Evaluation of Cytotoxicity and α-Glucosidase Inhibitory Activity of Amide and Polyamino-Derivatives of Lupane Triterpenoids
Abstract
1. Introduction
2. Results and Discussion
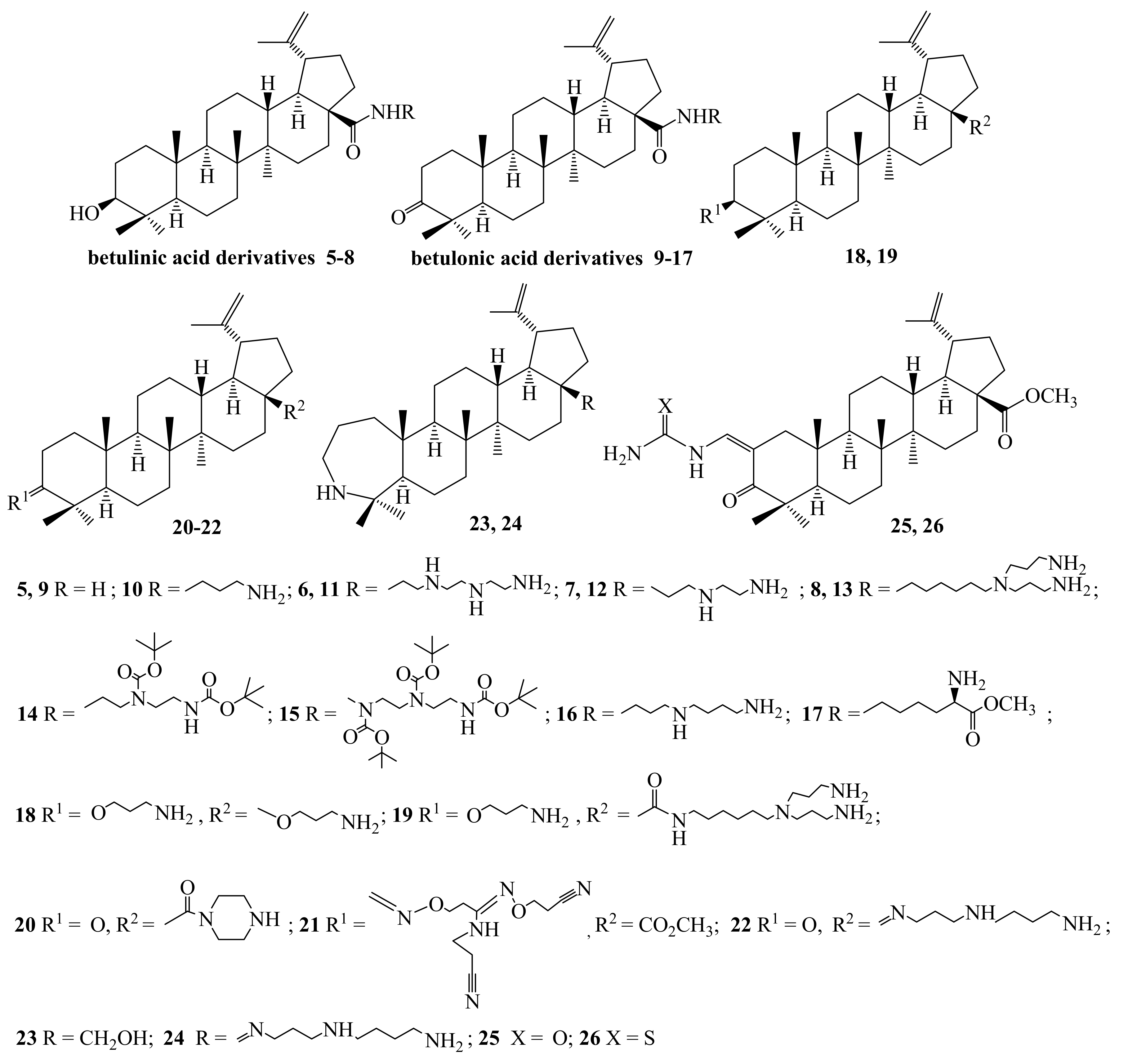
2.1. Chemistry
2.2. Biological Evaluation
2.2.1. Evaluation of In Vitro Antiproliferative Activity by NCI
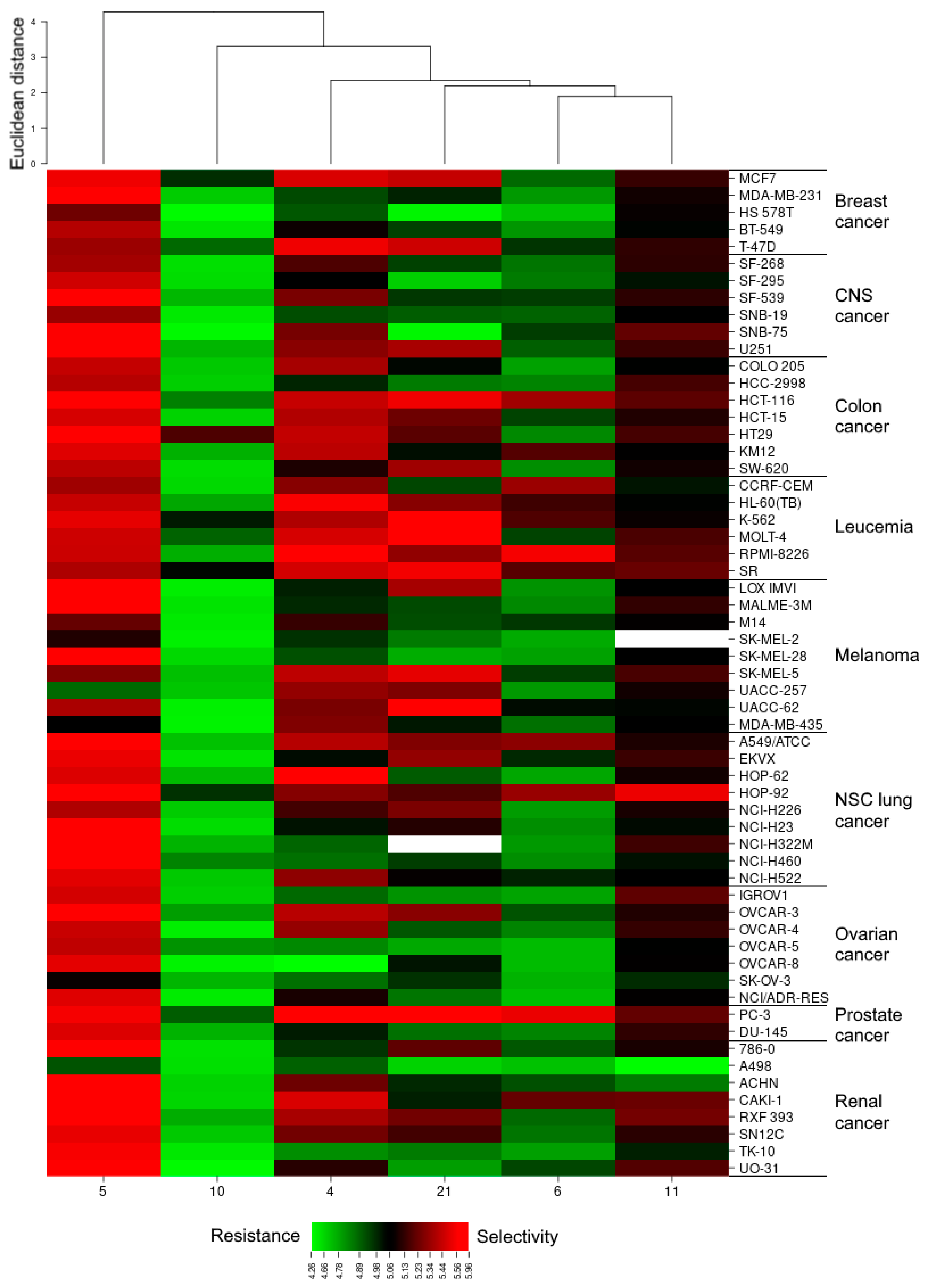
2.2.2. CellMinerTM and Gene Enrichment Analysis
2.2.3. α-Glucosidase Inhibition
2.2.4. Antibacterial and Fungicidal Activities
3. Materials and Methods
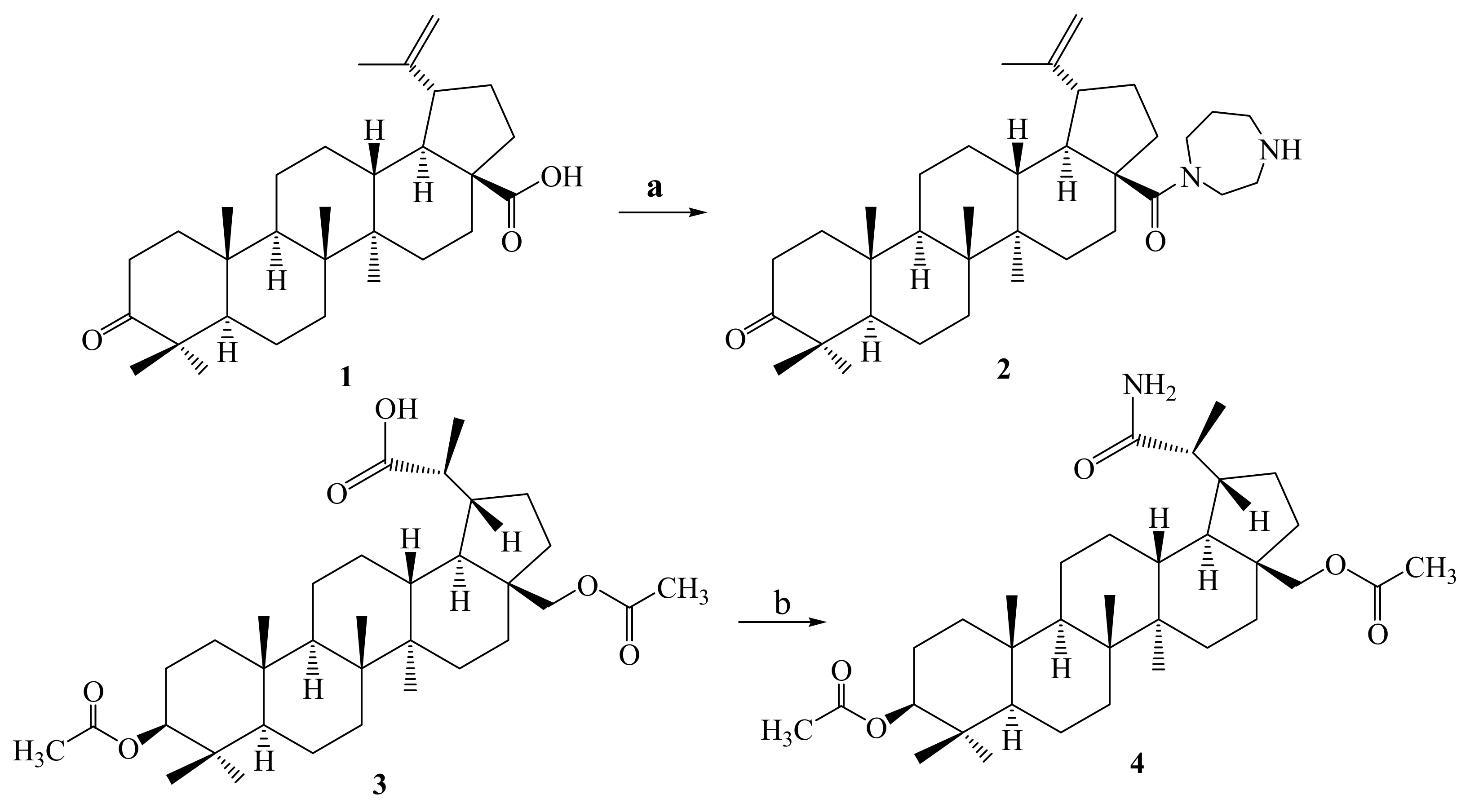
3.1. Chemistry
3.1.1. General
3.1.2. Synthesis of 28-(1,4-Diazepan-1-yl)-28-oxolup-20(29)-en-3-one 2
3.1.3. Synthesis of 3β,28-Diacetyloxy-(20R)-29-amino-29-oxolupane 4
3.2. Pharmacological Studies
3.2.1. In Vitro Cancer Screen in NCI, USA
3.2.2. CellMiner and Gene Ontology Enrichment Analysis
3.2.3. Yeast α-Glucosidase Activity Assay
3.2.4. Endoplasmic Reticulum α-Glucosidase Activity Assay
3.2.5. Antibacterial and Antifungal Assays
Antibacterial Assay
Antifungal Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kvasnica, M.; Urban, M.N.; Dickinson, J.; Sarek, J. Pentacyclic triterpenoids with nitrogen- and sulfur-containing heterocycles: Synthesis and medicinal significance. Nat. Prod. Rep. 2015, 32, 1303–1330. [Google Scholar] [CrossRef] [PubMed]
- Salvador, J.A.R.; Leal, A.S.; Valdeira, A.S.; Gonçalves, B.M.F.; Alho, D.P.S.; Figueiredo, S.A.C.; Silvestre, S.M.; Mendes, V.I.S. Oleanane-, ursane-, and quinone methide friedelane-type triterpenoid derivatives: Recent advances in cancer treatment. Eur. J. Med. Chem. 2017, 142, 95–130. [Google Scholar] [CrossRef] [PubMed]
- Sousa, J.L.C.; Freire, C.S.R.; Silvestre, A.J.D.; Silva, A.M.S. Recent Developments in the Functionalization of Betulinic Acid and Its Natural Analogues: A Route to New Bioactive Compounds. Molecules 2019, 24, 355. [Google Scholar] [CrossRef] [PubMed]
- Bildziukevich, U.; Kaletova, E.; Saman, D.; Sievanen, E.; Kolehmainen, E.T.; Slouf, M.; Wimmer, Z. Spectral and microscopic study of self-assambly of novel cationic spermine amides of betulinic acid. Steroids 2017, 117, 90–96. [Google Scholar] [CrossRef]
- Bildziukevich, U.; Rarova, L.; Janovska, L.; Saman, D.; Wimmer, Z. Enhancing effect of cystamine in its amides with betulinic acid as antimicrobial and antitumor agent in vitro. Steroids 2019, 148, 91–98. [Google Scholar] [CrossRef]
- Kazakova, O.B.; Brunel, J.M.; Khusnutdinova, E.F.; Negrel, S.; Giniyatullina, G.V.; Lopatina, T.V.; Petrova, A.V. A-ring modified triterpenoids and their spermidine-aldimines with strong antibacterial activity. Molbank 2019, M1078. [Google Scholar] [CrossRef]
- Khusnutdinova, E.F.; Apryshko, G.N.; Petrova, A.V.; Kukovinets, O.S.; Kazakova, O.B. The synthesis and selective cytotoxicity of new Mannich bases derivatives of 19- and 28-alkynyltriterpenoids. Russ. J. Org. Chem. 2018, 1, 123–127. [Google Scholar] [CrossRef]
- Kahnt, M.; Hoenke, S.; Fischer, L.; Al-Harrasi, A.; Csuk, R. Synthesis and cytotoxicity evaluation of DOTA-conjugates of ursolic acid. Molecules 2019, 24, E2254. [Google Scholar] [CrossRef]
- Medvedeva, N.I.; Kazakova, O.B.; Lopatina, T.V.; Smirnova, I.E.; Giniyatullina, G.V.; Baikova, I.P.; Kataev, V.E. Synthesis and antimycobacterial activity of triterpenic A-ring azepanes. Eur. J. Med. Chem. 2018, 143, 464–472. [Google Scholar] [CrossRef]
- Smirnova, I.E.; Kazakova, O.B. Structure—Anti-influenza Type a Activity Relationship among a Series of Nitrogen Lupane Triterpenoids. Nat. Prod. Commun. 2018, 13, 1267–1270. [Google Scholar] [CrossRef]
- Spivak, A.Y.; Khalitova, R.R.; Nedopekina, D.A.; Gubaidullin, R.R. Antimicrobial properties of amine- and guanidine-functionalized derivatives of betulinic, ursolic and oleanolic acids: Synthesis and structure/activity evaluation. Steroids 2020, 154, 108530. [Google Scholar] [CrossRef]
- Savage, P. Antibacterial properties of cationic steroid antibiotics. FEMS Microbiol. Lett. 2002, 217, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sills, A.K., Jr.; Williams, J.I.; Tyler, B.M.; Epstein, D.S.; Sipos, E.P.; Davis, J.D.; McLane, M.P.; Pitchford, S.; Cheshire, K.; Gannon, F.H.; et al. Squalamine inhibits angiogenesis and solid tumor growth in vivo and perturbs embryonic vasculature. Cancer Res. 1998, 58, 2784–2792. [Google Scholar] [PubMed]
