A Template-Based Approach for Guiding and Refining the Development of Cinnamon-Based Phenylpropanoids as Drugs
Abstract
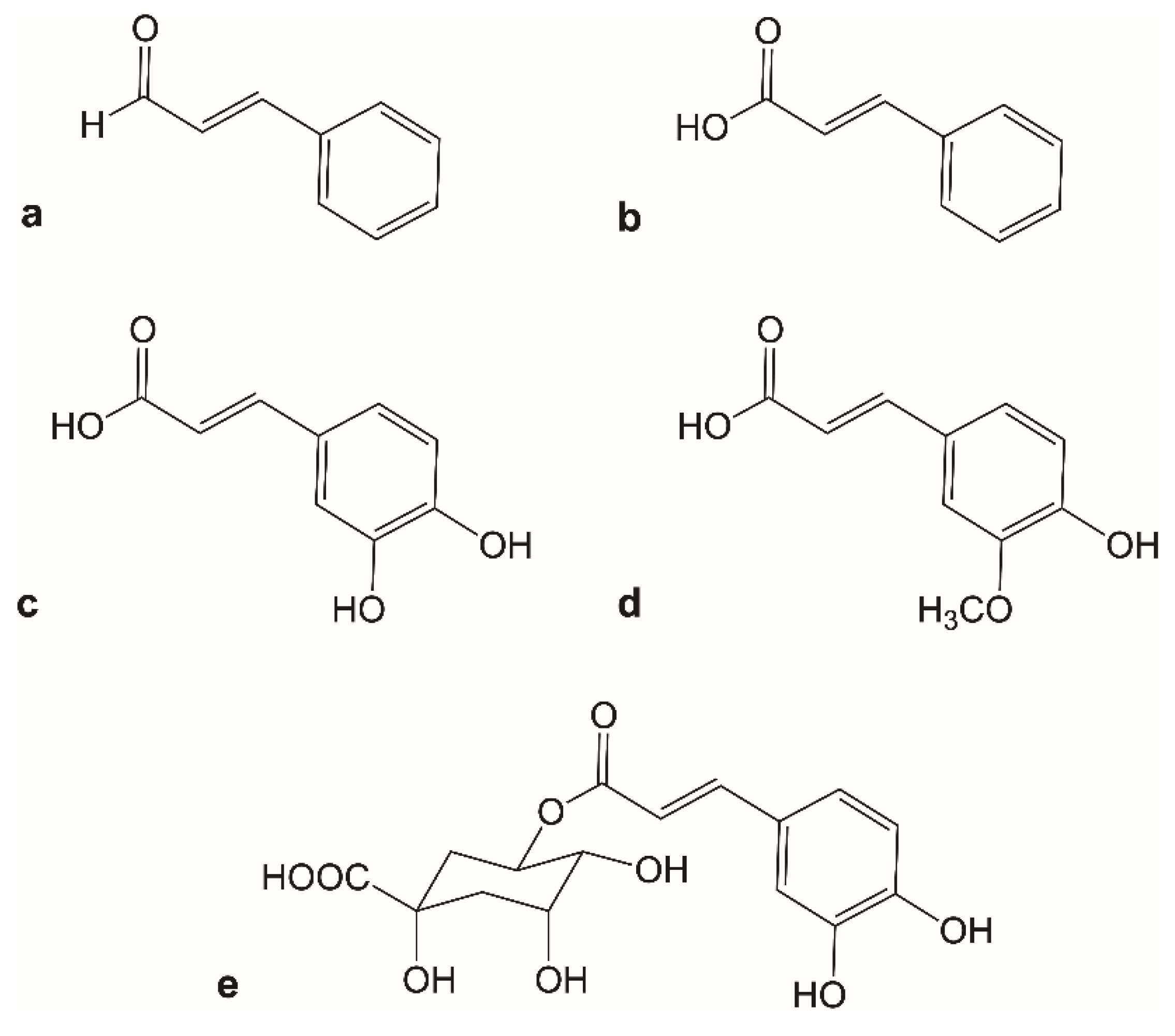
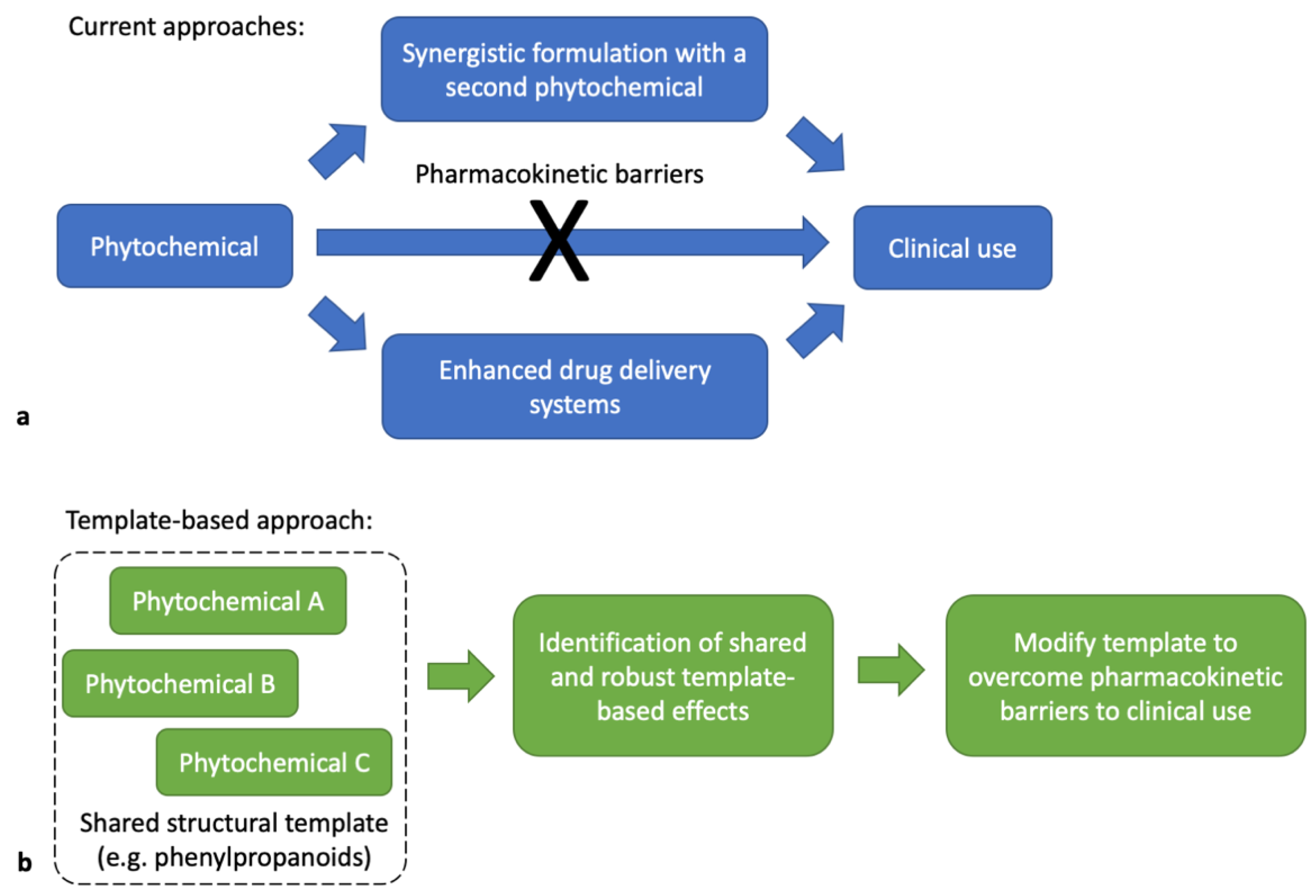
1. Introduction
2. Results
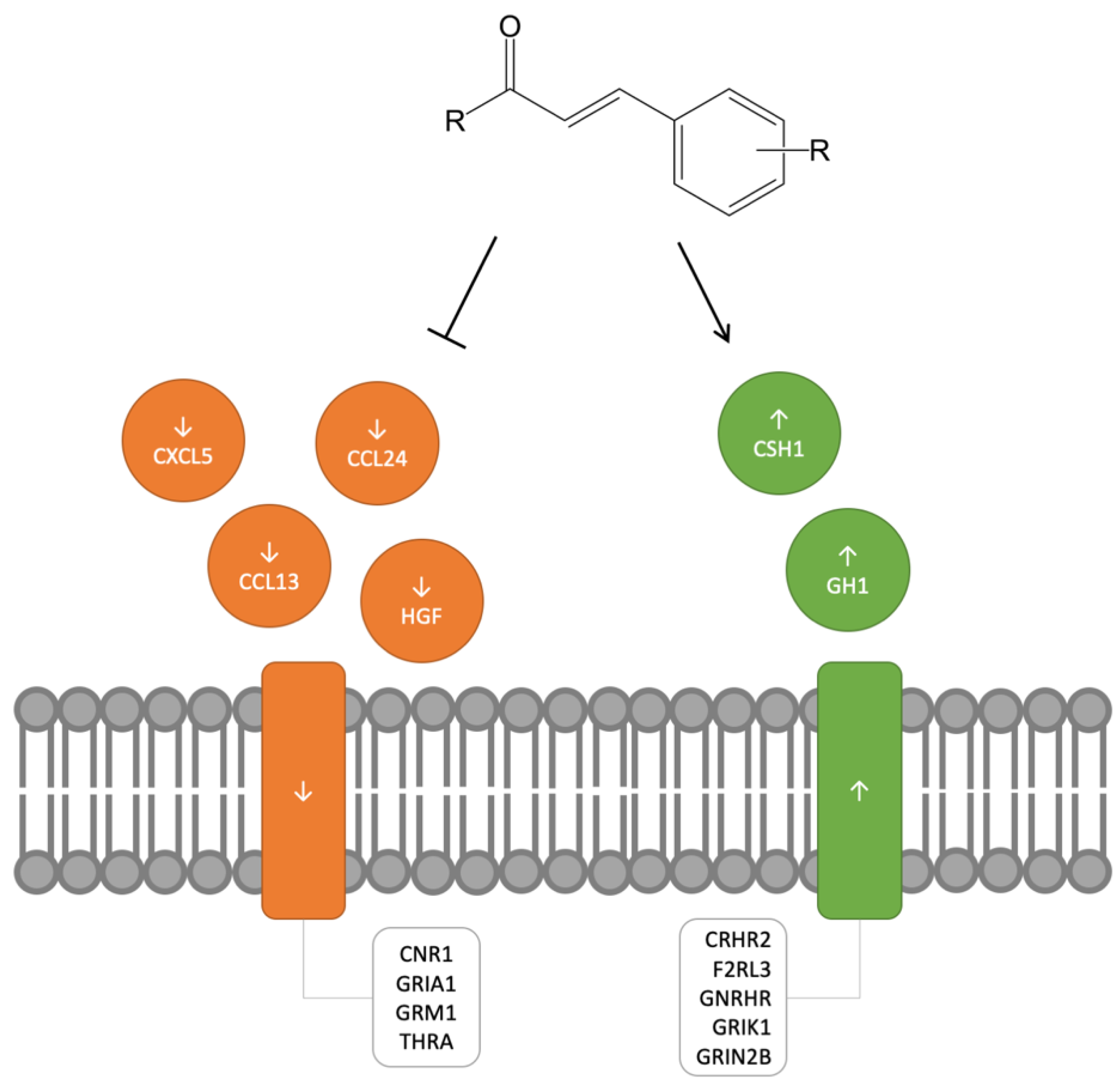
2.1. Downregulated Expression Data for Cinnamon-Based Phenylpropanoids
2.2. Upregulated Expression Data for Cinnamon-Based Phenylpropanoids
3. Discussion
4. Materials and Methods
4.1. Data Mining
4.2. Pathway Analysis
4.3. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Yuan, H.; Ma, Q.; Ye, L.; Piao, G. The Traditional Medicine and Modern Medicine from Natural Products. Molecules 2016, 21, 559. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.V.; Gan, S.H. Cinnamon: A Multifaceted Medicinal Plant. Evid. Based Complement. Altern. Med. 2014, 2014, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Pontiki, E.; Hadjipavlou-Litina, D. Antioxidant and anti-inflammatory activity of aryl-acetic and hydroxamic acids as novel lipoxygenase inhibitors. Med. Chem. 2006, 2, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Szwajgier, D.; Borowiec, K.; Pustelniak, K. The Neuroprotective Effects of Phenolic Acids: Molecular Mechanism of Action. Nutrients 2017, 9, 477. [Google Scholar] [CrossRef]
