Abstract
The purpose of this study is to investigate the effect of fungi on kimchi metabolites during fermentation. A gas chromatography-mass spectrometry (GC-MS) based metabolite profiling approach in combination with principal component analysis (PCA) is performed to differentiate metabolites produced by fungi or bacteria. To avoid bacterial growth, kimchi is treated with 100 μg/mL of ampicillin every three days from 30 to 50 days of fermentation. The relative content of the major fungi at 50 days of fermentation, between the control group and the ampicillin treatment group, was not significantly different. The administration of ampicillin changed the metabolites in kimchi by affecting the growth of kimchi bacteria. Based on the pattern of change of each metabolite, the changed metabolites are grouped into four categories: (1) metabolites produced or consumed by fungi, (2) metabolites involving both fungi and bacteria, (3) metabolites produced or consumed by bacteria, and (4) metabolites of undetermined origin. Alanine, thymine, galacturonic acid, and malonic acid can be regarded as the metabolites produced by fungi between 30 and 50 days of fermentation. In contrast, malic acid, oxaloacetic acid, galactitol, glucose, and mannitol are presumed to be the metabolites mainly consumed by fungi. This study is meaningful as the first study conducted by inhibiting growth of bacteria to identify the metabolites contributed by fungi or bacteria in the kimchi fermentation process. These results could be used to make customized kimchi that controls the production of desired metabolites by selectively controlling the formation of microbial communities in the kimchi industry.
1. Introduction
Kimchi is a traditional Korean cuisine made from kimchi cabbage and various seasonings including ginger, garlic, red pepper, spring onion, jeotgal (salted seafood), and salts [1]. There are many types of kimchi in Korea made with various vegetables as the main ingredients. In order to eat fresh vegetables that are not available in winter, there is a culture called ‘gimjang’ in Korea, which manufactures kimchi that can be consumed for a long time (more than a year) [2]. In general, kimchi made in early winter is stored at low temperatures and is consumed until the next year.
Spontaneous fermentation in kimchi leads to the formation of various microorganisms, affecting the sensory qualities of kimchi [3]. It is well known that the composition of metabolites important to kimchi taste and flavor, such as organic acids (lactic acid and acetic acid) and other flavoring compounds (mannitol and amino acids), are directly affected by the kimchi microbial community [4]. The microorganisms essential for kimchi production can be divided into lactic acid bacteria (LAB) and yeast. LAB are formed in the early stages of kimchi fermentation, whereas yeast is known to be formed in the middle stages of kimchi fermentation [5,6]. In many previous studies, LAB have been primarily studied as the key microorganisms responsible for kimchi fermentation [3,7,8,9,10]. Yeast, known as a symbolic microorganism in many fermented foods due to the production of alcoholic compounds and carbonic acid [11,12,13,14], can also significantly affect the quality of kimchi. However, the changes in the metabolites by yeast during fermentation and storage of kimchi have not been studied.
