. 2016, 1, 309–314. [Google Scholar] [CrossRef]
- Harikesh, P.C.; Mulmudi, H.K.; Ghosh, B.; Goh, T.W.; Teng, Y.T.; Thirumal, K.; Lockrey, M.; Weber, K.; Koh, T.M.; Li, S. Rb as an alternative cation for templating inorganic lead-free perovskites for solution processed photovoltaics. Chem. Mater. 2016, 28, 7496–7504. [Google Scholar] [CrossRef]
- Weber, S.; Rath, T.; Fellner, K.; Fischer, R.; Resel, R.; Kunert, B.; Dimopoulos, T.; Steinegger, A.; Trimmel, G. Influence of the iodide to bromide ratio on crystallographic and optoelectronic properties of rubidium antimony halide perovskites. ACS Appl. Energy Mater. 2018, 2, 539–547. [Google Scholar] [CrossRef]
- Zuo, C.; Ding, L. Lead-free Perovskite Materials (NH4) 3Sb2IxBr9−x. Angew. Chemie 2017, 129, 6628–6632. [Google Scholar] [CrossRef]
- Boopathi, K.M.; Karuppuswamy, P.; Singh, A.; Hanmandlu, C.; Lin, L.; Abbas, S.A.; Chang, C.C.; Wang, P.C.; Li, G.; Chu, C.W. Solution-processable antimony-based light-absorbing materials beyond lead halide perovskites. J. Mater. Chem. A 2017, 5, 20843–20850. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, C.; Cai, M.; Liao, Y.; Ding, Y.; Ma, S.; Liu, X.; Guli, M.; Dai, S.; Nazeeruddin, M.K. Dimension-Controlled Growth of Antimony-Based Perovskite-like Halides for Lead-Free and Semitransparent Photovoltaics. ACS Appl. Mater. Interfaces 2020, 12, 17062–17069. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, X.; Xia, X.; Wang, Z.; Huang, Z.; Lei, B.; Gao, Y. High-quality (CH3NH3) 3Bi2I9 film-based solar cells: Pushing efficiency up to 1.64%. J. Phys. Chem. Lett. 2017, 8, 4300–4307. [Google Scholar] [CrossRef]
