Synthesis of Block Copolymer Brush by RAFT and Click Chemistry and Its Self-Assembly as a Thin Film
Abstract
1. Introduction
2. Results
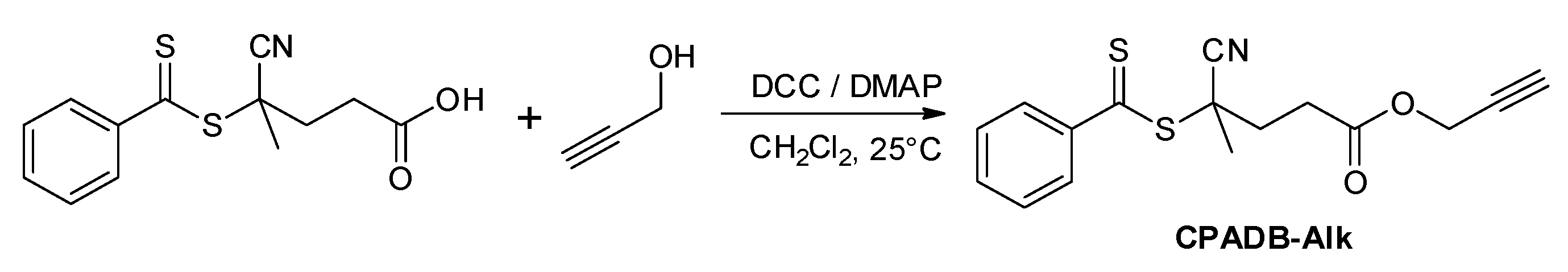
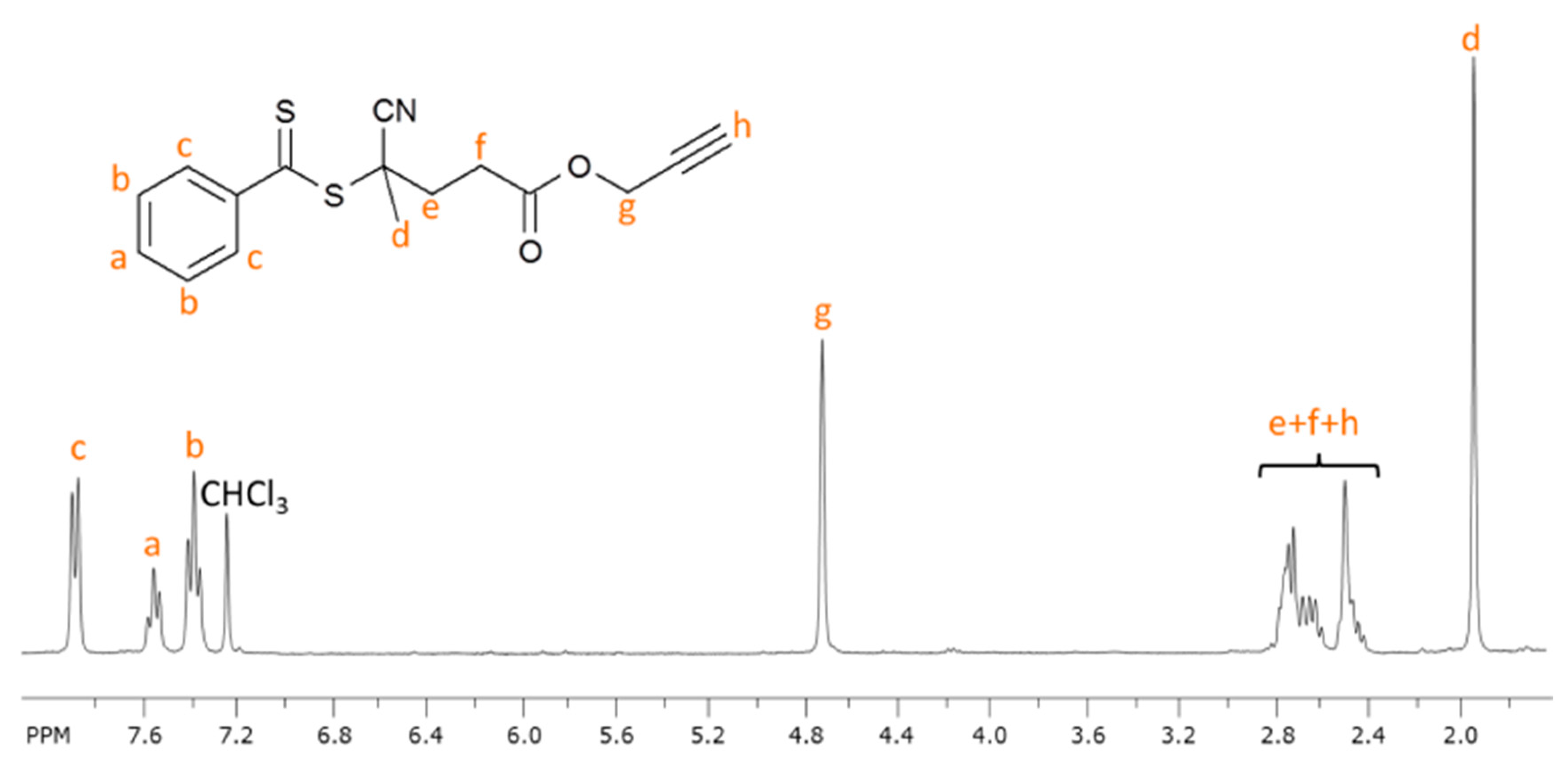
2.1. Synthesis of Alkyne Terminated RAFT Chain Transfer Agent (CPADB-Alk)
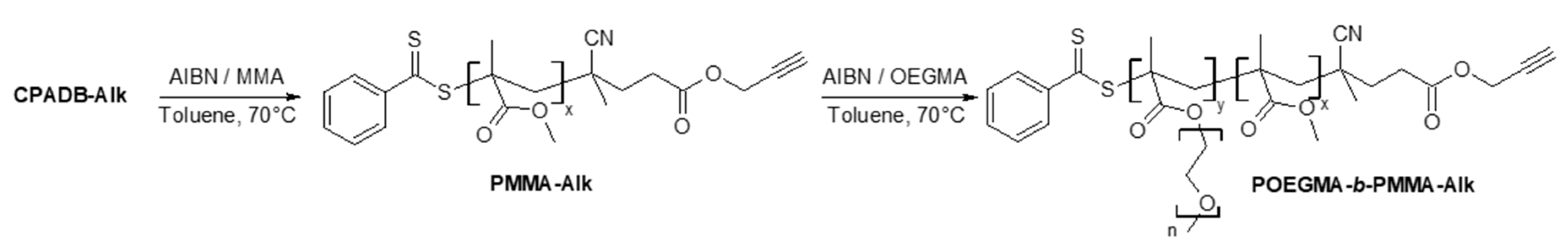
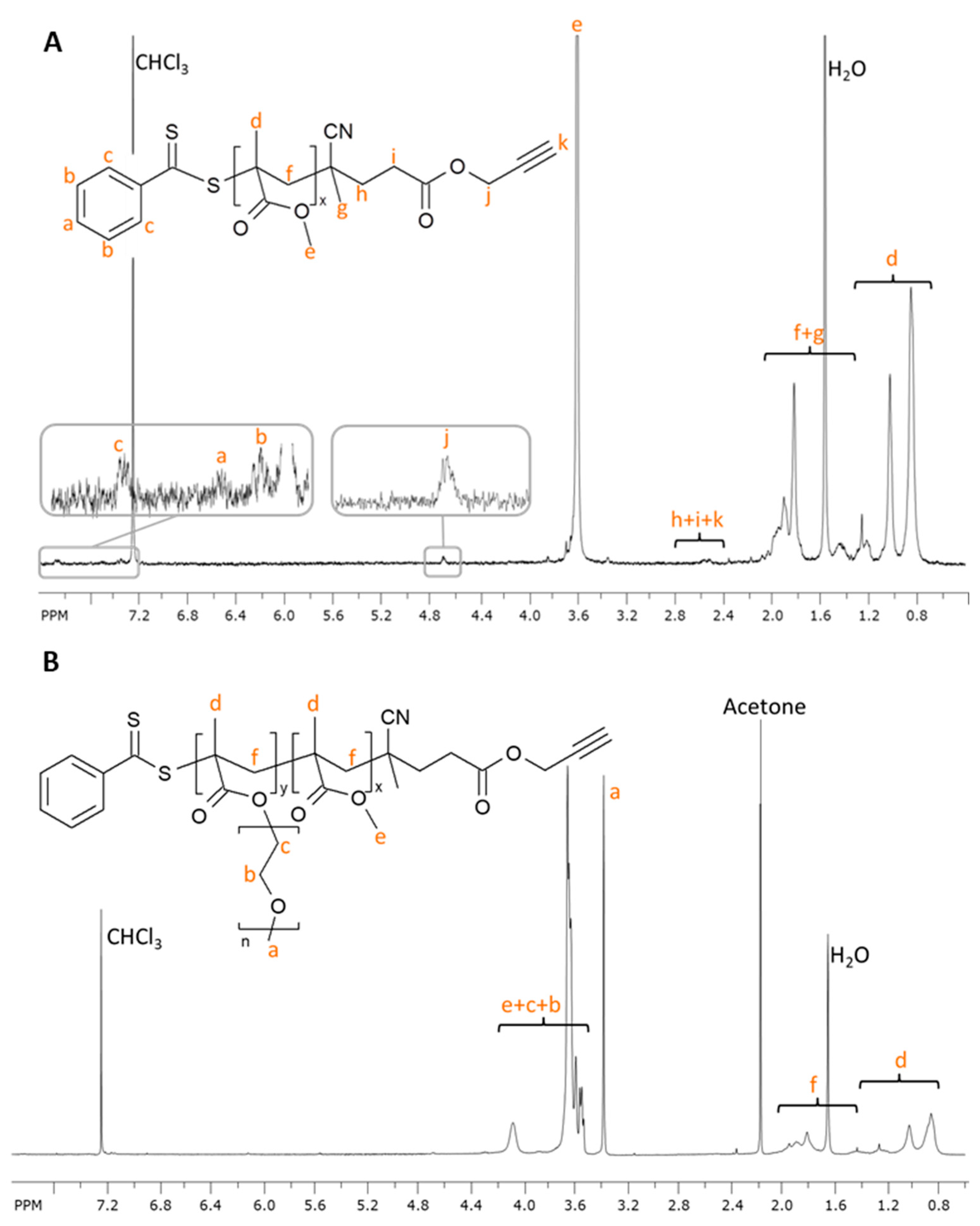
2.2. Synthesis of Poly(oligo(ethylene glycol) methyl ether methacrylate)-b-poly(methyl methacrylate) (POEGMA-b-PMMA-Alk) Block Copolymer
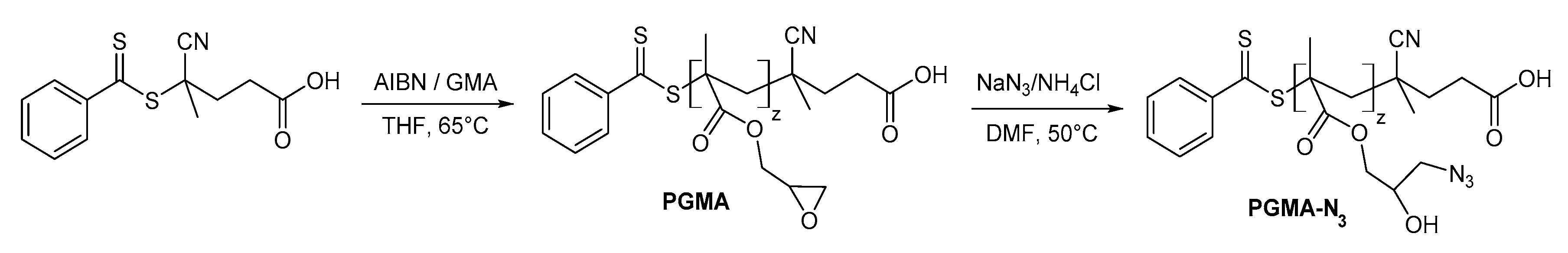
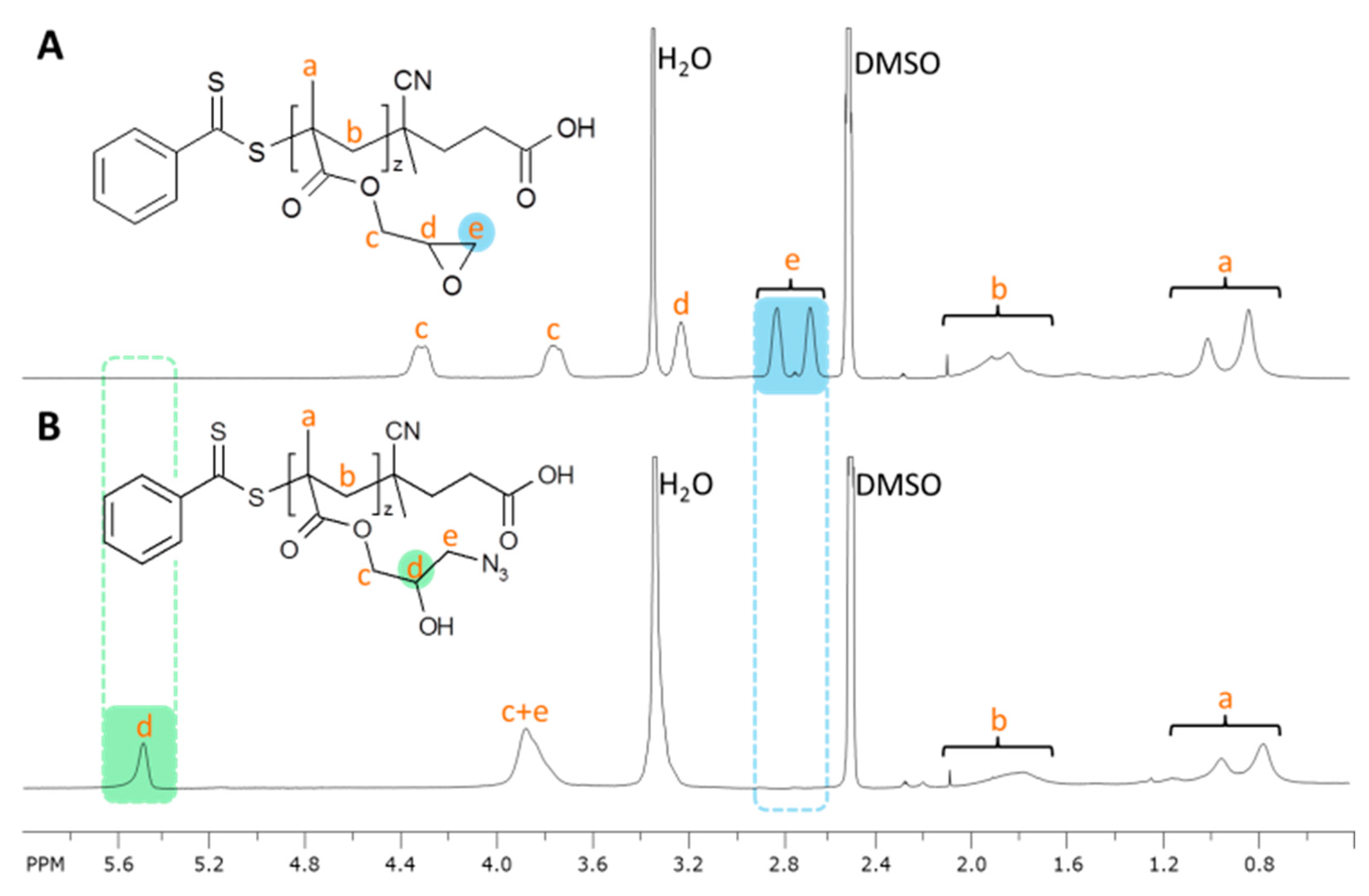
2.3. Synthesis of Poly(glycidyl methacrylate)(PGMA) and Poly(glycidyl methacrylate azide)(P(GMA-N3))
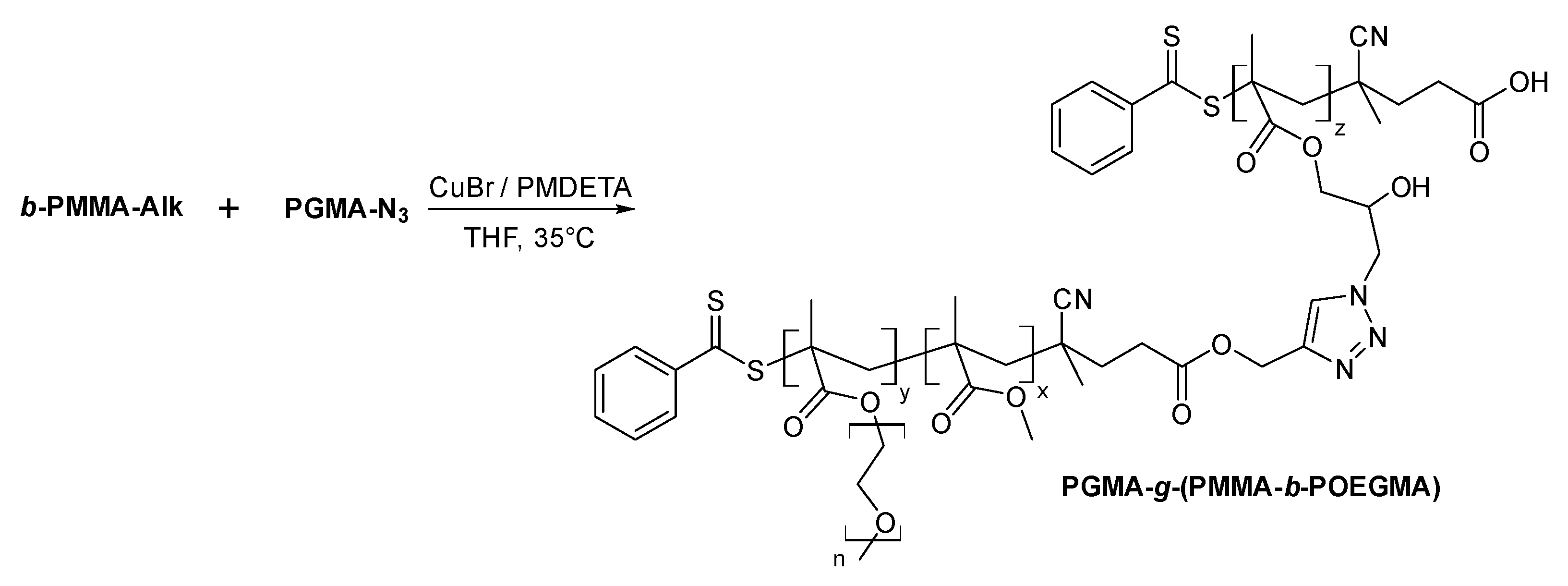
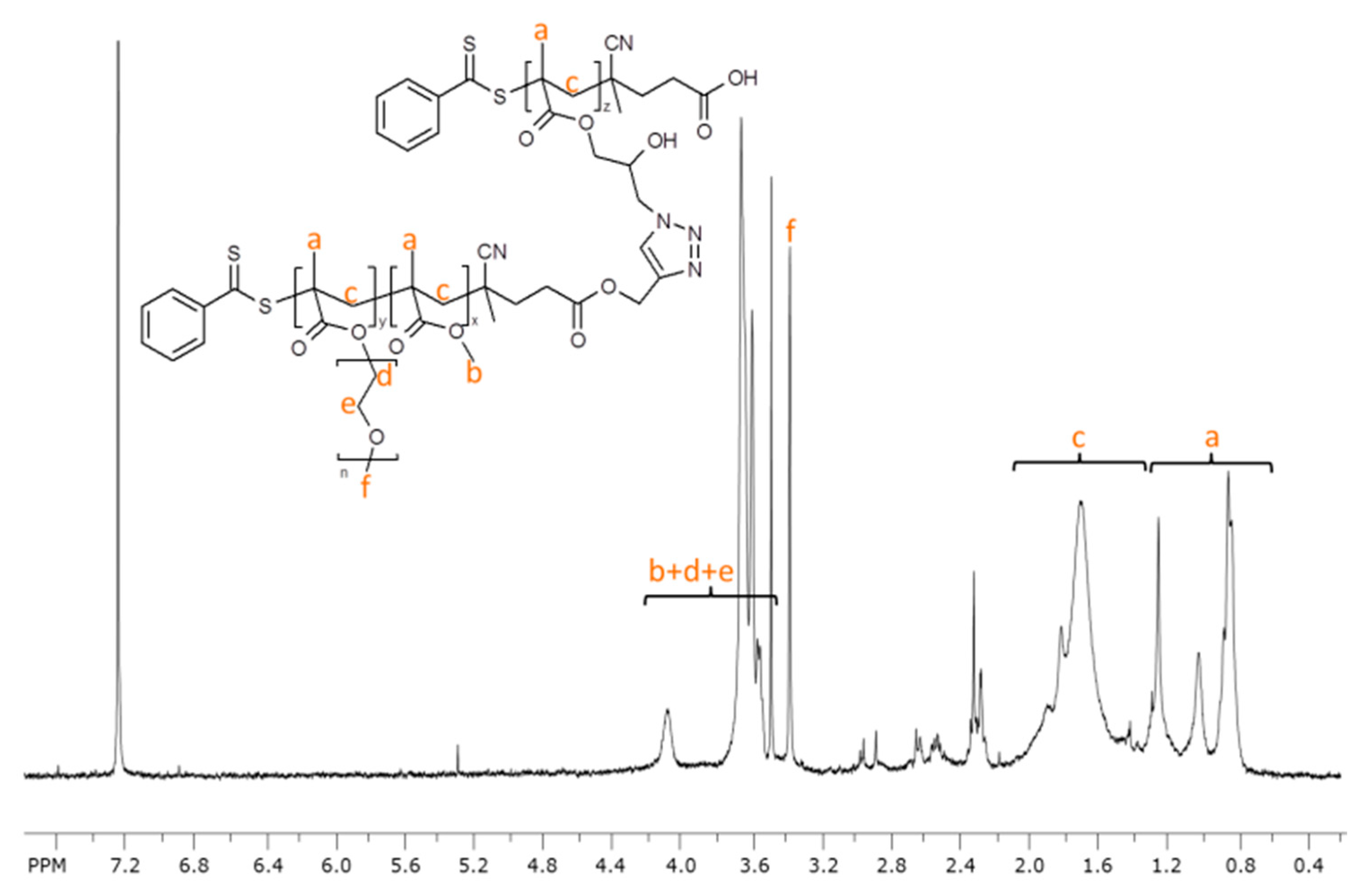
2.4. Block Copolymer Brush from Click Reaction between P(GMA-N3) and POEGMA-b-PMMA-Alk
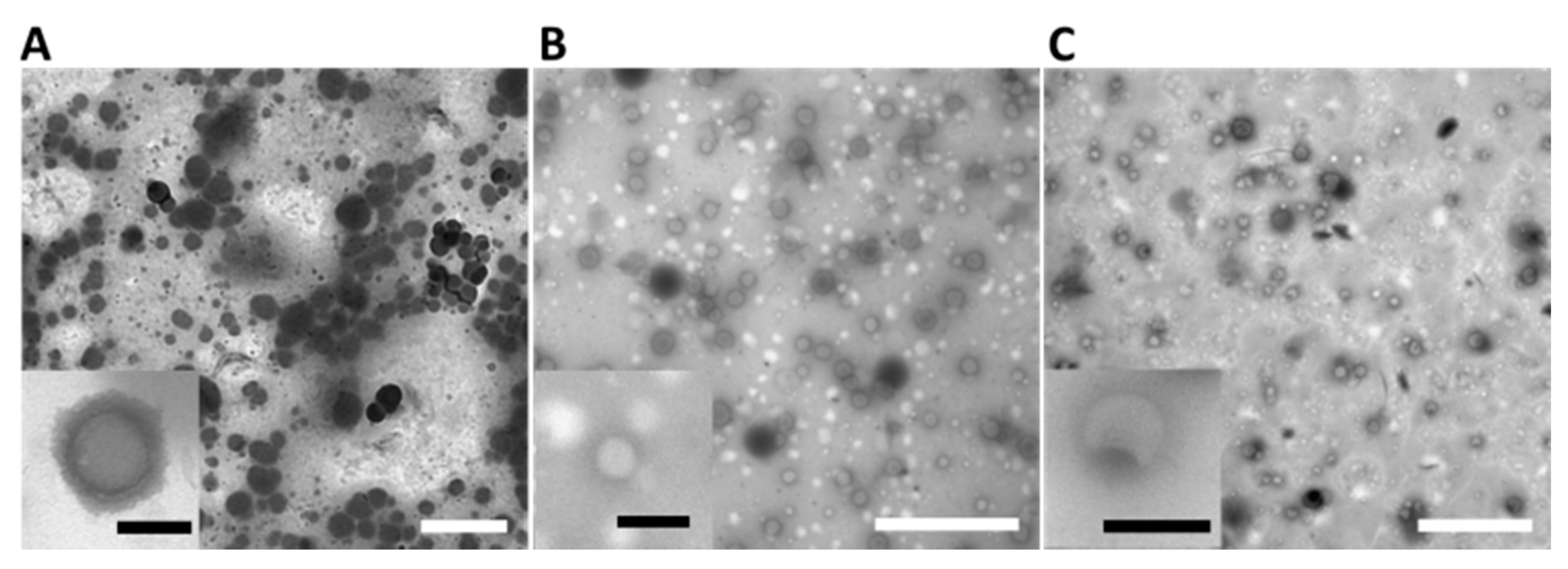
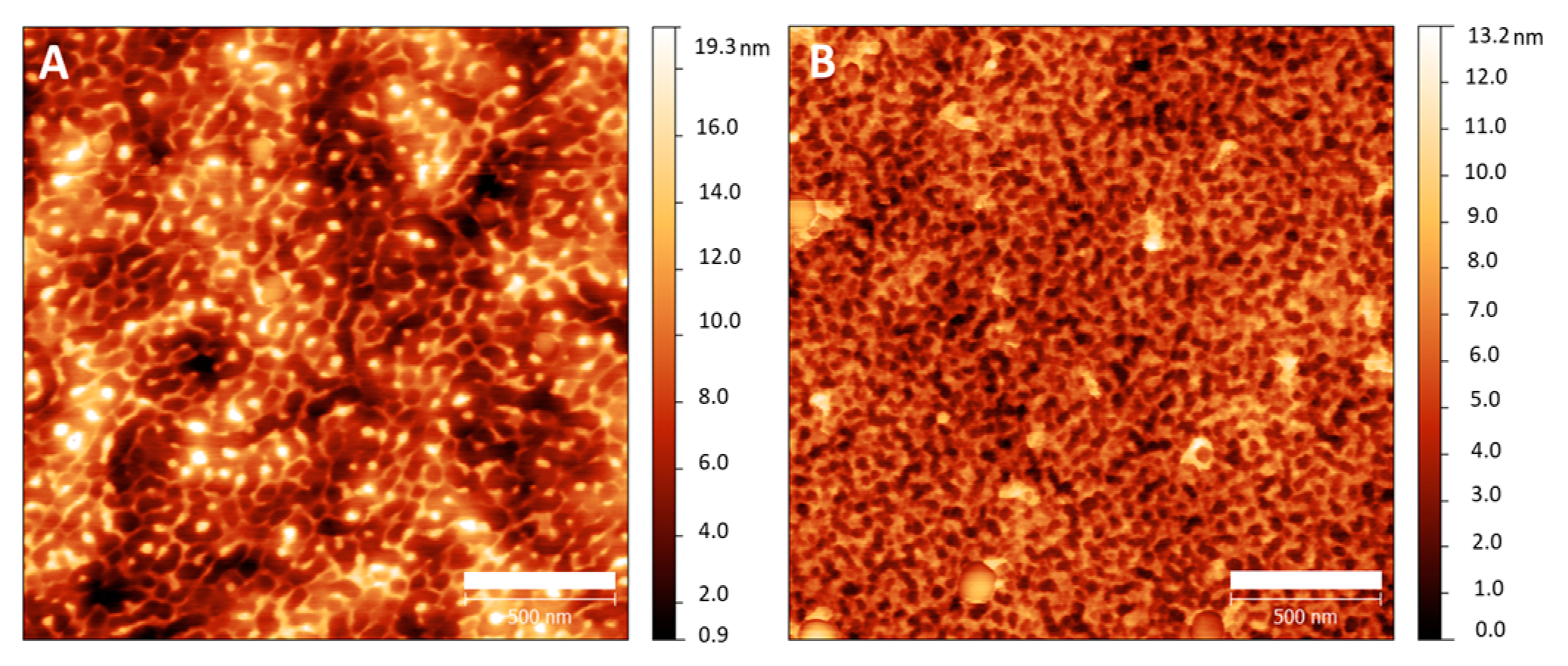
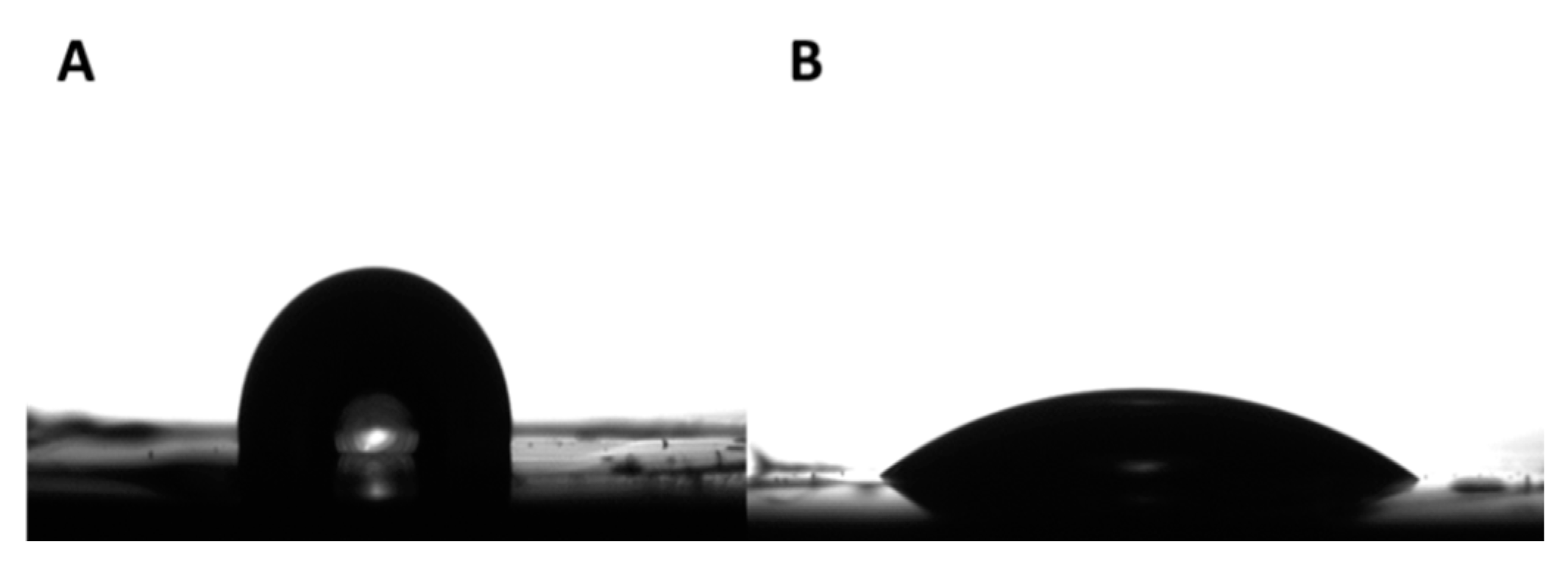
2.5. Thin Films’ Preparation
3. Materials and Methods
3.1. Materials
3.2. Characterization
3.3. Synthesis of POEGMA-b-PMMA-Alk, P(GMA-N3), and Block Copolymer Brush
3.3.1. Synthesis of Propargyl Modified RAFT Agent (CPADB-Alk)
3.3.2. Synthesis of Alkyne Terminated Poly(methyl methacrylate) Macro-Chain Transfer Agent (PMMA-Alk)
3.3.3. Synthesis of Poly(oligo(ethylene glycol) methyl ether methacrylate)-b-Poly(methyl methacrylate) (POEGMA-b-PMMA-Alk) Block Copolymer
3.3.4. Synthesis of Poly(glycidyl methacrylate) (PGMA)
3.3.5. Synthesis of Poly(glycidyl methacrylate-azide) (P(GMA-N3))