Metabolomics has been successfully applied to monitor metabolic changes caused by microorganisms during fermentation [15]. Recently, the combination of metabolomics and amplicon sequencing has been found to be a very comprehensive approach for investigating the relationship between microbial communities and metabolites [16,17,18,19,20]. Many studies have actively attempted to interpret changes in kimchi metabolites according to salinity [19,21] and starter culture [4,20] in connection with the kimchi microbial community. However, these kimchi studies only dealt with bacterial microbial communities and no studies have identified metabolic changes according to yeast microbial communities. Until now, there have been no studies on the sequencing of the internal transcribed spacer (ITS) region in kimchi, so nothing is known about the fungal or yeast community contained in kimchi.
Therefore, this study was conducted to investigate the effect of the fungal community, including yeast, on kimchi metabolites. After killing bacteria during kimchi fermentation, the effects of fungi on kimchi metabolites were investigated by comparing the metabolic differences compared to the control group. This study is meaningful as the first study to identify metabolites contributed by fungi or bacteria in the fermentation process of kimchi.
2. Results
2.1. Fungal Community Change during Kimchi Fermentation
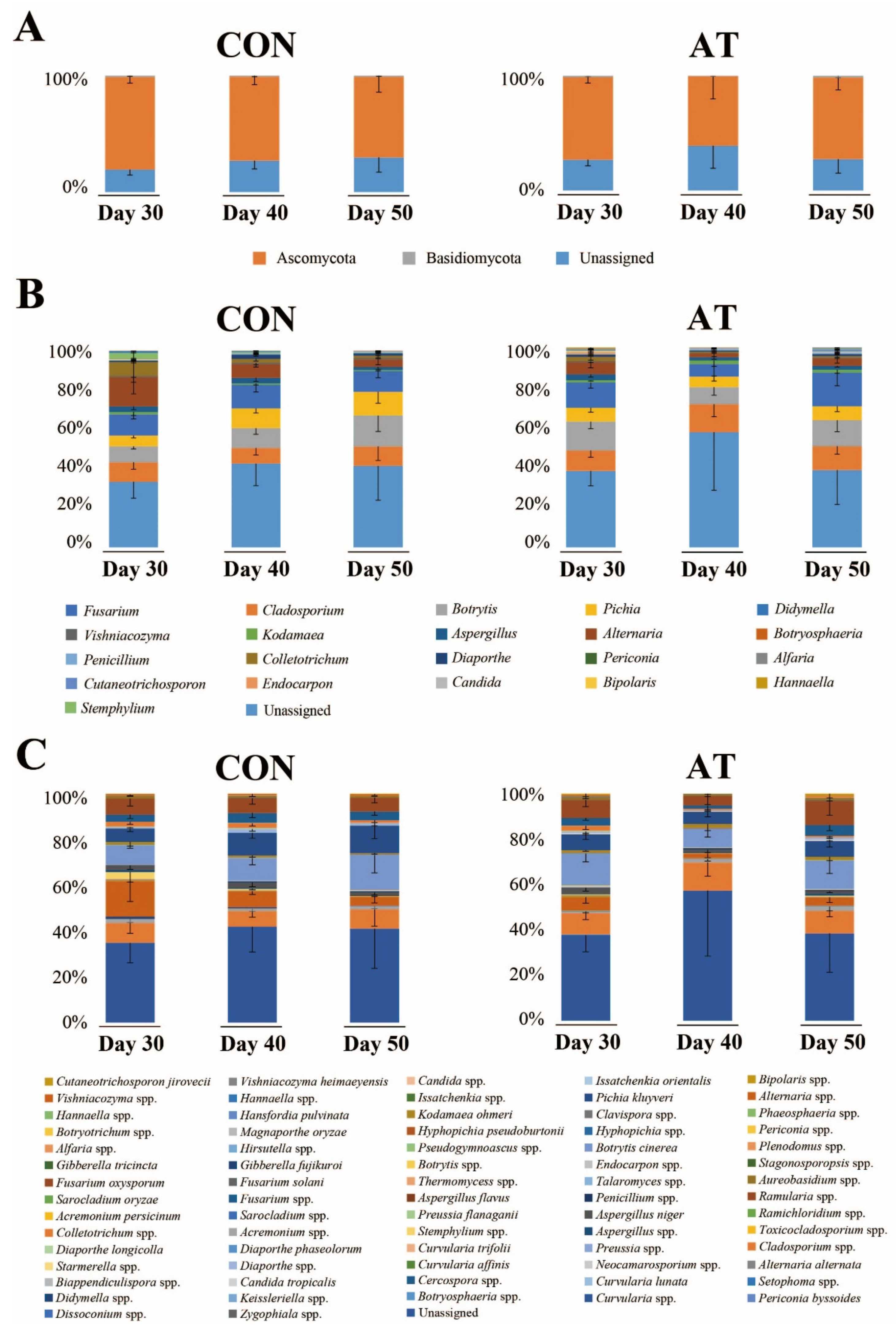
Figure 1 shows the results of the microbial community analysis between experimental groups at the phylum, genus, and species levels. The dominant phylum across the entire eukaryotic population was Ascomycota (72.70%), although it also contained Basidiomycota (1.12%) and unidentified fungal microorganisms (25.89%), on the 30th day of fermentation (Figure 1A). At the genus level, the dominant genera were Cladosporium, Fusarium, Pichia, Botrytis, and Alternaria (Figure 1B). The relative content of the five major genera (cutoff > 5%) at 50 days of fermentation between the control group (CON50) and the ampicillin-treated group (AT50) were not significantly different (data not shown, p < 0.05, Mann-Whitney U test), indicating that the fungal community was not significantly affected by ampicillin treatment. On the 30th day of fermentation, the dominant genera identified at the species level were Botrytis cinerea (4.64%), Fusarium oxysporum (3.87%), Pichia kluyveri (3.21%), Aspergillus niger (0.96%), and Kodamaea ohmeri (0.71%) (Figure 1C).
Figure 1.
The kimchi fungal community profiles at the phylum (A), genus (B), and species (C) levels in the control group (CON) and the ampicillin-treated group (AT) during 30 to 50 days of fermentation as revealed by internal transcribed spacer 2 (ITS2) sequencing. The x-axis represents different samples. The y-axis represents the percentage abundance of fungus in each sample.
2.2. Metabolic Change during Kimchi Fermentation
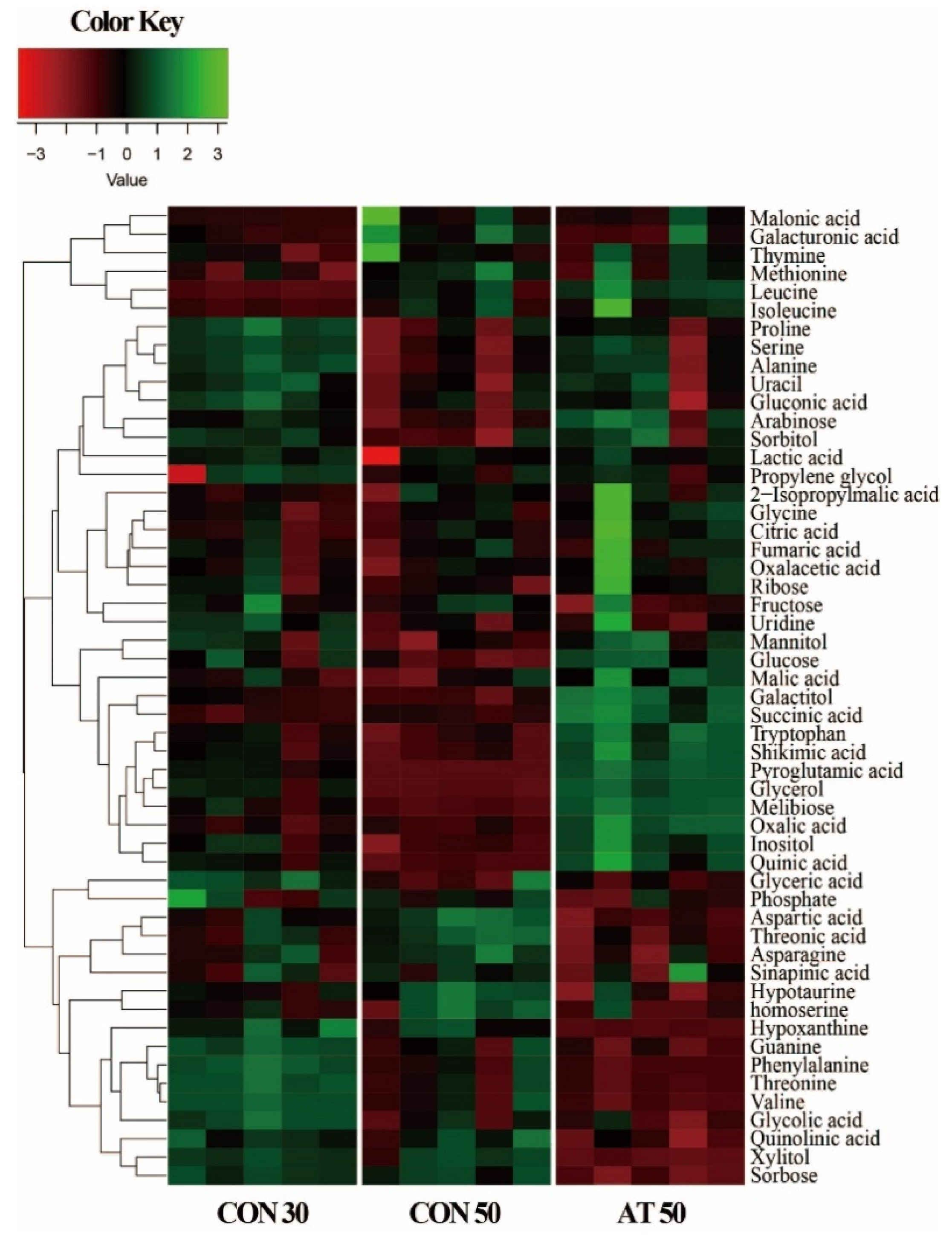
In this study, a total of 53 metabolites were identified in kimchi samples by gas chromatography-mass spectrometry (GC-MS) analysis. Variation in the identified metabolites for each group was depicted in a heatmap (Figure 2). As expected, some metabolite levels in kimchi were markedly changed according to the fermentation period. In a comparison between samples on the 50th day of fermentation, kimchi treated with ampicillin showed a metabolite pattern different from the control.