- Lan, C.; Liang, G.; Zhao, S.; Lan, H.; Peng, H.; Zhang, D.; Sun, H.; Luo, J.; Fan, P. Lead-free formamidinium bismuth perovskites (FA) 3Bi2I9 with low bandgap for potential photovoltaic application. Sol. Energy 2019, 177, 501–507. [Google Scholar] [CrossRef]
- Saparov, B.; Hong, F.; Sun, J.-P.; Duan, H.-S.; Meng, W.; Cameron, S.; Hill, I.G.; Yan, Y.; Mitzi, D.B. Thin-film preparation and characterization of Cs3Sb2I9: A lead-free layered perovskite semiconductor. Chem. Mater. 2015, 27, 5622–5632. [Google Scholar] [CrossRef]
- Correa-Baena, J.-P.; Nienhaus, L.; Kurchin, R.C.; Shin, S.S.; Wieghold, S.; Putri Hartono, N.T.; Layurova, M.; Klein, N.D.; Poindexter, J.R.; Polizzotti, A. A-Site Cation in Inorganic A 3Sb2I9 Perovskite Influences Structural Dimensionality, Exciton Binding Energy, and Solar Cell Performance. Chem. Mater. 2018, 30, 3734–3742. [Google Scholar] [CrossRef]
- Anyfantis, G.C.; Ioannou, A.; Barkaoui, H.; Abid, Y.; Psycharis, V.; Raptopoulou, C.P.; Mousdis, G.A. Hybrid halobismuthates as prospective light-harvesting materials: Synthesis, crystal, optical properties and electronic structure. Polyhedron 2020, 175, 114180. [Google Scholar] [CrossRef]
- Dammak, H.; Yangui, A.; Triki, S.; Abid, Y.; Feki, H. Structural characterization, vibrational, optical properties and DFT investigation of a new luminescent organic–inorganic material:(C6H14N) 3Bi2I9. J. Lumin. 2015, 161, 214–220. [Google Scholar] [CrossRef]
