Study on Structure Activity Relationship of Natural Flavonoids against Thrombin by Molecular Docking Virtual Screening Combined with Activity Evaluation In Vitro
Abstract
1. Introduction
2. Results and Discussion
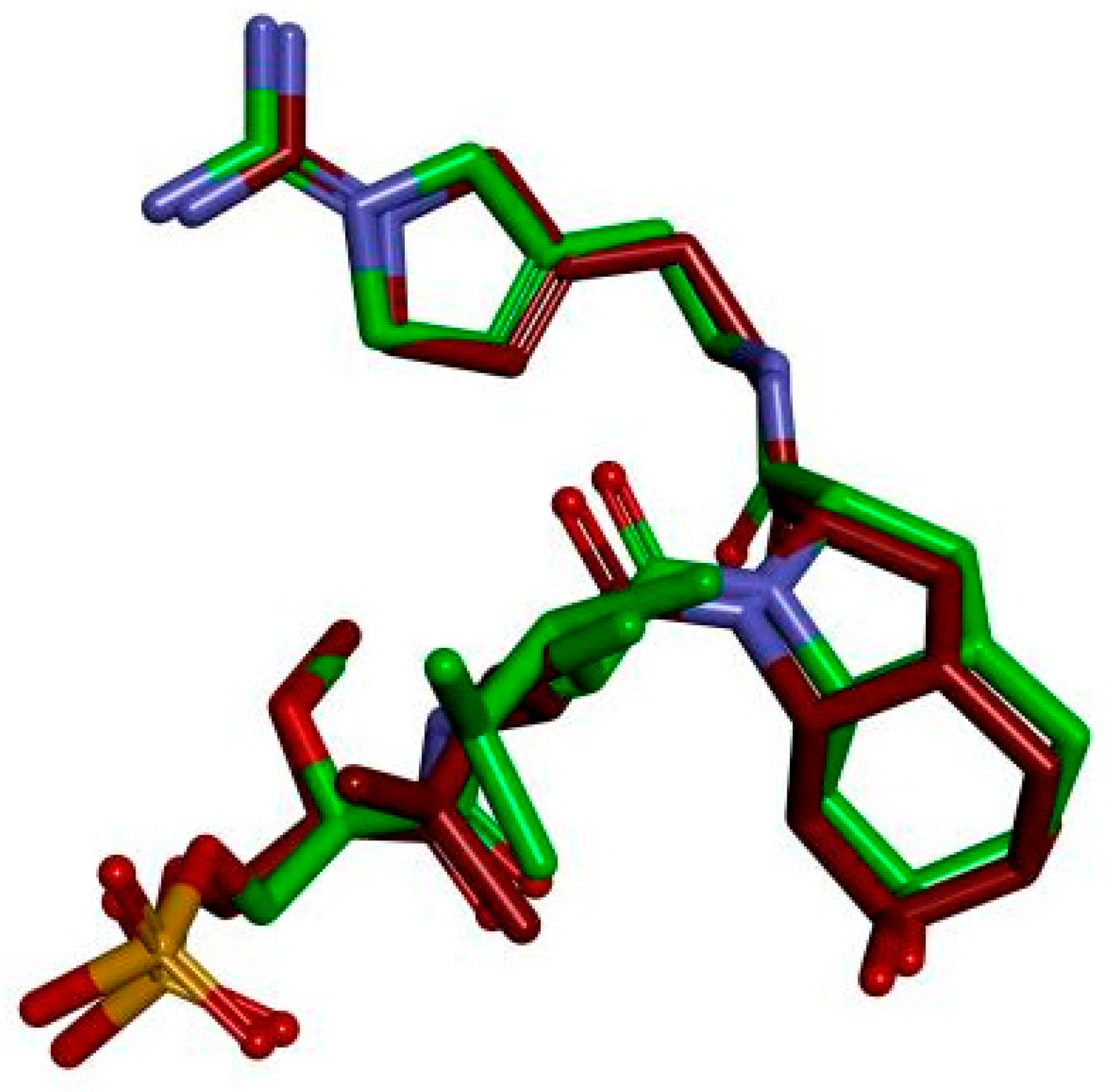
2.1. The Feasibility of the Established Docking Protocol
2.2. In Silico Screening for Thrombin Inhibitors
2.3. In Vitro Screening for Thrombin Inhibitors
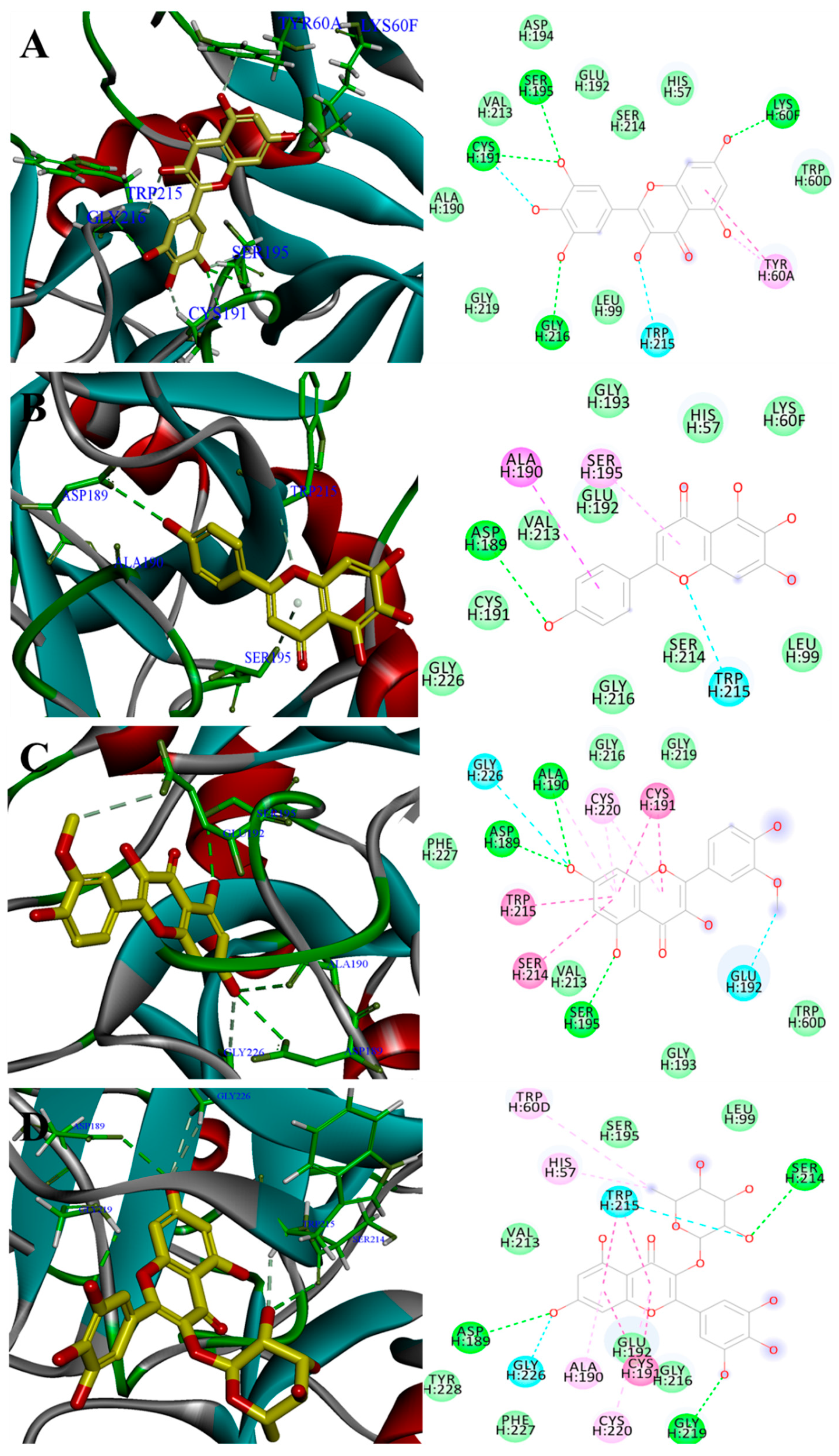
2.4. Binding Site of Myricetin, Scutellarein, Isorhamnetin and Myricitrin in Thrombin Model
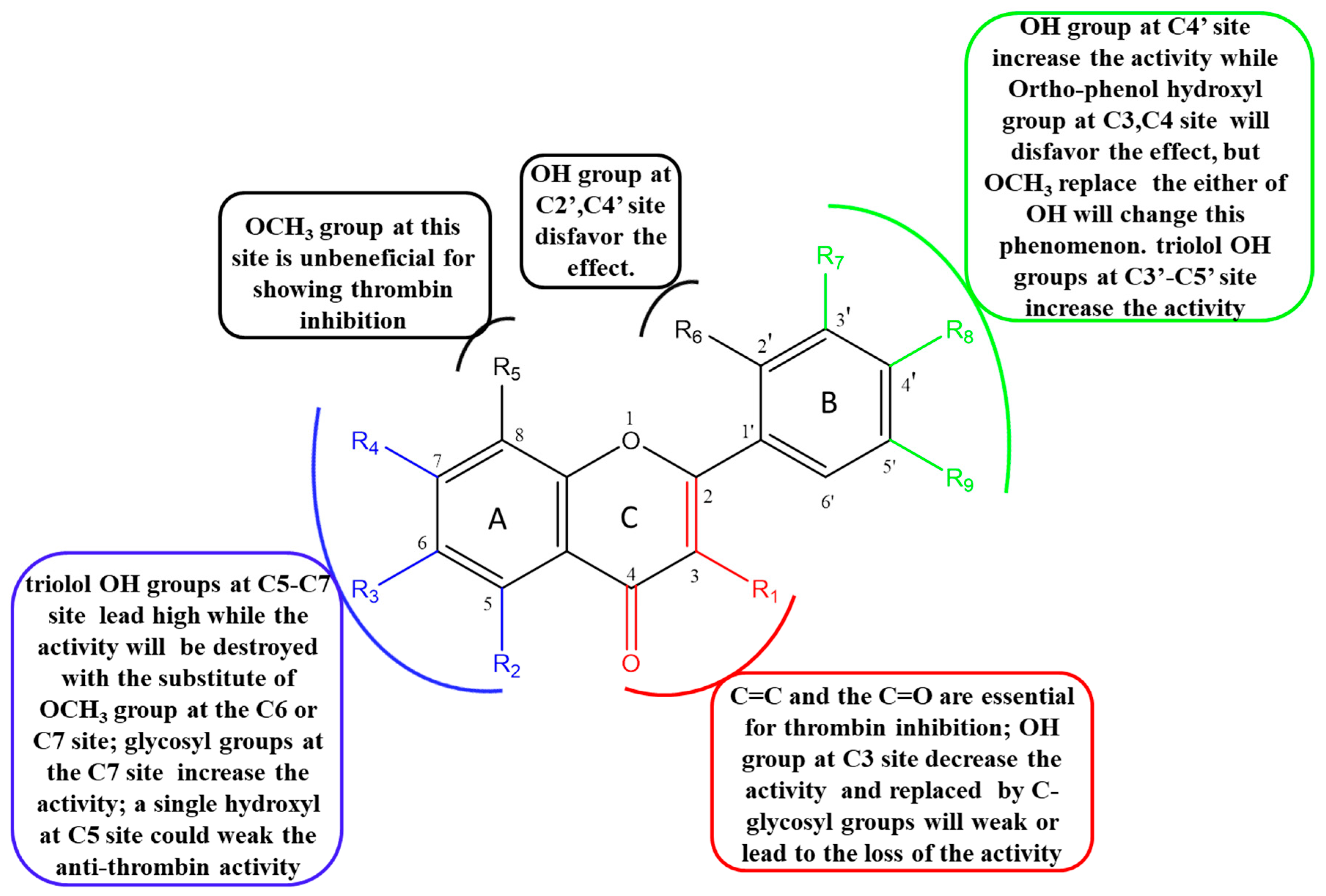
2.5. The Structure–Activity Relationship (SAR) of Flavonoids on Inhibition of Thrombin
2.5.1. The Influence of Double Bond, Carbonyl, Hydroxyl and O-glycosyl Groups at the C2, C3, C4 Sites of C Ring
2.5.2. The Influence of Hydroxyl, Methoxyl and O-glycosyl Groups at the C5, C6, C7, C8 Sites of A Ring
2.5.3. The Influence of Hydroxyl and Methoxyl Groups at the C2′, C3′, C4′ and C5′ Sites of B Ring
3. Materials and Methods
3.1. Materials and Chemicals
3.2. In Silico Molecular Docking
3.2.1. The Pretreatment of Receptor and Ligands
3.2.2. Molecular Docking and Virtual Screening
3.3. Thrombin Inhibitory Activity Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bijak, M.; Saluk, J.; Szelenberger, R. Popular naturally occurring antioxidants as potential anticoagulant drugs. Chem. Biol. Interact. 2016, 257, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Villoutreix, B.O.; Miteva, M.A. Discoidin domains as emerging therapeutic targets. Trends Pharmacol. Sci. 2016, 37, 641–659. [Google Scholar] [CrossRef] [PubMed]
