Kinetic Study of Glucosamine Production Using Aspergillus sydowii BCRC 31742 under Solid-State Fermentation
Abstract
1. Introduction
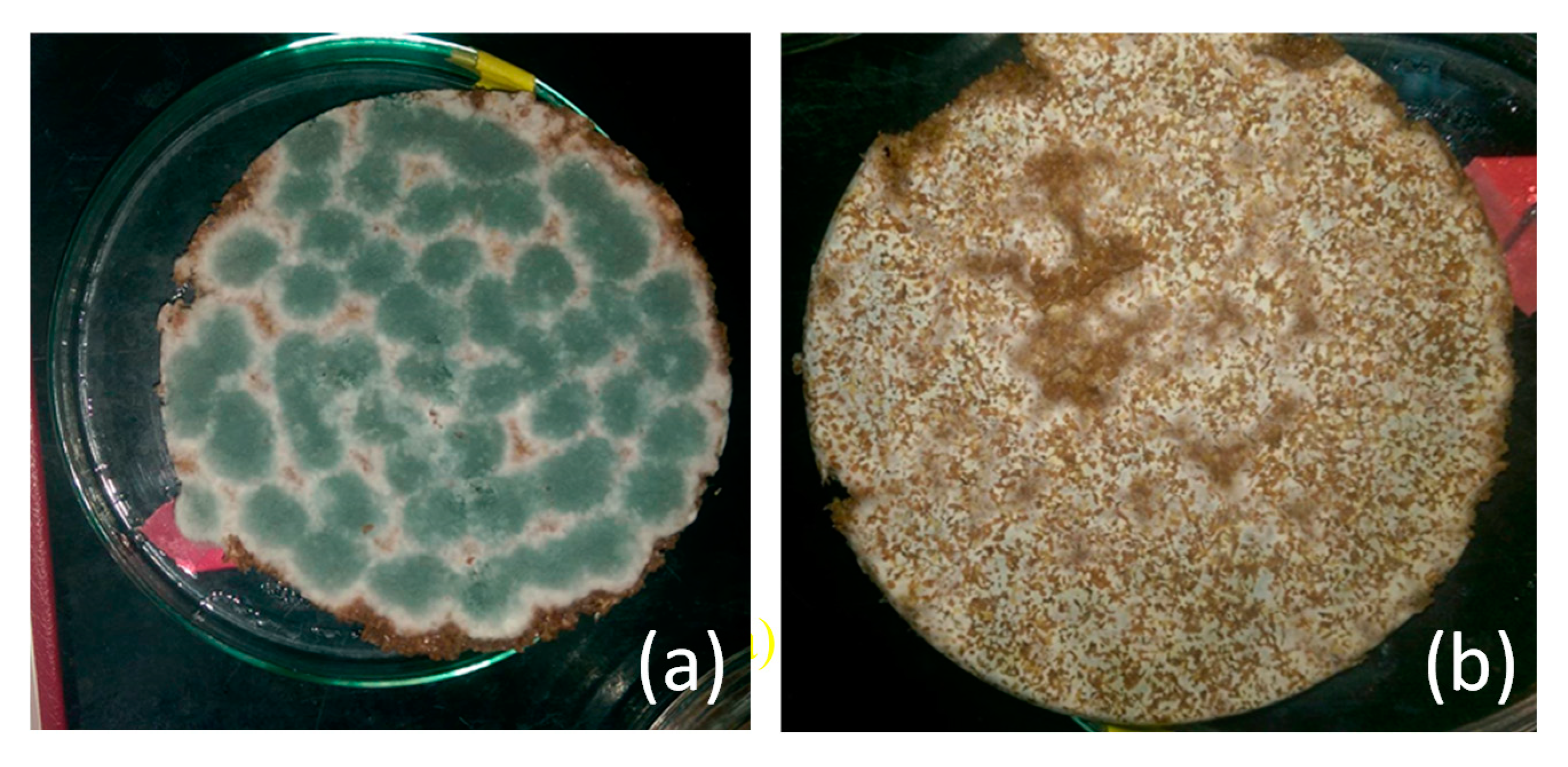
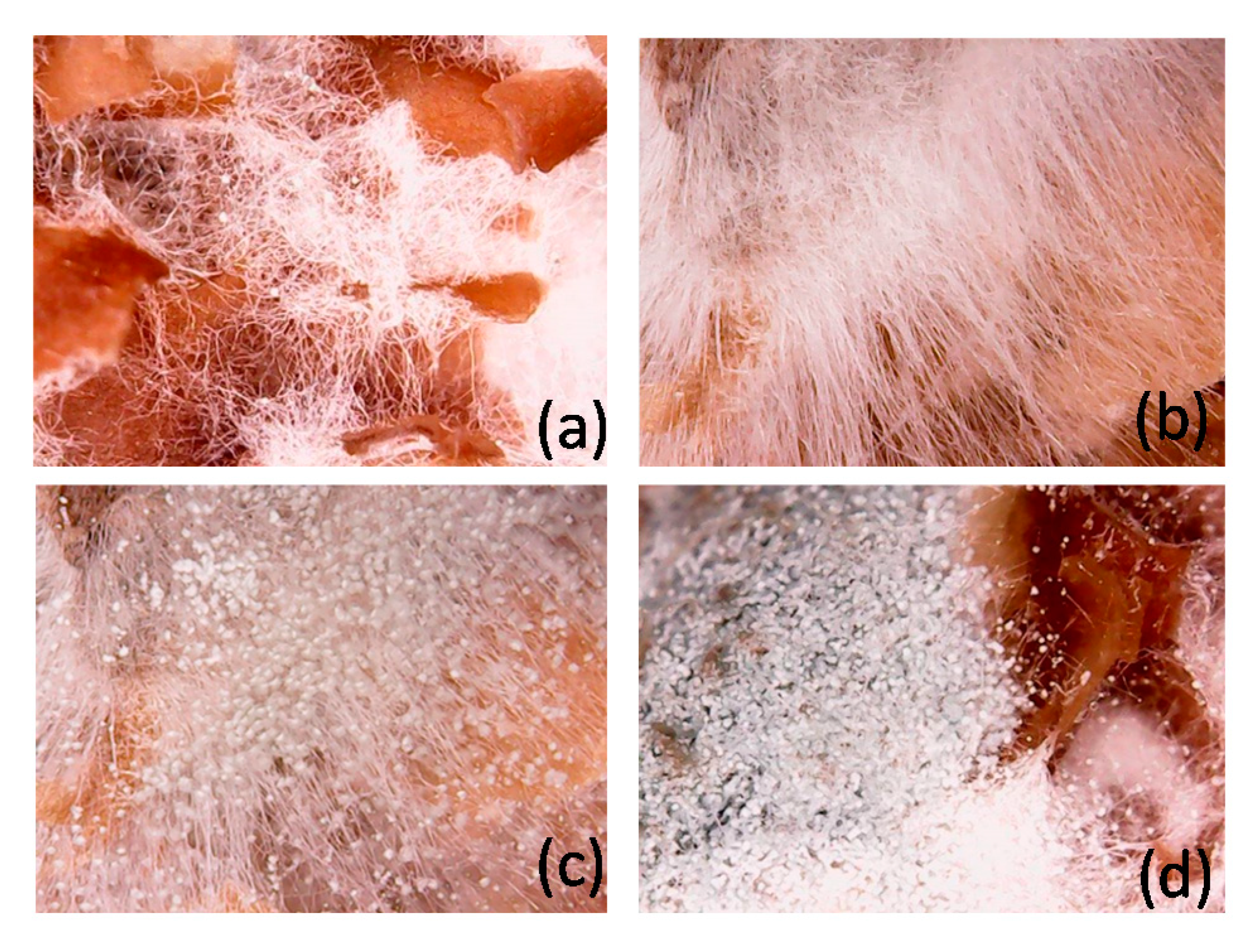
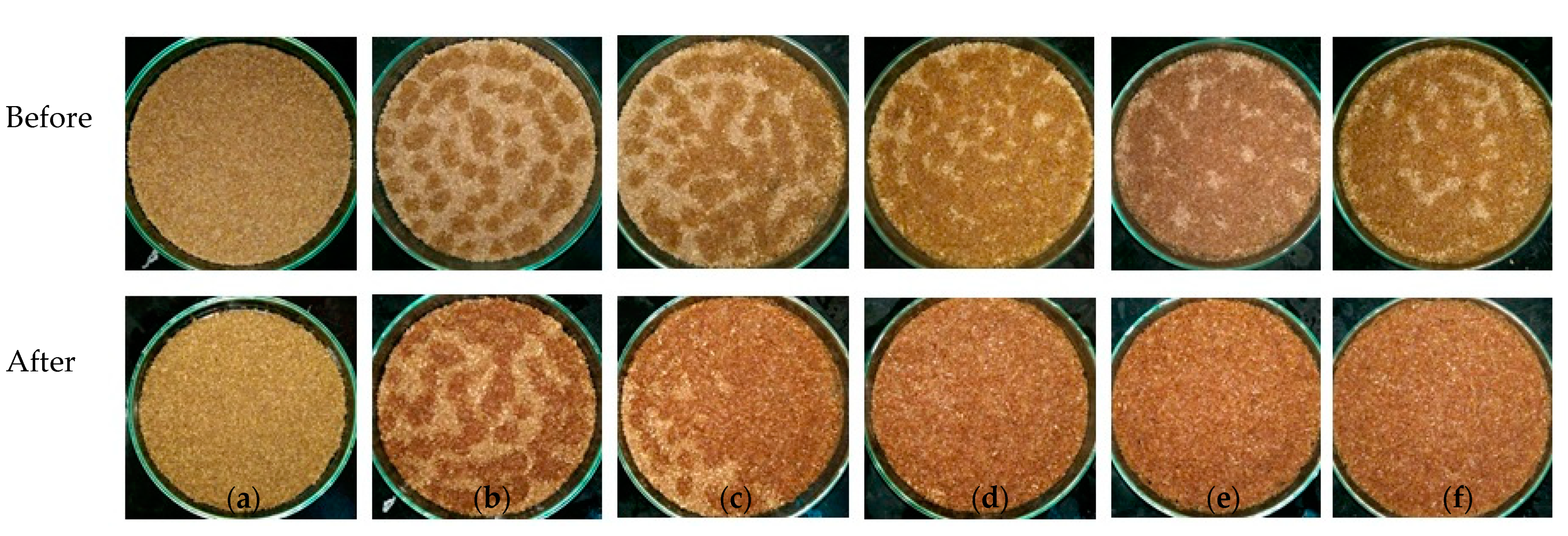
2. Results and Discussion
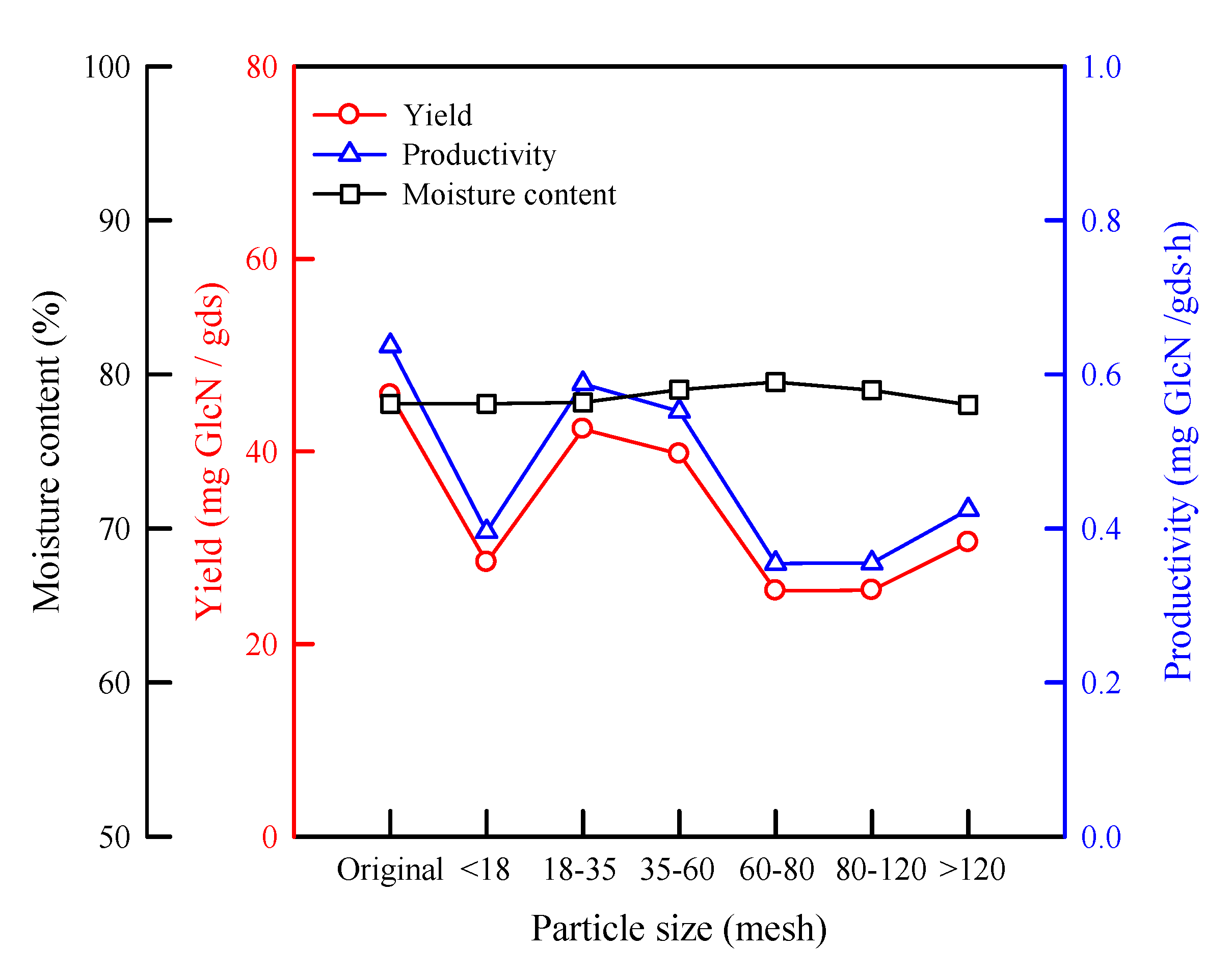
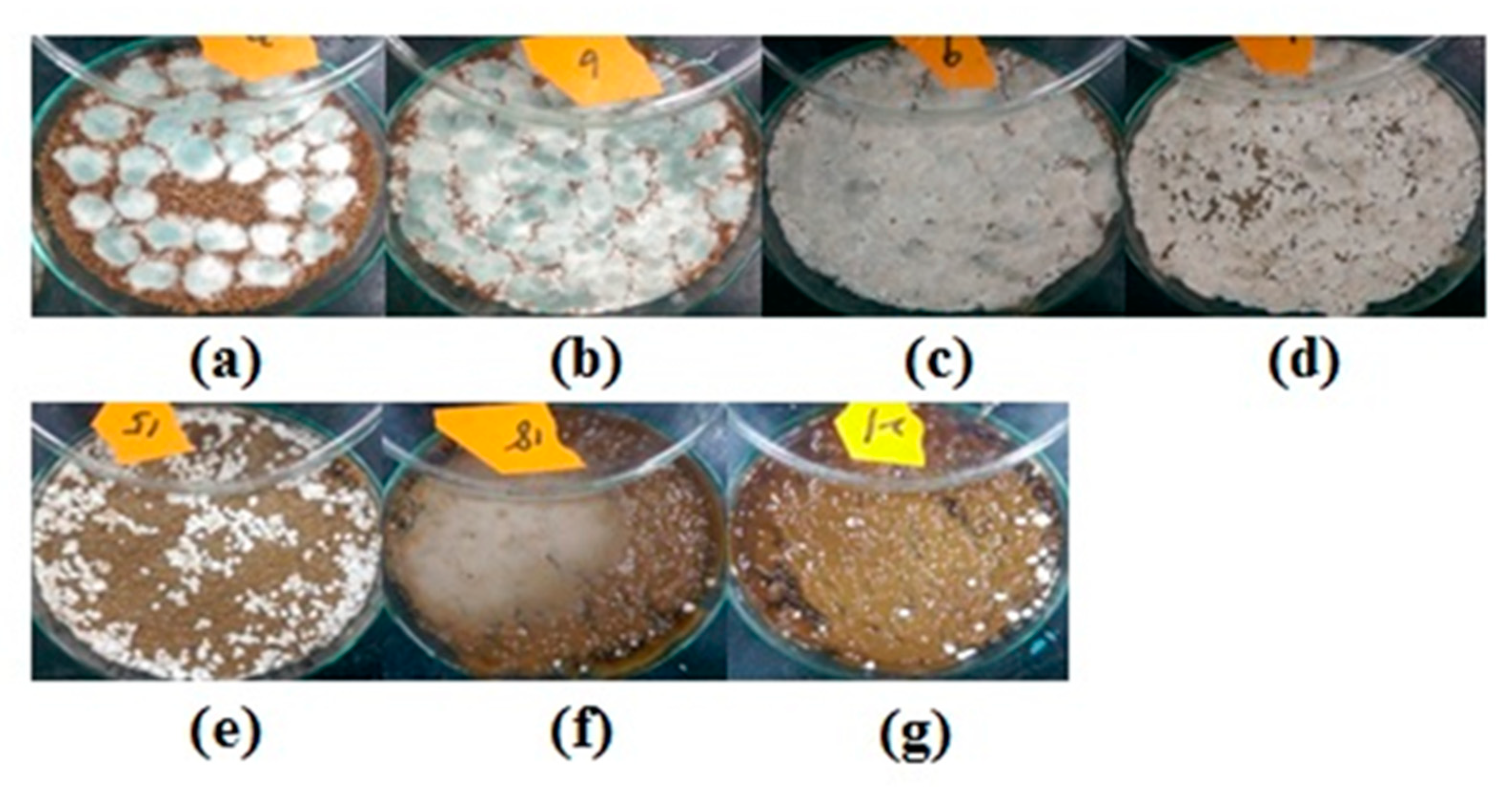
2.1. Effect of Particle Size of Wheat Bran
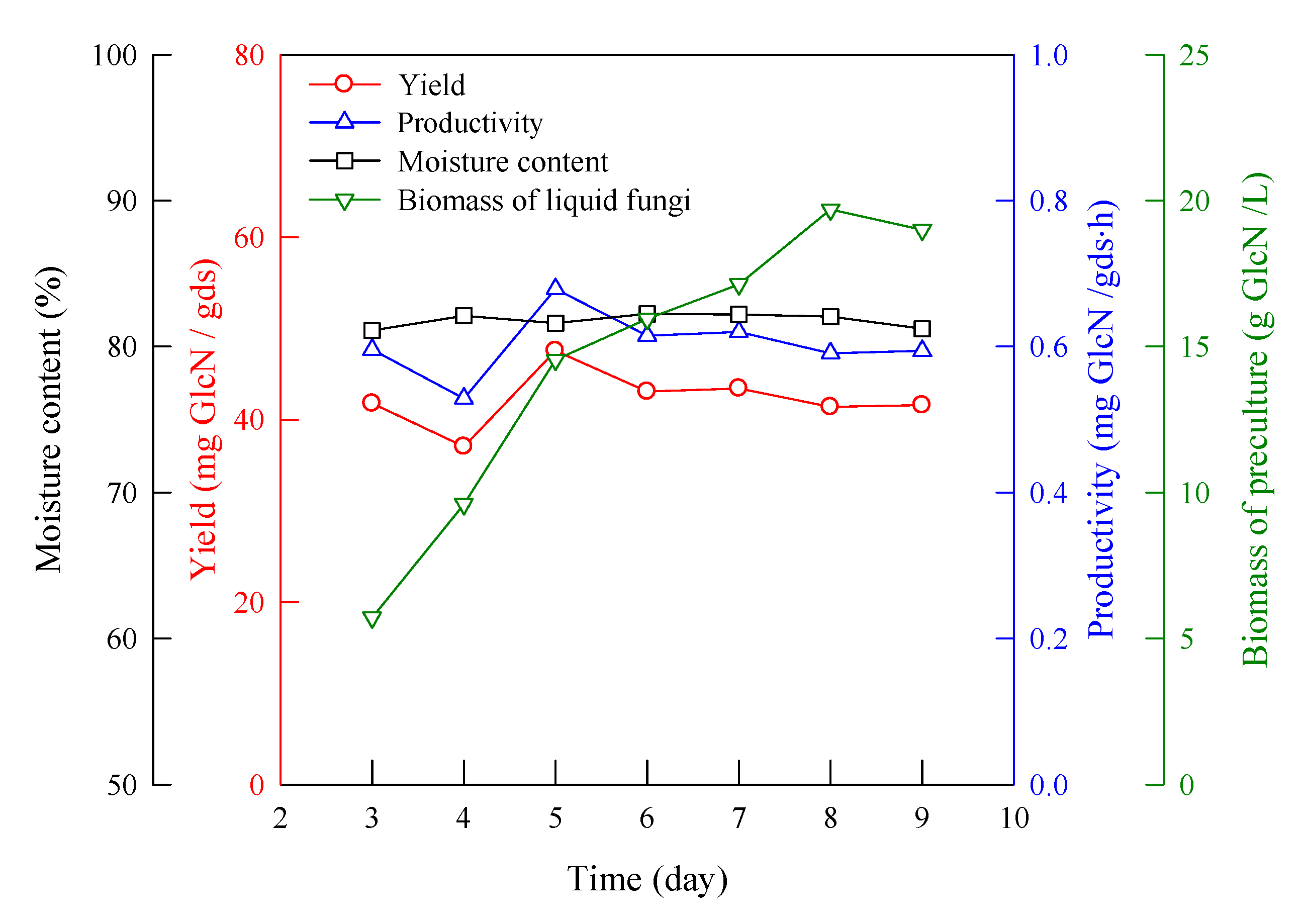
2.2. Effect of Preculture Time of Liquid Fermentation
2.3. Effect of Inoculum Biomass
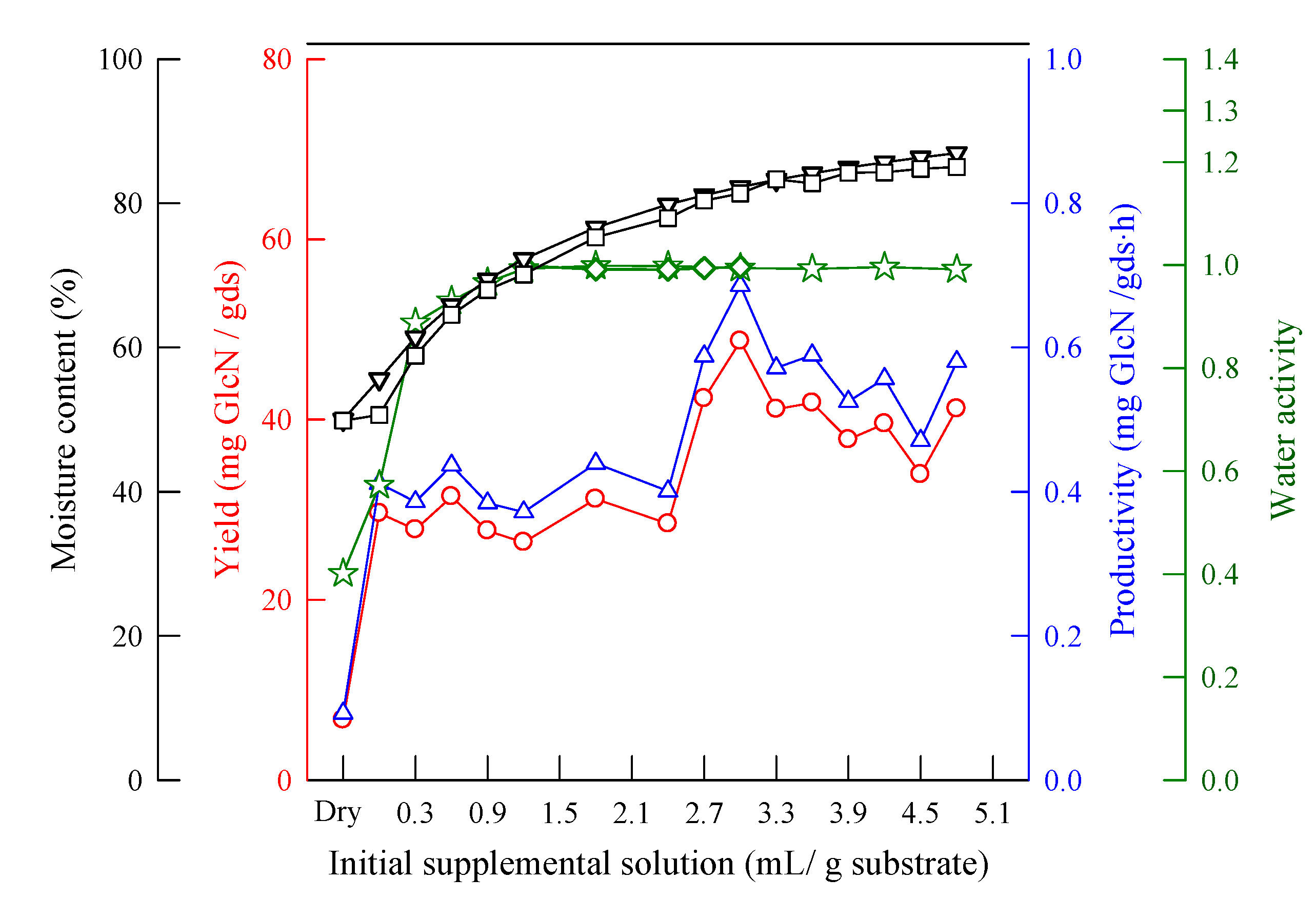
2.4. Effect of Initial Supplemental Solution Volume
2.5. Effect of Volume Ratio of Inoculum to Initial Supplemental Solution
2.6. Effect of Mineral Salts in Supplemental Solution
3. Materials and Methods
3.1. Materials
3.2. Preculture of Fungi
3.3. Solid-State Fermentation
3.4. Determination of Fungal GlcN