- Guzman, J.D. Natural Cinnamic Acids, Synthetic Derivatives and Hybrids with Antimicrobial Activity. Molecules 2014, 19, 19292–19349. [Google Scholar] [CrossRef]
- Ginsburg, I.; Kohen, R.; Koren, E. Microbial and host cells acquire enhanced oxidant-scavenging abilities by binding polyphenols. Arch. Biochem. Biophys. 2011, 506, 12–23. [Google Scholar] [CrossRef]
- Scalbert, A.; Williamson, G. Dietary Intake and Bioavailability of Polyphenols. J. Nutr. 2000, 130 (Suppl. S8), 2073S–2085S. [Google Scholar] [CrossRef]
- Aqil, F.; Munagala, R.; Jeyabalan, J.; Vadhanam, M.V. Bioavailability of phytochemicals and its enhancement by drug delivery systems. Cancer Lett. 2013, 334, 133–141. [Google Scholar] [CrossRef]
- Lv, C.; Wu, X.; Wang, X.; Su, J.; Zeng, H.; Zhao, J.; Lin, S.; Liu, R.; Li, H.; Li, X.; et al. The gene expression profiles in response to 102 traditional Chinese medicine (TCM) components: A general template for research on TCMs. Sci. Rep. 2017, 7, 352. [Google Scholar] [CrossRef]
- Clough, E.; Barrett, T. The Gene Expression Omnibus Database. Methods Mol. Biol. 2016, 1418, 93–110. [Google Scholar] [CrossRef]
- Huang Da, W.; Sherman, B.T.; Lempicki, R.A. Bioinformatics enrichment tools: Paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009, 37, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Peperidou, A.; Pontiki, E.; Hadjipavlou-Litina, D.; Voulgari, E.; Avgoustakis, K. Multifunctional Cinnamic Acid Derivatives. Molecules 2017, 22, 1247. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, K. Synergistic effects of thearubigin and genistein on human prostate tumor cell (PC-3) growth via cell cycle arrest. Cancer Lett. 2000, 151, 103–109. [Google Scholar] [CrossRef]
- Gao, X.; Luo, M.; Wang, B.-L.; Li, Y.-L.; Zhang, Q.; Qian, Z.-Y.; Shi, H.-S.; Fan, M.; Liu, Z.; Shen, Y.-M. Codelivery of curcumin and doxorubicin by MPEG-PCL results in improved efficacy of systemically administered chemotherapy in mice with lung cancer. Int. J. Nanomed. 2013, 8, 3521–3531. [Google Scholar] [CrossRef] [PubMed]
- Cabral, H.; Kataoka, K. Progress of drug-loaded polymeric micelles into clinical studies. J. Control. Release 2014, 190, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.D.; Nedjai, B.; Hurst, T.; Pennington, D.J. Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease. Biochim. Biophys. Acta 2014, 1843, 2563–2582. [Google Scholar] [CrossRef] [PubMed]
- Coussens, L.M.; Werb, Z. Inflammation and Cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Tung, Y.-T.; Chua, M.-T.; Wang, S.-Y.; Chang, S.-T. Anti-inflammation activities of essential oil and its constituents from indigenous cinnamon (Cinnamomum osmophloeum) twigs. Bioresour. Technol. 2008, 99, 3908–3913. [Google Scholar] [CrossRef]
