Sprouts vs. Microgreens as Novel Functional Foods: Variation of Nutritional and Phytochemical Profiles and Their In vitro Bioactive Properties
Abstract
1. Introduction
2. Results and Discussion
2.1. Basic Chemical Composition of Sprouts and Microgreens
2.2. L-ascorbic Acid Content in Sprouts and Microgreens
2.3. Chlorophyll and Carotenoid Contents in Sprouts and Microgreens
2.4. Polyphenol Content in Sprouts and Microgreens
2.5. Amino Acid Content in Sprouts and Microgreens
2.6. Biological Properties of Sprouts and Microgreens and Their Anti-Oxidant, Anti-Diabetic, Anti-Obesity and Anti-Cholinergic Activity
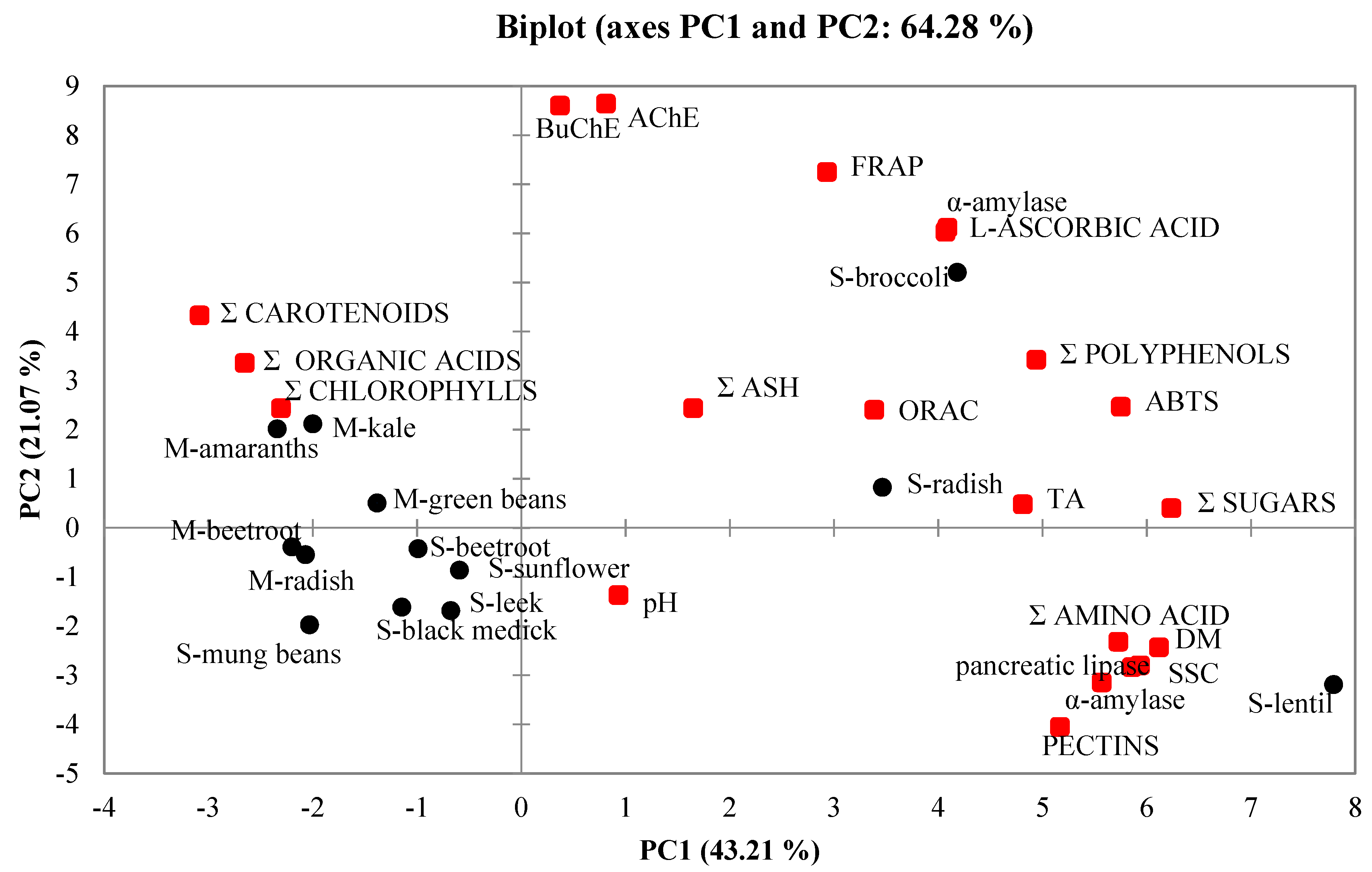
2.7. Principal Component Analysis (PCA)
3. Materials and Methods
3.1. Standards, Compounds and Chemicals
3.2. Plant Materials
3.3. Basic Physicochemical Analyses
3.4. Preparation of Sprout and Microgreen Extracts for Analysis
3.5. Estimation of Phenolic Compounds by LC-PDA-Qtof-ESI-MS (Identification) and UPLC-PDA-FL (Quantification)
3.6. Estimation of Carotenoids and Chlorophylls by LC-PDA-Qtof-ESI-MS (Identification) and UPLC-PDA (Quantification)
3.7. Estimation of Amino Acid by LC-PDA-Qtof-ESI-MS (Identification) and UPLC-PDA (Quantification)
3.8. Analysis of Biological Activity
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Samotyja, U.; Zdziebłowski, T.; Szlachta, M. Właściwości przeciwutleniają ce naturalnych ekstraktów polifenolowych z wybranych roślin w układach modelowych. Nauk. Technol. Żywność. 2012, 6, 41–53. [Google Scholar]
- Baenas, N.; Gómez-Jodar, I.; Moreno, D.A.; García-Viguera, C.; Periago, P.M. Broccoli and radish sprouts are safe and rich in bioactive phytochemicals. Postharvest Biol. Technol. 2017, 127, 60–67. [Google Scholar] [CrossRef]
- Silva, L.R.; Pereira, M.J.; Azevedo, J.; Gonçalves, R.F.; Valentão, P.; de Pinho, P.G.; Andrade, P.B. Glycine max L. Merr., Vigna radiata L. and Medicago sativa L. sprouts: A natural source of bioactive compounds. Food Res. Int. 2013, 50, 167–175. [Google Scholar] [CrossRef]
- Kyriacou, M.C.; El-Nakhel, C.; Graziani, G.; Pannico, A.; Soteriou, G.A.; Giordano, M.; Ritieni, A.; De Pascale, S.; Rouphael, Y. Functional quality in novel food sources: Genotypic variation in the nutritive and phytochemical composition of thirteen microgreens species. Food Chem. 2019, 277, 107–118. [Google Scholar] [CrossRef]
- Cevallos-Casals, B.A.; Cisneros-Zevallos, L. Impact of germination on phenolic content and antioxidant activity of 13 edible seed species. Food Chem. 2010, 119, 1485–1490. [Google Scholar] [CrossRef]
- Kyriacou, M.C.; Rouphael, Y.; Di Gioia, F.; Kyratzis, A.; Serio, F.; Renna, M.; De Pascale, S.; Santamaria, P. Micro-scale vegetable production and the rise of microgreens. Trends Food Sci. Technol. 2016, 57, 103–115. [Google Scholar] [CrossRef]
- Treadwell, D.D.; Hochmuth, R.; Landrum, L.; Laughlin, W. Microgreens: A new specialty crop. EDIS 2010, 3, 1–3. [Google Scholar] [CrossRef]
- Sun, J.; Xiao, Z.; Lin, L.; Lester, G.E.; Wang, Q.; Harnyl, J.M.; Chen, P. Profiling polyphenols in five Brassica species microgreens by UHPLC-PDA-ESI/HRMS. Bone 2008, 23, 10960–10970. [Google Scholar] [CrossRef]
- Xiao, Z.; Codling, E.E.; Luo, Y.; Nou, X.; Lester, G.E.; Wang, Q. Microgreens of Brassicaceae: Mineral composition and content of 30 varieties. J. Food Compos. Anal. 2016, 49, 87–93. [Google Scholar] [CrossRef]
- Choe, U.; Yu, L.L.; Wang, T.T.Y. The science behind microgreens as an exciting new food for the 21st Century. J. Agric. Food Chem. 2018, 66, 11519–11530. [Google Scholar] [CrossRef]
- Villaño, D.; López-Chillón, M.T.; Zafrilla, P.; Moreno, D.A. Bioavailability of broccoli sprouts in different human overweight populations. J. Funct. Foods 2019, 59, 337–344. [Google Scholar] [CrossRef]
