Evaluation of Structurally Different Ionic Liquid-Based Surfactants in a Green Microwave-Assisted Extraction for the Flavonoids Profile Determination of Mangifera sp. and Passiflora sp. Leaves from Canary Islands
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals, Reagents and Samples
2.2. Material, Instrumentation and Equipment
2.3. Procedures
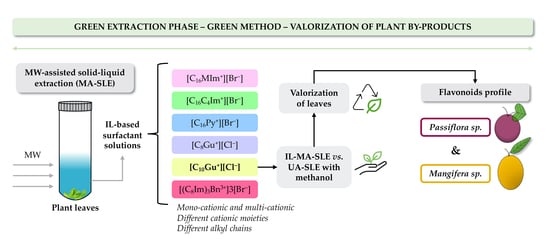
2.3.1. Synthesis of IL-Based Surfactants
Synthesis of Monocationic Imidazolium-Type IL-Based Surfactants
Synthesis of Tricationic Imidazolium IL-Based Surfactant
Synthesis of Guanidinium-Type IL-Based Surfactants
2.3.2. HPLC-PDA Method
2.3.3. IL-MA-SLE Method Using Ionic Liquid-Based Surfactants
2.3.4. UA-SLE Method Using Methanol
2.3.5. IL-MA-SLE Optimization using Experimental Designs
3. Results and Discussion
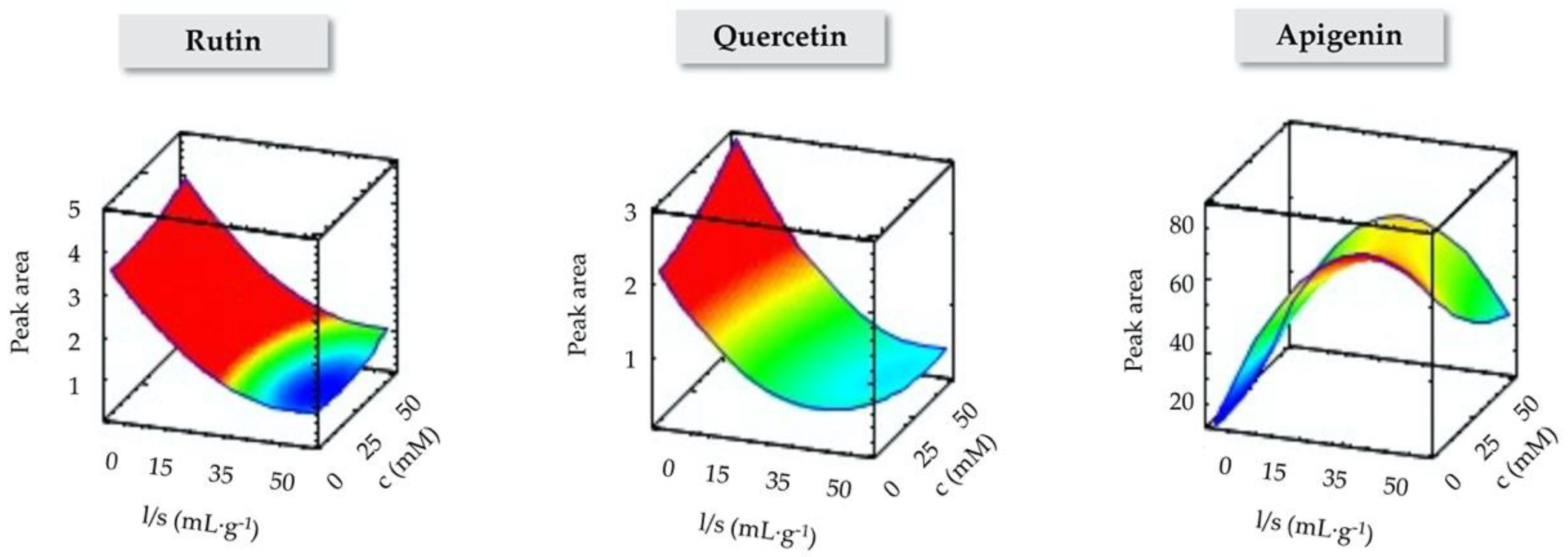
3.1. Optimization of IL-MA-SLE Method by RSM
3.2. Analytical Performance of the IL-MA-SLE-HPLC-PDA Method
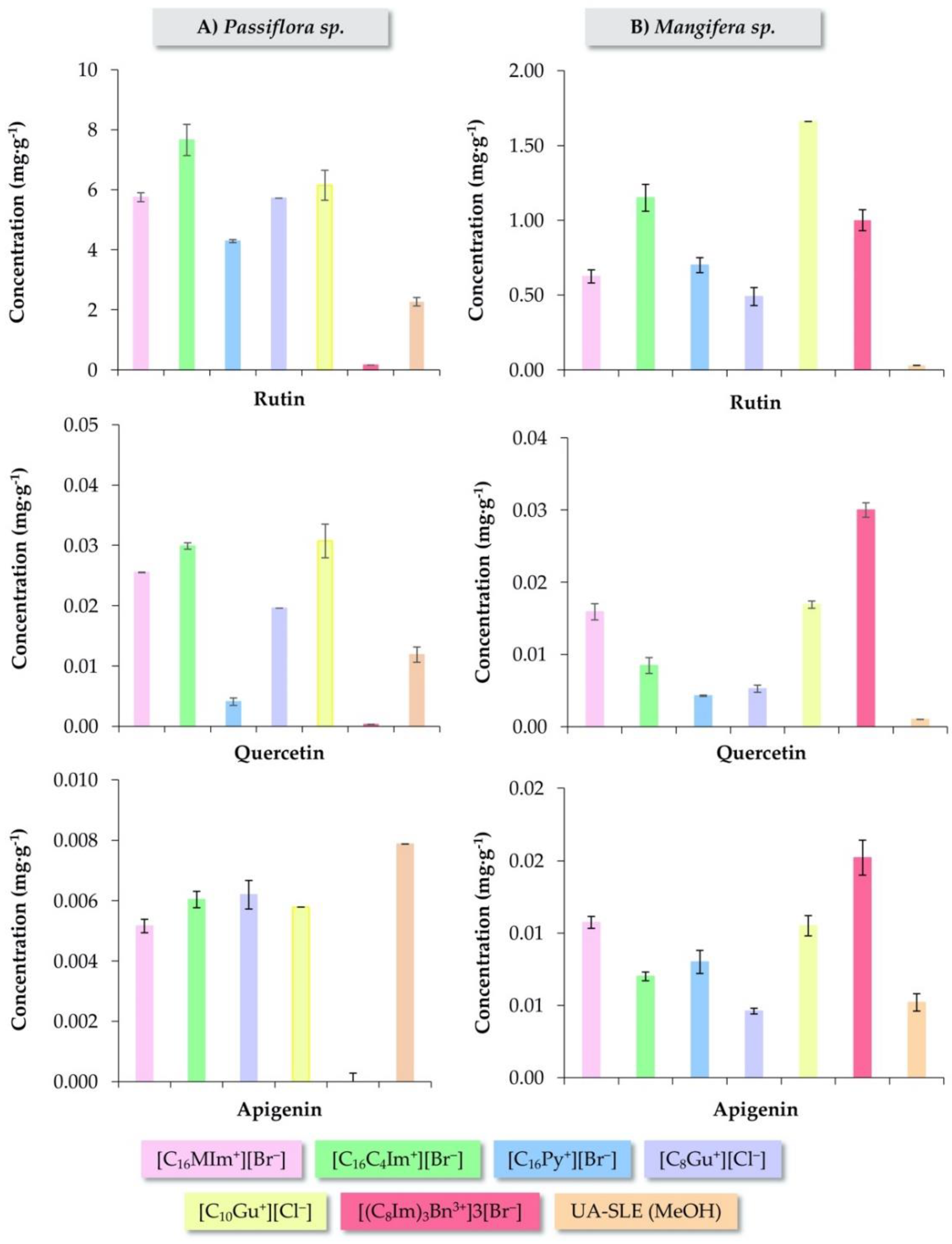
3.3. Evaluation of Different IL-Based Surfactants in the IL-MA-SLE-HPLC-PDA Method
3.4. Analysis of Plant Samples under Optimum IL-MA-SLE-HPLC-PDA Conditions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Armenta, S.; Garrigues, S.; De la Guardia, M. Green analytical chemistry. TrAC 2008, 27, 497–511. [Google Scholar] [CrossRef]
- Pacheco-Fernández, I.; Pino, V. Green solvents in analytical chemistry. Curr. Opin. Green Sustain. Chem. 2019, 18, 42–50. [Google Scholar] [CrossRef]
- Ullah, H.; Wilfred, C.D.; Shaharun, M.S. Ionic liquid-based extraction and separation trends of bioactive compounds from plant biomass. Sep. Sci. Technol. 2019, 54, 559–579. [Google Scholar] [CrossRef]
- Passos, H.; Freire, M.G.; Coutinho, J.A. Ionic liquid solutions as extractive solvents for value-added compounds from biomass. Green Chem. 2014, 16, 4786–4815. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Chen, Z.; Han, F.; Kang, X.; Gu, H.; Yang, L. Microwave-assisted method for simultaneous hydrolysis and extraction in obtaining ellagic acid, gallic acid and essential oil from Eucalyptus globulus leaves using Brönsted acidic ionic liquid [HO3S (CH2) 4mim] HSO4. Ind. Crop. Prod. 2016, 81, 152–161. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Kong, J.; Nie, C.; Lin, X. Application of ionic liquids in the microwave-assisted extraction of quercetin from Chinese herbal medicine. Anal. Methods 2012, 4, 1012–1018. [Google Scholar] [CrossRef]