- Moore, K.S.; Wehrli, S.; Roder, H.; Rogers, M.; Forrest, J.N.; McCrimmon, D.; Zasloff, M. Squalamine: An aminosterol antibiotic from the shark. Proc. Natl. Acad. Sci. USA 1993, 90, 1354–1358. [Google Scholar] [CrossRef]
- Lantz, K.A.; Hart, S.G.; Planey, S.L.; Roitman, M.F.; Ruiz-White, I.A.; Wolfe, H.R.; McLane, M.P. Inhibition of PTP1B by Trodusquemine (MSI-1436) Causes Fat-specific Weight Loss in Diet-induced Obese Mice. Obes. Silver Spring 2010, 18, 1516–1523. [Google Scholar] [CrossRef]
- Borselli, D.; Lieutaud, A.; Thefenne, H.; Garnotel, E.; Pagès, J.M.; Brunel, J.M.; Bolla, J.M. Polyamino-isoprenic derivatives block intrinsic resistance of P. aeruginosa to doxycycline and chloramphenicol in vitro. PLoS ONE 2016, 1–16. [Google Scholar] [CrossRef]
- Giniyatullina, G.V.; Flekhter, O.B.; Tolstikov, G.A. Synthesis of squalamine analogues on the basis of lupane triterpenoids. Mendeleev Commun. 2009, 19, 32–33. [Google Scholar] [CrossRef]
- Giniyatullina, G.V.; Kazakova, O.B.; Medvedeva, N.I.; Sorokina, I.V.; Zhukova, N.A.; Tolstikova, T.G.; Tolstikov, G.A. Synthesis of aminopropylamino derivatives of betulinic and oleanolic acids. Russ. J. Bioog. Chem. 2013, 39, 329–337. [Google Scholar] [CrossRef]
- Giniyatyllina, G.V.; Smirnova, I.E.; Kazakova, O.B.; Yavorskaya, N.P.; Golubeva, I.S.; Zhukova, O.S.; Pugacheva, R.B.; Apryshko, G.N.; Poroikov, V.V. Synthesis and anticancer activity of aminopropoxytriterpenoids. Med. Chem. Res. 2015, 24, 3423–3436. [Google Scholar] [CrossRef]
- Kazakova, O.B.; Giniyatullina, G.V.; Tolstikov, G.A. Synthesis of a-secomethylenamino- and substituted amidoximotriterpenoids. Russ. J. Bioog. Chem. 2011, 37, 619–625. [Google Scholar] [CrossRef]
- Kazakova, O.B.; Giniyatullina, G.V.; Medvedeva, N.I.; Tolstikov, G.A. Synthesis of a triterpene-spermidine conjugate. Russ. J. Org. Chem. 2012, 48, 1366–1369. [Google Scholar] [CrossRef]
- Bildziukevich, U.; Malík, M.; Özdemir, Z.; Rárová, L.; Janovská, L.; Šlouf, M.; Šaman, D.; Šarek, J.; Nonappa; Wimmer, Z. Spermine amides of selected triterpenoid acids: Dynamic supramolecular system formation influences the cytotoxicity of the drugs. J. Mater. Chem. B 2020, 8, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Giniyatullina, G.V.; Kazakova, O.B.; Salimova, E.V.; Tolstikov, G.A. Synthesis of new betulonic and oleanonic acid amides. Chem. Nat. Comp. 2011, 47, 68–72. [Google Scholar] [CrossRef]
- Kazakova, O.B.; Giniyatullina, G.V.; Tolstikov, G.A.; Baikova, I.P.; Zaprutko, L.; Apryshko, G.N. Synthesis and antitumor activity of aminopropoxy derivatives of betulin, erythrodiol, and uvaol. Rus. J. Bioorg. Chem. 2011, 37, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Kazakova, O.B.; Medvedeva, N.I.; Suponitskii, K.Y. Synthesis and molecular structure of 3β,28-diacetoxy-(20R)-lupan-29-oic acid. Chem. Nat. Comp. 2012, 41, 83–85. [Google Scholar] [CrossRef]
- Wiemann, J.; Heller, L.; Perl, V.; Kluge, R.; Ströhl, D.; Csuk, R. Betulinic acid derived hydroxamates and betulin derived carbamates are interesting scaffolds for the synthesis of novel cytotoxic compounds. Eur. J. Med. Chem. 2015, 106, 194–210. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Ganguly, A.; Chowdhuri, S.; Yousuf, M.; Ghosh, A.; Barui, A.K.; Kotcherlakota, R.; Adhikari, S.; Banerjee, R. Bis-Arylidene Oxindole–Betulinic Acid Conjugate: A Fluorescent Cancer Cell Detector with Potent Anticancer Activity. ACS Med. Chem. Lett. 2015, 6, 612–616. [Google Scholar] [CrossRef]
- Alakurtti, S.; Yli-Kauhaluoma, J.; Mäkelä, T.; Koskimies, S.; Bergström, S.; Hokkanen, H.; Menzler-Hokkanen, I. Betulin Derived Compounds as Anti-Feedants for Plant Pests. U.S. Patent 20120035224A1, 9 February 2012. [Google Scholar]
- Grever, M.R.; Schepartz, S.A.; Chabner, B.A. The National Cancer Institute: Cancer drug discovery and development program. Semin. Oncol. 1992, 19, 622–638. [Google Scholar]
- Boyd, M.R.; Paull, K.D. Some practical considerations and applications of the National Cancer Institute in vitro anticancer drug discovery screen. Drug Rev. Res. 1995, 34, 91–109. [Google Scholar] [CrossRef]
- Monks, A.; Scudiero, D.; Skehan, P.; Shoemaker, R.; Paull, K.; Vistica, D.; Hose, C.; Jangley, J.; Cronisie, P.; Viagro-Wolff, A.; et al. Feasibility of a highflux anticancer drug screen using a diverse panel of cultured human tumor cell lines. J. Natl. Cancer Inst. 1991, 83, 757–766. [Google Scholar] [CrossRef]
