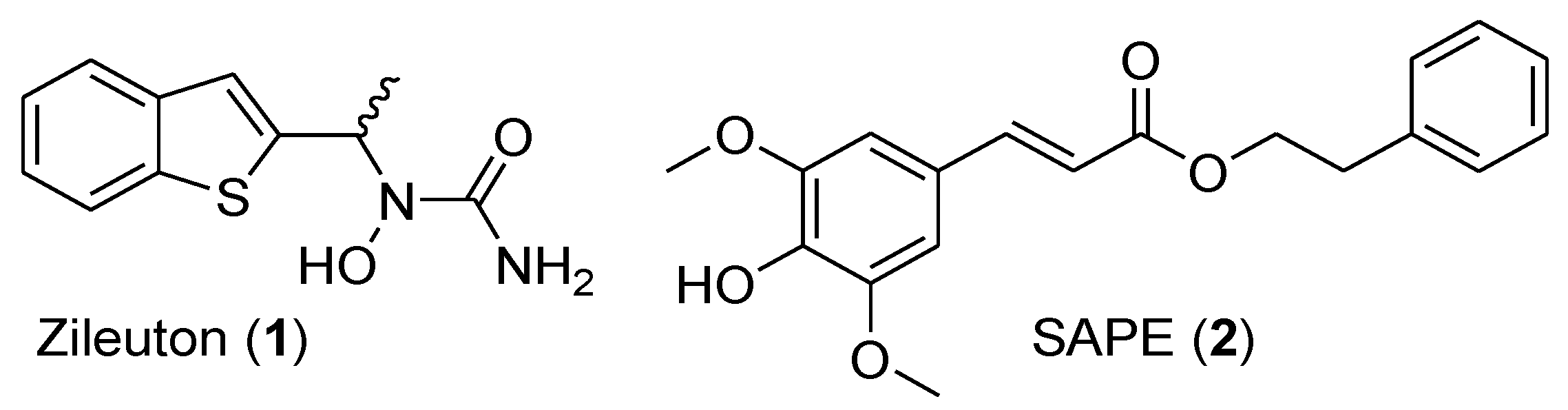
New Zileuton-Hydroxycinnamic Acid Hybrids: Synthesis and Structure-Activity Relationship towards 5-Lipoxygenase Inhibition
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis
2.2. Biological Evaluation
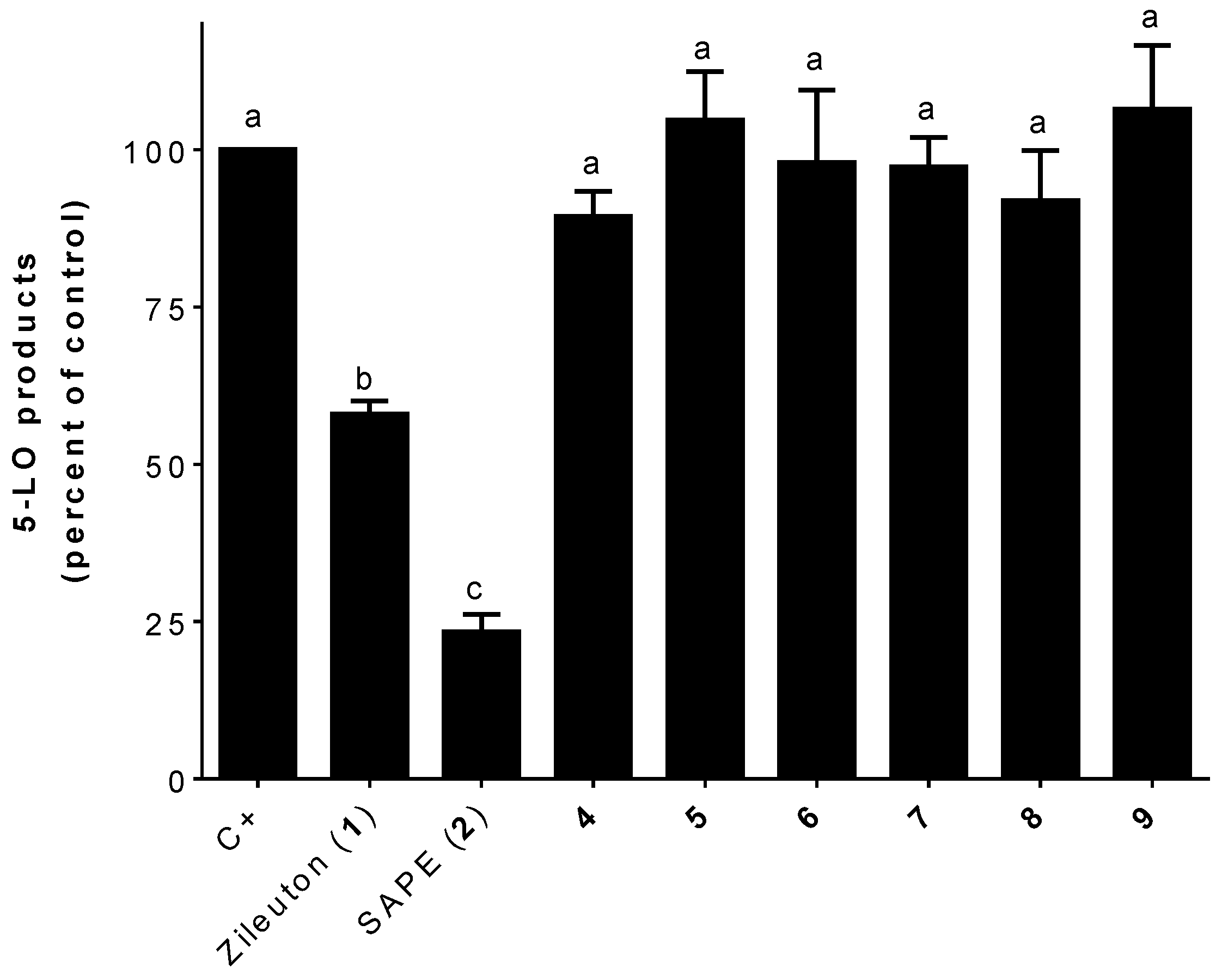
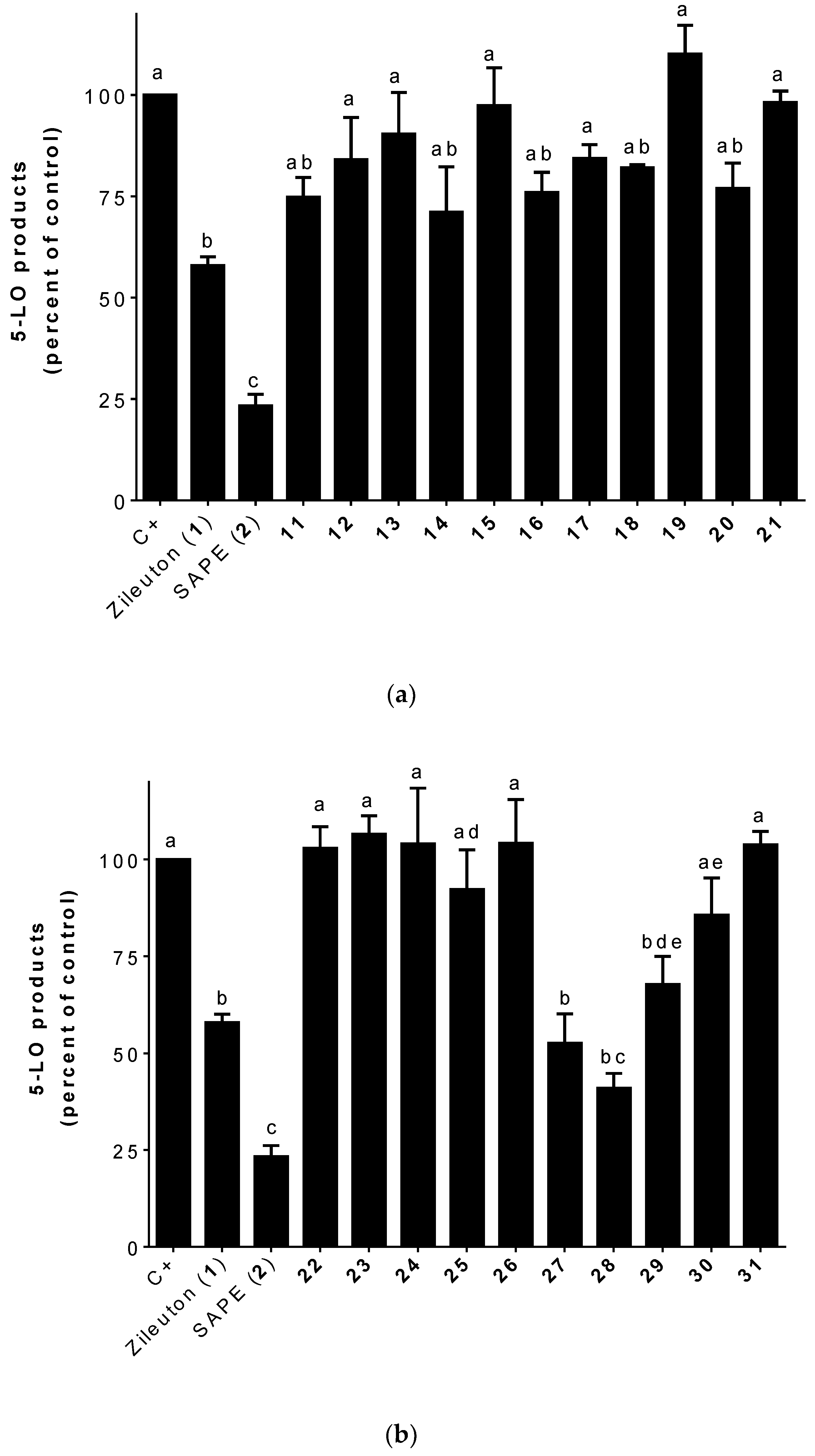
2.2.1. 5-LO Product Biosynthesis Assays
2.2.2. Free Radical Scavenging Activity Assay
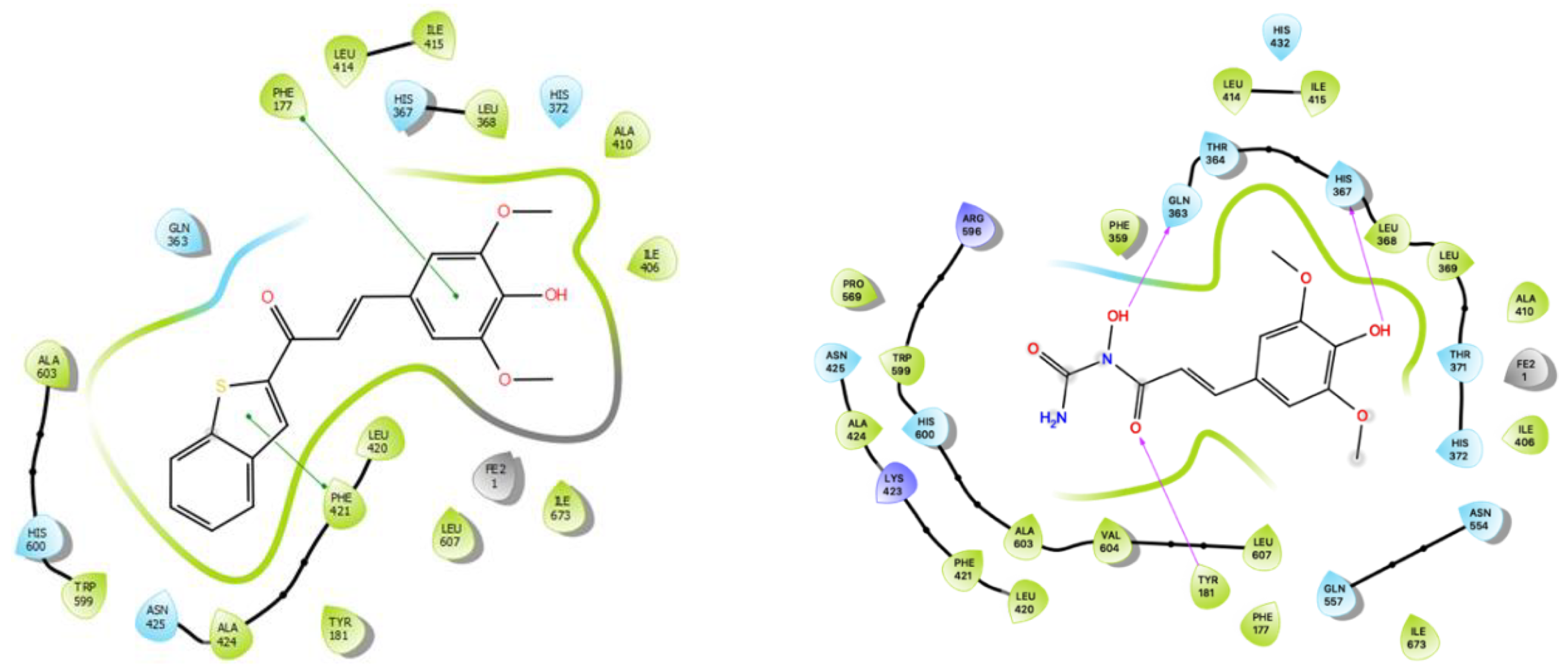
2.3. Molecular Docking
2.4. In Silico Physicochemical Properties and Druglikeness Evaluation
3. Materials and Methods
3.1. General Synthetic Experimental Procedures
3.1.1. General Procedure for the Esterification of Thianaphtene-2-carboxylic Acid
(2,4,6-Cycloheptatrien-1-yl)methyl 1-benzothiophene-2-carboxylate (4)
Phenethyl 1-benzothiophene-2-carboxylate (5)
3.1.2. General Procedure for the Aldol Condensation of 2-acetylbenzothiophene in Presence of Sodium Ethoxide