- Van Cott, E.; Roberts, A.; Dager, W. Laboratory monitoring of parenteral direct thrombin inhibitors. Semin. Thromb. Hemost. 2017, 43, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Song, H.P.; Li, P. Screening of direct thrombin inhibitors from Radix Salviae Miltiorrhizae by a peak fractionation approach. J. Pharm. Biomed. Anal. 2015, 109, 85. [Google Scholar] [CrossRef] [PubMed]
- Bijak, M.; Bobrowski, M.; Borowiecka, M. Anticoagulant effect of polyphenols-rich extracts from black chokeberry and grape seeds. Fitoterapia 2011, 82, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Park, S.E.; Kim, S.J. Kaempferol inhibits thrombosis and platelet activation. Biochimie 2015, 115, 177–186. [Google Scholar] [CrossRef]
- Lv, J.L.; Li, Z.Z.; Zhang, L.B. Two new flavonoids from Artemisia argyi with their anticoagulation activities. Nat. Prod. Res. 2018, 32, 632–639. [Google Scholar] [CrossRef]
- Cuccioloni, M.; Mozzicafreddo, M.; Bonfili, L. Natural occurring Polyphenols as template for drug design focus on serine proteases. Chem. Biol. Drug Des. 2010, 74, 1–15. [Google Scholar] [CrossRef]
- Hung, H.Y.; Wu, T.S. Recent progress on the traditional Chinese medicines that regulate the blood. J. Food Drug Anal. 2016, 24, 221–238. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, H.; Wu, H. Characterization and quantification by LC-MS/MS of the chemical components of the heating products of the flavonoids extract in Pollen Typhae for transformation rule exploration. Molecules 2015, 20, 18352. [Google Scholar] [CrossRef]