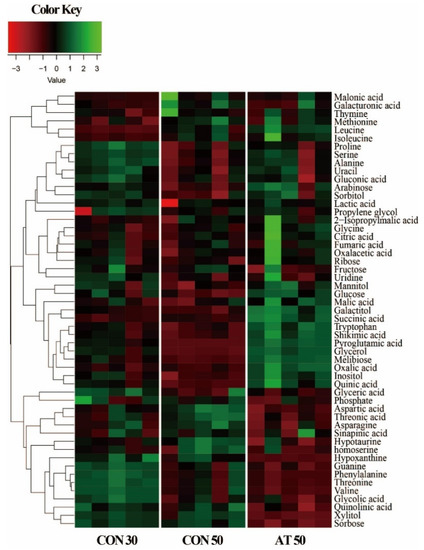
Figure 2.
A heatmap showing metabolite differences between the control groups (CON30 and CON50) and the ampicillin-treated group (AT50). CON30 and CON50 refer to the control group on the 30th and 50th day of fermentation, respectively. AT50 refers to the ampicillin-treatment group on the 50th day of fermentation.
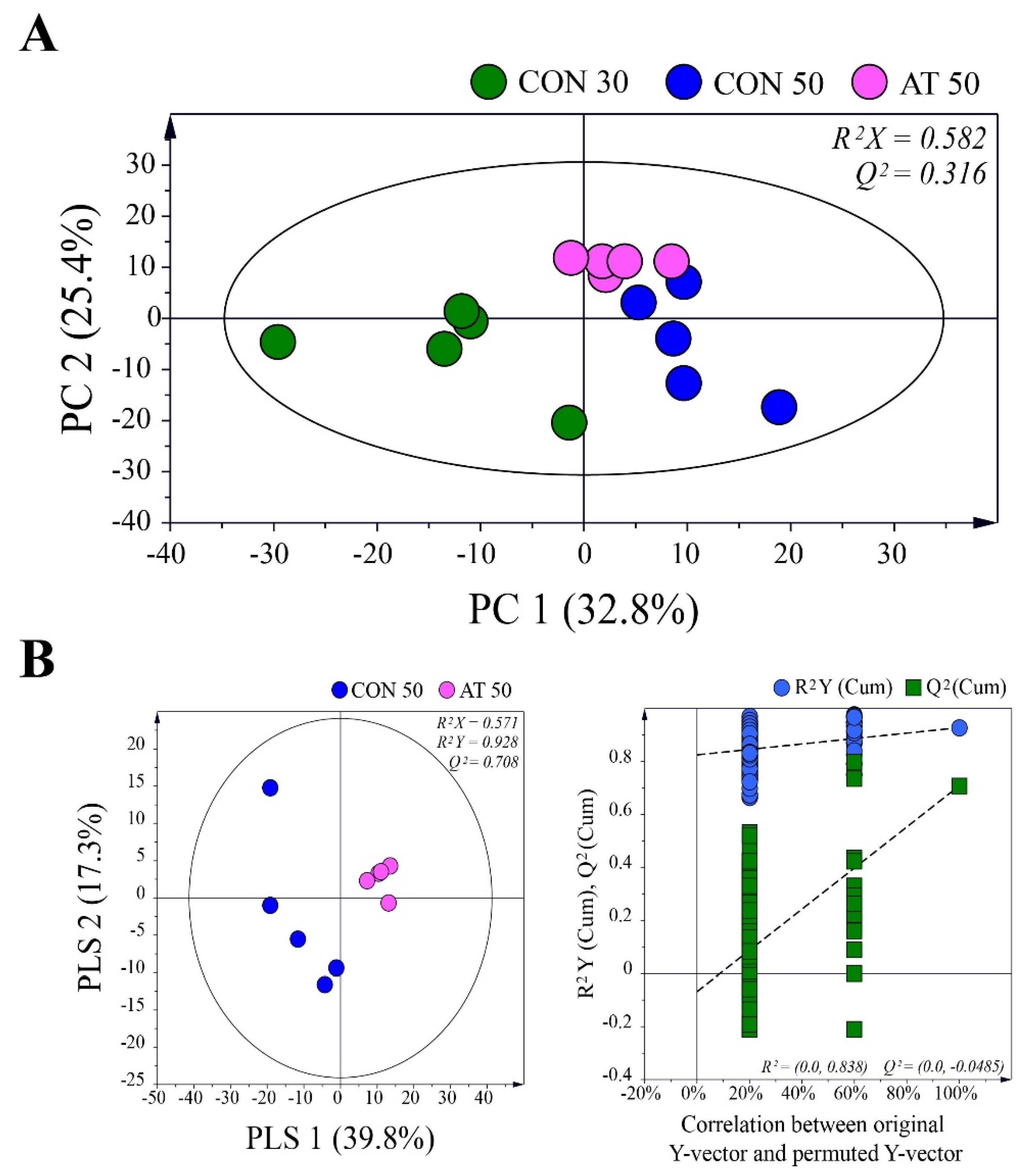
To investigate metabolic changes in the kimchi samples during fermentation by the administration of ampicillin, principal component analysis (PCA) was performed using GC-MS data for control groups (CON30 and CON50) and the ampicillin-treated group (AT50) (Figure 3A). A clear separation of samples between day 30 and day 50 was observed in the PCA score plot, indicating that the metabolic profile was changed by the fermentation period. On the 50th day of fermentation, kimchi samples were also separated by the administration of ampicillin, suggesting that the administration of ampicillin changed the metabolites in kimchi by affecting the growth of kimchi bacteria.