3.3.6. Synthesis of PGMA-g-(PMMA-b-POEGMA) Block Copolymer Brush
3.4. Thin Film Preparation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Matyjaszewski, K. Architecturally Complex Polymers with Controlled Heterogeneity. Science 2011, 333, 1104–1105. [Google Scholar] [CrossRef]
- Li, M.; Pester, C.W. Mixed Polymer Brushes for “Smart” Surfaces. Polymers 2020, 12, 1553. [Google Scholar] [CrossRef]
- Peng, S.; Bhushan, B. Smart polymer brushes and their emerging applications. RSC Adv. 2012, 2, 8557–8578. [Google Scholar] [CrossRef]
- Das, S.; Banik, M.; Chen, G.; Sinha, S.; Mukherjee, R. Polyelectrolyte brushes: Theory, modelling, synthesis and applications. Soft Matter 2015, 11, 8550–8583. [Google Scholar] [CrossRef]
- Johnson, J.A.; Lu, Y.Y.; Burts, A.O.; Lim, Y.-H.; Finn, M.G.; Koberstein, J.T.; Turro, N.J.; Tirrell, D.A.; Grubbs, R.H. Core-Clickable PEG-Branch-Azide Bivalent-Bottle-Brush Polymers by ROMP: Grafting-Through and Clicking-To. J. Am. Chem. Soc. 2011, 133, 559–566. [Google Scholar] [CrossRef]
- Djalali, R.; Hugenberg, N.; Fischer, K.; Schmidt, M. Amphipolar core-shell cylindrical brushes. Macromol. Rapid Commun. 1999, 20, 444–449. [Google Scholar] [CrossRef]
- Yamada, K.; Miyazaki, M.; Ohno, K.; Fukuda, T.; Minoda, M. Atom Transfer Radical Polymerization of Poly(vinyl ether) Macromonomers. Macromolecules 1999, 32, 290–293. [Google Scholar] [CrossRef]
- Wintermantel, M.; Gerle, M.; Fischer, K.; Schmidt, M.; Wataoka, I.; Urakawa, H.; Kajiwara, K.; Tsukahara, Y. Molecular Bottlebrushes. Macromolecules 1996, 29, 978–983. [Google Scholar] [CrossRef]