- Acton, E.M.; Narayanan, V.L.; Risbood, P.A.; Shoemaker, R.H.; Vistica, D.T.; Boyd, M.R. Anticancer Specificity of Some Ellipticinium Salts against Human Brain Tumors in vitro. J. Med. Chem. 1994, 37, 2185–2189. [Google Scholar] [CrossRef] [PubMed]
- Montoya, A.; Quiroga, J.; Abonia, R.; Nogueras, M.; Cobo, J.; Insuasty, B. Synthesis and in Vitro Antitumor Activity of a Novel Series of 2-Pyrazoline Derivatives Bearing the 4-Aryloxy-7-chloroquinoline Fragment. Molecules 2014, 19, 18656–18675. [Google Scholar] [CrossRef] [PubMed]
- Reinhold, W.C.; Sunshine, M.; Liu, H.; Varma, S.; Kohn, K.W.; Morris, J.; Doroshow, J.; Pommier, Y. CellMiner: A Web-Based Suite of Genomic and Pharmacologic Tools to Explore Transcript and Drug Patterns in the NCI-60 Cell Line Set. Cancer Res. 2012, 72, 3499–3511. [Google Scholar] [CrossRef] [PubMed]
- Yates, M.S.; Tauchi, M.; Katsuoka, F.; Flanders, K.C.; Liby, K.T.; Honda, T.; Gribble, G.W.; Johnson, D.A.; Johnson, J.A.; Burton, N.C.; et al. Pharmacodynamic characterization of chemopreventive triterpenoids as exceptionally potent inducers of Nrf2-regulated genes. Mol. Cancer Ther. 2007, 6, 154–162. [Google Scholar] [CrossRef]
- Samudio, I.; Konopleva, M.; Hail, N.; Shi, Y.X.; McQueen, T.; Hsu, T.; Evans, R.; Honda, T.; Gribble, G.W.; Sporn, M.; et al. 2-Cyano-3,12-dioxooleana-1,9-dien-28-imidazolide (CDDO-Im) directly targets mitochondrial glutathione to induce apoptosis in pancreatic cancer. J. Biol. Chem. 2005, 280, 36273–36282. [Google Scholar] [CrossRef]
- Cuadrado, A.; Rojo, A.I.; Wells, G.; Hayes, J.D.; Cousin, S.P.; Rumsey, W.L.; Attucks, O.C.; Franklin, S.; Levonen, A.L.; Kensler, T.W.; et al. Therapeutic targeting of the NRF2 and KEAP1 partnership in chronic diseases. Nat. Rev. Drug Discov. 2019, 14–16. [Google Scholar] [CrossRef]
- Stadtmauer, E.A.; Siegel, D.; Vogl, D.T.; Stinnett, J.; Narayanan, G.; Bapsy, P.P.; Ganesan, T.; Jain, M.; Prasad, S.V.S.; McNair, D.S.; et al. A Phase 1 Trial of Fluphenazine HCl (Fz), a Serotonin Antagonist, in Relapsed and Refractory Multiple Myeloma. Blood 2008, 112, 5188. [Google Scholar] [CrossRef]
- Di Carlo, F.; Conti, G.; Reboani, C. Interference of gestagens and androgens with rat uterine oestrogen receptors. J. Endocrinol. 1978, 77, 49–55. [Google Scholar] [CrossRef]
- Rocha, W.; Sanchez, R.; Deschênes, J.; Auger, A.; Hébert, E.; White, J.H.; Mader, S. Opposite Effects of Histone Deacetylase Inhibitors on Glucocorticoid and Estrogen Signaling in Human Endometrial Ishikawa Cells. Mol. Pharmacol. 2005, 68, 1852–1862. [Google Scholar] [CrossRef]
- Nocentini, G.; Giunchi, L.; Ronchetti, S.; Krausz, L.T.; Bartoli, A.; Moraca, R.; Migliorati, G.; Riccardi, C. A new member of the tumor necrosis factor/nerve growth factor receptor family inhibits T cell receptor-induced apoptosis. Proc. Natl. Acad. Sci. USA 1997, 94, 6216–6221. [Google Scholar] [CrossRef]
- Liu, B.; Li, Z.; Mahesh, S.P.; Pantanelli, S.; Hwang, F.S.; Siu, W.O.; Nussenblatt, R.B. Glucocorticoid-induced Tumor Necrosis Factor Receptor Negatively Regulates Activation of Human Primary Natural Killer (NK) Cells by Blocking Proliferative Signals and Increasing NK Cell Apoptosis. J. Biol. Chem. 2008, 283, 8202–8210. [Google Scholar] [CrossRef]
- Moroz, A.; Eppolito, C.; Li, Q.; Tao, J.; Clegg, C.H.; Shrikant, P.A. IL-21 Enhances and Sustains CD8 + T Cell Responses to Achieve Durable Tumor Immunity: Comparative Evaluation of IL-2, IL-15, and IL-21. J. Immunol. 2004, 173, 900–909. [Google Scholar] [CrossRef]
- Zhao, C.; Inoue, J.; Imoto, I.; Otsuki, T.; Iida, S.; Ueda, R.; Inazawa, J. POU2AF1, an amplification target at 11q23, promotes growth of multiple myeloma cells by directly regulating expression of a B-cell maturation factor, TNFRSF17. Oncogene 2008, 27, 63–75. [Google Scholar] [CrossRef]
- Chapellier, M.; Peña-Martínez, P.; Ramakrishnan, R.; Eriksson, M.; Talkhoncheh, M.S.; Orsmark-Pietras, C.; Lilljebjörn, H.; Högberg, C.; Hagström-Andersson, A.; Fioretos, T.; et al. Arrayed molecular barcoding identifies TNFSF13 as a positive regulator of acute myeloid leukemia-initiating cells. Haematologica 2019, 104, 2006–2016. [Google Scholar] [CrossRef] [PubMed]
- Lundin, M.; Baltscheffsky, H.; Ronne, H. Yeast PPA2 gene encodes a mitochondrial inorganic pyrophosphatase that is essential for mitochondrial function. J. Biol. Chem. 1991, 266, 12168–12172. [Google Scholar] [PubMed]