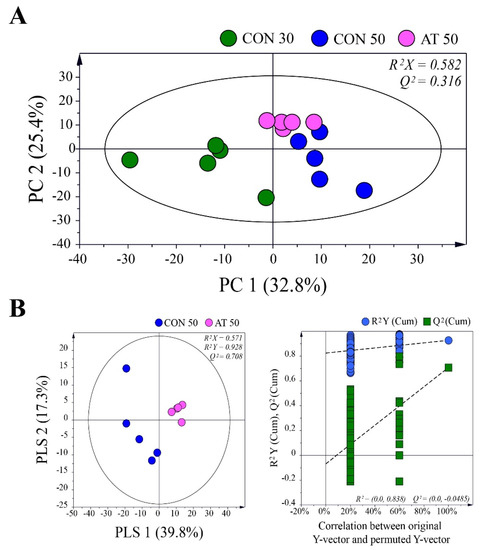
Figure 3.
(A) Principal component analysis (PCA) score plots derived from gas chromatography-mass spectrometry (GC-MS) data for control groups (CON30 and CON50) and the ampicillin-treated group (AT50) during kimchi fermentation. (B) Partial least squares-discriminant analysis (PLS-DA) score plot between the CON50 and AT50 groups after 50 days of fermentation validated by a permutation test.
The partial least squares-discriminant analysis (PLS-DA) method was used to refine the separation between the CON50 and AT50 groups already established via PCA (Figure 3B). No over-fitting was observed in the cross-validation performed using 200 repeated permutation tests. According to the criterion of variable importance in projection (VIP) > 1.0 and p < 0.05, a total of 25 metabolites were identified as metabolites contributing to the differential PLS-DA model between the groups. Table 1 shows the difference in metabolites in each group. A false discovery rate was applied to all tests to correct for multiple testing. Based on the pattern of change of each metabolite, they were largely grouped into the following four categories: (1) metabolites produced or consumed by fungi, (2) metabolites involving both fungi and bacteria, (3) metabolites produced or consumed by bacteria, and (4) metabolites of undetermined origin.

Table 1.
Significantly different metabolites between the control and ampicillin-treated group after 50 days of kimchi fermentation.
3. Discussion
Since household kimchi manufactured in Korea does not use a starter culture, it is fermented through various microorganisms derived from the raw materials [22]. LAB are formed from the beginning of kimchi fermentation, whereas yeast is known to appear in the middle and late stages of fermentation [23]. The organic acids or antimicrobial compounds produced by LAB inhibit yeast growth, making it difficult for yeast to grow during kimchi fermentation [24,25,26]. According to Jeong et al. [27], the abundance of bacteria and yeast showed a negative correlation during kimchi fermentation, suggesting that there may be antagonism between bacteria and yeast. The appearance time of yeast and the number of viable cells differ depending on the kimchi manufacturing conditions. Lee et al. [5] reported that yeast began to be detected in kimchi from the 10th week of storage and reached 5.91 log CFU/g on the 20th week. According to the results of Jeong et al. [6], yeast increased from 19 days of fermentation with a decrease in bacteria, reaching a maximum value of about 7 log CFU/mL at 45 days of fermentation. It is predicted that various fungal communities, including yeast, are involved in the fermentation of kimchi but there have been no studies analyzing the fungal communities.
To the best of our knowledge, this is the first study to investigate fungal community in kimchi. Fungal community present in kimchi can contribute or adversely affect kimchi quality, such as texture, taste, food safety, nutrition, and functionality. Fai et al. [28] reported that Pichia kluyveri strain showed to be the best candidate for probiotic use among the yeasts tested, taking into account properties such as colonization and survival in the digestive tract, and the ability of antagonism against enteropathogenic bacteria. In the current study, Aspergillus niger, Aspergillus flavus, and Fusarium, known to produce mycotoxins associated with multiple human and animal diseases, were also detected in kimchi. There is no research on the mycotoxins contained in kimchi. However, since the main ingredient of kimchi is red pepper powder that is sensitive to mycotoxins contamination [29], the possibility of the presence of mycotoxins in kimchi cannot be excluded. Kimchi can be considered generally safe because it has been consumed safely for a long time. However, kimchi products manufactured by spontaneous fermentation can be exposed to various sources of contaminants, including pathogens, during the manufacturing process. Therefore, the Korea Food and Drug Administration adapted Hazard Analysis Critical Control Point rules to ensure that the hygiene of commercially manufactured kimchi is acceptably safe [30].