- Miller, N.C.; Bernechea, M. Research Update: Bismuth based materials for photovoltaics. APL Mater. 2018, 6, 84503. [Google Scholar] [CrossRef]
- Park, B.; Philippe, B.; Zhang, X.; Rensmo, H.; Boschloo, G.; Johansson, E.M.J. Bismuth based hybrid perovskites A3Bi2I9 (A: methylammonium or cesium) for solar cell application. Adv. Mater. 2015, 27, 6806–6813. [Google Scholar] [CrossRef]
- Ma, Z.; Peng, S.; Wu, Y.; Fang, X.; Chen, X.; Jia, X.; Zhang, K.; Yuan, N.; Ding, J.; Dai, N. Air-stable layered bismuth-based perovskite-like materials: Structures and semiconductor properties. Phys. B Condens. Matter 2017, 526, 136–142. [Google Scholar] [CrossRef]
- Johansson, M.B.; Zhu, H.; Johansson, E.M.J. Extended photo-conversion spectrum in low-toxic bismuth halide perovskite solar cells. J. Phys. Chem. Lett. 2016, 7, 3467–3471. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, B.; Wu, B.; Mulmudi, H.K.; Guet, C.; Weber, K.; Sum, T.C.; Mhaisalkar, S.; Mathews, N. Limitations of Cs3Bi2I9 as lead-free photovoltaic absorber materials. ACS Appl. Mater. Interfaces 2018, 10, 35000–35007. [Google Scholar] [CrossRef] [PubMed]
- Öz, S.; Hebig, J.-C.; Jung, E.; Singh, T.; Lepcha, A.; Olthof, S.; Jan, F.; Gao, Y.; German, R.; van Loosdrecht, P.H.M. Zero-dimensional (CH3NH3) 3Bi2I9 perovskite for optoelectronic applications. Sol. Energy Mater. Sol. Cells 2016, 158, 195–201. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, G.; Gu, Z.; Guo, B.; Liu, W.; Yang, S.; Ye, T.; Chen, C.; Tu, W.; Chen, H. Active-layer evolution and efficiency improvement of (CH3NH 3 3Bi2I9-based solar cell on TiO2-deposited ITO substrate. Nano Res. 2016, 9, 2921–2930. [Google Scholar] [CrossRef]
- Ran, C.; Wu, Z.; Xi, J.; Yuan, F.; Dong, H.; Lei, T.; He, X.; Hou, X. Construction of compact methylammonium bismuth iodide film promoting lead-free inverted planar heterojunction organohalide solar cells with open-circuit voltage over 0.8 V. J. Phys. Chem. Lett. 2017, 8, 394–400. [Google Scholar] [CrossRef]
- Jain, S.M.; Phuyal, D.; Davies, M.L.; Li, M.; Philippe, B.; De Castro, C.; Qiu, Z.; Kim, J.; Watson, T.; Tsoi, W.C. An effective approach of vapour assisted morphological tailoring for reducing metal defect sites in lead-free,(CH3NH3) 3Bi2I9 bismuth-based perovskite solar cells for improved performance and long-term stability. Nano Energy 2018, 49, 614–624. [Google Scholar] [CrossRef]
- Sun, S.; Tominaka, S.; Lee, J.-H.; Xie, F.; Bristowe, P.D.; Cheetham, A.K. Synthesis, crystal structure, and properties of a perovskite-related bismuth phase,(NH4) 3Bi2I9. APL Mater. 2016, 4, 31101. [Google Scholar] [CrossRef]
- Li, T.; Wang, Q.; Nichol, G.S.; Morrison, C.A.; Han, H.; Hu, Y.; Robertson, N. Extending lead-free hybrid photovoltaic materials to new structures: Thiazolium, aminothiazolium and imidazolium iodobismuthates. Dalt. Trans. 2018, 47, 7050–7058. [Google Scholar] [CrossRef] [PubMed]