- Zewail, A.; Xie, M.W.; Xing, Y.; Lin, L.; Zhang, P.F.; Zou, W.; Saxe, J.P.; Huang, J. Novel functions of the phosphatidylinositol metabolic pathway discovered by a chemical genomics screen with wortmannin. Proc. Natl. Acad. Sci. USA 2003, 100, 3345–3350. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.Z.; Akter, R.; Kleve, M.G.; Gealt, M.A. Wortmannin induces MCF-7 breast cancer cell death via the apoptotic pathway, involving chromatin condensation, generation of reactive oxygen species, and membrane blebbing. Breast Cancer Targets Ther. 2012, 4, 103–113. [Google Scholar] [CrossRef]
- Blommaart, E.F.C.; Krause, U.; Schellens, J.P.M.; Vreeling-Sindelarova, H.; Meijer, A.J. The Phosphatidylinositol 3-Kinase Inhibitors Wortmannin and LY294002 Inhibit Autophagy in Isolated Rat Hepatocytes. Eur. J. Biochem. 1997, 243, 240–246. [Google Scholar] [CrossRef]
- Pietras, R.J.; Marquez-Garban, D.C. Membrane-Associated Estrogen Receptor Signaling Pathways in Human Cancers. Clin. Cancer Res. 2007, 13, 4672–4676. [Google Scholar] [CrossRef]
- Bennett, L.L.; Smithers, D.; Rose, L.M.; Adamson, D.J.; Thomas, H.J. Inhibition of synthesis of pyrimidine nucleotides by 2-hydroxy-3-(3,3-dichloroallyl)-1,4-naphthoquinone. Cancer Res. 1979, 39, 4868–4874. [Google Scholar]
- Shah, N.; Thomas, T.J.; Lewis, J.S.; Klinge, C.M.; Shirahata, A.; Gelinas, C.; Thomas, T. Regulation of estrogenic and nuclear factor κB functions by polyamines and their role in polyamine analog-induced apoptosis of breast cancer cells. Oncogene 2001, 20, 1715–1729. [Google Scholar] [CrossRef] [PubMed]
- Thomas, T.; Kulkarni, G.D.; Gallo, M.A.; Greenfield, N.; Lewis, J.S.; Shirahata, A.; Thomas, T.J. Effects of natural and synthetic polyamines on the conformation of an oligodeoxyribonucleotide with the estrogen response element. Nucl. Acids Res. 1997, 25, 2396–2402. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vijayanathan, V.; Thomas, T.J.; Nair, S.K.; Shirahata, A.; Gallo, M.A.; Thomas, T. Bending of the estrogen response element by polyamines and estrogen receptors α and β: A fluorescence resonance energy transfer study. Int. J. Biochem. Cell Biol. 2006, 38, 1191–1206. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Xing, Y.; Wen, C.; Yu, X.; Sun, W.; Xiu, Z.; Dong, Y. Pentacyclic triterpenes as α-glucosidase and α-amylase inhibitors: Structure-activity relationships and the synergism with acarbose. Bioorg. Med. Chem. Lett. 2017, 27, 5065–5070. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Gornostai, T.G.; Penzina, T.A.; Borovskii, G.B. Lupane triterpenoids and sterols from Inonotus rheades Mycelium and their anti-glucosidase activity. Chem. Nat. Compd. 2017, 53, 988–990. [Google Scholar] [CrossRef]
- Khusnutdinova, E.F.; Petrova, A.V.; Thu, H.N.T.; Tu, A.L.T.; Thanh, T.N.; Thi, C.B.; Babkov, D.A.; Kazakova, O.B. Structural modifications of 2,3-indolobetulinic acid: Design and synthesis of highly potent α-glucosidase inhibitors. Bioorg. Chem. 2019, 88, 102957. [Google Scholar] [CrossRef] [PubMed]
- Gundoju, N.; Bokam, R.; Yalavarthi, N.R.; Azad, R.; Ponnapalli, M.G. Betulinic acid derivatives: A new class of α-glucosidase inhibitors and LPS-stimulated nitric oxide production inhibition on mouse macrophage RAW 264.7 cells. Nat. Prod. Res. 2019, 33, 2618–2622. [Google Scholar] [CrossRef]
- Ouyang, J.-K.; Dong, L.-M.; Xu, Q.-L.; Wang, J.; Liu, S.-B.; Qian, T.; Yuan, Y.-F.; Tan, J.-W. Triterpenoids with α-glucosidase inhibitory activity and cytotoxic activity from the leaves of Akebia trifoliate. RSC Adv. 2018, 8, 40483–40489. [Google Scholar] [CrossRef]
- Nguyen, T.P.; Le, T.D.; Phan, N.M.; Bui, T.D.; Mai, D.T. Triterpene saponins with α-glucosidase inhibition and cytotoxic activity from the leaves of Schefflera sessiliflora. J. Asian Nat. Prod. Res. 2016, 18, 542–550. [Google Scholar] [CrossRef]
- Gerber-Lemaire, S.; Juillerat-Jeanneret, L. Glycosylation Pathways as Drug Targets for Cancer: Glycosidase Inhibitors. Mini-Rev. Med. Chem. 2006, 6, 104–1052. [Google Scholar] [CrossRef]
- Liu, X.; Zang, X.; Yin, X.; Yang, W.; Huang, J.; Huang, J.; Yu, C.; Ke, C.; Hong, Y. Semi-synthesis of C28-modified triterpene acid derivatives from maslinic acid or corosolic acid as potential α-glucosidase inhibitors. Bioorg. Chem. 2020, 97, 103694. [Google Scholar] [CrossRef] [PubMed]
- Pili, R.; Chang, J.; Partis, R.A.; Mueller, R.A.; Chrest, F.J.; Passaniti, A. The α-Glucosidase I Inhibitor Castanospermine Alters Endothelial Cell Glycosylation, Prevents Angiogenesis, and Inhibits Tumor Growth. Cancer Res. 1995, 55, 2920. [Google Scholar] [PubMed]