3.5. Determination of Water Activity Using the Diffusion Method
3.6. Moisture Content
3.7. Determination of Particle Size of Wheat Bran
3.8. Calculation of Yield, Productivity, and Content of GlcN
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wen, Z. Application and research progress of glucosamine. In Proceedings of the International Symposium on the Frontiers of Biotechnology and Bioengineering, Hangzhou, China, 20–21 April 2019. [Google Scholar]
- Arbia, W.; Arbia, L.; Adour, L.; Amrane, A. Chitin extraction from crustacean shells using biological methods—A review. Food Technol. Biotechnol. 2013, 51, 12–25. [Google Scholar]
- Sitanggang, A.B.; Wu, H.-S.; Wang, S.S.; Ho, Y.-C. Effect of pellet size and stimulating factor on the glucosamine production using Aspergillus sp. BCRC 31742. Bioresour. Technol. 2010, 101, 3595–3601. [Google Scholar] [CrossRef] [PubMed]
- Nirmal, N.P.; Santivarangkna, C.; Rajput, M.S.; Benjakul, S. Trends in shrimp processing waste utilization: An industrial prospective. Trends in Food Sci. Technol. 2020, 103, 20–35. [Google Scholar] [CrossRef]
- Lopata, A.L.; Kleine-Tebbe, J.; Kamath, S.D. Allergens and molecular diagnostics of shellfish allergy. Allergo J. 2016, 25, 24–32. [Google Scholar] [CrossRef]
- Hsieh, J.W.; Wu, H.S.; Wei, Y.H.; Wang, S.S. Determination and kinetics of producing glucosamine using fungi. Biotechnol. Prog. 2007, 23, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Sitanggang, A.B.; Sophia, L.; Wu, H. MiniReview Aspects of glucosamine production using microorganisms. Int. Food Res. J. 2012, 19, 393–404. [Google Scholar]
- Habibi, A.; Karami, S.; Varmira, K.; Hadadi, M. Key parameters optimization of chitosan production from Aspergillus terreus using apple waste extract as sole carbon source. Bioprocess Biosyst. Eng. 2020. [Google Scholar] [CrossRef]