- Xiao, Z.; Lester, G.E.; Luo, Y.; Wang, Q. Assessment of vitamin and carotenoid concentrations of emerging food products: Edible microgreens. J. Agric. Food Chem. 2012, 60, 7644–7651. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Maeda, T.; Sarker, M.Z.I.; Takigawa, S.; Matsuura-Endo, C.; Yamauchi, H.; Mukasa, Y.; Saito, K.; Hashimoto, N.; Noda, T.; et al. Identification of anthocyanins in the sprouts of buckwheat. J. Agric. Food Chem. 2007, 55, 6314–6318. [Google Scholar] [CrossRef] [PubMed]
- Turkiewicz, I.P.; Wojdyło, A.; Tkacz, K.; Nowicka, P.; Hernández, F. Antidiabetic, anticholinesterase and antioxidant activity vs. terpenoids and phenolic compounds in selected new cultivars and hybrids of artichoke cynara scolymus L. Molecules 2019, 24. [Google Scholar] [CrossRef] [PubMed]
- Tkacz, K.; Wojdyło, A.; Turkiewicz, I.P.; Bobak, Ł.; Nowicka, P. Anti-oxidant and anti-enzymatic activities of sea buckthorn (Hippophaë rhamnoides L.) fruits modulated by chemical components. Antioxidants 2019, 8, 618. [Google Scholar] [CrossRef]
- Samuoliene, G.; Brazaityte, A.; Jankauskiene, J.; Viršile, A.; Sirtautas, R.; Novičkovas, A.; Sakalauskiene, S.; Sakalauskaite, J.; Duchovskis, P. LED irradiance level affects growth and nutritional quality of Brassica microgreens. Cent. Eur. J. Biol. 2013, 8, 1241–1249. [Google Scholar] [CrossRef]
- Marton, M.; Mandoki, Z.; Caspo, J.; Caspo-Kiss, Z.; Marton, M.; Mándoki, Z.; Marton, M.; Mandoki, Z.; Caspo, J.; Caspo-Kiss, Z. The role of sprouts in human nutrition. A review. Aliment. Hungarian Univ. Transylvania 2010, 3, 81–117. [Google Scholar]
- Cefola, M.; Pace, B. Application of Oxalic Acid to Preserve the Overall Quality of Rocket and Baby Spinach Leaves during Storage. J. Food Process. Preserv. 2015, 39, 2523–2532. [Google Scholar] [CrossRef]
- Ruíz-Jiménez, J.M.; Zapata, P.J.; Serrano, M.; Valero, D.; Martínez-Romero, D.; Castillo, S.; Guillén, F. Effect of oxalic acid on quality attributes of artichokes stored at ambient temperature. Postharvest Biol. Technol. 2014, 95, 60–63. [Google Scholar] [CrossRef]
- Riga, P.; Benedicto, L.; Gil-Izquierdo, Á.; Collado-González, J.; Ferreres, F.; Medina, S. Diffuse light affects the contents of vitamin C, phenolic compounds and free amino acids in lettuce plants. Food Chem. 2019, 272, 227–234. [Google Scholar] [CrossRef]
- Waterland, N.L.; Moon, Y.; Tou, J.C.; Kim, M.J.; Pena-Yewtukhiw, E.M.; Park, S. Mineral content differs among microgreen, baby leaf, and adult stages in three cultivars of kale. Hort. Sci. 2017, 52, 566–571. [Google Scholar] [CrossRef]
- Weber, C.F. Broccoli Microgreens: A mineral-rich crop that can diversify food systems. Front. Nutr. 2017, 4, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.J.; Dong, J.F.; Zhu, M.Y. Effects of germination conditions on ascorbic acid level and yield of soybean sprouts. J. Sci. Food Agric. 2005, 85, 943–947. [Google Scholar] [CrossRef]
- Samuolienė, G.; Viršilė, A.; Brazaitytė, A.; Jankauskienė, J.; Sakalauskienė, S.; Vaštakaitė, V.; Novičkovas, A.; Viškelienė, A.; Sasnauskas, A.; Duchovskis, P. Blue light dosage affects carotenoids and tocopherols in microgreens. Food Chem. 2017, 228, 50–56. [Google Scholar] [CrossRef]
- Alrifai, O.; Hao, X.; Marcone, M.F.; Tsao, R. Current review of the modulatory effects of LED lights on photosynthesis of secondary metabolites and future perspectives of microgreen vegetables. J. Agric. Food Chem. 2019, 67, 6075–6090. [Google Scholar] [CrossRef]
- Brazaityte, A.; Sakalauskiene, S.; Samuoliene, G.; Jankauskiene, J.; Viršile, A.; Novičkovas, A.; Sirtautas, R.; Miliauskiene, J.; Vaštakaite, V.; Dabašinskas, L.; et al. The effects of LED illumination spectra and intensity on carotenoid content in Brassicaceae microgreens. Food Chem. 2015, 173, 600–606. [Google Scholar] [CrossRef]