(E)-1-(1-Benzothiophen-2-yl)-3-phenyl-2-propen-1-one (11)
3.1.3. General Procedure for the Base-Catalyzed Aldol Condensation of 2-acetylbenzothiophene
(E)-1-(1-Benzothiophen-2-yl)-3-(p-methoxyphenyl)-2-propen-1-one (13)
(E)-1-(1-Benzothiophen-2-yl)-3-(o-hydroxyphenyl)-2-propen-1-one (14)
3.1.4. General Procedure of Hydroxyurea Analogs by Peptide Coupling
1-Hydroxy-1-((E)-3-phenylacryloyl)urea (33)
1-Hydroxy-1-((E)-3-(3-hydroxy-4-methoxyphenyl)acryloyl)urea (34)
3.1.5. General Procedure of Hydroxyurea Analogs Synthesis with Acyl Chlorides
1-Hydroxy-1-(benzoyl)urea (38)
1-Hydroxy-1-(2-phenylacetyl)urea (39)
3.2. 5-LO Product Biosynthesis Assays in HEK293 Cells
3.3. 5-LO Products Biosynthesis Assays in Human PMNL Cells
3.4. Free Radical Scavenging Activity Assay
3.5. Molecular Docking
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Fullerton, J.N.; Gilroy, D.W. Resolution of inflammation: A new therapeutic frontier. Nat. Rev. Drug Discov. 2016, 15, 551–567. [Google Scholar] [CrossRef] [PubMed]