In the case of kimchi fermented under similar conditions, the number of viable yeast increases rapidly between 30 and 50 days of fermentation [6]. In the current study, to identify the metabolites produced or consumed by yeast during kimchi fermentation, kimchi was treated with ampicillin to selectively kill only bacteria. Ampicillin is known to inhibit bacterial cell wall synthesis through binding to penicillin-binding proteins [31]. Among the metabolites that changed significantly during 30 and 50 days of fermentation (Table 1), those that were not affected by treatment with ampicillin are presumed to be the metabolites related to fungi. Although alanine, thymine, galacturonic acid, and malonic acid increased in both the CON50 and AT50 groups compared to the CON30 group, there was no significant difference between the CON50 and AT50 groups. Therefore, these metabolites can be regarded as the metabolites produced by fungi between 30 and 50 days of fermentation. Likewise, malic acid, oxaloacetic acid, galactitol, glucose, and mannitol are presumed to be the metabolites mainly consumed by fungi. Among them, galacturonic acid [32,33], glucose, and mannitol [27] have been reported to be correlated with Pichia and Candida in other kimchi studies. Both yeasts were also detected in this study. Interestingly, a reduction in the sugar alcohols (galactitol and mannitol) was observed. Mannitol is known to be an important component providing a refreshing taste to kimchi. Mannitol can be produced by LAB using fructose as an electron acceptor [34]. According to a kimchi study using Leuconstoc mesenteroides as a starter, mannitol increased between 10 and 30 days of kimchi fermentation [4]. Jeong et al. [27] reported that the concentration of mannitol increased until 30 days of fermentation, and then gradually decreased, which was presumed to be due to metabolism by yeast. However, it is very difficult to identify which fungi species of kimchi are mainly involved in the production of metabolites.
In contrast, citric acid, fructose, uridine, and ribose decreased in both the CON50 and AT50 groups compared to the CON30 group, but the AT50 group was significantly higher than the CON50 group. These metabolites can be considered the metabolites consumed by both bacteria and fungi. Similarly, it is presumed that both bacteria and fungi were involved in the production of propylene glycol during kimchi fermentation. The metabolites with significant increases or decreases in the CON50 group but no significant difference from the AT50 group were lactic acid, gluconic acid, sinapinic acid, fumaric acid, and tryptophan. These metabolites indicate that bacteria were mainly produced or consumed during the kimchi fermentation. There was no change in the CON50 group, but significant increases and decreases in several amino acids were observed in the AT50 group. It is presumed that these metabolites were caused by changes in the microbial ecosystem in kimchi caused by ampicillin treatment.
Recently, studies have attempted to understand the relationship between microbial communities and metabolites through a combination of metabolomics and amplicon sequencing. For example, Kim et al. [35] revealed that the metabolic profile of kimchi was not significantly affected by the difference in white colony-forming yeast diversity using high-throughput DNA sequencing and metabolomics techniques. Most of the studies that focused on the fungi of kimchi, not yeast, dealt with the safety of kimchi [36,37,38]. Therefore, this study is meaningful as the first study conducted by killing bacteria with ampicillin to determine the metabolites contributed by fungi in the kimchi fermentation process. However, the changes in the bacterial ecosystem of kimchi caused by ampicillin may affect the microbial growth environment of the fungi, so the results of the metabolites derived from this study may differ from those in kimchi. To determine which fungi actually produce or consume metabolites during kimchi fermentation, it is necessary to analyze changes by selectively adding individual strains.
4. Materials and Methods
4.1. Kimchi Preparation and Antibiotic Treatment
Kimchi was prepared by modifying the method of the World Institute of Kimchi (Gwangju, Korea) [39]. Briefly, a seasoning mixture was prepared by mixing jeotgal, garlic, ginger, onion, radish, glutinous rice porridge, red pepper powder, and water in the weight ratio of 70:3.75:1.2:1.85:2.4:4.5:1.2:4.5:2:2:6.6. This seasoning mixture was added to kimchi cabbage at a ratio of 9:1 (w/w). The final salt concentration in kimchi was adjusted to 2.5%. This kimchi mixture was homogenized using a blender and then placed in a 20 kg plastic container. The kimchi was placed at room temperature for 24 h for active fermentation and then stored at 4 °C for 30 days. On the 30th day of fermentation, the kimchi was divided into 10 batches of 2 kg each. To prevent bacterial growth, 100 μg/mL of ampicillin [40] was added to five batches of kimchi every three days from 30 to 50 days of fermentation. According to the results of antibiotic susceptibility testing for Lactobacillus and Bifidobacterium [41], the minimum inhibitory concentration of ampicillin was 1 μg/mL or less for all tested strains. During the experimental period, bacteria were cultured using de Man, Rogasa and Sharpe (MRS) broth every three days in the kimchi samples, but no viable cells were detected. Kimchi samples were taken at 30, 40, and 50 days of fermentation for analyses. CON30, CON40, and CON50 refer to the control group on the 30th, 40th and 50th day of kimchi fermentation. AT30, AT40, and AT50 refer to the ampicillin-treatment group on the 30th, 40th and 50th day of kimchi fermentation. The supernatants were separated from the kimchi by centrifugation (4 °C, 10 min, 12,000 rpm) and stored at −80 °C prior to microbial community and metabolite analyses. The research overall design and flow process used in this study are depicted in Figure S1.
4.2. DNA Extraction and ITS2 Sequencing
DNA was extracted using an AccuFAST automation system (AccuGene, Incheon, Korea) according to the manufacturer’s instructions. The internal transcribed spacer 2 (ITS2) regions were amplified from the DNA extracts through 25 PCR cycles using KAPA HiFi HotStart ReadyMix (Roche, Basel, Switzerland) and fusion primers fITS9/ITS4 [42] containing Nextera adaptor sequences. The PCR products were purified with HiAccuBeads (AccuGene). The amplicon libraries were pooled at an equimolar ratio and the pooled libraries were sequenced on an Illumina MiSeq system using a MiSeq Reagent Kit v2 for 500 cycles (Illumina, San Diego, CA, USA).
For all raw data sets, VSEARCH v2.10.3 was used to remove chimeric PCR products from the filtered reads [43]. Downstream analyses of the quality and chimera filters were performed using the QIIME 1.9.2 software package [44]. Each of the quality-filtered sequencing read datasets was assigned to operational taxonomic units (OTUs) with a threshold of 97% pairwise identity using QIIME´s reference-based workflow scripts and a UNITE reference database [45].