- O’Neill, M.E.; Carroll, Y.; Corridan, B.; Olmedilla, B.; Granado, F.; Blanco, I.; Van den Berg, H.; Hininger, I.; Rousell, A.-M.; Chopra, M.; et al. A European carotenoid database to assess carotenoid intakes and its use in a five-country comparative study. Br. J. Nutr. 2001, 85, 499–507. [Google Scholar] [CrossRef]
- Mir, S.A.; Shah, M.A.; Mir, M.M. Microgreens: Production, shelf life, and bioactive components. Crit. Rev. Food Sci. Nutr. 2017, 57, 2730–2736. [Google Scholar] [CrossRef]
- Wojdyło, A.; Nowicka, P.; Bąbelewski, P. Phenolic and carotenoid profile of new goji cultivars and their anti-hyperglycemic, anti-aging and antioxidant properties. J. Funct. Foods 2018, 48, 632–642. [Google Scholar] [CrossRef]
- Silva, B.M.; Casal, S.; Andrade, P.B.; Seabra, R.M.; Oliveira, M.B.P.P.; Ferreira, M.A. Free Amino acid composition of quince (Cydonia oblonga Miller) fruit (pulp and peel) and jam. J. Agric. Food Chem. 2004, 52, 1201–1206. [Google Scholar] [CrossRef]
- Tarasevičiene, Ž.; Danilčenko, H.; Jariene, E.; Paulauskiene, A.; Gajewski, M. Changes in some chemical components during germination of broccoli seeds. Not. Bot. Horti Agrobot. Cluj-Napoca 2009, 37, 173–176. [Google Scholar] [CrossRef]
- Collado-González, J.; Cruz, Z.N.; Medina, S.; Mellisho, C.D.; Rodríguez, P.; Galindo, A.; Egea, I.; Romojaro, F.; Ferreres, F.; Torrecillas, A.; et al. Effects of water deficit during maturation on amino acids and jujube fruit eating quality. Maced. J. Chem. Chem. Eng. 2014, 33, 105–119. [Google Scholar] [CrossRef]
- Kobus-Cisowska, J.; Szymanowska, D.; Maciejewska, P.; Kmiecik, D.; Gramza-Michałowska, A.; Kulczyński, B.; Cielecka-Piontek, J. In vitro screening for acetylcholinesterase and butyrylcholinesterase inhibition and antimicrobial activity of chia seeds (Salvia hispanica). Electron. J. Biotechnol. 2019, 37, 1–10. [Google Scholar] [CrossRef]
- Nowicka, P.; Wojdyło, A. Anti-hyperglycemic and anticholinergic effects of natural antioxidant contents in edible flowers. Antioxidants 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Kanetro, B. Amino acid profile of soybean (Glicine max) sprout protein for determining insulin stimulation amino acids. Int. Food Res. J. 2018, 25, 2497–2502. [Google Scholar]
- Bahadoran, Z.; Mirmiran, P.; Hosseinpanah, F.; Rajab, A.; Asghari, G.; Azizi, F. Broccoli sprouts powder could improve serum triglyceride and oxidized LDL/LDL-cholesterol ratio in type 2 diabetic patients: A randomized double-blind placebo-controlled clinical trial. Diabetes Res. Clin. Pract. 2012, 96, 348–354. [Google Scholar] [CrossRef]
- Wojdyło, A.; Nowicka, P.; Grimalt, M.; Legua, P.; Almansa, M.S.; Amorós, A.; Carbonell-Barrachina, Á.A.; Hernández, F. Polyphenol compounds and biological activity of caper (Capparis spinosa L.) flowers buds. Plants 2019, 8, 539. [Google Scholar] [CrossRef]
- Wojdyło, A.; Jáuregui, P.N.N.; Carbonell-Barrachina, Á.A.; Oszmiański, J.; Golis, T. Variability of phytochemical properties and content of bioactive compounds in Lonicera caerulea L. var. kamtschatica Berries. J. Agric. Food Chem. 2013, 61, 12072–12084. [Google Scholar] [CrossRef]
- Wojdyło, A.; Nowicka, P.; Carbonell-Barrachina, Á.A.; Hernández, F. Phenolic compounds, antioxidant and antidiabetic activity of different cultivars of Ficus carica L. fruits. J. Funct. Foods 2016, 25, 421–432. [Google Scholar] [CrossRef]
- Wojdyło, A.; Oszmiański, J.; Bielicki, P. Polyphenolic composition, antioxidant activity, and polyphenol oxidase (PPO) activity of quince (Cydonia oblonga Miller) varieties. J. Agric. Food Chem. 2013, 61, 2762–2772. [Google Scholar] [CrossRef]