- Ruokolainen, J.; Saariaho, M.; Ikkala, O.; Brinke, G.T.; Thomas, E.L.; Torkkeli, M.; Serimaa, R. Supramolecular Routes to Hierarchical Structures: Comb-Coil Diblock Copolymers Organized with Two Length Scales. Macromolecules 1999, 32, 1152–1158. [Google Scholar] [CrossRef]
- Beers, K.L.; Gaynor, S.G.; Matyjaszewski, K.; Sheiko, S.S.; Möller, M. The Synthesis of Densely Grafted Copolymers by Atom Transfer Radical Polymerization. Macromolecules 1998, 31, 9413–9415. [Google Scholar] [CrossRef]
- Braunecker, W.A.; Matyjaszewski, K. Controlled/living radical polymerization: Features, developments, and perspectives. Prog. Polym. Sci. 2007, 32, 93–146. [Google Scholar] [CrossRef]
- Moad, G.; Rizzardo, E.; Thang, S.H. Living Radical Polymerization by the RAFT Process. Aust. J. Chem. 2005, 58, 379–410. [Google Scholar] [CrossRef]
- Matyjaszewski, K.; Xia, J. Atom Transfer Radical Polymerization. Chem. Rev. 2001, 101, 2921–2990. [Google Scholar] [CrossRef] [PubMed]
- Hawker, C.J.; Bosman, A.W.; Harth, E. New Polymer Synthesis by Nitroxide Mediated Living Radical Polymerizations. Chem. Rev. 2001, 101, 3661–3688. [Google Scholar] [CrossRef] [PubMed]
- Chiefari, J.; Chong, Y.K.B.; Ercole, F.; Krstina, J.; Jeffery, J.; Le, T.P.T.; Mayadunne, R.T.A.; Meijs, G.F.; Moad, C.L.; Moad, G.; et al. Living Free-Radical Polymerization by Reversible Addition−Fragmentation Chain Transfer: The RAFT Process. Macromolecules 1998, 31, 5559–5562. [Google Scholar] [CrossRef]