- Teicher, B.A.; Williams, J.I.; Takeuchi, H.; Ara, G.; Herbst, R.S.; Buxton, D. Potential of the aminosterol, squalamine in combination therapy in the rat 13,762 mammary carcinoma and the murine Lewis lung carcinoma. Anticancer Res. 1998, 18, 2567. [Google Scholar]
- Akhter, S.; Nath, S.K.; Tse, G.M.; Williams, J.; Zasloff, M.; Donowitz, M. Squalamine, a novel cationic steroid, specifically inhibits the brush- border Na+/H+ exchanger isoform NHE3. Am. J. Physiol. Cell Physiol. 1999, 276, C136–C144. [Google Scholar] [CrossRef]
- Hiramatsu, R.; Fukuhara, S.; Mitsuda, S.; Yokomichi, T.; Kataoka, T. Betulinic acid and oleanolic acid, natural pentacyclic triterpenoids, interfere with N-linked glycan modifications to intercellular adhesion molecule-1, but not its intracellular transport to the cell surface. Eur. J. Pharmacol. 2015, 767, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.W.; Dye, D.E.; Coombe, D.R. The role of immunoglobulin superfamily cell adhesion molecules in cancer metastasis. Int. J. Cell Biol. 2012, 340296. [Google Scholar] [CrossRef] [PubMed]
- Brunel, J.M.; Salmi, C.; Loncle, C.; Vidal, N.; Letourneux, Y. Squalamine: A Polyvalent Drug of the Future? Curr. Cancer Drug Targets 2005, 5, 267–272. [Google Scholar] [CrossRef]
- Flekhter, O.B.; Nigmatullina, L.R.; Baltina, L.A.; Karachurina, L.T.; Galin, F.Z.; Zarudii, F.S.; Tolstikov, G.A.; Boreko, E.I.; Pavlova, N.I.; Nikolaeva, S.N.; et al. Synthesis of betulinic acid from betulin extract and study of the antiviral and antiulcer activity of some related terpenoids. Pharm. Chem. J. 2002, 36, 484–487. [Google Scholar] [CrossRef]
- Maere, S.; Heymans, K.; Kuiper, M. BiNGO: A Cytoscape plugin to assess overrepresentation of Gene Ontology categories in Biological Networks. Bioinformatics 2005, 21, 3448–3449. [Google Scholar] [CrossRef]
| Compounds | Mean Growth, % | Range of Growth, % | Sensitive Cell Lines, % |
|---|---|---|---|
| 2 | 89.47 | 8.04 to 126.62 | Leukemia: SR (8.04) |
| 7 | 99.22 | 53.80 to 146.79 | not active |
| 8 | 80.54 | 20.45 to 135.21 | Colon Cancer: HT29 (24.32); Breast Cancer: MDA-MB-231/ATCC (20.45) |
| 9 | 72.02 | 25.88 to 115.11 | Leukemia: HL-60(TB) (25.88) |
| 12 | 98.94 | 59.08 to 140.73 | not active |
| 13 | 104.76 | 85.83 to 118.03 | not active |
| 14 | 36.65 | ‒33.78 to 73.79 | Leukemia: CCRF-CEM (14.95), HL-60(TB) (−14.34), MOLT-4 (11.67), RPMI-8226 (22.20); Non-Small Cell Lung Cancer: HOP-92 (8.07), NCI-460 (20.60); Colon Cancer: HCT-116 (17.53), HT29 (23.68), SW-620 (28.83); CNS Cancer: SF-295 (31.97), SNB-75 (23.86), U251 (27.93); Melanoma: SK-MEL-5 (−8.44); Renal Cancer: ACHN (23.51), CAKI-1 (18.57), UO-31 (17.41); Prostate Cancer: PC-3 (19.12); Breast Cancer: MCF7 (22.21), MDA-MB-231/ATCC (−33.78), T-47D (28.02) |
| 15 | 90.11 | 56.79 to 114.03 | not active |
| 16 | 100.79 | 59.32 to 140.95 | not active |
| 17 | 98.74 | 79.25 to 111.36 | not active |
| 19 | 63.02 | −39.45 to 140.99 | Leukemia: CCRF-CEM (11.76), K-562 (1.52), MOLT-4 (10.06), RPMI-8226 (29.30), SR (18.41); Non-Small Cell Lung Cancer: NCI-460 (−16.23); Colon Cancer: COLO 205 (18.88), HCT-116 (12.60), HT29 (4.21), KM12 (31.48), SW-620 (7.33); CNS Cancer: U251 (−39.45); Melanoma: LOX IMVI (5.71); Ovarian cancer: IGROV1 (27.33), OVCAR-8 (−28.27); Breast Cancer: MCF7 (14.49) |
| 20 | 100.53 | 87.76 to 111.05 | not active |
| 22 | 101.76 | 80.54 to 123.09 | not active |
| 24 | 93.62 | 21.69 to 131.79 | Leukemia: SR (21.69) |
| 25 | 91.74 | 21.22 to 112.08 | Leukemia: SR (21.22) |
| 26 | 90.51 | 60.92 to 125.91 | not active |
| Subpanel Tumor Cell Lines | Percentage Cell Growth for Compounds | |||||
|---|---|---|---|---|---|---|
| 4 | 5 | 6 | 10 | 11 | 21 | |
| Leukemia | ||||||
| CCRF-CEM | −12.36 | 3.11 | 5.18 | 1.39 | −11.29 | 7.42 |
| HL-60(TB) | −23.06 | 1.17 | −23.98 | 1.25 | −35.58 | 0.90 |
| K-562 | 3.10 | 4.57 | −15.78 | 1.10 | −16.99 | 5.42 |
| MOLT-4 | −29.06 | 1.86 | 1.18 | 2.76 | −16.31 | −26.81 |
| RPMI-8226 | −18.26 | −19.77 | −8.15 | −6.66 | −30.74 | 3.28 |
| SR | 10.81 | 15.50 | −4.72 | 23.66 | 4.96 | 1.86 |
| NSC Lung Cancer | ||||||
| A549/ATCC | 9.89 | −69.33 | −64.63 | −4.70 | −76.50 | 15.82 |
| EKVX | −25.21 | −10.31 | −11.36 | −15.68 | 11.37 | 53.13 |
| HOP-62 | −72.98 | −72.18 | −69.12 | −26.80 | 43.09 | 32.54 |
| HOP−92 | −83.15 | −62.83 | −65.35 | −55.49 | −65.19 | −14.95 |
| NCI-H226 | −58.51 | −50.47 | −23.56 | 36.64 | 74.30 | 2.42 |