- Ou, B.; Huang, D.; Hampsch-Woodill, M.; Flanagan, J.A.; Deemer, E.K. Analysis of antioxidant activities of common vegetables employing oxygen radical absorbance capacity (ORAC) and ferric reducing antioxidant power (FRAP) assays: A comparative study. J. Agric. Food Chem. 2002, 50, 3122–3128. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
| Compounds | Sprouts | Microgreens | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Radish (Raphanus sativus) | Lentil (Lens culinaris) | Black Medick (Medicago lupulina) | Broccoli (Brassica oleracea var. Italica) | Sunflower (Helianthus annuus L.) | Leek (Allium porrum) | Beetroot (Beta vulgaris) | Mung Beans (Vigna radiata) | Kale (Brassica oleracea) | Radish (Raphanus sativus) | Beetroot (Beta vulgaris) | Green Peas (Pisum sativum) | Amaranths (Amaranthus) | |
| Dry matter | 16.5 ± 0.1 ‡b† | 46.4 ± 0.2a | 6.2 ± 0.0g | 15.8 ± 0.4c | 8.0 ± 0.5e | 8.6 ± 0.1d | 6.9 ± 0.6 f | 4.7 ± 0.2i | 4.1 ± 0.2j | 5.0 ± 0.3i | 5.5 ± 0.4h | 8.1 ± 0.1e | 5.4 ± 0.1h |
| SSC | 11.5 ± 0.2b | 33.2 ± 0.2a | 4.2 ± 0.1g | 9.0 ± 0.1c | 4.8 ± 0.9f | 5.5 ± 0.4e | 3.4 ± 0.5hi | 3.4 ± 0.2hi | 3.1 ± 0.3i | 3.2 ± 0.4i | 3.5 ± 0.2hi | 6.3 ± 0.3d | 3.8 ± 0.2gh |
| pH | 5.3 ± 0.1i | 6.6 ± 0.0a | 5.6 ± 0.1g | 5.5 ± 0.3h | 5.5 ± 0.4h | 5.6 ± 0.2g | 6.4 ± 0.5b | 5.3 ± 0.3i | 6.2 ± 0.4c | 5.7 ± 0.2f | 6.1 ± 0.3d | 6.0 ± 0.4e | 6.0 ± 0.3e |
| TA | 0.7 ± 0.1a | 0.5 ± 0.0b | 0.4 ± 0.0c | 0.5 ± 0.0b | 0.2 ± 0.1gh | 0.4 ± 0.1c | 0.2 ± 0.0fg | 0.3 ± 0.0d | 0.2 ± 0.0h | 0.3 ± 0.2e | 0.2 ± 0.0f | 0.4 ± 0.1c | 0.2 ± 0.0f |
| Pectins | 0.3 ± 0.0b | 3.1 ± 0.0a | 0.0 ± 0.0c | 0.0 ± 0.0c | 0.0 ± 0.0c | 0.0 ± 0.0c | 0.0 ± 0.0c | 0.0 ± 0.0c | 0.0 ± 0.0c | 0.0 ± 0.0c | 0.0 ± 0.0c | 0.0 ± 0.0c | 0.0 ± 0.0c |
| Σ Ash | 0.8 ± 0.1e | 1.3 ± 0.0b | 0.4 ± 0.1i | 0.7 ± 0.0e | 0.3 ± 0.0i | 0.5 ± 0.2g | 0.7 ± 0.0f | 0.2 ± 0.3j | 1.1 ± 0.1c | 0.7 ± 0.0f | 0.9 ± 0.4d | 0.4 ± 0.0h | 1.6 ± 0.2a |
| L-ascorbic acid | 93.4 ± 2.0b | 28.3 ± 1.0d | 11.0 ± 0.8g | 114.0 ± 2.7a | 9.7 ± 0.3g | 9.1 ± 0.7gh | 2.8 ± 0.1j | 7.1 ± 0.2hi | 20.3 ± 0.7f | 24.7 ± 1.2e | 6.6 ± 0.3i | 31.1 ± 1.4c | 8.9 ± 0.8gh |
| Sugars (g/100 g fw) | |||||||||||||
| Glucose | 0.9 ± 0.1ab | 0.1 ± 0.0c | 0.0 ± 0.0d | 1.0 ± 0.1a | 0.0 ± 0.0d | 0.0 ± 0.0d | 0.0 ± 0.0d | 0.0 ± 0.0d | 0.0 ± 0.0d | 0.0 ± 0.0d | 0.0 ± 0.0d | 0.0 ± 0.0d | 0.0 ± 0.0d |
| Fructose | 0.3 ± 0.1b | 0.2 ± 0.0c | 0.1 ± 0.0d | 0.5 ± 0.0a | 0.2 ± 0.0c | 0.4 ± 0.1a | 0.0 ± 0.0e | 0.2 ± 0.0c | 0.0 ± 0.0e | 0.0 ± 0.0e | 0.0 ± 0.0e | 0.0 ± 0.0e | 0.0 ± 0.0e |
| Saccharose | 0.0 ± 0.0c | 1.4 ± 0.1a | 0.1 ± 0.0b | 0.0 ± 0.0c | 0.0 ± 0.0c | 0.0 ± 0.0c | 0.0 ± 0.0c | 0.0 ± 0.0c | 0.0 ± 0.0c | 0.0 ± 0.0c | 0.0 ± 0.0c | 0.0 ± 0.0c | 0.0 ± 0.0c |
| Sorbitol | 0.0 ± 0.0c | 0.0 ± 0.0c | 0.0 ± 0.0c | 0.0 ± 0.0c | 0.6 ± 0.1a | 0.1 ± 0.0b | 0.0 ± 0.0a | 0.0 ± 0.0c | 0.0 ± 0.0c | 0.0 ± 0.0c | 0.0 ± 0.0c | 0.0 ± 0.0c | 0.0 ± 0.0c |
| Mannose | 0.0 ± 0.0b | 0.0 ± 0.0b | 0.0 ± 0.0b | 0.0 ± 0.0b | 0.1 ± 0.0a | 0.0 ± 0.0b | 0.0 ± 0.0b | 0.0 ± 0.0b | 0.0 ± 0.0b | 0.0 ± 0.0b | 0.0 ± 0.0b | 0.0 ± 0.0b | 0.0 ± 0.0b |
| Rhamnose | 0.0 ± 0.0b | 0.1 ± 0.00a | 0.0 ± 0.0b | 0.0 ± 0.0b | 0.0 ± 0.0b | 0.0 ± 0.0b | 0.0 ± 0.0b | 0.0 ± 0.0b | 0.0 ± 0.0b | 0.0 ± 0.0b | 0.0 ± 0.0b | 0.0 ± 0.0b | 0.0 ± 0.0b |
| Σ Sugars | 1.2c | 1.8a | 0.2f | 1.5b | 0.9d | 0.5e | 0.0 ± 0.0g | 0.2f | 0.0 ± 0.0g | 0.0 ± 0.0g | 0.0 ± 0.0g | 0.0 ± 0.0g | 0.0 ± 0.0g |
| Organic acids (g/100 g fw) | |||||||||||||
| Phytic acid | 0.0 ± 0.0d | 0.0 ± 0.0d | 0.1 ± 0.0c | 0.0 ± 0.0d | 0.7 ± 0.1b | 0.0 ± 0.0d | 2.8 ± 0.1a | 0.0 ± 0.0d | 0.0 ± 0.0d | 0.0 ± 0.0d | 0.0 ± 0.0d | 0.0 ± 0.0d | 0.0 ± 0.0d |