- Singh, M.; Kaur, M.; Silakari, O. Flavones: An important scaffold for medicinal chemistry. Eur. J. Med. Chem. 2014, 84, 206. [Google Scholar] [CrossRef]
- Kuang, X.H. Chinese Medicine Chemistry; China Press of Traditional Chinese Medicine: Beijing, China, 2003. (In Chinese) [Google Scholar]
- Lee, W.; Bae, J.S. Antithrombotic and antiplatelet activities of orientin in vitro, and in vivo. J. Funct. Foods. 2015, 17, 388–398. [Google Scholar] [CrossRef]
- Pal, S.; Saha, C. A review on structure–affinity relationship of dietary flavonoids with serum albumins. J. Biomol. Struct. Dyn. 2014, 32, 1132–1147. [Google Scholar] [CrossRef] [PubMed]
- Sarbu, L.G.; Bahrin, L.G.; Babii, C.; Stefan, M.; Birsa, M.L. Synthetic flavonoids with antimicrobial activity: A review. J. Appl. Microbiol. 2019, 127, 1282–1290. [Google Scholar] [CrossRef] [PubMed]
- Alam, S.; Khan, F. 3D-QSAR, docking, ADME/Tox studies on flavone analogs reveal anticancer activity through tankyrase inhibition. Sci. Rep. 2019, 9, 5414. [Google Scholar] [CrossRef]
- Liu, L.; Ma, H.; Yang, N. A Series of natural flavonoids as thrombin inhibitors: Structure-activity relationships. Thromb. Res. 2010, 126, e365–e378. [Google Scholar] [CrossRef]
- Brahma, R.K.; Blanchet, G.; Kaur, S. Expression and characterization of haemathrins, madanin-like thrombin inhibitors, isolated from the salivary gland of tick Haemaphysalis bispinosa (Acari: Ixodidae). Thromb. Res. 2017, 152, 20–29. [Google Scholar] [CrossRef]