- Zhao, C.; Lu, Z.; Li, C.; He, X.; Li, Z.; Shi, K.; Yang, L.; Fu, Y.; Zu, Y. Optimization of ionic liquid based simultaneous ultrasonic-and microwave-assisted extraction of rutin and quercetin from leaves of velvetleaf (Abutilon theophrasti) by response surface methodology. Sci. World J. 2014, 2014. [Google Scholar] [CrossRef]
- Pacheco-Fernández, I.; González-Hernández, P.; Pino, V.; Ayala, J.H.; Afonso, A.M. Ionic Liquid-Based Surfactants: A Step forward. In Ionic Liquid Devices; The Royal Society of Chemistry: Croydon, UK, 2017; pp. 53–78. [Google Scholar]
- Villacís-Chiriboga, J.; Elst, K.; Van Camp, J.; Vera, E.; Ruales, J. Valorization of byproducts from tropical fruits: Extraction methodologies, applications, environmental, and economic assessment: A review (Part 1: General overview of the byproducts, traditional biorefinery practices, and possible applications). Compr. Rev. Food Sci. Food Saf. 2020, 19, 405–447. [Google Scholar] [CrossRef]
- Altınok, E.; Palabiyik, I.; Gunes, R.; Toker, O.S.; Konar, N.; Kurultay, S. Valorisation of grape by-products as a bulking agent in soft candies: Effect of particle size. LWT Food Sci. Technol. 2020, 118, 108776. [Google Scholar] [CrossRef]
- Jangra, A.; Pawar, B. Quantification of Flavonoids from different Parts of Grapefruit (Citrus x Paradisi) from different Extraction Methods. JASFT 2019, 6, 75–78. [Google Scholar]
- Pimentel-Moral, S.; de la Luz Cádiz-Gurrea, M.; Rodríguez-Pérez, C.; Segura-Carretero, A. Recent advances in extraction technologies of phytochemicals applied for the revaluation of agri-food by-products. In Functional and Preservative Properties of Phytochemicals; Elsevier: Amsterdam, The Netherlands, 2020; pp. 209–239. [Google Scholar]
- Hanganu, D.; Olah, N.K.; Pop, C.E.; Vlase, L.; Oniga, I.; Ciocarlan, N.; Matei, A.; Puscas, C.; Silaghi-Dumitrescu, R.; Benedec, D. Evaluation of Polyphenolic Profile and Antioxidant Activity for Some Salvisa Species. Farmacia 2019, 67, 801–805. [Google Scholar] [CrossRef]
- Fonseca, L.R.D.; Rodrigues, R.D.A.; Ramos, A.D.S.; da Cruz, J.D.; Ferreira, J.L.P.; Silva, J.R.D.A.; Amaral, A.C.F. Herbal Medicinal Products from Passiflora for Anxiety: An Unexploited Potential. Sci. World J. 2020, 2020, 6598434. [Google Scholar] [CrossRef] [PubMed]
- Sakalem, M.E.; Negri, G.; Tabach, R. Chemical composition of hydroethanolic extracts from five species of the Passiflora genus. Rev. Bras. Farmacogn. 2012, 22, 1219–1232. [Google Scholar] [CrossRef]
- Abourashed, E.A.; Vanderplank, J.R.; Khan, I.A. High-speed extraction and HPLC fingerprinting of medicinal plants—I. Application to Passiflora flavonoids. Pharm. Biol. 2002, 40, 81–91. [Google Scholar] [CrossRef]