| Oxalic acid | 1.4 ± 0.2f | 1.5 ± 0.2f | 0.5 ± 0.0g | 1.9 ± 0.3e | 0.5 ± 0.1g | 2.2 ± 0.3e | 2.3 ± 0.3e | 0.7 ± 0.1g | 98.8 ± 1.6b | 79.3 ± 1.3c | 13.4 ± 0.3d | 0.3 ± 0.0g | 120.8 ± 1.3a |
| Citric acid | 0.1 ± 0.0c | 0.5 ± 0.1b | 0.2 ± 0.0c | 0.2 ± 0.0c | 0.1 ± 0.0c | 1.6 ± 0.2a | 0.2 ± 0.0c | 0.1 ± 0.0c | 0.0 ± 0.0d | 0.0 ± 0.0d | 0.0 ± 0.0d | 0.5 ± 0.0b | 0.0 ± 0.0d |
| Malic acid | 0.2 ± 0.0c | 0.3 ± 0.0b | 0.4 ± 0.1a | 0.3 ± 0.0b | 0.1 ± 0.0d | 0.2 ± 0.0c | 0.1 ± 0.0d | 0.0 ± 0.0e | 0.0 ± 0.0e | 0.0 ± 0.0e | 0.0 ± 0.0e | 0.0 ± 0.0e | 0.0 ± 0.0e |
| Quinic acid | 0.1 ± 0.0c | 0.0 ± 0.0d | 0.3 ± 0.0b | 0.1 ± 0.0c | 0.4 ± 0.0a | 0.0 ± 0.0d | 0.0 ± 0.0d | 0.1 ± 0.0c | 0.0 ± 0.0d | 0.0 ± 0.0d | 0.0 ± 0.0d | 0.0 ± 0.0d | 0.0 ± 0.0d |
| Succinic | 0.5 ± 0.0c | 0.8 ± 0.1ab | 0.0 ± 0.0e | 1.0 ± 0.1a | 0.4 ± 0.0c | 0.4 ± 0.0c | 0.0 ± 0.0e | 0.0 ± 0.0e | 0.0 ± 0.0e | 0.0 ± 0.0e | 0.0 ± 0.0e | 0.2 ± 0.0d | 0.0 ± 0.0e |
| Other organic acid | 0.5 ± 0.1c | 0.2 ± 0.0e | 0.6 ± 0.1c | 0.4 ± 0.0d | 0.1 ± 0.0e | 0.2 ± 0.0e | 0.0 ± 0.0f | 1.7 ± 0.2ab | 0.0 ± 0.0f | 0.0 ± 0.0f | 0.0 ± 0.0f | 2.0 ± 0.1a | 0.0 ± 0.0f |
| Σ Organic acids | 2.6e | 3.2e | 2.2e | 3.9e | 2.3e | 4.5e | 5.4e | 2.6e | 98.8b | 79.3c | 13.4d | 3.0e | 120.8a |
| ANOVA test † | Dry matter | Soluble solid | pH | Titratable acidity | L-ascorbic acid | Σ Ash | Pectins | Σ Sugars | Σ Organic acids | ||||
| Sprouts | A | A | B | A | A | B | A | A | B | ||||
| Microgreens | B | B | A | B | B | A | B | B | A | ||||
| Compounds | Sprouts | Microgreens | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Radish (Raphanus sativus) | Lentil (Lens culinaris) | Black Medick (Medicago lupulina) | Broccoli (Brassica oleracea var. Italica) | Sunflower (Helianthus annuus L.) | Leek (Allium porrum) | Beetroot (Beta vulgaris) | Mung Beans (Vigna radiata) | Kale (Brassica oleracea) | Radish (Raphanus sativus) | Beetroot (Beta vulgaris) | Green Peas (Pisum sativum) | Amaranths (Amaranthus) | |
| Polyphenolic compounds | |||||||||||||
| Flavan-3-ols | 40.3 ± 3.9b ‡ | 34.9 ± 2.7c | 29.9 ± 1.8c | 53.9 ± 1.1a | 24.9 ± 0.5d | 18.3 ± 0.6e | 24.7 ± 1.2d | 22.1 ± 0.9d | 21.2 ± 0.7d | 1.9 ± 0.1g | 9.8 ± 0.5f | 27.6 ± 2.4c | 8.8 ± 0.5f |
| Polymeric procyanidins | 39.8 ± 1.5b | 94.5 ± 3.1a | 6.3 ± 0.3f | 26.6 ± 1.3c | 2.1 ± 0.1i | 6.7 ± 0.1g | 3.3 ± 0.3g | 1.4 ± 0.3i | 8.3 ± 0.2e | 3.6 ± 0.2g | 7.3 ± 0.7ef | 14.4 ± 1.2d | 1.3 ± 0.1i |
| Phenolic acid | 100.0 ± 2.5c | 7.9 ± 0.3g | 6.4 ± 0.4g | 110.2 ± 1.4b | 125.6 ± 2.5a | 6.5 ± 0.2g | 5.8 ± 0.7g | 2.7 ± 0.2h | 15.1 ± 0.1f | 1.5 ± 0.4h | 16.6 ± 2.1f | 41.9 ± 3.3e | 58.6 ± 1.6d |
| Flavonols + flavones | 1.2 ± 0.0d | 2.0 ± 0.2c | 0.0 ± 0.0f | 0.0 ± 0.0f | 0.0 ± 0.0f | 0.0 ± 0.0f | 1.8 ± 0.1c | 0.5 ± 0.0e | 0.0 ± 0.0f | 0.0 ± 0.0f | 6.9 ± 0.5a | 4.6 ± 0.6b | 0.3 ± 0.0e |
| Isoflavones | 0.0 ± 0.0c | 40.5 ± 2.4a | 0.0 ± 0.0c | 0.0 ± 0.0c | 0.0 ± 0.0c | 0.0 ± 0.0c | 0.0 ± 0.0c | 0.0 ± 0.0c | 0.0 ± 0.0c | 0.0 ± 0.0c | 0.0 ± 0.0c | 20.0 ± 0.8b | 0.0 ± 0.0c |
| Anthocyanins | 2.1 ± 0.1d | 0.0 ± 0.0f | 0.1 ± 0.0e | 0.5 ± 0.0e | 0.0 ± 0.0f | 0.0 ± 0.0f | 2.3 ± 0.4d | 0.0 ± 0.0f | 0.0 ± 0.0f | 17.2 ± 0.3b | 5.5 ± 0.3c | 0.0 ± 0.0f | 63.9 ± 2.3a |
| Σ Polyphenols | 183.4b | 179.8b | 42.8f | 191.1a | 152.6c | 31.5hi | 35.6gh | 26.7ij | 44.7f | 24.3j | 40.7f | 108.5e | 132.9de |
| ANOVA test † | A | B | |||||||||||
| Chlorophylls | |||||||||||||
| Chlorophylls b | 3.5 ± 0.1i | 16.1 ± 0.6f | 1.6 ± 0.1g | 2.7 ± 0.4 | 13.8 ± 0.3g | 5.9 ± 0.2h | 2.6 ± 0.3j | 1.0 ± 0.2h | 57.0 ± 1.2e | 64.7 ± 1.5d | 68.3 ± 1.4c | 157.8 ± 2.4b | 186.3 ± 2.1a |
| Pheophytin b | 0.0 ± 0.0c | 0.0 ± 0.0c | 0.0 ± 0.0c | 0.0 ± 0.0c | 0.0 ± 0.0c | 0.0 ± 0.0c | 0.0 ± 0.0c | 0.0 ± 0.0c | 6.4 ± 0.3a | 0.0 ± 0.0c | 5.7 ± 0.2b | 0.0 ± 0.0c | 0.0 ± 0.0c |