- Delduc, P.; Tailhan, C.; Zard, S.Z. A convenient source of alkyl and acyl radicals. J. Chem. Soc. Chem. Commun. 1988, 308–310. [Google Scholar] [CrossRef]
- Perrier, S. 50th Anniversary Perspective: RAFT Polymerization—A User Guide. Macromolecules 2017, 50, 7433–7447. [Google Scholar] [CrossRef]
- Hawker, C.J.; Wooley, K.L. The Convergence of Synthetic Organic and Polymer Chemistries. Science 2005, 309, 1200–1205. [Google Scholar] [CrossRef]
- Zhang, X.; Lian, X.; Liu, L.; Zhang, J.; Zhao, H. Synthesis of Comb Copolymers with Pendant Chromophore Groups Based on RAFT Polymerization and Click Chemistry and Formation of Electron Donor−Acceptor Supramolecules. Macromolecules 2008, 41, 7863–7869. [Google Scholar] [CrossRef]
- Zhao, C.; Wu, D.; Lian, X.; Zhang, Y.; Song, X.; Zhao, H. Amphiphilic Asymmetric Comb Copolymer with Pendant Pyrene Groups and PNIPAM Side Chains: Synthesis, Photophysical Properties, and Self-Assembly. J. Phys. Chem. B 2010, 114, 6300–6308. [Google Scholar] [CrossRef]
- Lian, X.; Wu, D.; Song, X.; Zhao, H. Synthesis and Self-Assembly of Amphiphilic Asymmetric Macromolecular Brushes. Macromolecules 2010, 43, 7434–7445. [Google Scholar] [CrossRef]
- Quémener, D.; Davis, T.P.; Barner-Kowollik, C.; Stenzel, M.H. RAFT and click chemistry: A versatile approach to well-defined block copolymers. Chem. Commun. 2006, 5051–5053. [Google Scholar] [CrossRef]
- Ranjan, R.; Brittain, W.J. Synthesis of High Density Polymer Brushes on Nanoparticles by Combined RAFT Polymerization and Click Chemistry. Macromol. Rapid Commun. 2008, 29, 1104–1110. [Google Scholar] [CrossRef]