- Umamahesh, K.; Sivudu, S.N.; Reddy, O.V.S. Evaluation of antioxidant activity, total phenolics and total flavonoids in peels of five cultivars of mango (Mangifera indica) fruit. J. Med. Plants Stud. 2016, 4, 200–203. [Google Scholar]
- Kanwal, Q.; Hussain, I.; Siddiqui, H.L.; Javaid, A. Flavonoids from mango leaves with antibacterial activity. J. Serb. Chem. Soc. 2009, 74, 1389–1399. [Google Scholar] [CrossRef]
- Seal, T. Quantitative HPLC analysis of phenolic acids, flavonoids and ascorbic acid in four different solvent extracts of two wild edible leaves, Sonchus arvensis and Oenanthe linearis of North-Eastern region in India. J. Appl. Pharm. Sci. 2016, 6, 157–166. [Google Scholar] [CrossRef]
- Mastellone, G.; Pacheco-Fernández, I.; Rubiolo, P.; Pino, V.; Cagliero, C. Sustainable Micro-Scale Extraction of Bioactive Phenolic Compounds from Vitis vinifera Leaves with Ionic Liquid-Based Surfactants. Molecules 2020, 25, 3072. [Google Scholar] [CrossRef]
- Pacheco-Fernández, I.; Pino, V.; Lorenzo-Morales, J.; Ayala, J.H.; Afonso, A.M. Salt-induced ionic liquid-based microextraction using a low cytotoxic guanidinium ionic liquid and liquid chromatography with fluorescence detection to determine monohydroxylated polycyclic aromatic hydrocarbons in urine. Anal. Bioanal. Chem. 2018, 410, 4701–4713. [Google Scholar] [CrossRef] [PubMed]
- Baltazar, Q.Q.; Chandawalla, J.; Sawyer, K.; Anderson, J.L. Interfacial and micellar properties of imidazolium-based monocationic and dicationic ionic liquids. Colloids Surf. A 2007, 302, 150–156. [Google Scholar] [CrossRef]
- Nacham, O.; Martín-Pérez, A.; Steyer, D.J.; Trujillo-Rodríguez, M.J.; Anderson, J.L.; Pino, V.; Afonso, A.M. Interfacial and aggregation behavior of dicationic and tricationic ionic liquid-based surfactants in aqueous solution. Colloids Surf. A 2015, 469, 224–234. [Google Scholar] [CrossRef]
- El Hankari, S.; Hesemann, P. Guanidinium vs. Ammonium Surfactants in Soft-Templating Approaches: Nanostructured Silica and Zwitterionic i-Silica from Complementary Precursor–Surfactant Ion Pairs. Eur. J. Inorg. Chem. 2012, 2012, 5288–5298. [Google Scholar] [CrossRef]
- Gomes, S.V.; Portugal, L.A.; dos Anjos, J.P.; de Jesus, O.N.; de Oliveira, E.J.; David, J.P.; David, J.M. Accelerated solvent extraction of phenolic compounds exploiting a Box-Behnken design and quantification of five flavonoids by HPLC-DAD in Passiflora species. Microchem. J. 2017, 132, 28–35. [Google Scholar] [CrossRef]
- Li, C.; Lu, Z.; Zhao, C.; Yang, L.; Fu, Y.; Shi, K.; He, X.; Li, Z.; Zu, Y. Ionic-liquid-based ultrasound/microwave-assisted extraction of 2, 4-dihydroxy-7-methoxy-1, 4-benzoxazin-3-one and 6-methoxy-benzoxazolin-2-one from maize (Zea mays L.) seedlings. J. Sep. Sci. 2015, 38, 291–300. [Google Scholar] [CrossRef]