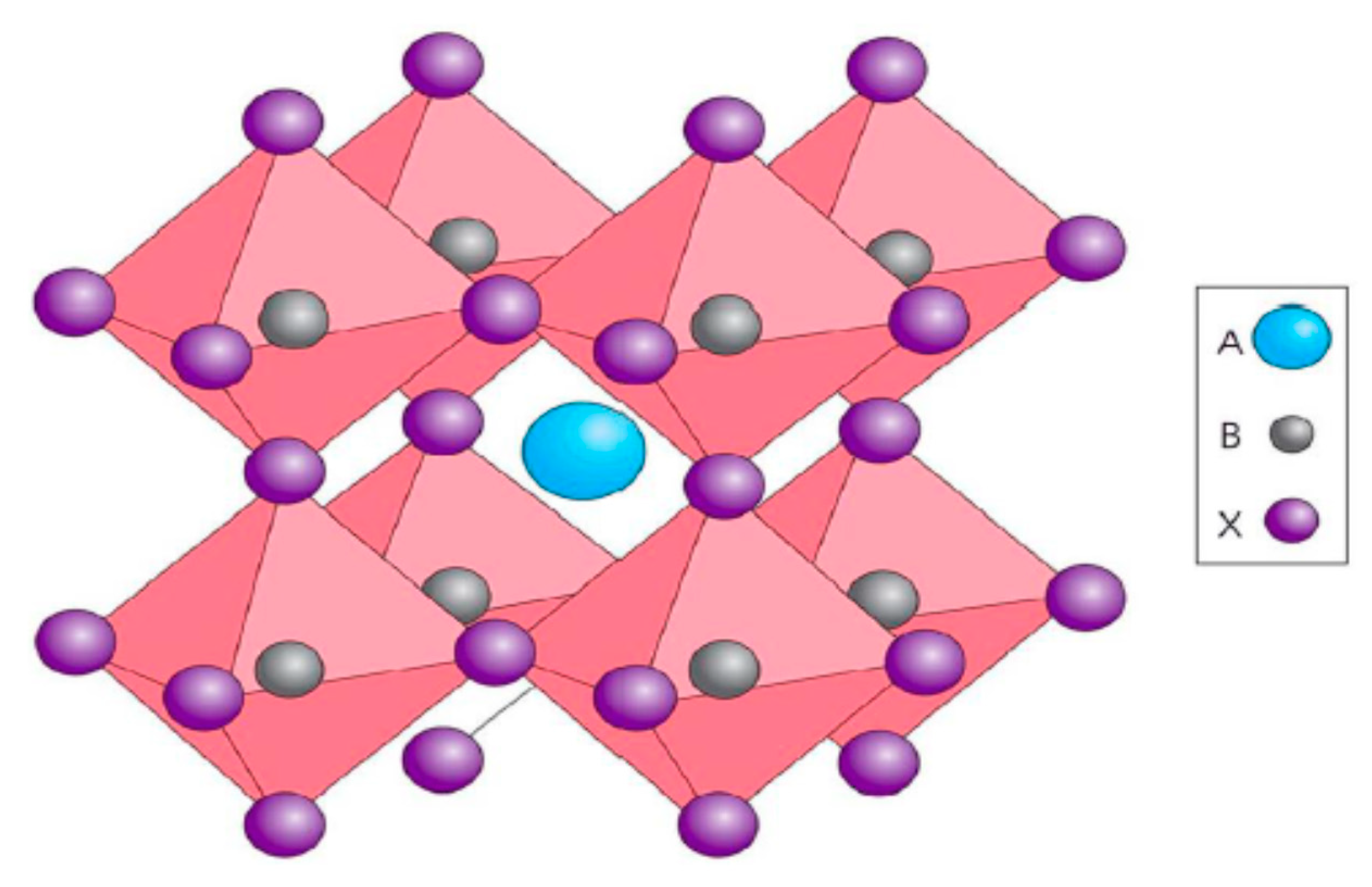
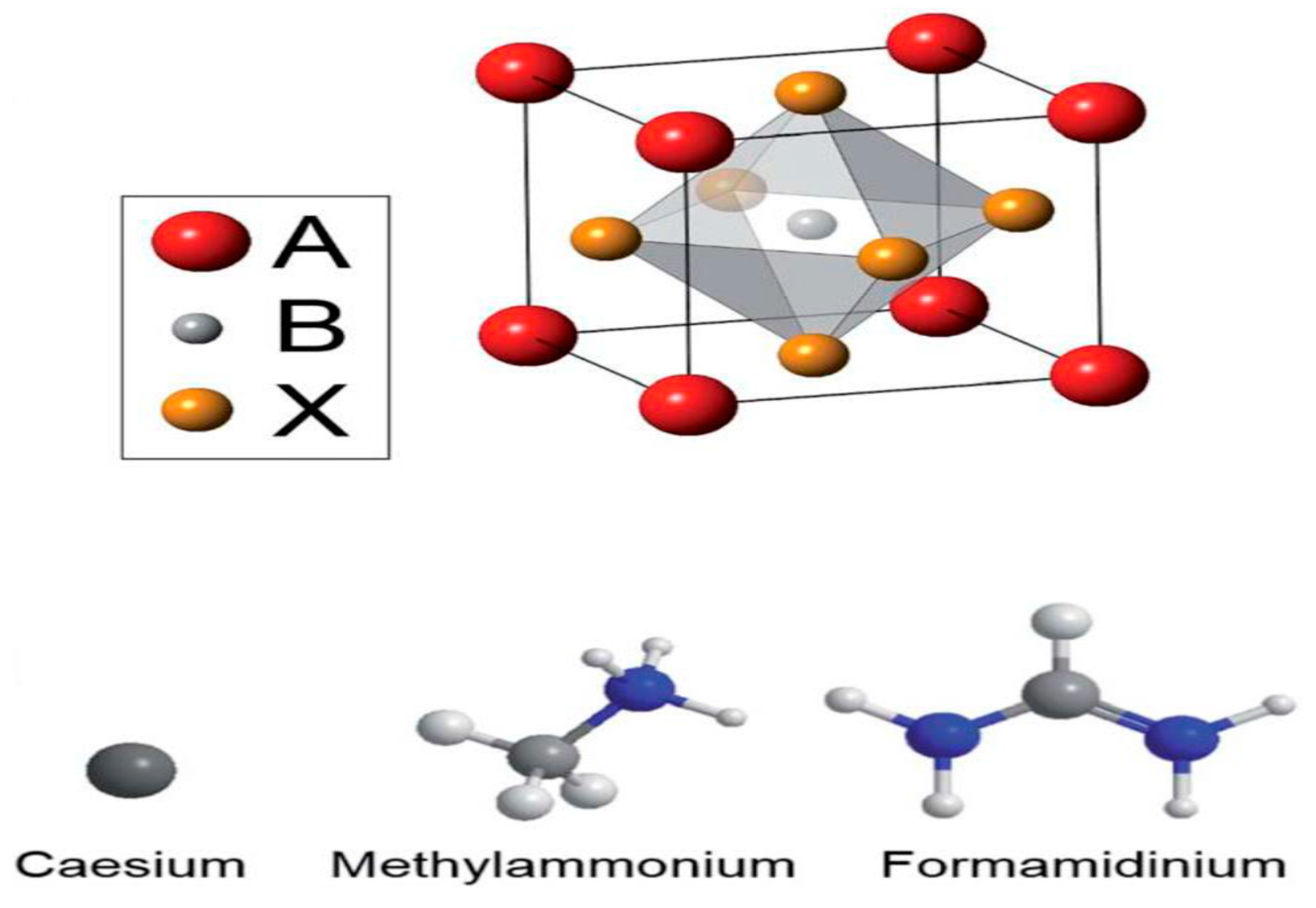
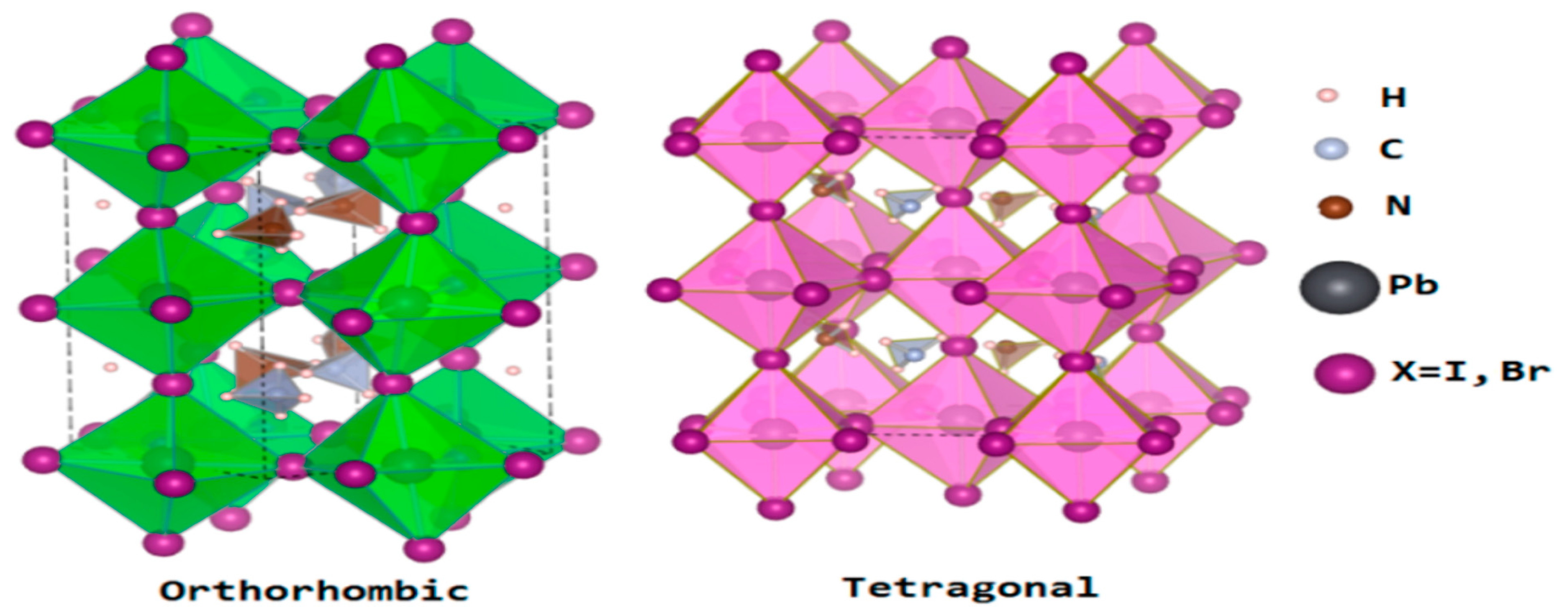
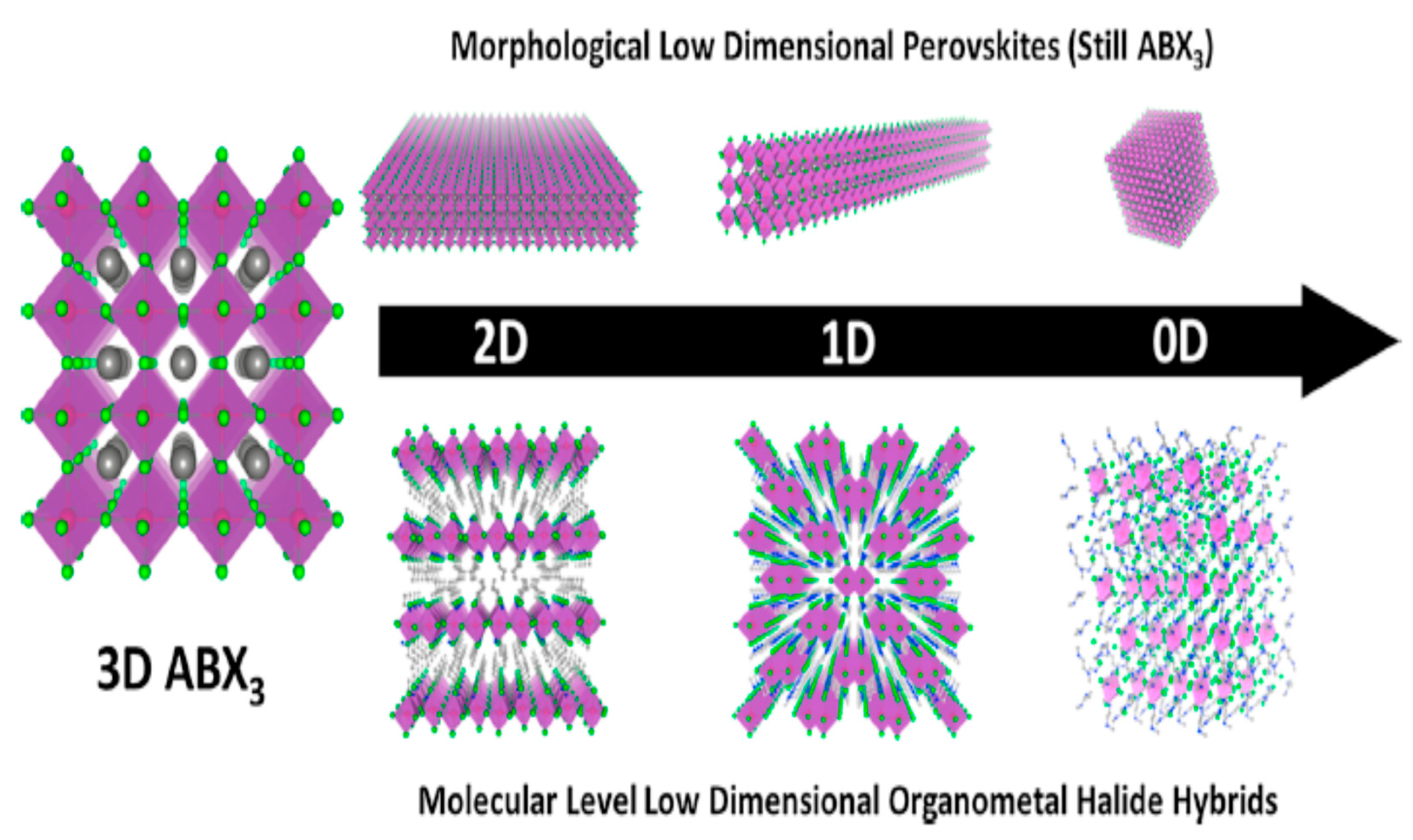

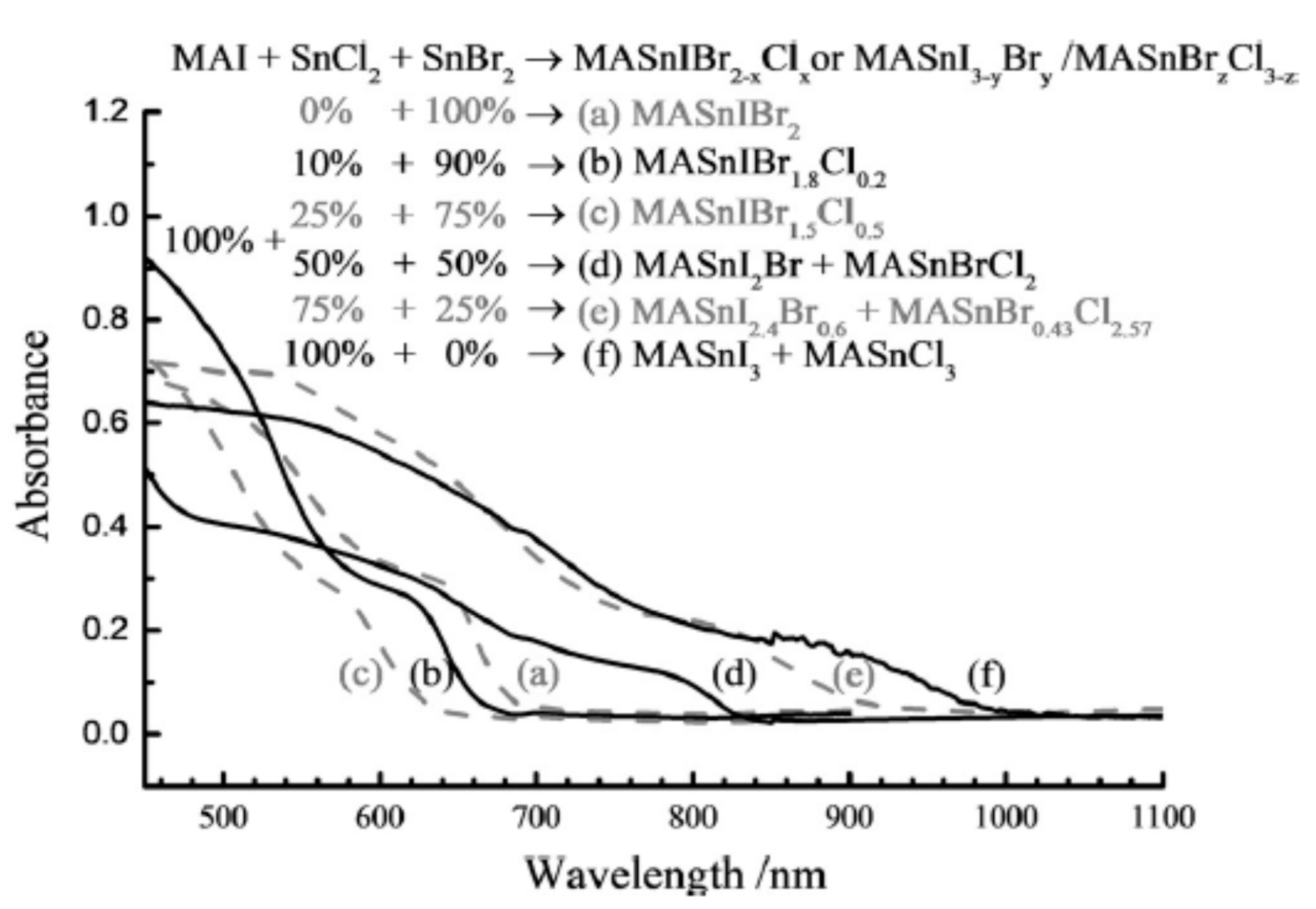

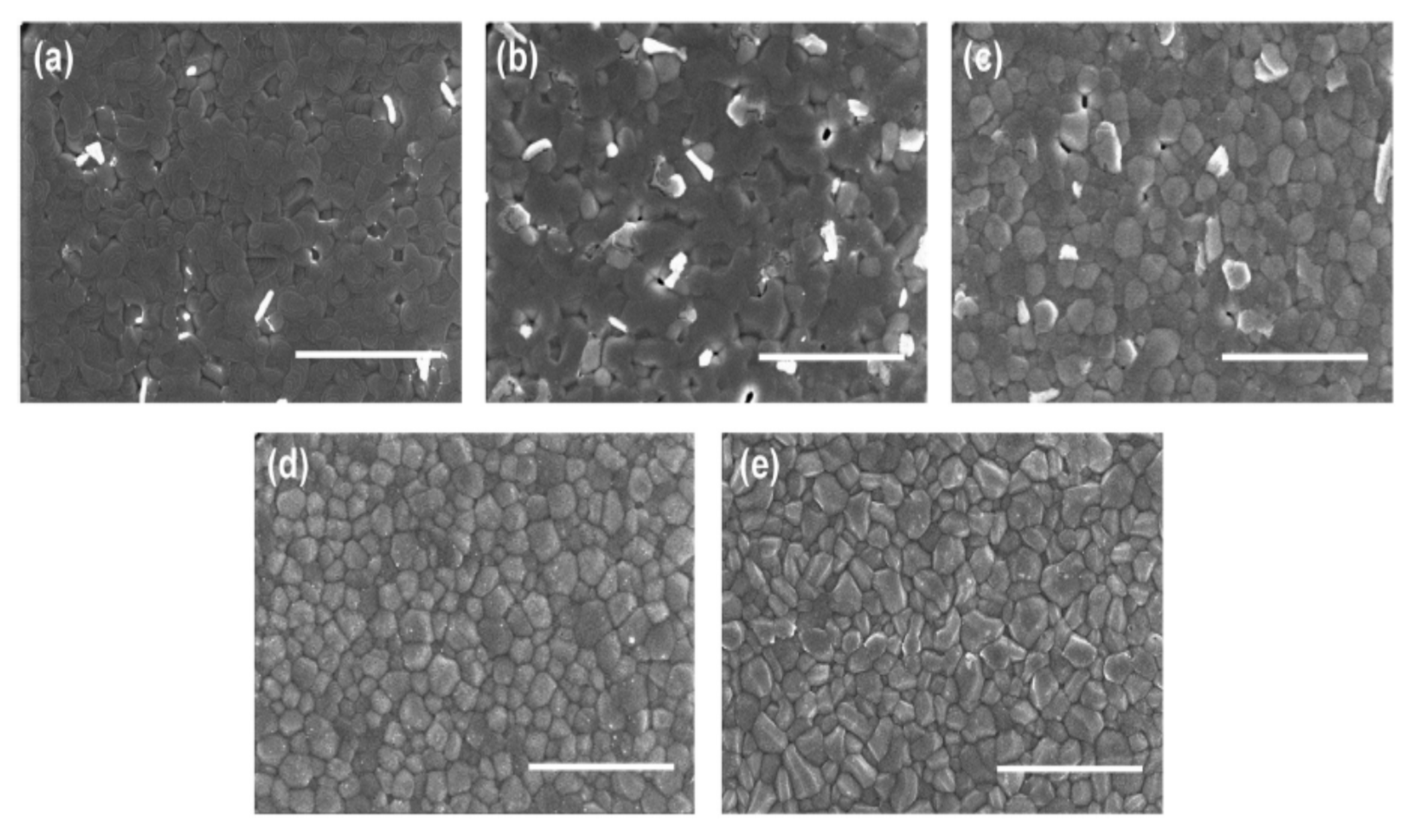
| Lead-Free Halide Perovskite | Eg (eV) | Voc (V) | Jsc (mAcm−2) | FF | PCE (%) | Ref. |
|---|---|---|---|---|---|---|
| FA0.75MA0.25SnI3: SF2 | 1.33 | 0.61 | 21.2 | 0.63 | 8.12 | [37] |
| MASnI3 | 1.3 | 0.68 | 16.30 | 0.48 | 5.23 | [54] |
| MASnI3−xBrx | 1.75 | 0.82 | 12.30 | 0.57 | 5.73 | [54] |
| MASnIBr0.8Cl0.2 | 1.25 | 0.38 | 14.0 | 0.57 | 3.1 | [55] |
| MA0.8HA0.2SnI3 | - | 0.38 | 14.1 | 0.47 | 2.6 | [56] |
| FASnI3:SF2 | 1.41 | 0.238 | 24.45 | 0.36 | 2.10 | [57] |
| FASnI3:10% en | 1.51 | 0.48 | 22.54 | 0.66 | 7.14 | [58] |
| FASnI3:N2H5Cl | 1.37 | 0.455 | 17.64 | 0.67 | 5.4 | [59] |
| Cs0.08FA0.92SnI3 | - | 0.44 | 20.70 | 0.67 | 6.08 | [60] |
| (3D)FASnI3:(2D)Sn:SF2 | - | 0.525 | 24.1 | 0.71 | 9.0 | [61] |
| 20% SF2-CsSnI3 | - | 0.24 | 22.70 | 0.37 | 2.02 | [62] |
| CsSnI2.9Br0.1 | - | 0.22 | 24.16 | 0.33 | 1.76 | [63] |
| CsSnI3:Co(C2H5) | - | 0.36 | 18.32 | 0.46 | 3.0 | [64] |
| 20% SF2-CsSnBr3 | 1.75 | 0.41 | 9.0 | 0.58 | 2.1 | [65] |
| CsGeI3 | 1.63 | 0.074 | 5,7 | 0.27 | 0.11 | [66] |
| MAGeI3 | 2.0 | 0.15 | 4.0 | 0.30 | 0.20 | [66] |
| MAGeI2.7Br0.3 | - | 0.46 | 3.11 | 0.48 | 0.57 | [67] |
| Metal Double Halide Perovskite | Eg (eV) | Voc (V) | Jsc (mAcm−2) | FF | PCE (%) | Ref. |