- Samuelsson, B. Leukotrienes: Mediators of immediate hypersensitivity reactions and inflammation. Science 1983, 220, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Shimizu, T. Leukotriene Receptors. Chem. Rev. 2011, 111, 6231–6298. [Google Scholar] [CrossRef] [PubMed]
- Rådmark, O.; Werz, O.; Steinhilber, D.; Samuelsson, B. 5-Lipoxygenase: Regulation of expression and enzyme activity. Trends Biochem. Sci. 2007, 32, 332–341. [Google Scholar] [CrossRef]
- Bruno, F.; Spaziano, G.; Liparulo, A.; Roviezzo, F.; Nabavi, S.M.; Sureda, A.; Filosa, R.; D’Agostino, B. Recent advances in the search for novel 5-lipoxygenase inhibitors for the treatment of asthma. Eur. J. Med. Chem. 2018, 153, 65–72. [Google Scholar] [CrossRef]
- Bader, A.; Martini, F.; Schinella, G.R.; Rios, J.L.; Prieto, J.M. Modulation of Cox-1, 5-, 12- and 15-Lox by Popular Herbal Remedies Used in Southern Italy Against Psoriasis and Other Skin Diseases: Modulation of Eicosanoid Synthesis by Italian Antipsoriatic Plants. Phytother. Res. 2015, 29, 108–113. [Google Scholar] [CrossRef]
- Singh, R.K.; Tandon, R.; Dastidar, S.G.; Ray, A. A review on leukotrienes and their receptors with reference to asthma. J. Asthma 2013, 50, 922–931. [Google Scholar] [CrossRef]
- Montuschi, P.; Sala, A.; Dahlen, S.; Folco, G. Pharmacological modulation of the leukotriene pathway in allergic airway disease. Drug Discov. Today 2007, 12, 404–412. [Google Scholar] [CrossRef]
- Khan, R.; Spagnoli, V.; Tardif, J.-C.; L’Allier, P.L. Novel anti-inflammatory therapies for the treatment of atherosclerosis. Atherosclerosis 2015, 240, 497–509. [Google Scholar] [CrossRef]
- Chu, J.; Praticò, D. The 5-Lipoxygenase as modulator of Alzheimer’s γ-secretase and therapeutic target. Brain Res. Bull. 2016, 126, 207–212. [Google Scholar] [CrossRef]
- Chen, Y.; Li, D.; Li, S. The Alox5 gene is a novel therapeutic target in cancer stem cells of chronic myeloid leukemia. Cell Cycle 2009, 8, 3488–3492. [Google Scholar] [CrossRef]
- Peters-Golden, M.; Henderson, W.R., Jr. Leukotrienes. N. Eng. J. Med. 2007, 357, 1841–1854. [Google Scholar] [CrossRef]
- Roos, J.; Grösch, S.; Werz, O.; Schröder, P.; Ziegler, S.; Fulda, S.; Paulus, P.; Urbschat, A.; Kühn, B.; Maucher, I.; et al. Regulation of tumorigenic Wnt signaling by cyclooxygenase-2, 5-lipoxygenase and their pharmacological inhibitors: A basis for novel drugs targeting cancer cells? Pharmacol. Ther. 2016, 157, 43–64. [Google Scholar] [CrossRef] [PubMed]
- FDA Briefing Document, Pediatric Advisory Committee Meeting Neuropsychiatric Events with Use of Montelukast in Pediatric Patients. Available online: https://www.fda.gov/media/131035/download (accessed on 23 April 2020).