- Wei, Z.; Zu, Y.; Fu, Y.; Wang, W.; Luo, M.; Zhao, C.; Pan, Y. Ionic liquids-based microwave-assisted extraction of active components from pigeon pea leaves for quantitative analysis. Sep. Pur. Technol. 2013, 102, 75–81. [Google Scholar] [CrossRef]
- Xu, W.; Chu, K.; Li, H.; Zhang, Y.; Zheng, H.; Chen, R.; Chen, L. Ionic liquid-based microwave-assisted extraction of flavonoids from Bauhinia championii (Benth.) Benth. Molecules 2012, 17, 14323–14335. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Wang, Y.; Kong, J.; Nie, C.; Yuan, Y. Ionic liquid-based microwave-assisted extraction of rutin from Chinese medicinal plants. Talanta 2010, 83, 582–590. [Google Scholar] [CrossRef]
- Li, C.; Zhang, J.; Zhao, C.; Yang, L.; Zhao, W.; Jiang, H.; Ren, X.; Su, W.; Li, Y.; Guan, J. Separation of the main flavonoids and essential oil from seabuckthorn leaves by ultrasonic/microwave-assisted simultaneous distillation extraction. R. Soc. Open Sci. 2018, 5, 180133. [Google Scholar] [CrossRef]
- Ferreira, S.C.; Bruns, R.; Ferreira, H.; Matos, G.; David, J.; Brandao, G.; da Silva, E.P.; Portugal, L.; Dos Reis, P.; Souza, A. Box-Behnken design: An alternative for the optimization of analytical methods. Anal. Chim. Acta 2007, 597, 179–186. [Google Scholar] [CrossRef]
- Vanyur, R.; Biczok, L.; Miskolczy, Z. Micelle formation of 1-alkyl-3-methylimidazolium bromide ionic liquids in aqueous solution. Colloids Surf. A 2007, 299, 256–261. [Google Scholar] [CrossRef]
- Asakawa, T.; Kitano, H.; Ohta, A.; Miyagishi, S. Convenient estimation for counterion dissociation of cationic micelles using chloride-sensitive fluorescence probe. J. Colloid Interface Sci. 2001, 242, 284–287. [Google Scholar] [CrossRef]
- Pacheco-Fernández, I.; Pino, V.; Ayala, J.H.; Afonso, A.M. Guanidinium ionic liquid-based surfactants as low cytotoxic extractants: Analytical performance in an in-situ dispersive liquid–liquid microextraction method for determining personal care products. J. Chromatogr. A 2018, 1559, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Qiao, L.; Gu, H.; Yang, F.; Yang, L. Development of Brönsted acidic ionic liquid based microwave assisted method for simultaneous extraction of pectin and naringin from pomelo peels. Sep. Pur. Technol. 2017, 172, 326–337. [Google Scholar] [CrossRef]
- Gu, H.; Chen, F.; Zhang, Q.; Zang, J. Application of ionic liquids in vacuum microwave-assisted extraction followed by macroporous resin isolation of three flavonoids rutin, hyperoside and hesperidin from Sorbus tianschanica leaves. J. Chromatogr. B 2016, 1014, 45–55. [Google Scholar] [CrossRef]
