Polyphenol Extracts from Three Colombian Passifloras (Passion Fruits) Prevent Inflammation-Induced Barrier Dysfunction of Caco-2 Cells
Abstract
:1. Introduction
2. Results
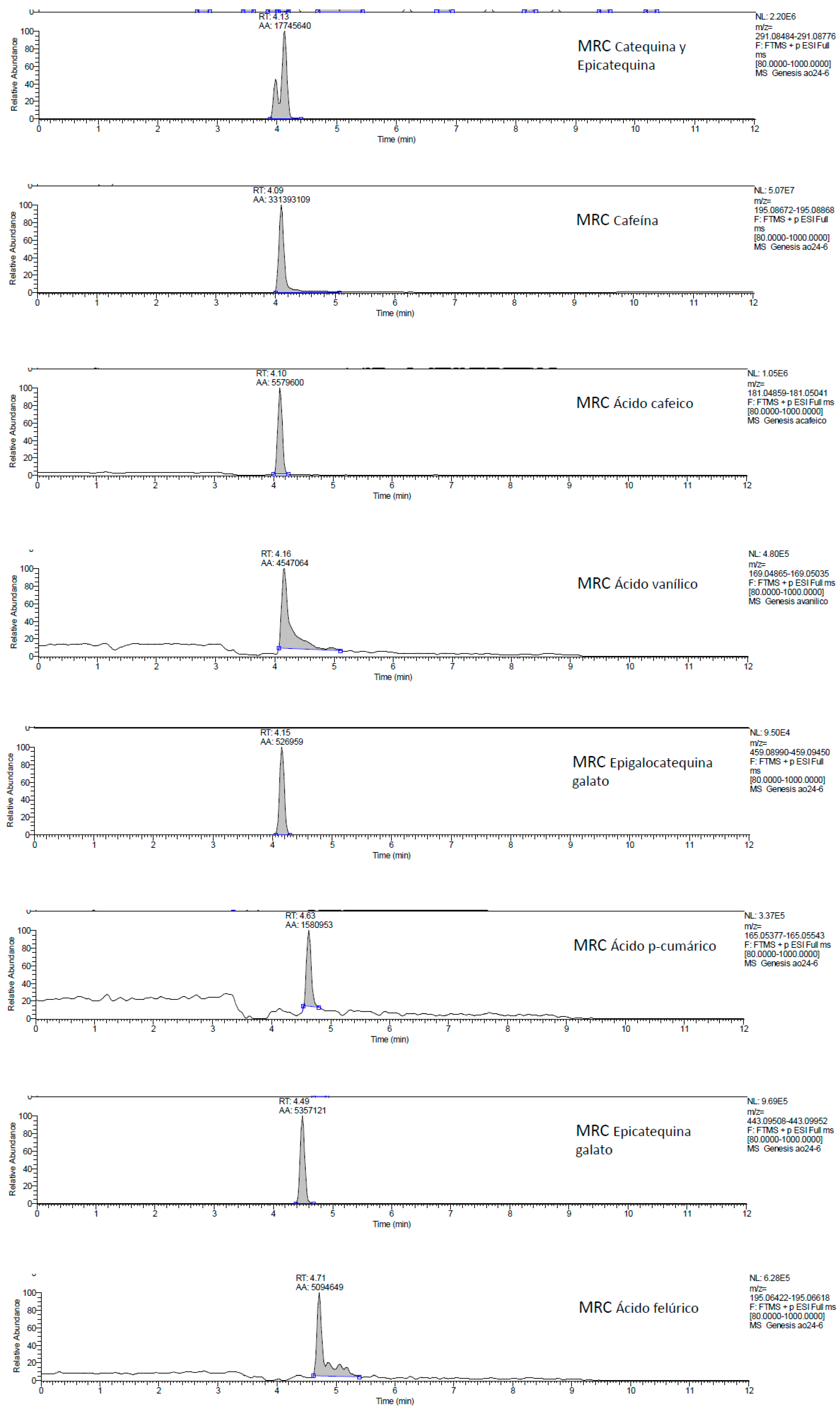
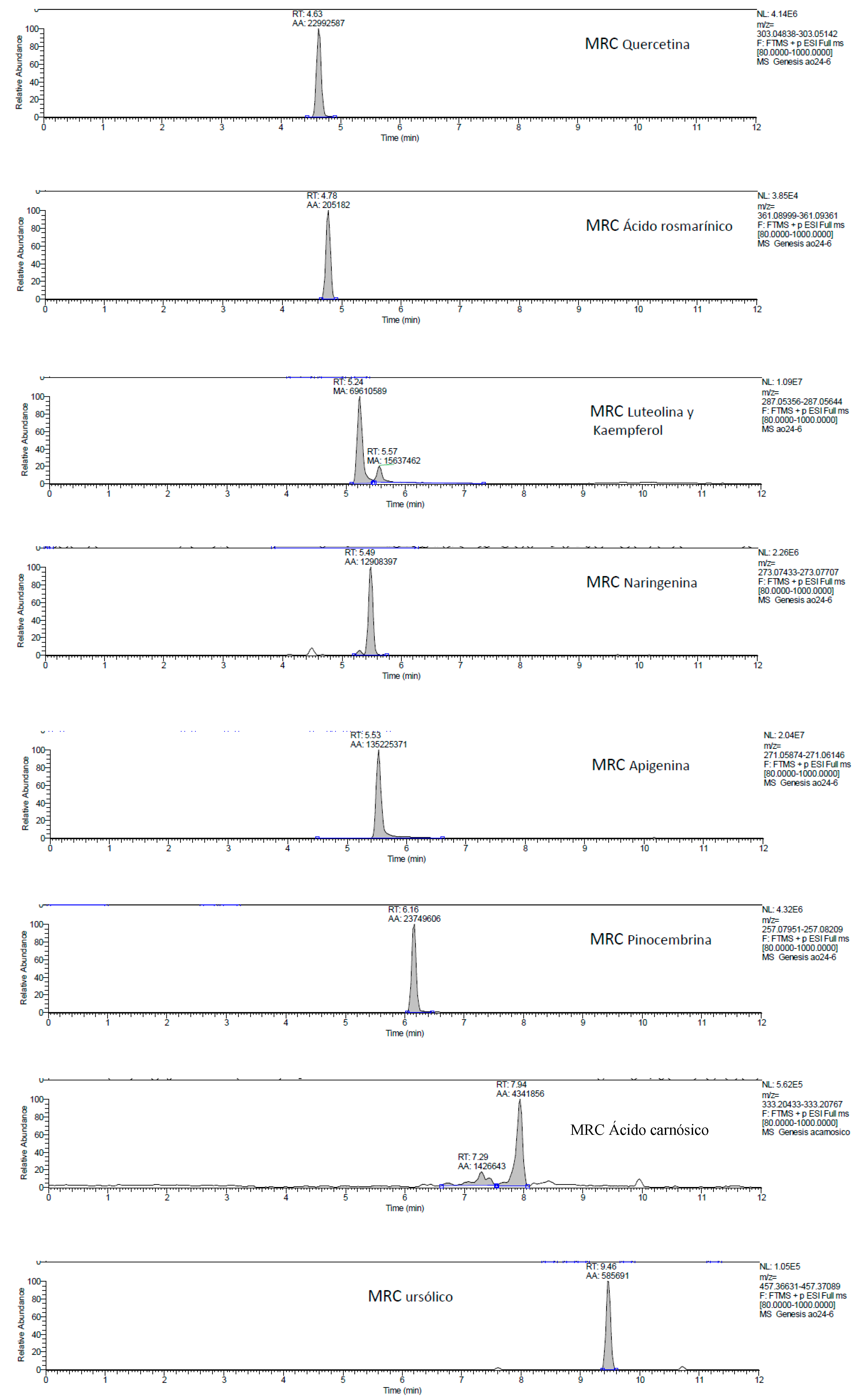
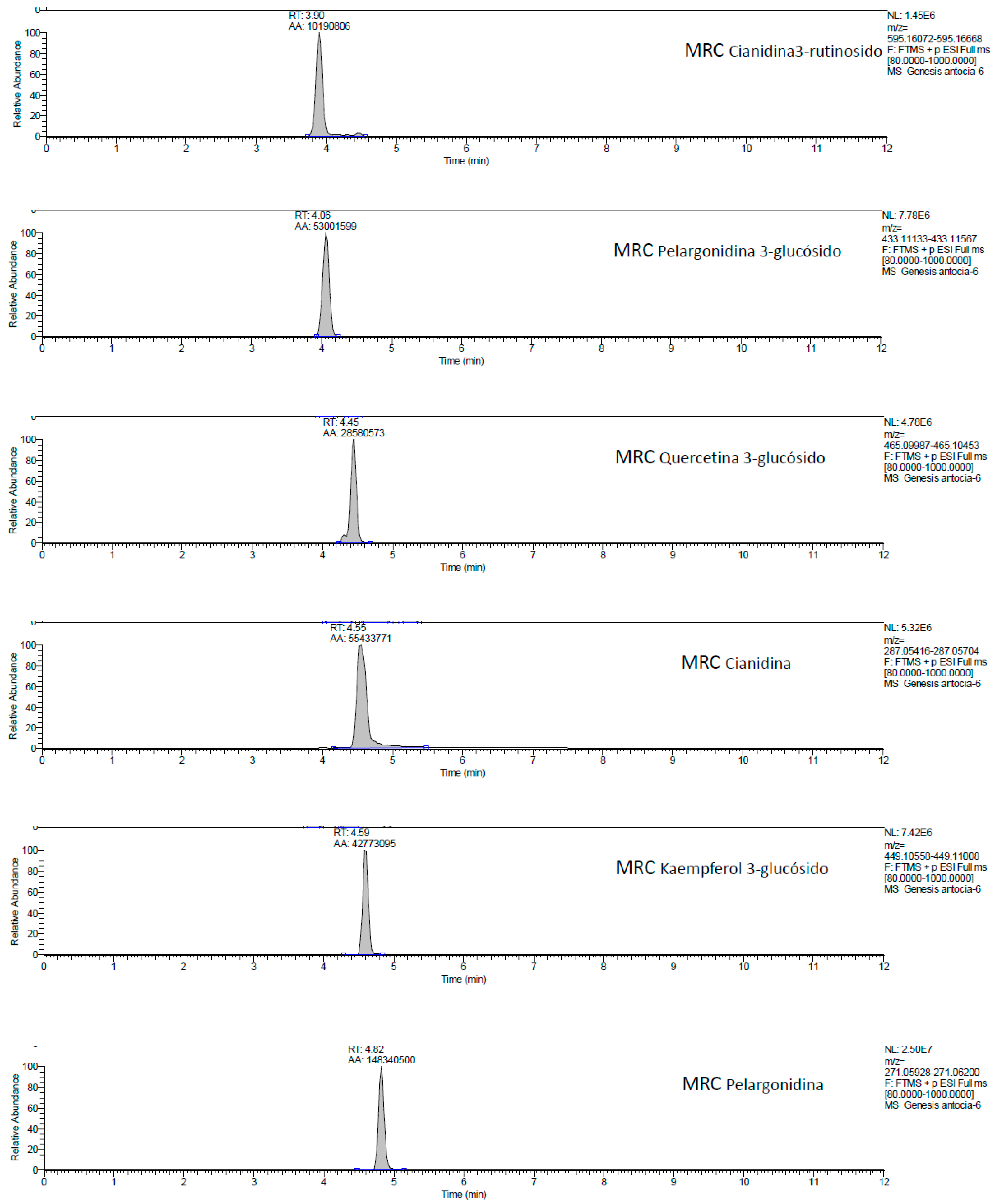
2.1. UHPLC-MS Analysis of Passiflora Extracts