- Ruiz-Terán, F.; David Owens, J. Chemical and enzymic changes during the fermentation of bacteria-free soya bean tempe. J. Sci. Food Agric. 1996, 71, 523–530. [Google Scholar] [CrossRef]
- Margulies, M.; Egholm, M.; Altman, W.E.; Attiya, S.; Bader, J.S.; Bemben, L.A.; Berka, J.; Braverman, M.S.; Chen, Y.-J.; Chen, Z.; et al. Genome sequencing in microfabricated high-density picolitre reactors. Nature 2005, 437, 376–380. [Google Scholar] [CrossRef]
- Wilhelm, S.M.; Carter, C.; Tang, L.; Wilkie, D.; McNabola, A.; Rong, H.; Chen, C.; Zhang, X.; Vincent, P.; McHugh, M.; et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004, 64, 7099–7109. [Google Scholar] [CrossRef]
- Chang, Y.F.; Sitanggang, A.B.; Wu, H.S. Optimizing biotechnological production of glucosamine as food ingredient from Aspergillus sp. BCRC 31742. J. Food Technol. 2011, 9, 75–82. [Google Scholar] [CrossRef]
- Wu, H.S.; Lin, B.C. Effect of oxygen transfer and pellet size for producing of glucosamine using Aspergillus sydowii BCRC 31742 cultivated in a fermenter. J. Food Process. Technol. 2017, 8. [Google Scholar] [CrossRef]
- Couto, S.R.; Sanromán, M.Á. Application of solid-state fermentation to food industry—A review. J. Food Eng. 2006, 76, 291–302. [Google Scholar] [CrossRef]
- Wu, H.S.; Peng, J.W. Solid Medium for Producing Glucosamine and Its Application. U.S. Patent 20200087693 A1, 19 March 2020. [Google Scholar]
- Zheng, Z.; Shetty, K. Solid-state production of beneficial fungi on apple processing wastes using glucosamine as the indicator of growth. J. Agric. Food Chem. 1998, 46, 783–787. [Google Scholar] [CrossRef]