- Mena, I.F.; Diaz, E.; Palomar, J.; Rodriguez, J.J.; Mohedano, A.F. Cation and anion effect on the biodegradability and toxicity of imidazolium- and choline-based ionic liquids. Chemosphere 2020, 240, 124947. [Google Scholar] [CrossRef] [PubMed]
- Lou, Z.; Wang, H.; Zhu, S.; Chen, S.; Zhang, M.; Wang, Z. Ionic liquids based simultaneous ultrasonic and microwave assisted extraction of phenolic compounds from burdock leaves. Anal. Chim. Acta 2012, 716, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, H.; Zu, Y.-G.; Zhao, C.; Zhang, L.; Chen, X.; Zhang, Z. Ultrasound-assisted extraction of the three terpenoid indole alkaloids vindoline, catharanthine and vinblastine from Catharanthus roseus using ionic liquid aqueous solutions. Chem. Eng. J. 2011, 172, 705–712. [Google Scholar] [CrossRef]
- Remsing, R.C.; Swatloski, R.P.; Rogers, R.D.; Moyna, G. Mechanism of cellulose dissolution in the ionic liquid 1-n-butyl-3-methylimidazolium chloride: A 13 C and 35/37 Cl NMR relaxation study on model systems. Chem. Commun. 2006, 1271–1273. [Google Scholar] [CrossRef]
- Liang, H.; Wang, W.; Xu, J.; Zhang, Q.; Shen, Z.; Zeng, Z.; Li, Q. Optimization of ionic liquid-based microwave-assisted extraction technique for curcuminoids from Curcuma longa L. Food Bioprod. Process. 2017, 104, 57–65. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, S.H.; Chen, J.; Zhang, L.W. Application of ionic liquid-based microwave-assisted extraction of flavonoids from Scutellaria baicalensis Georgi. J. Chromatogr. B 2015, 1002, 411–417. [Google Scholar] [CrossRef]
- Huddleston, J.G.; Visser, A.E.; Reichert, W.M.; Willauer, H.D.; Broker, G.A.; Rogers, R.D. Characterization and comparison of hydrophilic and hydrophobic room temperature ionic liquids incorporating the imidazolium cation. Green Chem. 2001, 3, 156–164. [Google Scholar] [CrossRef]
- Acquadro, S.; Appleton, S.; Marengo, A.; Bicchi, C.; Sgorbini, B.; Mandrone, M.; Gai, F.; Peiretti, P.G.; Cagliero, C.; Rubiolo, P. Grapevine Green Pruning Residues as a Promising and Sustainable Source of Bioactive Phenolic Compounds. Molecules 2020, 25, 464. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.-H.; Yusoff, R.; Ngoh, G.-C.; Kung, F.W.-L. Microwave-assisted extractions of active ingredients from plants. J. Chromatogr. A 2011, 1218, 6213–6225. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Xu, C.; Li, J.; Zhang, L.; Yang, L.; Zhou, Z.; Zhu, Y.; Zhao, D. Ionic liquid-based microwave-assisted extraction of verbascoside from Rehmannia root. Ind. Crop. Prod. 2018, 124, 59–65. [Google Scholar] [CrossRef]