- Śledź, P.; Caflisch, A. Protein structure-based drug design: From docking to molecular dynamics. Curr. Opin. Struct. Biol. 2017, 48, 93–103. [Google Scholar] [CrossRef]
- Weng, Z.M.; Ge, G.B.; Dou, T.Y. Characterization and structure-activity relationship studies of flavonoids as inhibitors against human carboxylesterase 2. Bioorg. Chem. 2018, 77, 320. [Google Scholar] [CrossRef]
- Wang, X.; Ren, Z.z.; He, Y.s.; Xiang, Y.h.; Zhang, Y.l.; Qiao, Y.J. A combination of pharmacophore modeling, molecular docking and virtual screening for iNOS inhibitors from Chinese herbs. Biomed. Mater. Eng. 2014, 24, 1315–1322. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Yang, Y. Identification of berberine as a direct thrombin inhibitor from traditional Chinese medicine through structural, functional and binding studies. Sci. Rep. 2017, 7, 44040. [Google Scholar] [CrossRef] [PubMed]
- Kongpichitchoke, T.; Hsu, J.L.; Huang, T.C. Number of hydroxyl groups on the B-ring of flavonoids affects their antioxidant activity and interaction with phorbol ester binding site of PKCδ C1B domain: In vitro and in silico studies. J. Agric. Food Chem. 2015, 63, 4580–4586. [Google Scholar] [CrossRef] [PubMed]
- Adcock, D.M.; Strandberg, K.; Shima, M.; Marlar, R.A. Advantages, disadvantages and optimization of one-stage and chromogenic factor activity assays in haemophilia A and B. Int. J. Lab. Hematol. 2018, 40, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Peyvandi, F.; Oldenburg, J.; Friedman, K.D. A critical appraisal of one-stage and chromogenic assays of factor VIII activity. J. Thromb. Haemost. 2016, 14, 248–261. [Google Scholar] [CrossRef] [PubMed]