4.3. Metabolite Analysis and Data Processing
The sample preparation methods for metabolite analysis were similar to those described in a previous study [46]. Briefly, after centrifugation (13,000 rpm, 4 °C, 15 min), 50 μL of supernatant was pooled in 1.5 mL Eppendorf tubes. Then, 10 μL of ribitol solution (0.5 mg/L) was added as an internal standard (IS). After freeze-drying, O-methoxyamine hydrochloride (20 mg/mL) was added to each sample and incubated (75 rpm, 30 °C, 90 min) in the dark. Silylation was performed by adding 50 μL of N-methyl-N-trimethylsilyl-trifluoroacetamide. Each sample was vortex mixed, shaken (75 rpm), and incubated at 37 °C for 30 min. After centrifuging the sample at 13,000 rpm for 10 min, GC-MS analysis was performed on the supernatant. To validate the stability and performance of the GC-MS analysis, a quality control (QC) sample was prepared by pooling equal volumes (10 μL) of kimchi from each sample. QC samples were analyzed every 5 samples during the run. Generally, the relative standard deviation (RSD) of each metabolite across the QC samples was used as an indicator of the reproducibility and the repeatability of the analysis. In this study, the average RSD of the QC samples was 4.65%. The signal features used in this study were filtered using RSD values below 30% in QC samples.
The derivatized samples were analyzed using a QP 2020 GC-MS (Shimadzu, Kyoto, Japan). The GC oven temperature was initially held at 80 °C for 2 min and finally increased to 330 °C at a rate of 15 °C/min and held for 6 min. The m/z range was set to 50–600 with electron impact ionization (70 eV). The sample was injected in the split mode (1:50).
The raw GC-MS data were converted into a netCDF format and processed with MetAlign software for peak detection, noise removal, normalization, and alignment [47]. The resulting data was imported into AIoutput software for peak identification and prediction [46]. The identification of metabolites was performed by comparing the mass spectrum to the AIoutput software library, the NIST 14.0 library, and the human metabolome database (HMDB, http://www.hmdb.ca). Multivariate analysis, such as principal component analysis (PCA), was conducted using SIMCA-P version 14.0 software package (Umetrics, Umea, Sweden). Cross-validation was performed using a permutation test (n = 200). Metabolites with variable importance in projection (VIP) scores greater than 1.0 and p-values lower than 0.05 were considered metabolites capable of discriminating groups.
4.4. Availability of Data
The ITS2 gene amplicon datasets presented in this study were deposited in National Center for Biotechnology Information (NCBI) under accession numbers PRJNA670060.
5. Conclusions
Although many kimchi studies report changes in metabolites during fermentation, they are interpreted in relation to bacteria, such as LAB. Therefore, this study is meaningful as the first study conducted by inhibiting the growth of bacteria to identify the metabolites contributed by fungi or bacteria in the kimchi fermentation process. The administration of ampicillin changed the metabolites in kimchi by not affecting the relative content of the major fungi. Based on the pattern of change of each metabolite, the changed metabolites were grouped into four categories: (1) metabolites produced or consumed by fungi, (2) metabolites involving both fungi and bacteria, (3) metabolites produced or consumed by bacteria, and (4) metabolites of undetermined origin. These results could be used to make customized kimchi by selectively controlling the formation of microbial communities in the kimchi industry.
Supplementary Materials
The following are available online: Figure S1: Schematic diagram of this study.
Author Contributions
Conceptualization, S.-H.S., and H.-S.S.; data curation, S.-H.S., S.-E.P., E.-J.K., and K.-M.C.; investigation, E.-J.K.; methodology, H.-S.S.; Software, S.-E.P. and E.-J.K.; writing—original draft, S.-H.S.; writing—review and editing, S.J.K. and H.-S.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (No. 2019R1C1C1002208 and 2020R1I1A1A0106992011).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jung, J.Y.; Lee, S.H.; Kim, J.M.; Park, M.S.; Bae, J.W.; Hahn, Y.; Madsen, E.L.; Jeon, C.O. Metagenomic analysis of kimchi, a traditional Korean fermented food. Appl. Environ. Microbiol. 2011, 77, 2264–2274. [Google Scholar] [CrossRef] [PubMed]
- Jang, D.J.; Chung, K.R.; Yang, H.J.; Kim, K.S.; Kwon, D.Y. Discussion on the origin of kimchi, representative of Korean unique fermented vegetables. J. Ethn. Foods 2015, 2, 126–136. [Google Scholar] [CrossRef]
- Jeong, S.H.; Lee, H.J.; Jung, J.Y.; Lee, S.H.; Seo, H.Y.; Park, W.S.; Jeon, C.O. Effects of red pepper powder on microbial communities and metabolites during kimchi fermentation. Int. J. Food Microbiol. 2013, 160, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.Y.; Lee, S.H.; Lee, H.J.; Seo, H.Y.; Park, W.S.; Jeon, C.O. Effects of Leuconostoc mesenteroides starter cultures on microbial communities and metabolites during kimchi fermentation. Int. J. Food Microbiol. 2012, 153, 378–387. [Google Scholar] [CrossRef]