- Zorzetto, C.; Sánchez-Mateo, C.C.; Rabanal, R.M.; Lupidi, G.; Petrelli, D.; Vitali, L.A.; Bramucci, M.; Quassinti, L.; Caprioli, G.; Papa, F. Phytochemical analysis and in vitro biological activity of three Hypericum species from the Canary Islands (Hypericum reflexum, Hypericum canariense and Hypericum grandifolium). Fitoterapia 2015, 100, 95–109. [Google Scholar] [CrossRef] [PubMed]
- Papoulias, E.; Siomos, A.S.; Koukounaras, A.; Gerasopoulos, D.; Kazakis, E. Effects of genetic, pre-and post-harvest factors on phenolic content and antioxidant capacity of white asparagus spears. Int. J. Mol. Sci. 2009, 10, 5370–5380. [Google Scholar] [CrossRef] [PubMed]
- Gimeno, E.; Castellote, A.; Lamuela-Raventós, R.; De la Torre, M.; López-Sabater, M. The effects of harvest and extraction methods on the antioxidant content (phenolics, α-tocopherol, and β-carotene) in virgin olive oil. Food Chem. 2002, 78, 207–211. [Google Scholar] [CrossRef]
- Bilgin, M.; Şahin, S. Effects of geographical origin and extraction methods on total phenolic yield of olive tree (Olea europaea) leaves. J. Taiwan Inst. Chem. Eng. 2013, 44, 8–12. [Google Scholar] [CrossRef]
- Iqbal, S.; Bhanger, M. Effect of season and production location on antioxidant activity of Moringa oleifera leaves grown in Pakistan. J. Food Compos. Anal. 2006, 19, 544–551. [Google Scholar] [CrossRef]
- Orphanides, A.; Goulas, V.; Gekas, V. Effect of drying method on the phenolic content and antioxidant capacity of spearmint. Czech J. Food Sci. 2013, 31, 509–513. [Google Scholar] [CrossRef]
- Hossain, M.; Barry-Ryan, C.; Martin-Diana, A.B.; Brunton, N. Effect of drying method on the antioxidant capacity of six Lamiaceae herbs. Food Chem. 2010, 123, 85–91. [Google Scholar] [CrossRef]
- El-Hawary, S.S.; Ashour, R.M.S.; El-Gayed, S.H.; Gad, H.A.; Jaleel, G.A.A.; El Gedaily, R.A. Genetic, chemical, and biological diversity in Mangifera indica L. cultivars. Pharmacogn. Res 2020, 12, 186–193. [Google Scholar]
| IL Full Name [IL Abbreviation] | Structure | State | Molecular Weight (g∙mol−1) | CMC a (mM)/Ref. |
|---|---|---|---|---|
| 1-hexadecyl−3-methyl imidazolium bromide | ||||
| [C16MIm+][Br–] | 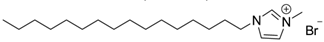 | Solid | 386.9 | 0.61/[32] |
| 1-hexadecyl-3-butyl imidazolium bromide | ||||
| [C16C4Im+][Br–] |  | Solid | 428.3 | 0.10/[22] |
| Hexadecyl pyridinium bromide | ||||
| [C16Py+][Br–] | 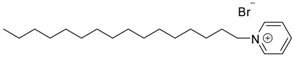 | Solid | 384.4 | 0.72/[33] |
| Octyl guanidinium chloride | ||||