- Harenberg, J.; Mar, x.S.; Erdle, S.; Krämer, R. Determination of the anticoagulant effects of new oral anticoagulants: An unmet need. Expert Rev. Hematol. 2012, 5, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.N.; Head, M.S.; Kulkarni, A. Validation studies of the site-directed Docking Program LibDock. J. Chem. Inf. Model. 2007, 47, 2159–2171. [Google Scholar] [CrossRef]
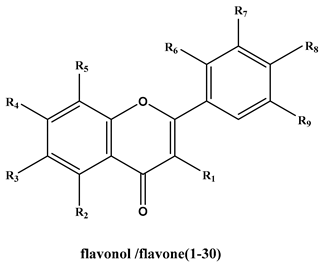
| No. | Compound | R1 | R2 | R3 | R4 | R5 | R6 | R7 | R8 | R9 | IC50(μM) | -CDOCKER_ Energy (Kcal/mol) | -CDOCKER_INTERACTION_ Energy (Kcal/mol) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| flavonol | |||||||||||||
| 1 | Galangin | OH | OH | H | OH | H | H | H | H | H | 159 ± 0 | 27.5 | 32.1 |
| 2 | Herbacetin | OH | OH | H | OH | OH | H | H | OH | H | 133 ± 4 | 31.0 | 31.2 |
| 3 | Kaempferol | OH | OH | H | OH | H | H | H | OH | H | 107 ± 2 | 33.6 | 40.2 |
| 4 | Astragaline | O-glc | OH | H | OH | H | H | H | OH | H | 217 ± 5 | 7.92 | 46.8 |
| 5 | Kaempferol-7-O-β-D- glucopyranoside | OH | OH | H | O-glc | H | H | H | OH | H | >500 | 22.6 | 48.5 |
| 6 | Morin | OH | OH | H | OH | H | OH | H | OH | H | 237 ± 26 | 33.0 | 39.3 |
| 7 | Fisetin | OH | H | H | OH | H | H | OH | OH | H | 175 ± 18 | 33.4 | 36.2 |
| 8 | Quercetin | OH | OH | H | OH | H | H | OH | OH | H | 205 ± 14 | 32.2 | 34.3 |
| 9 | Quercitrin | O-rha | OH | H | OH | H | H | OH | OH | H | >500 | 17.5 | 51.2 |
| 10 | Isoquercitrin | O-glc | OH | H | OH | H | H | OH | OH | H | >500 | 13.6 | 58.8 |