| chlorophylls b’ | 0.0 ± 0.0h | 0.0 ± 0.0h | 0.0 ± 0.0h | 0.0 ± 0.0h | 2.4 ± 0.2e | 1.2 ± 0.3f | 0.4 ± 0.1g | 0.0 ± 0.0h | 10.9 ± 0.7c | 7.5 ± 0.8d | 9.7 ± 0.2c | 14.8 ± 1.4b | 17.3 ± 0.7a |
| Pheophytin b’ | 0.0 ± 0.0d | 0.0 ± 0.0d | 0.0 ± 0.0d | 0.0 ± 0.0d | 0.0 ± 0.0d | 0.0 ± 0.0d | 0.0 ± 0.0d | 0.0 ± 0.0d | 1.9 ± 0.1b | 0.0 ± 0.0d | 0.9 ± 0.1c | 3.1 ± 0.3a | 2.1 ± 0.2b |
| Chlorophylls a | 27.9 ± 1.1f | 77.6 ± 1.5e | 10.5 ± 0.7h | 11.0 ± 0.6h | 29.9 ± 1.1f | 7.7 ± 0.2i | 23.0 ± 0.5g | 3.9 ± 0.1j | 88.1 ± 1.4d | 121.2 ± 2.1c | 125.6 ± 2.4c | 288.3 ± 3.6b | 336.2 ± 3.2a |
| Chlorophylls a’ | 3.5 ± 0.2g | 7.6 ± 0.3e | 1.3 ± 0.3h | 1.0 ± 0.1i | 3.1 ± 0.1g | 4.7 ± 0.4f | 1.8 ± 0.2h | 0.0 ± 0.0j | 12.7 ± 1.2c | 6.4 ± 0.3e | 9.5 ± 0.1d | 20.3 ± 1.5a | 16.9 ± 1.1b |
| Pheophytin a | 2.3 ± 0.3f | 7.2 ± 0.7d | 1.0 ± 0.1h | 1.7 ± 0.0g | 3.0 ± 0.2f | 5.5 ± 0.2e | 2.7 ± 0.1f | 1.1 ± 0.3h | 16.0 ± 0.7c | 14.7 ± 0.7c | 35.2 ± 0.5b | 35.3 ± 01b | 75.4 ± 0.3a |
| Pheophytin a’ | 0.0 ± 0.0e | 0.0 ± 0.0e | 0.0 ± 0.0e | 0.0 ± 0.0e | 0.0 ± 0.0e | 1.5 ± 0.5d | 0.0 ± 0.0e | 0.0 ± 0.0e | 2.6 ± 0.2c | 1.2 ± 0.1d | 3.8 ± 0.5b | 3.2 ± 0.1c | 4.3 ± 0.2a |
| Σ Chlorophylls | 37.2fg | 108.5e | 14.3hi | 16.4ghi | 52.2f | 26.5ghi | 30.5fgh | 6.0i | 195.6d | 215.7d | 258.7c | 522.7b | 638.5a |
| ANOVA test† | B | A | |||||||||||
| Carotenoids | |||||||||||||
| Neochrome | 21.0 ± 0.4g | 0.0 ± 0.0l | 5.4 ± 0.1j | 10.6 ± 1.0i | 29.1 ± 1.2f | 18.3 ± 1.1g | 15.7 ± 1.3h | 1.2 ± 0.3k | 107.5 ± 1.3c | 99.9 ± 1.1d | 86.4 ± 1.2e | 255.0 ± 2.9b | 301.3 ± 4.0a |
| Neoxanthin | 98.5 ± 1.6c | 2.7 ± 0.3h | 45.7 ± 1.7e | 56.4 ± 2.7d | 32.4 ± 2.1f | 18.4 ± 0.9g | 55.2 ± 2.5d | 3.0 ± 0.2h | 3.5 ± 0.5h | 90.5 ± 2.6c | 2.8 ± 1.1h | 256.8 ± 4.1b | 293.8 ± 1.3a |
| Zeoxanthin | 38.1 ± 1.1d | 0.0 ± 0.0j | 6.7 ± 0.2g | 23.5 ± 1.8e | 20.2 ± 1.4e | 21.7 ± 1.3e | 36.9 ± 1.1d | 1.1 ± 0.1i | 3.2 ± 0.2h | 42.7 ± 1.4c | 17.4 ± 0.3f | 57.1 ± 2.8b | 132.2 ± 2.6a |
| Lutein | 570.6 ± 2.7d | 31.0 ± 1.4j | 113.2 ± 2.1i | 193.2 ± 3.6g | 382.0 ± 3.5e | 168.6 ± 2.5h | 305.2 ± 3.8f | 13.2 ± 0.7k | 629.6 ± 41c | 565.2 ± 1.8d | 684.4 ± 4.2b | 1435.7 ± 2.6a | 1478.9 ± 1.2a |
| Violaxanthin | 37.9 ± 1.1a | 0.0 ± 0.0g | 4.3 ± 0.1e | 37.2 ± 1.2a | 14.0 ± 1.0d | 21.5 ± 0.7b | 12.1 ± 1.3d | 0.5 ± 0.0f | 35.8 ± 1.1a | 8.3 ± 2.5e | 37.0 ± 1.1a | 16.9 ± 1.1c | 36.6 ± 1.9a |
| (α+β)-Carotene | 41.7 ± 0.5g | 0.0 ± 0.0j | 10.1 ± 0.2i | 23.3 ± 2.6i | 26.4 ± 1.0i | 31.7 ± 1.0h | 139.8 ± 1.8f | 0.9 ± 0.1k | 2239.0 ± 5.9a | 699.9 ± 4.8e | 1249.1 ± 5.2c | 728.4 ± 4.2d | 1769.2 ± 5.2b |
| other carotenoids | 141.0 ± 1.1a | 2.8 ± 0.3g | 12.1 ± 1.3f | 107.6 ± 3.6b | 25.4 ± 1.0e | 60.9 ± 2.1c | 41.0 ± 1.1d | 2.5 ± 0.2g | 68.5 ± 1.7c | 3.7 ± 0.2g | 22.8 ± 1.1e | 44.4 ± 0.5d | 61.6 ± 1.1c |
| Σ Carotenoids | 948.8f | 36.4j | 197.5i | 451.7gh | 529.5g | 341.0hi | 605.8g | 22.5j | 3087.1b | 1510.1e | 2099.9d | 2794.4c | 4073.5a |
| ANOVA test † | B | A | |||||||||||
| Amino acid | Sprouts | Microgreens | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Radish (Raphanus sativus) | Lentil (Lens culinaris) | Black Medick (Medicago lupulina) | Broccoli (Brassica oleracea var. Italica) | Sunflower (Helianthus annuus L.) | Leek (Allium porrum) | Beetroot (Beta vulgaris) | Mung Beans (Vigna radiata) | Kale (Brassica oleracea) | Radish (Raphanus sativus) | Beetroot (Beta vulgaris) | Green Peas (Pisum sativum) | Amaranths (Amaranthus) | |
| L-Histidine | 43.3 ± 2.1b | 92.6 ± 2.4a | 20.9 ± 1.3c | 41.5 ± 3.2b | 13.8 ± 1.5e | 16.9 ± 1.1d | 12.8 ± 1.4e | 22.8 ± 2.1c | 10.6 ± 0.9e | 13.1 ± 1.1e | 2.3 ± 0.3g | 7.7 ± 1.1f | 1.5 ± 0.2g |
| L-Asparagine | 30.3 ± 2.8i | 438.5 ± 4.2a | 317.8 ± 8.6b | 46.3 ± 1.3h | 56.1 ± 2.4g | 181.8 ± 5.2d | 16.2 ± 0.3j | 211.7 ± 7.3c | 69.7 ± 3.6f | 221.4 ± 5.2c | 4.3 ± 0.1l | 106.2 ± 4.1e | 6.7 ± 0.3k |