| [C8Gu+][Cl–] |  | Liquid | 206.5 | 44.6/[34] |
| Decyl guanidinium chloride | ||||
| [C10Gu+][Cl–] | 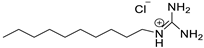 | Liquid | 234.5 | 18.6/[21] |
| 3,3′,3″-octyl-1,1′,1″-(1,3,5)tris(methylene) benzene imidazolium bromide | ||||
| [(C8Im)3Bn3+]3[Br-] | 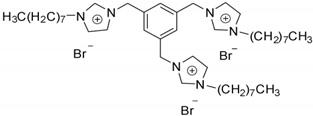 | Solid | 849.8 | 2.30/[23] |
| Plant | Rutin (RSD *) | Quercetin (RSD *) | Apigenin (RSD *) |
|---|---|---|---|
| Passiflora sp. | |||
| PS032 | 6.15 (8.0%) | 0.031 (9.0%) | 0.006 (5.0%) |
| 17PS009 | 4.15 (8.0%) | 0.046 (7.0%) | 0.006 (0.5%) |
| PS003 | 4.51 (5.0%) | 0.021 (9.0%) | 0.010 (8.0%) |
| 17PS008 | 2.59 (7.0%) | 0.090 (2.0%) | 0.017 (2.0%) |
| 18PS003 | 2.35 (3.0%) | 0.036 (6.0%) | 0.008 (6.5%) |
| Mangifera sp. | |||
| Sweet Tart | 0.163 (2.0%) | 0.031 (1.5%) | 0.015 (8.5%) |
| Mun | 0.239 (0.3%) | 0.044 (3.0%) | 0.011 (9.0%) |
| Gomera 1 | 0.082 (2.0%) | 0.006 (1.7%) | 0.007 (1.2%) |
| Gomera 3 | 0.082 (1.2%) | 0.011 (2.0%) | 0.008 (2.0%) |
Sample Availability: Samples of the compounds are not available. Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moučková, K.; Pacheco-Fernández, I.; Ayala, J.H.; Bajerová, P.; Pino, V. Evaluation of Structurally Different Ionic Liquid-Based Surfactants in a Green Microwave-Assisted Extraction for the Flavonoids Profile Determination of Mangifera sp. and Passiflora sp. Leaves from Canary Islands. Molecules 2020, 25, 4734. https://doi.org/10.3390/molecules25204734
Moučková K, Pacheco-Fernández I, Ayala JH, Bajerová P, Pino V. Evaluation of Structurally Different Ionic Liquid-Based Surfactants in a Green Microwave-Assisted Extraction for the Flavonoids Profile Determination of Mangifera sp. and Passiflora sp. Leaves from Canary Islands. Molecules. 2020; 25(20):4734. https://doi.org/10.3390/molecules25204734
Chicago/Turabian StyleMoučková, Kristýna, Idaira Pacheco-Fernández, Juan H. Ayala, Petra Bajerová, and Verónica Pino. 2020. "Evaluation of Structurally Different Ionic Liquid-Based Surfactants in a Green Microwave-Assisted Extraction for the Flavonoids Profile Determination of Mangifera sp. and Passiflora sp. Leaves from Canary Islands" Molecules 25, no. 20: 4734. https://doi.org/10.3390/molecules25204734
APA StyleMoučková, K., Pacheco-Fernández, I., Ayala, J. H., Bajerová, P., & Pino, V. (2020). Evaluation of Structurally Different Ionic Liquid-Based Surfactants in a Green Microwave-Assisted Extraction for the Flavonoids Profile Determination of Mangifera sp. and Passiflora sp. Leaves from Canary Islands. Molecules, 25(20), 4734. https://doi.org/10.3390/molecules25204734