- Sadeghi, S.; Davoodvandi, A.; Pourhanifeh, M.H.; Sharifi, N.; Arefnezhad, R.; Sahebnasagh, R.; Moghadam, S.A.; Sahebkar, A.; Mirzaei, H. Anti-cancer effects of cinnamon: Insights into its apoptosis effects. Eur. J. Med. Chem. 2019, 178, 131–140. [Google Scholar] [CrossRef]
- Emamghoreishi, M.; Farrokhi, M.R.; Amiri, A.; Keshavarz, M. The neuroprotective mechanism of cinnamaldehyde against amyloid-β in neuronal SHSY5Y cell line: The role of N-methyl-D-aspartate, ryanodine, and adenosine receptors and glycogen synthase kinase-3β. Avicenna J. Phytomed. 2019, 9, 271–280. [Google Scholar]
- Gaique, T.G.; Lopes, B.P.; Souza, L.L.; Paula, G.S.M.; Oliveira, K.J.; Pazos-Moura, C.C. Cinnamon intake reduces serum T3 level and modulates tissue-specific expression of thyroid hormone receptor and target genes in rats. J. Sci. Food Agric. 2016, 96, 2889–2895. [Google Scholar] [CrossRef] [PubMed]
- Albayram, O.; Alferink, J.; Pitsch, J.; Piyanova, A.; Neitzert, K.; Poppensieker, K.; Mauer, D.; Michel, K.; Legler, A.; Becker, A.; et al. Role of CB1 cannabinoid receptors on GABAergic neurons in brain aging. Proc. Natl. Acad. Sci. USA 2011, 108, 11256–11261. [Google Scholar] [CrossRef] [PubMed]
- Heuberger, M.D.; Schuepbach, R.A. Protease-activated receptors (PARs): Mechanisms of action and potential therapeutic modulators in PAR-driven inflammatory diseases. Thromb. J. 2019, 17, 4. [Google Scholar] [CrossRef] [PubMed]