| NCI-H23 | −40.35 | −27.08 | −51.70 | −8.47 | −35.23 | 21.79 |
| NCI-H322M | −78.19 | −58.91 | −83.84 | −68.83 | −81.08 | 46.12 |
| NCI-H460 | −61.81 | −37.45 | −100.00 | −43.19 | −90.93 | 4.23 |
| NCI-H522 | −61.13 | −53.76 | −57.83 | −16.98 | −69.70 | 13.36 |
| Colon Cancer | ||||||
| COLO 205 | −52.98 | −66.21 | −19.47 | 7.82 | −47.48 | 17.60 |
| HCC-2998 | −68.10 | −39.02 | −90.36 | −23.40 | −90.04 | 26.92 |
| HCT-116 | −84.18 | −24.80 | −26.53 | 1.97 | −80.70 | 4.16 |
| HCT-15 | −44.10 | 6.36 | −24.81 | −22.06 | −36.56 | 34.28 |
| HT29 | −77.68 | −49.47 | −39.56 | 3.90 | −77.42 | 3.88 |
| KM12 | −62.05 | −63.59 | −52.23 | −25.31 | −81.93 | 10.89 |
| SW-620 | −55.13 | −4.49 | −86.66 | −36.18 | −76.71 | 21.96 |
| CNS Cancer | ||||||
| SF-268 | −61.04 | 4.41 | −30.99 | −16.88 | −38.68 | 29.14 |
| SF-295 | −50.75 | −57.70 | −84.72 | −26.39 | −54.47 | 36.56 |
| SF-539 | −83.91 | −70.46 | −100.00 | −36.20 | −92.71 | −2.29 |
| SNB-19 | −39.95 | 1.30 | −74.52 | −29.49 | −78.54 | 28.93 |
| SNB-75 | −47.83 | −39.09 | −79.32 | −51.56 | −41.55 | 18.44 |
| U251 | −51.40 | −69.32 | −71.65 | −13.77 | −81.82 | −39.66 |
| Melanoma | ||||||
| LOX IMVI | −85.52 | −72.80 | −41.03 | 1.37 | −68.23 | 14.56 |
| MALME-3M | −88.97 | −57.05 | −100.00 | −18.76 | −72.82 | 25.27 |
| M14 | −44.25 | 8.80 | −30.98 | 5.07 | −62.82 | 26.57 |
| MDA-MB-435 | −63.78 | −4.44 | −89.21 | −32.41 | −49.89 | 14.55 |
| SK-MEL-2 | −77.63 | −31.18 | −65.39 | −24.21 | 56.36 | 36.23 |
| SK-MEL-28 | −73.19 | 0.38 | −98.68 | −31.42 | −73.91 | 29.25 |
| SK-MEL-5 | −71.94 | −39.59 | −90.10 | −23.93 | −86.18 | −84.68 |
| UACC-257 | −46.24 | −6.08 | −39.58 | −46.67 | −10.66 | 8.82 |
| UACC-62 | −82.97 | −23.23 | −100.00 | −22.62 | −77.94 | −25.76 |
| Ovarian Cancer | ||||||
| IGROV1 | −71.25 | −58.08 | −81.43 | −6.94 | −84.81 | 18.78 |
| OVCAR−3 | −76.19 | −46.59 | −66.02 | −45.89 | −72.48 | −34.55 |
| OVCAR-5 | −77.37 | −21.57 | −83.37 | −47.68 | −44.11 | 50.59 |
| OVCAR-8 | −31.70 | −64.82 | −12.90 | −3.81 | −75.03 | 13.17 |
| NCI/ADR-RES | −57.29 | −8.91 | −13.05 | −9.52 | −73.33 | 17.06 |
| SK-OV-3 | −11.89 | −45.72 | −14.17 | −27.22 | 70.49 | 34.60 |
| Renal Cancer | ||||||
| 786-0 | −73.99 | −72.16 | −83.51 | −22.61 | −84.46 | −11.47 |
| A498 | −81.70 | −28.33 | −32.64 | −44.82 | 80.81 | −0.38 |
| ACHN | −75.68 | −80.29 | −100.00 | −49.24 | −83.29 | 13.94 |
| CAKI-1 | −45.80 | −64.30 | −93.59 | −54.52 | −8.79 | 18.52 |
| RXF 393 | −64.94 | −49.35 | −82.88 | −42.28 | −71.88 | −43.14 |
| SN12C | −91.61 | −45.76 | −53.68 | −34.74 | −88.48 | −71.91 |
| TK-10 | −35.40 | −69.84 | −67.30 | −32.23 | −77.68 | 37.88 |
| UO-31 | −97.54 | −100.00 | −100.00 | −34.32 | −96.35 | 19.03 |
| Prostate Cancer | ||||||
| PC-3 | −47.42 | −24.47 | 1.07 | −53.78 | −24.64 | 4.49 |
| DU-145 | −69.87 | −32.96 | −8.34 | −100.00 | −56.67 | 27.53 |
| Breast Cancer | ||||||
| MCF7 | −45.58 | −12.52 | −60.54 | −8.46 | −57.97 | 12.44 |
| MDA-MB-231/ATCC | −88.58 | −28.40 | −56.94 | −47.78 | −92.38 | 5.09 |
| HS 578T | −32.99 | −1.70 | −23.26 | −4.98 | 47.76 | 24.26 |
| BT-549 | −55.13 | −21.60 | −51.94 | −2.14 | 58.31 | 4.40 |
| T-47D | −8.82 | −24.75 | −32.56 | −12.76 | −4.36 | 15.11 |
| MDA-MB-468 | −40.40 | −56.03 | −48.60 | −19.87 | −49.06 | 4.41 |
| Panel/Cell Line | 4 | 5 | 6 | 10 | 11 | 21 | DRB | 5-FU |
|---|---|---|---|---|---|---|---|---|
| GI50 a (µM) | GI50 (µM) | GI50 (µM) | GI50 (µM) | GI50 (µM) | GI50 (µM) | GI50 (µM) | GI50 (µM) | |
| Leukemia | ||||||||
| CCRF-CEM | 5.19 | 4.49 | 4.55 | 22.1 | 9.65 | 11.7 | 0.08 | 9.97 |
| HL-60(TB) | 2.76 | 3.59 | 7.33 | 16.5 | 8.88 | 5.18 | 0.19 | 2.30 |
| K-562 | 4.09 | 3.04 | 6.90 | 9.84 | 8.20 | 2.23 | - | 3.58 |
| MOLT-4 | 3.29 | 3.43 | 11.5 | 12.9 | 6.96 | 2.52 | 0.03 | 0.35 |
| RPMI-8226 | 2.63 | 3.53 | 2.81 | 17.2 | 6.78 | 4.91 | 0.08 | 0.04 |
| SR | 3.32 | 4.14 | 6.76 | 9.05 | 5.91 | 2.87 | 0.03 | - |
| Non-Small Cell Lung Cancer | ||||||||
| A549/ATCC | 3.97 | 2.11 | 4.97 | 19.6 | 7.69 | 5.27 | 0.06 | 0.18 |
| EKVX | 9.28 | 2.96 | 10.5 | 23.3 | 7.34 | 4.77 | 0.41 | - |
| HOP-62 | 1.10 | 3.21 | 16.5 | 18.3 | 7.91 | 12.5 | 0.07 | 0.39 |
| HOP-92 | 5.21 | 2.69 | 4.61 | 10.8 | 2.93 | 6.90 | 0.10 | 77.9 |
| NCI-H226 | 7.28 | 4.11 | 15.5 | 21.2 | 7.74 | 5.43 | 0.05 | 54.7 |
| NCI-H23 | 9.42 | 2.26 | 14.7 | 22.6 | 9.14 | 7.59 | 0.15 | 0.33 |
| NCI-H322M | 13.0 | 2.59 | 15.1 | 17.6 | 7.32 | >50.0 | - | - |
| NCI-H460 | 13.4 | 2.12 | 14.7 | 14.0 | 9.40 | 11.1 | 0.02 | 0.05 |