| L-Arginine | 10.0 ± 1.0g | 36.1 ± 1.2d | 39.8 ± 2.3d | 39.8 ± 2.2d | 70.2 ± 1.2b | 53.6 ± 1.3c | 55.9 ± 2.5c | 52.5 ± 4.1c | 32.3 ± 1.3d | 18.2 ± 1.1f | 28.3 ± 0.4e | 83.2 ± 2.5a | 27.5 ± 1.1e |
| L-Serine | 30.2 ± 2.1b | 25.9 ± 1.2c | 8.7 ± 1.1f | 36.8 ± 1.0ab | 39.5 ± 3.1a | 29.2 ± 6.3b | 22.9 ± 1.1c | 16.0 ± 1.2d | 23.2 ± 1.1c | 10.7 ± 0.5e | 4.9 ± 0.2 | 12.9 ± 1.0e | 7.6 ± 0.4f |
| L-Glutamine | 203.3 ± 4.9c | 73.7 ± 3.2f | 32.1 ± 4.2h | 330.6 ± 5.7a | 145.5 ± 5.3d | 260.1 ± 7.3b | 90.6 ± 4.2e | 11.4 ± 1.7i | 98.8 ± 3.8e | 4.9 ± 0.2j | 25.7 ± 0.5h | 53.5 ± 2.6g | 48.0 ± 1.1g |
| L-Glycine | 18.8 ± 3.1b | 20.8 ± 1.1b | 5.4 ± 0.5e | 20.0 ± 1.1b | 10.2 ± 1.1c | 12.3 ± 1.1c | 7.7 ± 0.2d | 3.3 ± 0.5f | 1.3 ± 0.2g | 35.4 ± 1.5a | 3.9 ± 0.1f | 10.3 ± 1.0c | 4.0 ± 0.2f |
| L-Aspartic acid | 17.1 ± 1.1f | 151.4 ± 2.5a | 12.3 ± 1.4g | 20.0 ± 0.7e | 11.7 ± 0.6g | 23.0 ± 2.1e | 32.5 ± 1.1c | 6.6 ± 0.6h | 13.3 ± 0.1g | 25.2 ± 2.1d | 48.0 ± 2.1b | 27.0 ± 1.0d | 36.1 ± 1.2c |
| L-Glutamic acid | 21.1 ± 2.4b | 77.2 ± 3.2a | 18.3 ± 1.3b | 20.8 ± 0.5b | 4.7 ± 0.2f | 23.4 ± 1.2b | 6.9 ± 0.4e | 2.0 ± 0.2g | 13.5 ± 0.5c | 16.7 ± 1.0c | 12.3 ± 1.9d | 16.6 ± 2.10c | 14.8 ± 0.5c |
| L-Threonine | 27.3 ± 2.3b | 40.6 ± 2.0a | 20.7 ± 1.0c | 40.5 ± 1.2a | 12.3 ± 0.9d | 21.0 ± 2.0c | 5.0 ± 0.7e | 13.8 ± 0.9d | 4.8 ± 0.2e | 4.4 ± 0.5e | 1.7 ± 0.2g | 5.8 ± 1.1e | 2.9 ± 0.0f |
| L-Alanine | 14.5 ± 1.3e | 47.5 ± 1.1a | 16.8 ± 0.6d | 41.3 ± 2.1ab | 17.4 ± 1.1d | 21.5 ± 1.6c | 9.7 ± 1.3f | 7.5 ± 0.4f | 4.6 ± 0.1g | 6.7 ± 0.7f | 6.2 ± 0.1f | 6.9 ± 1.0f | 10.0 ± 0.1f |
| γ-Amino n-butyric acid | 7.3 ± 0.9c | 46.3 ± 0.8a | 5.3 ± 0.2e | 10.3 ± 1.1b | 9.2 ± 0.5b | 6.4 ± 0.4d | 6.2 ± 0.4d | nd | 0.5 ± 0.0h | 8.7 ± 0.4c | 1.6 ± 1.1g | 3.4 ± 2.1f | 5.7 ± 0.5e |
| L-Ornithine | 29.2 ± 2.1a | nd | nd | nd | nd | nd | nd | nd | 2.4 ± 0.2b | nd | nd | nd | nd |
| L-Proline | 29.9 ± 1.5b | 113.2 ± 4.6a | 5.4 ± 0.7d | 28.7 ± 1.4b | 13.6 ± 1.3c | 3.6 ± 0.1e | nd | 10.6 ± 0.8c | 1.5 ± 0.4f | 1.4 ± 0.0f | 0.7 ± 0.1g | 1.8 ± 1.1f | 1.5 ± 0.3f |
| L-Cystine | 1.7 ± 0.2f | nd | 1.0 ± 0.1g | 2.7 ± 0.3e | 16.8 ± 0.4a | 4.6 ± 0.4d | 13.4 ± 1.3b | nd | 0.7 ± 0.1g | 17.4 ± 0.7a | 0.3 ± 0.0h | 8.5 ± 0.3c | 5.8 ± 0.1d |
| L-Lysine | 15.8 ± 1.2c | 14.7 ± 1.2c | 5.5 ± 0.3e | 27.7 ± 0.7a | 8.5 ± 0.5d | 17.5 ± 1.3b | 7.1 ± 0.4d | 14.2 ± 1.1c | 4.3 ± 0.2e | 8.4 ± 0.3d | 3.8 ± 0.4e | 13.5 ± 0.9c | 3.5 ± 0.3e |
| L-Tyrosine | 10.1 ± 0.7d | 10.4 ± 2.1d | 3.5 ± 0.2f | 19.3 ± 1.4a | 5.1 ± 0.4e | 13.6 ± 2.1c | 3.1 ± 0.2f | 16.7 ± 1.6b | 2.6 ± 0.1g | 4.6 ± 0.1e | 1.6 ± 0.1h | 3.2 ± 0.1f | 3.7 ± 0.1f |
| L-Methionine | 1.3 ± 0.2c | 1.5 ± 1.1b | 0.8 ± 0.1e | 1.2 ± 0.1cd | 1.5 ± 1.1b | 1.1 ± 0.1d | nd | 3.9 ± 0.4a | 1.6 ± 0.1b | 0.9 ± 0.0e | nd | 1.0 ± 0.2d | nd |
| L-Valine | 28.6 ± 0.5c | 45.9 ± 3.2a | 21.6 ± 1.1d | 47.1 ± 3.2a | 15.7 ± 2.1e | 9.8 ± 0.3f | 7.7 ± 0.6g | 34.6 ± 2.7b | 14.5 ± 0.4e | 19.1 ± 0.7d | 4.3 ± 0.4h | 11.7 ± 0.2f | 4.5 ± 0.4h |
| L-Homocysteine | nd | nd | 1.5 ± 0.1a | nd | nd | nd | nd | nd | 0.2 ± 0.0b | nd | nd | nd | nd |
| L-Isoleucine | 18.4 ± 0.2b | 12.0 ± 1.1d | 9.6 ± 0.6e | 30.8 ± 2.1a | 14.7 ± 1.6c | 7.3 ± 0.4f | 7.5 ± 0.9f | 28.0 ± 3.1a | 5.4 ± 0.3g | 8.9 ± 0.3e | 2.5 ± 0.1h | 5.0 ± 0.1g | 3.4 ± 0.1h |
| L-Leucine | 11.1 ± 0.6b | 8.1 ± 0.6c | 5.3 ± 0.2d | 22.8 ± 0.4a | 13.5 ± 2.4b | 19.6 ± 1.4a | 5.3 ± 0.4d | 21.0 ± 1.1a | 3.0 ± 0.1e | 5.1 ± 0.1d | 3.5 ± 0.6e | 3.2 ± 0.3e | 5.3 ± 0.2d |
| L-Phenylalanine | 12.2 ± 1.2e | 31.4 ± 1.5b | 17.8 ± 1.4d | 21.6 ± 1.1c | 7.2 ± 1.6f | 21.3 ± 2.1c | 5.0 ± 0.6g | 51.8 ± 1.4a | 23.2 ± 0.6c | 17.1 ± 0.7d | 4.6 ± 0.2g | 11.1 ± 0.4e | 3.8 ± 0.4h |