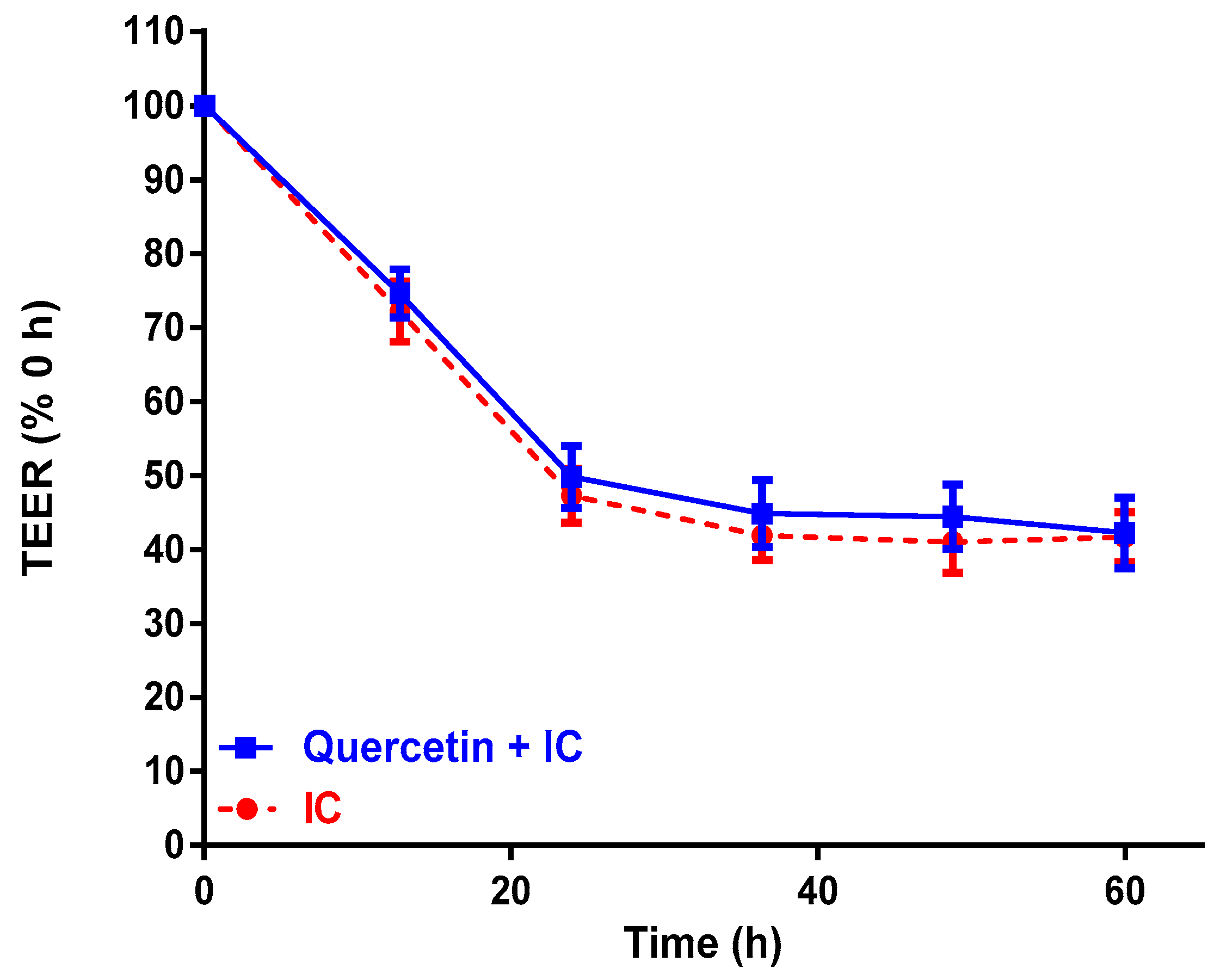
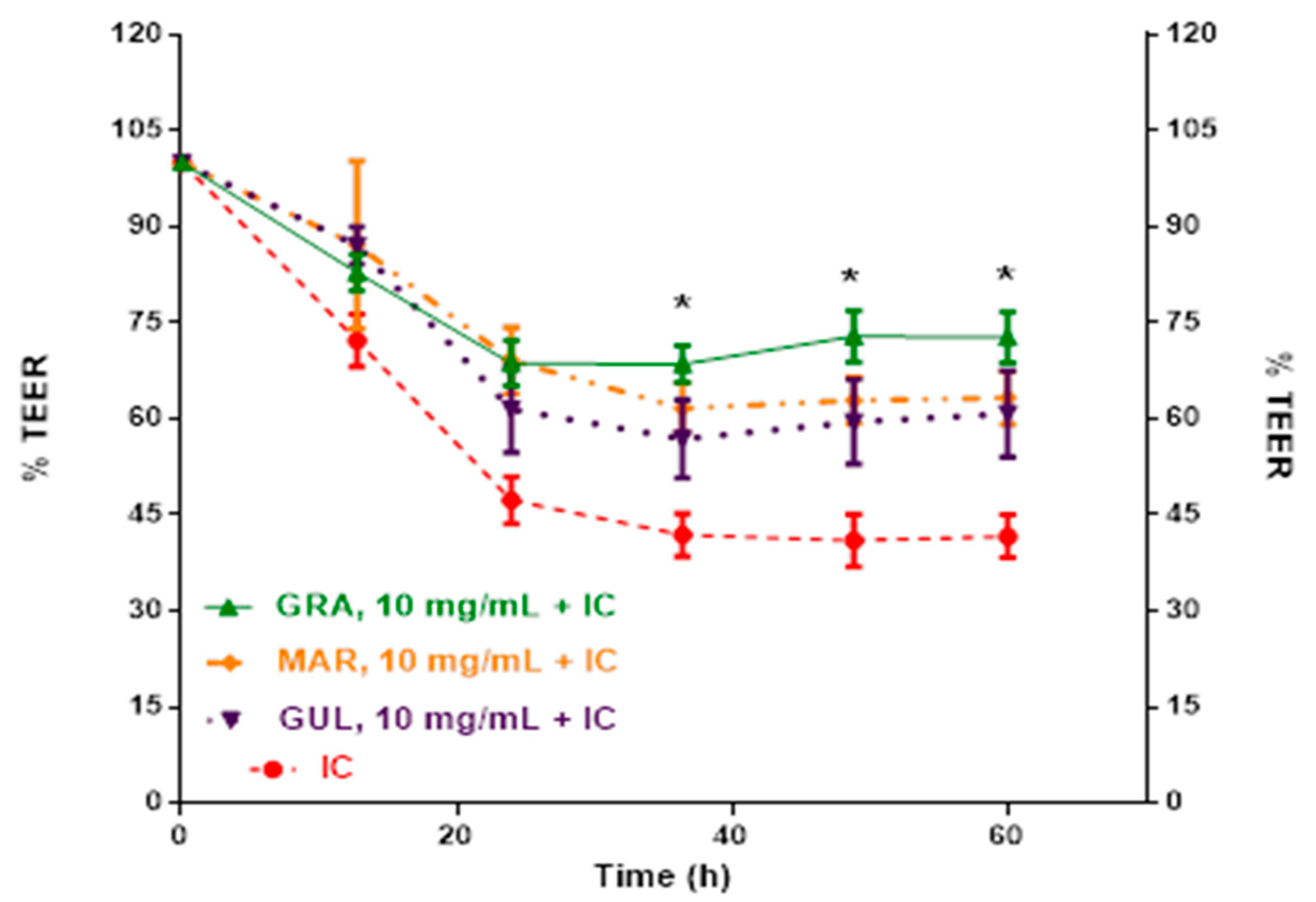
2.2. Effect of Passion Fruit on Caco-2 Barrier Function
3. Discussion
4. Materials and Methods
4.1. Standard Reference Solutions
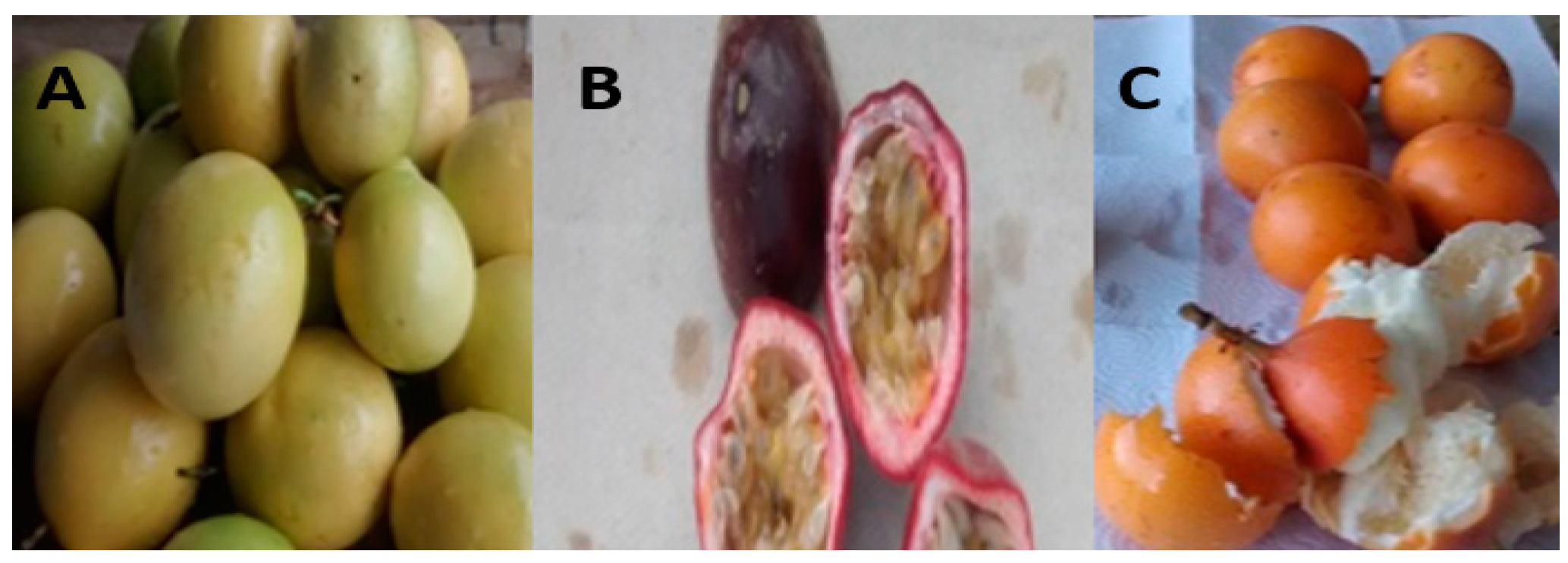
4.2. Sample Preparation
4.3. Polyphenol Extraction