|---|---|---|---|---|---|---|
| Cs2AgBiBr6 | 1.91 | 1.01 | 3.19 | 0.66 | 2.2 | [81] |
| Cs2NaBiI6 | 1.66 | 0.47 | 1.99 | 0.44 | 0.42 | [82] |
| Cs2SnI4Br2 | 1.40 | 0.563 | 6.225 | 0.58 | 2.025 | [83] |
| Material Compositions | Morphology | Bandgap (eV) | Synthetic Method | References |
|---|---|---|---|---|
| Cs2BiAgCl6 | Crystal | 2.2 | Conventional solid-state reaction | [85] |
| Cs2Ag(SbxBi1−x)Br6 | Smaller grains of mixed alloys | 2.08 | Solution-based route | [86] |
| Cs2AgBiBr6 | Single crystal | 1.72 | Crystal engineering strategy | [87] |
| Cs2AgSbBr6 | Single crystal | 1.64 | Hydrothermal methods | [88] |
| Cs2NaVCl6 | Red crystals | 2.64 | Solid-state reaction and hydrothermal method | [89] |
| Cs2AgInCl6 | Nanocrystals | 3.57 | Colloidal synthesis | [90] |
| Cs2AgSbCl6 | Nanocrystals | 2.57 | Colloidal synthesis | [90] |
| Cs2CuSbCl6 | Nanocrystals. | 1.66 | Modified one-pot hot injection of colloidal synthesis | [91] |
| Cs2NaBiI6 | Single crystal | 1.5 | Solution-based method | [92] |
| (MA)2Au2X6, (X = Br, I) | Tetragonal crystal | 1.0 | Solution-processed route | [93] |
| Material Compositions | Morphology | Bandgap (eV) | Synthetic Method | References |
|---|---|---|---|---|
| Cs2SnI6 | Powders | 1.84 | Facile hydrothermal method | [105] |
| Cs2SnBr6 | Powders | 1.42 | Facile hydrothermal method | [105] |
| Cs2SnCl6 | Single-phase structures | 4.89 | Solution processing method | [106] |