| L-Tryptophan | 10.7 ± 1.1b | 4.3 ± 0.9e | 9.3 ± 0.9c | 14.9 ± 1.0a | 10.0 ± 2.1b | 7.8 ± 1.1d | 5.9 ± 0.2c | 9.3 ± 0.6c | 10.3 ± -0.9b | 6.6 ± 0.3c | 2.0 ± 0.1f | 5.5 ± 0.1e | 2.9 ± 0.1f |
| Σ AA | 592.2d | 1292.1a | 579.5fd | 864.7b | 497.1fg | 755.4c | 321.4i | 537.7ef | 342.5i | 455.0g | 162.3j | 397.9h | 199.4j |
| ANOVA test † | A | B | |||||||||||
| Sample | Anti-Oxidant Capacity | Anti-Diabetic Activity | Anti-Obesity Activity | Anti-Cholinergic Activity | |||||
|---|---|---|---|---|---|---|---|---|---|
| ORAC | FRAP | ABTS | α-Amylase | α-Glucosidase | AChE | BuChE | |||
| Sprouts | radish (Raphanus sativus) | 4.5 ± 0.3c ‡ | 0.6 ± 0.1b | 1.8 ± 0.2b | 50.8 ± 4.8g | 6.7 ± 0.7e | 0.5 ± 0.0g | 21.3 ± 2.4d | 11.7 ± 1.2fg |
| lentil (Lens culinaris) | 4.4 ± 0.6c | 0.1 ± 0.0g | 2.0 ± 0.3a | 88.4 ± 5.3h | 15.9 ± 2.1g | 1.5 ± 0.2h | 10.1 ± 1.0fg | 1.0 ± 0.3h | |
| black medick (Medicago lupulina) | 2.5 ± 0.3d | 0.1 ± 0.0h | 0.8 ± 0.1e | 4.9 ± 0.8b | 0.6 ± 0.2a | 0.1 ± 0.0bc | 8.6 ± 0.6g | 3.6 ± 0.2h | |
| broccoli (Brassica oleracea var. italica) | 5.7 ± 0.3a | 0.8 ± 0.1a | 1.8 ± 0.4b | 6.9 ± 0.5cd | 51.0 ± 3.5h | 0.4 ± 0.1f | 98.0 ± 2.8a | 92.0 ± 3.8a | |
| sunflower (Helianthus annuus L.) | 2.4 ± 0.1d | 0.2 ± 0.0f | 0.6 ± 0.0f | 9.8 ± 1.0e | 3.2 ± 0.2cd | 0.3 ± 0.0 | 22.0 ± 0.7d | 9.7 ± 0.6g | |
| leek (Allium porrum) | 1.1 ± 0.2g | 0.1 ± 0.0gh | 0.8 ± 0.1e | 10.4 ± 0.5e | 2.0 ± 0.3bc | 0.3 ± 0.0e | 12.8 ± 0.8ef | 10.0 ± 1.2g | |
| beetroot (Beta vulgaris) | 5.9 ± 0.5a | 0.3 ± 0.0c | 1.3 ± 0.2c | 5.4 ± 0.2bc | 2.0 ± 0.2bc | 0.2 ± 0.0cd | 22.7 ± 1.3d | 14.3 ± 1.3fg | |
| mung beans (Vigna radiata) | 1.1 ± 0.1fg | >0.01i | >0.01h | 5.3 ± 0.3b | 0.9 ± 0.3bc | 0.2 ± 0.0d | 14.2 ± 1.2e | 3.0 ± a0.5h | |
| Microgreens | kale (Brassica oleracea) | 2.4 ± 0.2d | 0.3 ± 0.0d | 1.0 ± 0.2d | 4.8 ± 0.3b | 4.3 ± 0.6d | 0.1 ± 0.0a | 59.2 ± 3.6b | 79.4 ± 2.5b |
| radish (Raphanus sativus) | 1.8 ± 0.2e | 0.1 ± 0.0f | 0.6 ± 0.2f | 7.7 ± 0.6d | 1.3 ± 0.2bc | 0.2 ± 0.0d | 9.0 ± a1.1g | 16.4 ± 1.1de | |
| beetroot (Beta vulgaris) | 5.3 ± 0.3b | 0.2 ± 0.0e | 0.2 ± 0.0g | 2.9 ± 0.1a | 1.4 ± 0.1bc | 0.1 ± 0.0a | 12.0 ± 0.8efg | 8.3 ± 0.4g | |
| green peas (Pisum sativum) | 1.2 ± 0.3fg | 0.1 ± 0.0g | 0.7 ± 0.1e | 8.3 ± 0.5d | 8.0 ± 0.1f | 0.1 ± 0.0bc | 52.5 ± 0.4c | 19.6 ± 2.4d | |
| amaranth (Amaranthus) | 1.4 ± 0.2f | 0.1 ± 0.0f | 0.6 ± 0.1f | 12.5 ± 0.9f | 4.3 ± 0.2d | 0.3 ± 0.0e | 50.5 ± 1.4c | 54.9 ± 3.5c | |
| ANOVA test † | |||||||||
| Sprouts | A | A | A | B | B | B | B | B | |
| Microgreens | B | B | B | A | A | A | A | A | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wojdyło, A.; Nowicka, P.; Tkacz, K.; Turkiewicz, I.P. Sprouts vs. Microgreens as Novel Functional Foods: Variation of Nutritional and Phytochemical Profiles and Their In vitro Bioactive Properties. Molecules 2020, 25, 4648. https://doi.org/10.3390/molecules25204648
Wojdyło A, Nowicka P, Tkacz K, Turkiewicz IP. Sprouts vs. Microgreens as Novel Functional Foods: Variation of Nutritional and Phytochemical Profiles and Their In vitro Bioactive Properties. Molecules. 2020; 25(20):4648. https://doi.org/10.3390/molecules25204648
Chicago/Turabian StyleWojdyło, Aneta, Paulina Nowicka, Karolina Tkacz, and Igor Piotr Turkiewicz. 2020. "Sprouts vs. Microgreens as Novel Functional Foods: Variation of Nutritional and Phytochemical Profiles and Their In vitro Bioactive Properties" Molecules 25, no. 20: 4648. https://doi.org/10.3390/molecules25204648
APA StyleWojdyło, A., Nowicka, P., Tkacz, K., & Turkiewicz, I. P. (2020). Sprouts vs. Microgreens as Novel Functional Foods: Variation of Nutritional and Phytochemical Profiles and Their In vitro Bioactive Properties. Molecules, 25(20), 4648. https://doi.org/10.3390/molecules25204648