| 11 | Rutin | O-rut | OH | H | OH | H | H | OH | OH | H | 274 ± 23 | 5.76 | 61.7 |
| 12 | Isorhamnetin | OH | OH | H | OH | H | H | OCH3 | OH | H | 72.2 ± 4.8 | 29.2 | 35.1 |
| 13 | Isorhamnetin-3-O- neohesperidoside | O-glc-rha | OH | H | OH | H | H | OCH3 | OH | H | >500 | 5.76 | 57.2 |
| 14 | Typhaneoside | O-rha-(glc)2 | OH | H | OH | H | H | OCH3 | OH | H | >500 | 2.97 | 75.8 |
| 15 | Myricetin | OH | OH | H | OH | H | H | OH | OH | OH | 56.5 ± 2.1 | 36.2 | 37.7 |
| 16 | Myricitrin | O-rha | OH | H | OH | H | H | OH | OH | OH | 79.5 ± 3.4 | 17.4 | 50.3 |
| flavone | |||||||||||||
| 17 | Chrysin | H | OH | H | OH | H | H | H | H | H | >500 | 25.5 | 30.3 |
| 18 | Baicalein | H | OH | OH | OH | H | H | H | H | H | 249 ± 31 | 28.7 | 28.5 |
| 19 | Baicalin | H | OH | OH | O-glc | H | H | H | H | H | 88.6 ± 8.2 | 20.5 | 40.0 |
| 20 | Wogonin | H | OH | H | OH | OCH3 | H | H | H | H | >500 | 20.1 | 28.6 |
| 21 | Wogonoside | H | OH | H | O-glu | OCH3 | H | H | H | H | >500 | 29.5 | 38.2 |
| 22 | Hispidulin | H | OH | OCH3 | OH | H | H | H | OH | H | 126 ± 6 | 29.5 | 38.2 |
| 23 | Scutellarein | H | OH | OH | OH | H | H | H | OH | H | 70.8 ± 2.7 | 35.1 | 35.7 |
| 24 | Apigenin | H | OH | H | OH | H | H | H | OH | H | 96.2 ± 10.0 | 25.9 | 30.8 |
| 25 | Genkwanin | H | OH | H | OCH3 | H | H | H | OH | H | 212 ± 30 | 26.1 | 33.2 |
| 26 | Hydroxygenkwanin | H | OH | H | OCH3 | H | H | OH | OH | H | 99.7 ± 5.5 | 33.6 | 38.6 |
| 27 | Luteolin | H | OH | H | OH | H | H | OH | OH | H | 146 ± 10 | 31.5 | 36.6 |
| 28 | Luteoloside | H | OH | H | O-glc | H | H | OH | OH | H | 155 ± 18 | 14.1 | 45.4 |