- Stamper, B.D. The Utilization of Online Gene Expression Data Repositories to Generate Testable Hypotheses in the Laboratory. Transcriptomics 2013, 1, 104. [Google Scholar] [CrossRef]
- Huang Da, W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: The microarray samples used in this study are readily available through the Gene Expression Omnibus Series GSE85871. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, N.U.; Stamper, B.D. A Template-Based Approach for Guiding and Refining the Development of Cinnamon-Based Phenylpropanoids as Drugs. Molecules 2020, 25, 4629. https://doi.org/10.3390/molecules25204629
Nguyen NU, Stamper BD. A Template-Based Approach for Guiding and Refining the Development of Cinnamon-Based Phenylpropanoids as Drugs. Molecules. 2020; 25(20):4629. https://doi.org/10.3390/molecules25204629
Chicago/Turabian StyleNguyen, Ngoc Uy, and Brendan David Stamper. 2020. "A Template-Based Approach for Guiding and Refining the Development of Cinnamon-Based Phenylpropanoids as Drugs" Molecules 25, no. 20: 4629. https://doi.org/10.3390/molecules25204629
APA StyleNguyen, N. U., & Stamper, B. D. (2020). A Template-Based Approach for Guiding and Refining the Development of Cinnamon-Based Phenylpropanoids as Drugs. Molecules, 25(20), 4629. https://doi.org/10.3390/molecules25204629