- Amanullah, A.; Christensen, L.H.; Hansen, K.; Nienow, A.W.; Thomas, C.R. Dependence of morphology on agitation intensity in fed-batch cultures of Aspergillus oryzae and its implications for recombinant protein production. Biotechnol. Bioeng. 2002, 77, 815–826. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, M.; Zamani, A.; Karimi, K. Effect of phosphate on glucosamine production by ethanolic fungus Mucor indicus. Appl. Biochem. Biotechnol. 2013, 171, 1465–1472. [Google Scholar] [CrossRef]
- Wu, H.S.; Chen, J.H. Medium for Producing Glucosamine. U.S. Patent 20190292574A1, 26 September 2019. [Google Scholar]
- Sitanggang, A.B.; Wu, H.S.; Wang, S.S. Determination of fungal glucosamine using HPLC with 1-napthyl isothiocyanate derivatization and microwave heating. Biotechnol. Bioprocess Eng. 2009, 14, 819–827. [Google Scholar] [CrossRef]
- Gervais, P.; Molin, P. The role of water in solid-state fermentation. Biochem. Eng. J. 2003, 13, 85–101. [Google Scholar] [CrossRef]
- Koop, T.; Luo, B.; Tsias, A.; Peter, T. Water activity as the determinant for homogeneous ice nucleation in aqueous solutions. Nature 2000, 406, 611–614. [Google Scholar] [CrossRef]
- Labuza, T.P. The effect of water activity on reaction kinetics of food deterioration. Food Technol 1980, 34, 36–41. [Google Scholar]
- Zhao, S.M. Determination of water activity in buckwheat flour by way of Conway dish diffusion method. J. Anhui Agri. Sci. 2012, 40, 2901–2902. [Google Scholar]


| Water Addition to the Medium (mL/g Wheat Bran) | MC in Wheat Bran (%, Wet Basis) | aw |
|---|---|---|
| Dry | 0 | 0.401 |
| 0 | 0.112 | 0.572 |
| 0.3 | 0.317 | 0.887 |
| 0.6 | 0.445 | 0.930 |
| 0.9 | 0.532 | 0.966 |
| 1.2 | 0.596 | 0.992 |
| 1.8 | 0.683 | 0.999 |
Sample Availability: Samples of the compound is not available from the authors. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, J.W.; Wu, H.S. Kinetic Study of Glucosamine Production Using Aspergillus sydowii BCRC 31742 under Solid-State Fermentation. Molecules 2020, 25, 4832. https://doi.org/10.3390/molecules25204832
Peng JW, Wu HS. Kinetic Study of Glucosamine Production Using Aspergillus sydowii BCRC 31742 under Solid-State Fermentation. Molecules. 2020; 25(20):4832. https://doi.org/10.3390/molecules25204832
Chicago/Turabian StylePeng, Jia Wei, and Ho Shing Wu. 2020. "Kinetic Study of Glucosamine Production Using Aspergillus sydowii BCRC 31742 under Solid-State Fermentation" Molecules 25, no. 20: 4832. https://doi.org/10.3390/molecules25204832
APA StylePeng, J. W., & Wu, H. S. (2020). Kinetic Study of Glucosamine Production Using Aspergillus sydowii BCRC 31742 under Solid-State Fermentation. Molecules, 25(20), 4832. https://doi.org/10.3390/molecules25204832