| 29 | Diosmetin | H | OH | H | OH | H | H | OH | OCH3 | H | 131 ± 14 | 31.41 | 38.4 |
| 30 | 6-Methoxyluteolin | H | OH | OCH3 | OH | H | H | OH | OH | H | >500 | 31.0 | 38.7 |
 | |||||||||||||
| flavanonol | |||||||||||||
| 31 | Dihydroquercetin | OH | OH | H | OH | H | H | OH | OH | H | >500 | 34.4 | 39.7 |
| 32 | Dihydromyricetin flavanone | OH | OH | H | OH | H | H | OH | OH | OH | >500 | 40.3 | 43.3 |
| 33 | Naringenin | H | OH | H | OH | H | H | H | OH | H | >500 | 30.3 | 35.5 |
| 34 | Naringin | H | OH | H | O-glu-rha | H | H | H | OH | H | 444 ± 59 | 7.6 | 56.3 |
| 35 | Narirutin | H | OH | H | O-rut | H | H | H | OH | H | >500 | 10.8 | 55.7 |
| 36 | Hesperetin | H | OH | H | OH | H | H | OH | OCH3 | H | >500 | 35.5 | 41.9 |
| 37 | Hesperidin | H | OH | H | O-glu-rha | H | H | OH | OCH3 | H | >500 | 6.28 | 60.1 |
| 38 | Neohesperidin | H | OH | H | O-(glc)2 | H | H | OH | H | OCH3 | >500 | 8.64 | 55.2 |
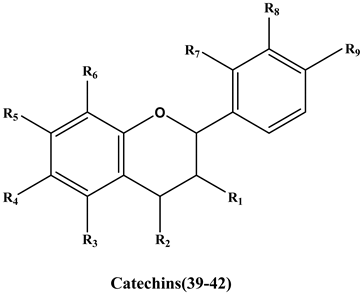 | |||||||||||||
| Catechins | |||||||||||||
| 39 | Epicatechin | OH↑ | H | OH | H | OH | H | H | OH | OH | >500 | 31.5 | 36.5 |
| 40 | (-)-epigallocatechin | OH↓ | H | OH | H | OH | H | H | OH | OH | >500 | 33.4 | 39.3 |
| 41 | (-)-Epicatechin gallate | 1* | H | OH | H | OH | H | H | OH | OH | >500 | 52.1 | 54.6 |
| 42 | Proanthocyanidin B1 | OH | 2* | OH | H | OH | H | H | OH | OH | >500.0 | 42.4 | 55.9 |
| Argatroban | - | - | - | - | - | - | - | - | - | 1.86 ± 0.10 | - | - | |
 ; 2*
; 2*  ; glc, glucose; glu, glucuronic acid; rha, rhamnose; rut, rutinose.