- Quémener, D.; Hellaye, M.L.; Bissett, C.; Davis, T.P.; Barner-Kowollik, C.; Stenzel, M.H. Graft block copolymers of propargyl methacrylate and vinyl acetate via a combination of RAFT/MADIX and click chemistry: Reaction analysis. J. Polym. Sci. Part Polym. Chem. 2008, 46, 155–173. [Google Scholar] [CrossRef]
- Zhao, B.; Brittain, W.J. Polymer brushes: Surface-immobilized macromolecules. Prog. Polym. Sci. 2000, 25, 677–710. [Google Scholar] [CrossRef]
- Zhao, B.; Brittain, W.J. Synthesis of Tethered Polystyrene-block-Poly(methyl methacrylate) Monolayer on a Silicate Substrate by Sequential Carbocationic Polymerization and Atom Transfer Radical Polymerization. J. Am. Chem. Soc. 1999, 121, 3557–3558. [Google Scholar] [CrossRef]
- Boyes, S.G.; Brittain, W.J.; Weng, X.; Cheng, S.Z.D. Synthesis, Characterization, and Properties of ABA Type Triblock Copolymer Brushes of Styrene and Methyl Acrylate Prepared by Atom Transfer Radical Polymerization. Macromolecules 2002, 35, 4960–4967. [Google Scholar] [CrossRef]
- Verduzco, R.; Li, X.; Pesek, S.L.; Stein, G.E. Structure, function, self-assembly, and applications of bottlebrush copolymers. Chem. Soc. Rev. 2015, 44, 2405–2420. [Google Scholar] [CrossRef]
- Alexander, S.; Cosgrove, T.; de Vos, W.M.; Castle, T.C.; Prescott, S.W. Aggregation Behavior of Polyisoprene–Pluronic Graft Copolymers in Selective Solvents. Langmuir 2014, 30, 5747–5754. [Google Scholar] [CrossRef]
- Neises, B.; Steglich, W. Simple Method for the Esterification of Carboxylic Acids. Angew. Chem. Int. Ed. Engl. 1978, 17, 522–524. [Google Scholar] [CrossRef]