- Awni, W.M.; Braeckman, R.A.; Granneman, G.R.; Witt, G.; Dubé, L.M. Pharmacokinetics and Pharmacodynamics of Zileuton after Oral Administration of Single and Multiple Dose Regimens of Zileuton 600mg in Healthy Volunteers. Clin. Pharmacokinet. 1995, 29, 22–33. [Google Scholar] [CrossRef]
- Dubé, L.M.; Swanson, L.J.; Awni, W. Zileuton, a leukotriene synthesis inhibitor in the management of chronic asthma: Clinical pharmacokinetics and safety. Clin. Rev. Allergy Immunol. 1999, 17, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Boudreau, L.H.; Maillet, J.; LeBlanc, L.M.; Jean-François, J.; Touaibia, M.; Flamand, N.; Surette, M.E. Caffeic Acid Phenethyl Ester and Its Amide Analogue Are Potent Inhibitors of Leukotriene Biosynthesis in Human Polymorphonuclear Leukocytes. PLoS ONE 2012, 7, e31833. [Google Scholar] [CrossRef] [PubMed]
- Boudreau, L.H.; Lassalle-Claux, G.; Cormier, M.; Blanchard, S.; Doucet, M.S.; Surette, M.E.; Touaibia, M. New Hydroxycinnamic Acid Esters as Novel 5-Lipoxygenase Inhibitors That Affect Leukotriene Biosynthesis. Mediat. Inflamm. 2017, 2017, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mbarik, M.; Poirier, S.J.; Doiron, J.; Selka, A.; Barnett, D.A.; Cormier, M.; Touaibia, M.; Surette, M.E. Phenolic acid phenethylesters and their corresponding ketones: Inhibition of 5-lipoxygenase and stability in human blood and HepaRG cells. Pharmacol. Res. Perspect. 2019, 7, e00524. [Google Scholar] [CrossRef] [PubMed]
- Selka, A.; Doiron, J.A.; Lyons, P.; Dastous, S.; Chiasson, A.; Cormier, M.; Turcotte, S.; Surette, M.E.; Touaibia, M. Discovery of a novel 2,5-dihydroxycinnamic acid-based 5-lipoxygenase inhibitor that induces apoptosis and may impair autophagic flux in RCC4 renal cancer cells. Eur. J. Med. Chem. 2019, 179, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Touaibia, M.; Hébert, M.J.G.; Levesque, N.A.; Doiron, J.A.; Doucet, M.S.; Jean-François, J.; Cormier, M.; Boudreau, L.H.; Surette, M.E. Sinapic acid phenethyl ester as a potent selective 5-lipoxygenase inhibitor: Synthesis and structure-activity relationship. Chem. Biol. Drug Des. 2018, 92, 1876–1887. [Google Scholar] [CrossRef]
- Fakhr, I.M.I.; Radwan, M.A.A.; El-Batran, S.; Abd El-Salam, O.M.E.; El-Shenawy, S.M. Synthesis and pharmacological evaluation of 2-substituted benzo[b]thiophenes as anti-inflammatory and analgesic agents. Eur. J. Med. Chem. 2009, 44, 1718–1725. [Google Scholar] [CrossRef] [PubMed]
- Keri, R.S.; Chand, K.; Budagumpi, S.; Balappa Somappa, S.; Patil, S.A.; Nagaraja, B.M. An overview of benzo [b] thiophene-based medicinal chemistry. Eur. J. Med. Chem. 2017, 138, 1002–1033. [Google Scholar] [CrossRef] [PubMed]
- Martorana, A.; Gentile, C.; Perricone, U.; Piccionello, A.P.; Bartolotta, R.; Terenzi, A.; Pace, A.; Mingoia, F.; Almerico, A.M.; Lauria, A. Synthesis, antiproliferative activity, and in silico insights of new 3-benzoylamino-benzo[b]thiophene derivatives. Eur. J. Med. Chem. 2015, 90, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Lewis, T.A.; Bayless, L.; DiPesa, A.J.; Eckman, J.B.; Gillard, M.; Libertine, L.; Scannell, R.T.; Wypij, D.M.; Young, M.A. 5-Lipoxygenase inhibition by N-hydroxycarbamates in dual-function compounds. Bioorg. Med. Chem. Lett. 2005, 15, 1083–1085. [Google Scholar] [CrossRef] [PubMed]