; glc, glucose; glu, glucuronic acid; rha, rhamnose; rut, rutinose.| Group | Compound | IC50 ±SD (μM) | -Cdocker_ Energy | -Cdocker_ Interaction_ Energy | Number of Hydrogen Bonds | Residue |
|---|---|---|---|---|---|---|
| Strong inhibitor | Myricetin | 56.5 ± 2.1 | 36.2 | 37.7 | 5 | LYS60F, TYR60A, SER195, CYS191, GLY216 |
| Scutellarein | 70.8 ± 2.7 | 35.1 | 35.7 | 4 | ALA190, ASP189, TRP215, SER195 | |
| Isorhamnetin | 72.2 ± 4.8 | 29.2 | 35.1 | 9 | ALA190, ASP189, GLY226, GLU192, SER195, TRP215, SER214, CYS191, CYS220 | |
| Myricitrin | 79.5 ± 3.4 | 17.4 | 50.3 | 10 | GLY226, ASP189, SER214 *, GLY219, TRP215 *, ALA190, CYS191, CYS191, HIS57 *, TRP60D * | |
| Baicalin | 88.6 ± 8.2 | 20.5 | 40.0 | 5 | ALA190, CYS191, TRP215, GLY193, SER195 | |
| Apigenin | 96.2 ± 10.0 | 25.9 | 30.8 | 2 | TRP60D, TYR60A | |
| Hydroxygenkwanin | 99.7 ± 5.5 | 33.6 | 38.6 | 3 | HIS54, LYS60F, SER195 | |
| Moderate inhibitor | Kaempferol | 107 ± 2 | 33.6 | 40.2 | 6 | CYS220, CYS191, SER214, TRP215, ALA190, GLY226 |
| Hispidulin | 126 ± 6 | 29.5 | 38.2 | 7 | TRP60D, SER214, TRP215, CYS191, ALA190, HIS57, SER195 | |
| Diosmetin | 131 ± 14 | 31.41 | 38.4 | 5 | SER195, TYR60A, HIS57, LYS60F, HIS57, | |
| herbacetin | 133 ± 4 | 31.0 | 31.2 | 5 | CYS191, TRP215, GLU192, HIS57, TYR60A, | |
| Luteolin | 146 ± 10 | 31.5 | 36.6 | 6 | CYS220, CYS191, SER214, TRP215, ALA190, GLU192 | |
| luteoloside | 155 ± 18 | 14.1 | 45.4 | - | - | |
| galangin | 159 ± 0 | 27.5 | 32.1 | 5 | HIS57, HIS57, ALA190, CYS220, CYS191 | |
| fisetin | 175 ± 18 | 33.4 | 36.2 | CYS191, SER195, LYS60F, TYR60A, TRP215 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Yang, Z.; Su, F.; Li, J.; Boadi, E.O.; Chang, Y.-x.; Wang, H. Study on Structure Activity Relationship of Natural Flavonoids against Thrombin by Molecular Docking Virtual Screening Combined with Activity Evaluation In Vitro. Molecules 2020, 25, 422. https://doi.org/10.3390/molecules25020422
Wang X, Yang Z, Su F, Li J, Boadi EO, Chang Y-x, Wang H. Study on Structure Activity Relationship of Natural Flavonoids against Thrombin by Molecular Docking Virtual Screening Combined with Activity Evaluation In Vitro. Molecules. 2020; 25(2):422. https://doi.org/10.3390/molecules25020422
Chicago/Turabian StyleWang, Xiaoyan, Zhen Yang, Feifei Su, Jin Li, Evans Owusu Boadi, Yan-xu Chang, and Hui Wang. 2020. "Study on Structure Activity Relationship of Natural Flavonoids against Thrombin by Molecular Docking Virtual Screening Combined with Activity Evaluation In Vitro" Molecules 25, no. 2: 422. https://doi.org/10.3390/molecules25020422
APA StyleWang, X., Yang, Z., Su, F., Li, J., Boadi, E. O., Chang, Y.-x., & Wang, H. (2020). Study on Structure Activity Relationship of Natural Flavonoids against Thrombin by Molecular Docking Virtual Screening Combined with Activity Evaluation In Vitro. Molecules, 25(2), 422. https://doi.org/10.3390/molecules25020422