- Zhu, J.; Zhou, D.; Zhu, X.; Chen, G. Reversible addition–fragmentation chain transfer polymerization of glycidyl methacrylate with 2-cyanoprop-2-yl 1-dithionaphthalate as a chain-transfer agent. J. Polym. Sci. Part Polym. Chem. 2004, 42, 2558–2565. [Google Scholar] [CrossRef]
- Tsarevsky, N.V.; Bencherif, S.A.; Matyjaszewski, K. Graft Copolymers by a Combination of ATRP and Two Different Consecutive Click Reactions. Macromolecules 2007, 40, 4439–4445. [Google Scholar] [CrossRef]
- Yang, Q.; Balverde, S.; Dumur, F.; Lalevée, J.; Poly, J. Synergetic effect of the epoxide functional groups in the photocatalyzed atom transfer radical copolymerization of glycidyl methacrylate. Polym. Chem. 2016, 7, 6084–6093. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, X.; Zhu, H.; Wu, K.; Lu, M. Synthesis and self-assembly of well-defined binary graft copolymer and its use in superhydrophobic cotton fabrics preparation. RSC Adv. 2015, 5, 46132–46145. [Google Scholar] [CrossRef]
- Li, M.; Liu, C.; Hong, C.-Y.; Pan, C.-Y. Synthesis of graft copolymer with pendant macrocycles via combination of ATRP and click chemistry. Polymer 2015, 71, 23–30. [Google Scholar] [CrossRef]
- Cao, X.T.; Kim, Y.H.; Park, J.M.; Lim, K.T. One-pot syntheses of dual-responsive core cross-linked polymeric micelles and covalently entrapped drug by click chemistry. Eur. Polym. J. 2016, 78, 264–273. [Google Scholar] [CrossRef]