- McGann, P.T.; Ware, R.E. Hydroxyurea therapy for sickle cell anemia. Expert Opin. Drug Saf. 2015, 14, 1749–1758. [Google Scholar] [CrossRef] [PubMed]
- Pariente-Cohen, N.; Weitman, M.; Tania, N.; Major, D.T.; Gottlieb, H.E.; Hoz, S.; Nudelman, A. Acylation or phosphorylation of hydroxyurea unexpectedly takes place on N rather than on O, leading to the formation of amides instead of the expected esters. RSC Adv. 2015, 5, 24038–24043. [Google Scholar] [CrossRef]
- Thiessen, W.E.; Levy, H.A.; Flaig, B.D. Non-planarity of hydroxamic acids. Structures of 3-hydroxyxanthine dihydrate by X-ray diffraction and hydroxyurea by neutron diffraction. Acta Crystallogr. B 1978, 34, 2495–2502. [Google Scholar] [CrossRef]
- Allain, E.P.; Boudreau, L.H.; Flamand, N.; Surette, M.E. The Intracellular Localisation and Phosphorylation Profile of the Human 5-Lipoxygenase Δ13 Isoform Differs from That of Its Full Length Counterpart. PLoS ONE 2015, 10, e0132607. [Google Scholar] [CrossRef]
- Gilbert, N.C.; Bartlett, S.G.; Waight, M.T.; Neau, D.B.; Boeglin, W.E.; Brash, A.R.; Newcomer, M.E. The Structure of Human 5-Lipoxygenase. Science 2011, 331, 217–219. [Google Scholar] [CrossRef]
- Dinh, C.P.; Ville, A.; Neukirch, K.; Viault, G.; Temml, V.; Koeberle, A.; Werz, O.; Schuster, D.; Stuppner, H.; Richomme, P.; et al. Structure-based design, semi-synthesis and anti-inflammatory activity of tocotrienolic amides as 5-lipoxygenase inhibitors. Eur. J. Med. Chem. 2020, 202, 112518. [Google Scholar] [CrossRef]
- De Lucia, D.; Lucio, O.M.; Musio, B.; Bender, A.; Listing, M.; Dennhardt, S.; Koeberle, A.; Garscha, U.; Rizzo, R.; Manfredini, S.; et al. Design, synthesis and evaluation of semi-synthetic triazole-containing caffeic acid analogues as 5-lipoxygenase inhibitors. Eur. J. Med. Chem. 2015, 101, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Bartlett, S.G.; Newcomer, M.E. Identification of the Substrate Access Portal of 5-Lipoxygenase. Biochemistry 2015, 54, 6333–6342. [Google Scholar] [CrossRef] [PubMed]
- Hoobler, E.K.; Rai, G.; Warrilow, A.G.S.; Perry, S.C.; Smyrniotis, C.J.; Jadhav, A.; Simeonov, A.; Parker, J.E.; Kelly, D.E.; Maloney, D.J.; et al. Discovery of a Novel Dual Fungal CYP51/Human 5-Lipoxygenase Inhibitor: Implications for Anti-Fungal Therapy. PLoS ONE 2013, 8, e65928. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Robichaud, P.P.; Poirier, S.J.; Boudreau, L.H.; Doiron, J.A.; Barnett, D.A.; Boilard, E.; Surette, M.E. On the cellular metabolism of the click chemistry probe 19-alkyne arachidonic acid. J. Lipid Res. 2016, 57, 1821–1830. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Schrödinger Release 2019-2: Maestro, Schrödinger; LLC: New York, NY, USA, 2020.