| Cs2SnBr6 | Single-phase structures | 3.23 | Solution processing method | [106] |
| Cs2SnI6 | Single-phase structures | 1.35 | Solution processing method | [106] |
| Cs2PtI6 | Cubic crystal | 1.4 | Solution processing method | [107] |
| Cs2TiBr6 | Crystalline equiaxed grains | 1.8 | Two-step vapour deposition method | [108] |
| 2D Lead-Free Halide Perovskite | Eg (eV) | Voc (V) | Jsc (mAcm−2) | FF | PCE (%) | Ref. |
|---|---|---|---|---|---|---|
| MACuCl0.5Br3.5 | 1.8 | 0.29 | 21 × 10−6 | 0.28 | 0.017 | [123] |
| MAFeCl4 | 2.15 | 0.319 | 0.375 | 0.45 | 0.054 | [124] |
| Lead-Free Halide Perovskite-Like Absorbers | Eg (eV) | Voc (V) | Jsc (mAcm−2) | FF | PCE (%) | Ref. |
|---|---|---|---|---|---|---|
| (MA)3Sb2I9:antisolvent treatment | 1.9 | 0.77 | 6.64 | 0.60 | 2.77 | [135] |
| (MA)3(Sb1−xSnx)2I | 1.55 | 0.56 | 8.32 | 0.58 | 2.69 | [136] |
| Cs3Sb2I9 | 2.05 | 0.72 | 5.21 | 0.39 | 1.49 | [137] |
| (MA)3Sb2I9 | 2.14 | 0.89 | 1.0 | 0.55 | 0.5 | [138] |
| Rb3Sb2I9 | 2.24 | 0.55 | 2.12 | 0.66 | 0.66 | [139] |
| Rb3Sb2BR9−xIx (Px−0.9) | 2.02 | 0.55 | 4.25 | 0.595 | 1.37 | [140] |
| (NH4)3Sb2I9 | 2.27 | 1.03 | 1.15 | 0.43 | 0.57 | [141] |
| (MA)3Sb2I9 | 1.95 | 0.64 | 3.81 | 0.455 | 1.11 | [142] |
| (MA)3Sb2I9:HI | 1.95 | 0.62 | 5.41 | 0.68 | 2.04 | [142] |
| Cs3Sb2I9 | 2.0 | 0.62 | 2.34 | O.462 | 0.67 | [142] |
| Cs3Sb2I9:HI | 2.0 | 0.60 | 2.91 | 0.48 | 0.84 | [142] |
| (MA)3Sb2I9−xClx | 2.11 | 0.53 | 4.43 | 0.58 | 1.37 | [143] |
| (MA)3Sb2I9−xClx:LITFSI | 2.05 | 0.7 | 7.38 | 0.65 | 3.34 | [143] |