- Lewis, R.W.; Evans, R.A.; Malic, N.; Saito, K.; Cameron, N.R. Cleavage of macromolecular RAFT chain transfer agents by sodium azide during characterization by aqueous GPC. Polym. Chem. 2017, 8, 3702–3711. [Google Scholar] [CrossRef]
- Li, C.; Ge, Z.; Fang, J.; Liu, S. Synthesis and Self-Assembly of Coil−Rod Double Hydrophilic Diblock Copolymer with Dually Responsive Asymmetric Centipede-Shaped Polymer Brush as the Rod Segment. Macromolecules 2009, 42, 2916–2924. [Google Scholar] [CrossRef]
- Mo, Y.; Liu, G.; Tu, Y.; Lin, S.; Song, J.; Hu, J.; Liu, F. Morphological switching of unimolecular micelles of ternary graft copolymers in different solvents. J. Polym. Sci. Part Polym. Chem. 2017, 55, 1021–1030. [Google Scholar] [CrossRef]
- Pietrasik, J.; Sumerlin, B.S.; Lee, R.Y.; Matyjaszewski, K. Solution Behavior of Temperature-Responsive Molecular Brushes Prepared by ATRP. Macromol. Chem. Phys. 2007, 208, 30–36. [Google Scholar] [CrossRef]
- Nematollahzadeh, A.; Abdekhodaie, M.J.; Shojaei, A. Submicron nanoporous polyacrylamide beads with tunable size for verapamil imprinting. J. Appl. Polym. Sci. 2012, 125, 189–199. [Google Scholar] [CrossRef]
- Arshady, R. Suspension, emulsion, and dispersion polymerization: A methodological survey. Colloid Polym. Sci. 1992, 270, 717–732. [Google Scholar] [CrossRef]



| Sample Code | PMMA-Alky | PGMA | P(GMA-N3) | POEGMA-b-PMMA-Alk | Block Copolymer Brush | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mn (g/mol) | Ð | Mn (g/mol) | Ð | Mn (g/mol) | Ð | Mn (g/mol) | Ð | Mn (g/mol) | Ð | |
| 1: PGMA23-g-(PMMA18-b-POEGMA4) | 1800 | 1.09 | 3300 | 1.12 | 5200 | 1.13 | 3100 | 1.13 | 86,000 | 1.32 |