| NCI-H522 | 4.94 | 3.13 | 10.3 | 21.0 | 8.72 | 8.34 | 0.03 | 7.27 |
| Colon Cancer | ||||||||
| COLO 205 | 4.31 | 3.63 | 15.9 | 21.0 | 8.74 | 9.02 | 0.18 | 0.15 |
| HCC-2998 | 10.4 | 3.96 | 14.1 | 21.4 | 7.16 | 13.8 | 0.26 | 0.05 |
| HCT-116 | 3.57 | 1.97 | 4.40 | 13.9 | 6.65 | 2.89 | 0.08 | 0.22 |
| HCT-15 | 4.04 | 3.28 | 11.5 | 21.7 | 7.65 | 5.77 | 6.46 | 0.11 |
| HT29 | 3.73 | 2.56 | 14.6 | 6.95 | 7.17 | 6.70 | 0.12 | 0.17 |
| KM12 | 3.86 | 3.20 | 6.81 | 17.3 | 8.47 | 9.35 | 0.27 | 0.21 |
| SW-620 | 7.70 | 3.88 | 14.7 | 22.5 | 7.91 | 4.45 | 0.09 | 0.92 |
| CNS Cancer | ||||||||
| SF-268 | 6.95 | 4.34 | 13.5 | 23.0 | 7.53 | 11.4 | 0.10 | 1.62 |
| SF-295 | 8.57 | 3.39 | 13.8 | 22.6 | 9.42 | 21.4 | 0.10 | - |
| SF-539 | 5.52 | 1.78 | 11.1 | 17.9 | 7.53 | 11.0 | 0.12 | 0.06 |
| SNB-19 | 11.9 | 4.57 | 12.9 | 24.7 | 8.50 | 12.6 | 0.04 | 3.81 |
| SNB-75 | 5.59 | 2.74 | 11.1 | 32.1 | 6.26 | 31.2 | 0.07 | 78.7 |
| U251 | 5.10 | 2.54 | 12.7 | 17.7 | 7.34 | 4.30 | 0.04 | 0.92 |
| Melanoma | ||||||||
| LOX IMVI | 10.2 | 1.77 | 14.9 | 25.5 | 8.72 | 4.32 | 0.07 | 0.24 |
| MALME-3M | 10.5 | 2.39 | 14.6 | 23.3 | 7.40 | 11.8 | 0.12 | 0.05 |
| M14 | 7.38 | 6.05 | 11.0 | 24.7 | 8.38 | 11.9 | 0.18 | 0.98 |
| MDA-MB-435 | 5.23 | 8.65 | 13.4 | 28.7 | 8.70 | 9.70 | 0.25 | 0.07 |
| SK-MEL-2 | 10.8 | 7.63 | 16.8 | 27.3 | >50.0 | 13.8 | 0.17 | 56.7 |
| SK-MEL-28 | 12.0 | 1.09 | 16.0 | 22.1 | 8.49 | 17.1 | 0.21 | 1.03 |
| SK-MEL-5 | 3.80 | 5.22 | 11.1 | 19.4 | 6.98 | 3.02 | 0.08 | 0.46 |
| UACC-257 | 4.83 | 13.2 | 15.1 | 20.6 | 7.91 | 5.35 | 0.14 | 3.55 |
| UACC-62 | 5.46 | 4.20 | 9.29 | 27.4 | 8.96 | 2.32 | 0.12 | 0.52 |
| Ovarian Cancer | ||||||||
| IGROV1 | 13.2 | 3.32 | 16.1 | 21.5 | 6.42 | 14.9 | 0.17 | 1.22 |
| OVCAR-3 | 3.95 | 2.07 | 12.2 | 15.7 | 7.65 | 5.17 | 0.39 | 0.01 |
| OVCAR-4 | 4.72 | 3.54 | 14.1 | 25.6 | 7.39 | 12.3 | 0.37 | 4.43 |
| OVCAR-5 | 14.4 | 3.78 | 18.6 | 14.8 | 8.78 | 16.7 | 0.41 | 10.9 |
| OVCAR-8 | 54.4 | 3.12 | 18.8 | 27.7 | 8.41 | 9.66 | 0.10 | 1.74 |
| NCI/ADR-RES | 7.74 | 3.17 | 18.9 | 24.8 | 8.41 | 13.5 | 7.16 | 0.31 |
| SK-OV-3 | 13.4 | 8.18 | 17.5 | 17.7 | 10.6 | 10.8 | 0.22 | 21.8 |
| Renal Cancer | ||||||||
| 786-0 | 10.9 | 2.03 | 12.4 | 23.2 | 7.80 | 6.42 | 0.13 | 0.72 |
| A498 | 12.8 | 12.2 | 19.6 | 22.9 | 42.6 | 21.7 | 0.10 | 0.35 |
| ACHN | 5.78 | 2.44 | 12.0 | 21.7 | 13.8 | 10.5 | 0.08 | 0.27 |
| CAKI-1 | 3.24 | 1.98 | 5.92 | 21.9 | 5.87 | 10.2 | 0.95 | 0.07 |
| RXF 393 | 4.28 | 2.10 | 13.2 | 17.1 | 5.60 | 5.64 | 0.10 | 2.61 |
| SN12C | 5.65 | 3.00 | 13.5 | 20.8 | 7.56 | 7.28 | 0.07 | 0.49 |
| TK-10 | 14.7 | 2.79 | 15.8 | 23.9 | 10.1 | 13.8 | - | 1.12 |
| UO-31 | 7.57 | 1.72 | 11.7 | 38.9 | 6.85 | 15.6 | 0.49 | 1.42 |
| Prostate Cancer | ||||||||
| PC-3 | 2.67 | 2.83 | 2.95 | 12.6 | 6.26 | 2.26 | 0.32 | 2.36 |
| DU-145 | 9.94 | 3.21 | 14.1 | 17.5 | 7.47 | 13.4 | 0.11 | 0.36 |
| Breast Cancer | ||||||||
| MCF7 | 3.27 | 2.91 | 13.2 | 10.7 | 7.38 | 3.67 | 0.03 | 0.07 |
| MDA-MB-31/ATCC | 11.8 | 1.97 | 15.1 | 21.1 | 8.03 | 10.3 | 0.51 | 6.60 |
| HS 578T | 12.4 | 5.74 | 20.1 | 40.5 | 8.30 | 30.2 | 0.33 | 9.77 |
| BT-549 | 8.17 | 3.99 | 15.0 | 23.8 | 8.91 | 11.3 | 0.23 | 10.6 |
| T-47D | 2.91 | 4.55 | 10.9 | 13.1 | 7.52 | 3.47 | 0.06 | 8.12 |
| MDA-MB-468 | 3.96 | 3.20 | 8.88 | 12.6 | 8.09 | 2.02 | 0.05 | - |
| Panel | Compound 4 | Compound 5 | Compound 6 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| MIDa | MIDb | Selectivityc | MIDa | MIDb | Selectivityc | MIDa | MIDb | Selectivityc | |
| Leukemia | 7.87 | 3.55 | 2.22 | 3.67 | 3.20 | 1.15 | 12.15 | 6.64 | 1.83 |
| NSCL cancer | 7.50 | 1.05 | 2.79 | 1.32 | 11.88 | 1.02 | |||
| Colon Cancer | 5.37 | 1.47 | 3.21 | 1.14 | 11.72 | 1.04 | |||
| CNS cancer | 7.27 | 1.08 | 3.23 | 1.14 | 12.52 | 0.97 | |||
| Melanoma | 7.80 | 1.01 | 5.58 | 0.66 | 13.58 | 0.89 | |||
| Ovarian Cancer | 15.97 | 0.49 | 3.88 | 0.95 | 14.10 | 0.86 | |||
| Renal Cancer | 8.12 | 0.97 | 3.53 | 1.04 | 13.02 | 0.93 | |||
| Prostate cancer | 6.31 | 1.25 | 3.02 | 1.22 | 8.53 | 1.42 | |||
| Breast cancer | 7.09 | 1.11 | 3.73 | 0.98 | 13.86 | 0.88 | |||
| Panel | Compound 10 | Compound 11 | Compound 21 | ||||||
| MIDa | MIDb | Selectivityc | MIDa | MIDb | Selectivityc | MIDa | MIDb | Selectivityc | |
| Leukemia | 20.46 | 14.59 | 1.40 | 9.17 | 7.73 | 1.19 | 10.25 | 4.90 | 2.09 |
| NSCL cancer | 18.71 | 1.09 | 7.58 | 1.21 | 12.43 | 0.83 | |||
| Colon Cancer | 17.82 | 1.15 | 7.68 | 1.19 | 7.42 | 1.38 | |||