- Lee, K.W.; Shim, J.M.; Kim, D.W.; Yao, Z.; Kim, J.A.; Kim, H.J.; Kim, J.H. Effects of different types of salts on the growth of lactic acid bacteria and yeasts during kimchi fermentation. Food Sci. Biotechnol. 2018, 27, 489–498. [Google Scholar] [CrossRef]
- Jeong, S.H.; Lee, S.H.; Jung, J.Y.; Choi, E.J.; Jeon, C.O. Microbial succession and metabolite changes during long-term storage of kimchi. J. Food Sci. 2013, 78, M763–M769. [Google Scholar] [CrossRef]
- Bae, J.W.; Rhee, S.K.; Park, J.R.; Chung, W.H.; Nam, Y.D.; Lee, I.; Kim, H.; Park, Y.H. Development and evaluation of genome-probing microarrays for monitoring lactic acid bacteria. Appl. Environ. Microbiol. 2005, 71, 8825–8835. [Google Scholar] [CrossRef][Green Version]
- Chang, J.Y.; Chang, H.C. Improvements in the quality and shelf life of kimchi by fermentation with the induced bacteriocin-producing strain, Leuconostoc citreum GJ7 as a starter. J. Food Sci. 2010, 75, M103–M110. [Google Scholar] [CrossRef]
- Lee, K.; Lee, Y. Effect of Lactobacillus plantarum as a starter on the food quality and microbiota of kimchi. Food Sci. Biotechnol. 2010, 19, 641–646. [Google Scholar] [CrossRef]
- Lee, H.; Yoon, H.; Ji, Y.; Kim, H.; Park, H.; Lee, J.; Shin, H.; Holzapfel, W. Functional properties of Lactobacillus strains isolated from kimchi. Int. J. Food Microbiol. 2011, 145, 155–161. [Google Scholar] [CrossRef]
- Procopio, S.; Qian, F.; Becker, T. Function and regulation of yeast genes involved in higher alcohol and ester metabolism during beverage fermentation. Eur. Food Res. Technol. 2011, 233, 721. [Google Scholar] [CrossRef]
- Whitener, M.E.B.; Carlin, S.; Jacobson, D.; Weighill, D.; Divol, B.; Conterno, L.; Du Toit, M.; Vrhovsek, U. Early fermentation volatile metabolite profile of non-Saccharomyces yeasts in red and white grape must: A targeted approach. LWT-Food Sci. Technol. 2015, 64, 412–422. [Google Scholar] [CrossRef]
- Seo, S.H.; Park, S.E.; Yoo, S.A.; Lee, K.I.; Na, C.S.; Son, H.S. Metabolite profiling of Makgeolli for the understanding of yeast fermentation characteristics during fermentation and aging. Process Biochem. 2016, 51, 1363–1373. [Google Scholar] [CrossRef]
- Swart, C.W.; Dithebe, K.; Pohl, C.H.; Swart, H.C.; Coetsee, E.; van Wyk, P.W.; Swarts, J.C.; Lodolo, E.J.; Kock, J.L. Gas bubble formation in the cytoplasm of a fermenting yeast. FEMS Yeast Res. 2012, 12, 867–869. [Google Scholar] [CrossRef] [PubMed]
- Mozzi, F.; Ortiz, M.E.; Bleckwedel, J.; De Vuyst, L.; Pescuma, M. Metabolomics as a tool for the comprehensive understanding of fermented and functional foods with lactic acid bacteria. Food Res. Int. 2013, 54, 1152–1161. [Google Scholar] [CrossRef]
- Sogin, E.M.; Putnam, H.M.; Nelson, C.E.; Anderson, P.; Gates, R.D. Correspondence of coral holobiont metabolome with symbiotic bacteria, archaea and Symbiodinium communities. Environ. Microbiol. Rep. 2017, 9, 310–315. [Google Scholar] [CrossRef]
- Jiao, M.; Li, Z.; Zhang, F.; Qin, X. Comparison between Astragalus membranaceus var. mongholicus and Hedysarum polybotrys based on ITS sequences and metabolomics. Yao Xue Xue Bao 2015, 50, 1625–1631. [Google Scholar]
- Govinden-Soulange, J.; Lobine, D.; Frederich, M.; Kodja, H.; Coetzee, M.P.A.; Ranghoo-Sanmukhiya, V. Metabolomic and molecular signatures of Mascarene aloes using a multidisciplinary approach. S. Afr. J. Bot. 2017, 137–143. [Google Scholar] [CrossRef]
- Seo, S.H.; Park, S.E.; Kim, E.J.; Lee, K.I.; Na, C.S.; Son, H.S. A GC-MS based metabolomics approach to determine the effect of salinity on kimchi. Food Res. Int. 2018, 105, 492–498. [Google Scholar] [CrossRef]
- Park, S.E.; Seo, S.H.; Kim, E.J.; Byun, S.; Na, C.S.; Son, H.S. Changes of microbial community and metabolite in kimchi inoculated with different microbial community starters. Food Chem. 2019, 274, 558–565. [Google Scholar] [CrossRef]
- Kim, D.W.; Kim, B.M.; Lee, H.J.; Jang, G.J.; Song, S.H.; Lee, J.I.; Lee, S.B.; Shim, J.M.; Lee, K.W.; Kim, J.H. Effects of different salt treatments on the fermentation metabolites and bacterial profiles of kimchi. J. Food Sci. 2017, 82, 1124–1131. [Google Scholar] [CrossRef] [PubMed]
- Song, H.S.; Whon, T.W.; Kim, J.; Lee, S.H.; Kim, J.Y.; Kim, Y.B.; Choi, H.J.; Rhee, J.K.; Roh, S.W. Microbial niches in raw ingredients determine microbial community assembly during kimchi fermentation. Food Chem. 2020, 318, 126481. [Google Scholar] [CrossRef] [PubMed]
- Cheigh, H.S.; Park, K.Y.; Lee, C. Biochemical, microbiological, and nutritional aspects of kimchi (Korean fermented vegetable products). Crit. Rev. Food Sci. Nutr. 1994, 34, 175–203. [Google Scholar] [CrossRef]
- Schnürer, J.; Magnusson, J. Antifungal lactic acid bacteria as biopreservatives. Trends Food Sci. Technol. 2005, 16, 70–78. [Google Scholar] [CrossRef]
- Rouse, S.; Harnett, D.; Vaughan, A.; van Sinderen, D. Lactic acid bacteria with potential to eliminate fungal spoilage in foods. J. Appl. Microbiol. 2008, 104, 915–923. [Google Scholar] [CrossRef]