| 2: PGMA31-g-(PMMA33-b-POEGMA4) | 3300 | 1.15 | 4400 | 1.17 | 6500 | 1.22 | 4600 | 1.20 | 148,000 | 1.27 |
| 3: PGMA10-g-(PMMA33-b-POEGMA4) | 3300 | 1.15 | 1400 | 1.15 | 2150 | 1.19 | 4600 | 1.20 | 47,800 | 1.26 |
| 4: PGMA23-g-(PMMA33-b-POEGMA9) | 3300 | 1.15 | 3300 | 1.12 | 5200 | 1.13 | 6000 | 1.21 | 153,000 | 1.33 |
Sample Availability: Samples of the compounds are not available from the authors. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thankappan, H.; Semsarilar, M.; Li, S.; Chang, Y.; Bouyer, D.; Quemener, D. Synthesis of Block Copolymer Brush by RAFT and Click Chemistry and Its Self-Assembly as a Thin Film. Molecules 2020, 25, 4774. https://doi.org/10.3390/molecules25204774
Thankappan H, Semsarilar M, Li S, Chang Y, Bouyer D, Quemener D. Synthesis of Block Copolymer Brush by RAFT and Click Chemistry and Its Self-Assembly as a Thin Film. Molecules. 2020; 25(20):4774. https://doi.org/10.3390/molecules25204774
Chicago/Turabian StyleThankappan, Hajeeth, Mona Semsarilar, Suming Li, Yung Chang, Denis Bouyer, and Damien Quemener. 2020. "Synthesis of Block Copolymer Brush by RAFT and Click Chemistry and Its Self-Assembly as a Thin Film" Molecules 25, no. 20: 4774. https://doi.org/10.3390/molecules25204774
APA StyleThankappan, H., Semsarilar, M., Li, S., Chang, Y., Bouyer, D., & Quemener, D. (2020). Synthesis of Block Copolymer Brush by RAFT and Click Chemistry and Its Self-Assembly as a Thin Film. Molecules, 25(20), 4774. https://doi.org/10.3390/molecules25204774