| CNS cancer | 23.00 | 0.89 | 7.76 | 1.18 | 15.32 | 0.67 | |||
| Melanoma | 24.33 | 0.84 | 12.84 | 0.71 | 8.81 | 1.16 | |||
| Ovarian Cancer | 24.63 | 0.83 | 8.24 | 1.11 | 11.86 | 0.86 | |||
| Renal Cancer | 23.80 | 0.86 | 12.52 | 0.73 | 11.39 | 0.89 | |||
| Prostate cancer | 15.05 | 1.36 | 6.87 | 1.33 | 7.83 | 1.31 | |||
| Breast cancer | 20.30 | 1.01 | 8.04 | 1.14 | 10.16 | 1.00 | |||
| Compounds | Pearson’s Correlation b | P Value | NSC c | Name | Mechanism of Action | FDA Status |
|---|---|---|---|---|---|---|
| 4 | - | - | - | - | - | - |
| 5 | 0.545 | 0.000024 | 727038 | CDDO-Im | Nrf2 | - |
| 6 | 0.672 | 0 | 88536 | Calusterone | Hormone | FDA approved |
| 0.65 | 0 | 12198 | Dromostanolone propionate | Hormone | FDA approved | |
| 0.62 | 0 | 734945 | N-(4-Aminophenyl)-4-(3-(3,4-dihydroisoquinolin-2(1H)-yl)prop-1-en-2-yl)benzamide | HDAC | - | |
| 0.537 | 0.000012 | 736101 | 4-(3-(10H-Phenothiazin-10-yl)prop-1-en-2-yl)-N-(2-aminophenyl)benzamide | HDAC | - | |
| 0.565 | 0.000024 | 730001 | N-(2-Aminophenyl)-4-(3-(3,4-dihydro-1H-pyrido[3,4-b]indol-2(9H)-yl)prop-1-en-2-yl)benzamide | HDAC | - | |
| 0.507 | 0.000041 | 92339 | Fluphenazine | Antipsychotic | FDA approved | |
| 0.517 | 0.000063 | 734949 | 4-[3-(3,4-Dihydro-1H-isoquinolin-2-yl)prop-1-en-2-yl]-N-pyrazin-2-ylbenzamide | HDAC | - | |
| 10 | 0.563 | 0.000004 | 776422 | LDK-378 | ALK inhibitor | FDA approved |
| 0.537 | 0.000014 | 777193 | LDK-378 | ALK inhibitor | FDA approved | |
| 0.511 | 0.000035 | 12198 | Dromostanolone propionate | Hormone | FDA approved | |
| 11 | 0.507 | 0.000379 | 221019 | Wortmannin | PI3K inhibitor | Clinical trial |
| 21 | 0.578 | 0.000002 | 12198 | Dromostanolone propionate | Hormone | FDA approved |
| 0.537 | 0.000014 | 126771 | Dichloroallyl lawsone | DNA/RNA synthesis inhibitor | - |
| Compounds | Yeast α-Glucosidase, IC50 ± SE (μM) | Rat Liver ER Neutral α-Glucosidase Inhibition, m ± SD (%) | |
|---|---|---|---|
| 100 μM | 10 μM | ||
| 11 | 4.84 ± 1.02 | 43.60 ± 7.98 * | 15.03 ± 8.98 |
| 12 | >100 | 23.59 ± 1.34 * | 10.21 ± 3.49 |
| 16 | >100 | 14.94 ± 6.31 | 9.05 ± 6.68 |
| 18 | 5.70 ± 1.09 | 36.51 ± 14.90 * | 56.64 ± 4.24 * |
| 22 | >100 | 58.52 ± 8.21 * | 31.42 ± 7.96 * |
| 23 | >100 | 5.85 ± 8.03 | −1.34 ± 2.22 |
| 24 | >100 | 54.62 ± 15.38 * | 26.96 ± 2.33 * |
| 25 | 53.3 ± 5.7 | 9.62 ± 6.94 | 8.76 ± 9.63 |
| 26 | >100 | 26.60 ± 13.03 | 11.63 ± 9.09 |
| Acarbose | 436.7 ± 10.2 | 74.55 ± 3.76 * (1 mM) 24.09 ± 6.48 * (100 μM) | 5.68 ± 4.68 |
| Compounds | Gram-Positive Bacteria | Gram-Negative Bacteria | Fungi | ||||
|---|---|---|---|---|---|---|---|
| S. aureus | E. coli | K. pneumonia | P. aeruginosa | A. baumannii | C. albicans | C. neoformans | |
| Strain ATCC 43300 | Strain ATCC 25922 | Strain ATCC 700603 | Strain 19606 | Strain ATCC 27853 | Strain ATCC 90028 | Strain H99, ATCC 208821 | |
| 2 | 40.30 | 1.96 | 0.98 | 8.34 | 33.07 | 4.14 | −5.90 |
| 12 | 71.80 | −9.57 | 9.20 | 7.72 | 19.49 | 11.74 | 62.56 |
| 25 | 19.78 | −5.34 | 2.44 | −8.92 | 40.86 | 13.97 | −7.93 |
| 26 | 12.66 | −7.03 | −3.89 | −4.64 | 28.24 | 5.83 | −13.04 |
Simple Availability: Simples of the compounds 1–26 are available from the authors. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kazakova, O.B.; Giniyatullina, G.V.; Mustafin, A.G.; Babkov, D.A.; Sokolova, E.V.; Spasov, A.A. Evaluation of Cytotoxicity and α-Glucosidase Inhibitory Activity of Amide and Polyamino-Derivatives of Lupane Triterpenoids. Molecules 2020, 25, 4833. https://doi.org/10.3390/molecules25204833
Kazakova OB, Giniyatullina GV, Mustafin AG, Babkov DA, Sokolova EV, Spasov AA. Evaluation of Cytotoxicity and α-Glucosidase Inhibitory Activity of Amide and Polyamino-Derivatives of Lupane Triterpenoids. Molecules. 2020; 25(20):4833. https://doi.org/10.3390/molecules25204833
Chicago/Turabian StyleKazakova, Oxana B., Gul’nara V. Giniyatullina, Akhat G. Mustafin, Denis A. Babkov, Elena V. Sokolova, and Alexander A. Spasov. 2020. "Evaluation of Cytotoxicity and α-Glucosidase Inhibitory Activity of Amide and Polyamino-Derivatives of Lupane Triterpenoids" Molecules 25, no. 20: 4833. https://doi.org/10.3390/molecules25204833
APA StyleKazakova, O. B., Giniyatullina, G. V., Mustafin, A. G., Babkov, D. A., Sokolova, E. V., & Spasov, A. A. (2020). Evaluation of Cytotoxicity and α-Glucosidase Inhibitory Activity of Amide and Polyamino-Derivatives of Lupane Triterpenoids. Molecules, 25(20), 4833. https://doi.org/10.3390/molecules25204833