- Dalié, D.; Deschamps, A.; Richard-Forget, F. Lactic acid bacteria–potential for control of mould growth and mycotoxins: A review. Food Control 2010, 21, 370–380. [Google Scholar] [CrossRef]
- Jeong, S.H.; Jung, J.Y.; Lee, S.H.; Jin, H.M.; Jeon, C.O. Microbial succession and metabolite changes during fermentation of dongchimi, traditional Korean watery kimchi. Int. J. Food Microbiol. 2013, 164, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Fai, A.E.C.; Da Silva, J.B.; De Andrade, C.J.; Bution, M.L.; Pastore, G.M. Production of prebiotic galactooligosaccharides from lactose by Pseudozyma tsukubaensis and Pichia kluyveri. Biocatal. Agric. Biotechnol. 2014, 3, 343–350. [Google Scholar] [CrossRef]
- Cheon, S.H.; Kim, S.J.; Lee, S.I.; Chung, Y.; Kim, S.H.; Cho, J.; Seo, H.Y. Effect of lactic acid bacteria on changes of aflatoxin levels during kimchi fermentation. Korean J. Food Preserv. 2015, 22, 758–767. [Google Scholar] [CrossRef]
- Chang, H.C. Healthy and safe Korean traditional fermented foods: Kimchi and chongkukjang. J. Ethn. Foods 2018, 5, 161–166. [Google Scholar] [CrossRef]
- Rafailidis, P.I.; Ioannidou, E.N.; Falagas, M.E. Ampicillin/sulbactam. Drugs 2007, 67, 1829–1849. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.E.; Kim, M.J.; Kim, T.W. Diversity and role of yeast on kimchi fermentation. J. Korean Soc. Food Cult. 2019, 34, 201–207. [Google Scholar]
- Oh, J.Y.; Han, Y.S. Purification and characterization of L-galactono-gamma-lactone oxidase in Pichia sp. isolated from kimchi. Korean J. Food Sci. Technol. 2003, 35, 1135–1142. [Google Scholar]
- Wisselink, H.W.; Weusthuis, R.A.; Eggink, G.; Hugenholtz, J.; Grobben, G.J. Mannitol production by lactic acid bacteria: A review. Int. Dairy J. 2002, 12, 151–161. [Google Scholar] [CrossRef]
- Kim, M.J.; Lee, H.W.; Kim, J.Y.; Kang, S.E.; Roh, S.W.; Hong, S.W.; Yoo, S.R.; Kim, T.W. Impact of fermentation conditions on the diversity of white colony-forming yeast and analysis of metabolite changes by white colony-forming yeast in kimchi. Food. Res. Int. 2020, 136, 109315. [Google Scholar] [CrossRef]
- Kim, J.D. Antifungal activity of lactic acid bacteria isolated from kimchi against Aspergillus fumigatus. Mycobioloy 2005, 33, 210–214. [Google Scholar] [CrossRef]
- Ryu, E.H.; Yang, E.J.; Woo, E.R.; Chang, H.C. Purification and characterization of antifungal compounds from Lactobacillus plantarum HD1 isolated from kimchi. Food Microbiol. 2014, 41, 19–26. [Google Scholar] [CrossRef]
- Haque, M.A.; Lee, J.H.; Cho, K.M. Endophytic bacterial diversity in Korean kimchi made of Chinese cabbage leaves and their antimicrobial activity against pathogens. Food Control 2015, 56, 24–33. [Google Scholar] [CrossRef]
- Jung, M.Y.; Kim, T.W.; Lee, C.; Kim, J.Y.; Song, H.S.; Kim, Y.B.; Ahn, S.W.; Kim, J.S.; Roh, S.W.; Lee, S.H. Role of jeotgal, a Korean traditional fermented fish sauce, in microbial dynamics and metabolite profiles during kimchi fermentation. Food Chem. 2018, 265, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Clemente-Jimenez, J.M.; Mingorance-Cazorla, L.; Martínez-Rodríguez, S.; Heras-Vázquez, F.J.L.; Rodríguez-Vico, F. Molecular characterization and oenological properties of wine yeasts isolated during spontaneous fermentation of six varieties of grape must. Food Microbiol. 2004, 21, 149–155. [Google Scholar] [CrossRef]
- Georgieva, R.; Yocheva, L.; Tserovska, L.; Zhelezova, G.; Stefanova, N.; Atanasova, A.; Danguleva, A.; Ivanova, G.; Karapetkov, N.; Rumyan, N.; et al. Antimicrobial activity and antibiotic susceptibility of Lactobacillus and Bifidobacterium spp. intended for use as starter and probiotic cultures. Biotechnol. Biotechnol. Equip. 2015, 29, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Ihrmark, K.; Bödeker, I.; Cruz-Martinez, K.; Friberg, H.; Kubartova, A.; Schenck, J.; Strid, Y.; Stenlid, J.; Brandström-Durling, M.; Clemmensen, K.E. New primers to amplify the fungal ITS2 region–evaluation by 454-sequencing of artificial and natural communities. FEMS Microbiol. Ecol. 2012, 82, 666–677. [Google Scholar] [CrossRef] [PubMed]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. VSEARCH: A versatile open source tool for metagenomics. PeerJ 2016, 4, e2584. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pena, A.G.; Goodrich, J.K.; Gordon, J.I. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Abarenkov, K.; Tedersoo, L.; Nilsson, R.H.; Vellak, K.; Saar, I.; Veldre, V.; Parmasto, E.; Prous, M.; Aan, A.; Ots, M. PlutoF-a web based workbench for ecological and taxonomic research, with an online implementation for fungal ITS sequences. Evol. Bioinform. 2010, 6, 189–196. [Google Scholar] [CrossRef]
- Tsugawa, H.; Bamba, T.; Shinohara, M.; Nishiumi, S.; Yoshida, M.; Fukusaki, E. Practical non-targeted gas chromatography/mass spectrometry-based metabolomics platform for metabolic phenotype analysis. J. Biosci. Bioeng. 2011, 112, 292–298. [Google Scholar] [CrossRef]
- Lommen, A. MetAlign: Interface-driven, versatile metabolomics tool for hyphenated full-scan mass spectrometry data preprocessing. Anal. Chem. 2009, 81, 3079–3086. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).