- Laskowski, R.A.; Swindells, M.B. LigPlot+: Multiple Ligand–Protein Interaction Diagrams for Drug Discovery. J. Chem. Inf. Model. 2011, 51, 2778–2786. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available. |






| Compounds | IC50 (µM) [SEM] |
|---|---|
| 14 | 31.8 [0.016] |
| 16 | 68.9 [0.015] |
| 17 | 64.7 [0.013] |
| 18 | 68.8 [0.022] |
| 27 | 6.9 [0.023] |
| 28 | 48.9 [0.025] |
| 31 | 6.6 [0.030] |
| 37 | 13.3 [0.021] |
| Zileuton (1) | >100 |
| SAPE (2) | 25.4 [0.7] |
| Vitamin C | 21.8 [0.023] |
| Compound | Affinity (kcal/mol) | π–π Interactions | H-Bond | Distance (Å) |
|---|---|---|---|---|
| (R)-Zileuton (1) | −6.6 | Phe421 | Leu420 × 2, Asn425 | 2.50, 3.15, 3.29 |
| (S)-Zileuton (1) | −6.5 | - | Leu420 × 2, Ala424, Phe421 | 2.81, 3.09, 3.34, 2.97 |
| SAPE (2) | −8.7 | His372 | - | - |
| 28 | −8.4 | Phe177, Phe421 | - | - |
| 31 | −8.8 | His372, His367 | - | - |
| 37 | −7.6 | - | Tyr181, Gln363, His367 | 3.09, 3.09 3.30, 3.34 |
| Physicochemical Properties | Lipophilicity | Pharmacokinetics | ||||||
|---|---|---|---|---|---|---|---|---|
| MW (g/mol) | ROTB (n) | HBA (n) | HBD (n) | TPSA (Å) | CLogPo/w | GIA | BBBP | |
| Rule | 500 | 10 | 10 | 5 | 140 | 5 | - | - |
| Zileuton (1) | 236.29 | 3 | 2 | 2 | 94.80 | 1.81 | High | No |
| SAPE (2) | 328.36 | 8 | 5 | 1 | 64.99 | 3.35 | High | Yes |
| 28 | 340.39 | 5 | 4 | 1 | 84.00 | 3.99 | High | No |
| 37 | 282.25 | 6 | 6 | 3 | 122.32 | 0.47 | High | No |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiasson, A.I.; Robichaud, S.; Ndongou Moutombi, F.J.; Hébert, M.P.A.; Mbarik, M.; Surette, M.E.; Touaibia, M. New Zileuton-Hydroxycinnamic Acid Hybrids: Synthesis and Structure-Activity Relationship towards 5-Lipoxygenase Inhibition. Molecules 2020, 25, 4686. https://doi.org/10.3390/molecules25204686
Chiasson AI, Robichaud S, Ndongou Moutombi FJ, Hébert MPA, Mbarik M, Surette ME, Touaibia M. New Zileuton-Hydroxycinnamic Acid Hybrids: Synthesis and Structure-Activity Relationship towards 5-Lipoxygenase Inhibition. Molecules. 2020; 25(20):4686. https://doi.org/10.3390/molecules25204686
Chicago/Turabian StyleChiasson, Audrey Isabel, Samuel Robichaud, Fanta J. Ndongou Moutombi, Mathieu P. A. Hébert, Maroua Mbarik, Marc E. Surette, and Mohamed Touaibia. 2020. "New Zileuton-Hydroxycinnamic Acid Hybrids: Synthesis and Structure-Activity Relationship towards 5-Lipoxygenase Inhibition" Molecules 25, no. 20: 4686. https://doi.org/10.3390/molecules25204686
APA StyleChiasson, A. I., Robichaud, S., Ndongou Moutombi, F. J., Hébert, M. P. A., Mbarik, M., Surette, M. E., & Touaibia, M. (2020). New Zileuton-Hydroxycinnamic Acid Hybrids: Synthesis and Structure-Activity Relationship towards 5-Lipoxygenase Inhibition. Molecules, 25(20), 4686. https://doi.org/10.3390/molecules25204686