| Cs3Bi2I9 | 2.2 | 0.85 | 2.15 | 0.60 | 1.09 | [143] |
| (MA)3Bi2I9 | 2.1 | 0.68 | 0.52 | 0.33 | 0.12 | [143] |
| (MA)3Bi2I9−xClx | 2.4 | 0.04 | 0.18 | 0.38 | 0.003 | [143] |
| (MA)3Bi2I9 | - | 0.83 | 3.00 | 0.79 | 1.64 | [144] |
| (FA)3Bi2I9 | 2.19 | 0.48 | 0.11 | 0.46 | 0.022 | [145] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adjogri, S.J.; Meyer, E.L. A Review on Lead-Free Hybrid Halide Perovskites as Light Absorbers for Photovoltaic Applications Based on Their Structural, Optical, and Morphological Properties. Molecules 2020, 25, 5039. https://doi.org/10.3390/molecules25215039
Adjogri SJ, Meyer EL. A Review on Lead-Free Hybrid Halide Perovskites as Light Absorbers for Photovoltaic Applications Based on Their Structural, Optical, and Morphological Properties. Molecules. 2020; 25(21):5039. https://doi.org/10.3390/molecules25215039
Chicago/Turabian StyleAdjogri, Shadrack J., and Edson L. Meyer. 2020. "A Review on Lead-Free Hybrid Halide Perovskites as Light Absorbers for Photovoltaic Applications Based on Their Structural, Optical, and Morphological Properties" Molecules 25, no. 21: 5039. https://doi.org/10.3390/molecules25215039
APA StyleAdjogri, S. J., & Meyer, E. L. (2020). A Review on Lead-Free Hybrid Halide Perovskites as Light Absorbers for Photovoltaic Applications Based on Their Structural, Optical, and Morphological Properties. Molecules, 25(21), 5039. https://doi.org/10.3390/molecules25215039