4.4. UHPLC-MS Analysis of Passion Fruit Polyphenols
4.5. Maintenance of Caco-2 Cells
4.6. Assay for Cell Viability
4.7. Determination of Caco-2 Barrier Dysfunction
4.8. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
Appendix A

References
- Kunnumakkara, A.B.; Sailo, B.L.; Banik, K.; Harsha, C.; Prasad, S.; Gupta, S.C.; Bharti, A.C.; Aggarwal, B.B. Chronic diseases, inflammation, and spices: How are they linked? J. Transl. Med. 2018, 16, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forbes, A.; Escher, J.; Hébuterne, X.; Kłęk, S.; Krznaric, Z.; Schneider, S.; Shamir, R.; Stardelova, K.; Wierdsma, N.; Wiskin, A.E.; et al. ESPEN guideline: Clinical nutrition in inflammatory bowel disease. Clin. Nutr. 2017, 36, 321–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Odenwald, M.A.; Turner, J.R. The intestinal epithelial barrier: A therapeutic target? Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Pei, R.; Martin, D.A.; Valdez, J.C.; Liu, J.; Kerby, R.L.; Rey, F.E.; Smyth, J.A.; Liu, Z.; Bolling, B.W. Dietary prevention of colitis by aronia berry is mediated through increased Th17 and Treg. Mol. Nutr. Food. Res. 2019, 63, 1800985. [Google Scholar] [CrossRef] [PubMed]
- Koh, S.-J.; Choi, Y.-I.; Kim, Y.; Kim, Y.-S.; Choi, S.W.; Kim, J.W.; Kim, B.G.; Lee, K.L. Walnut phenolic extract inhibits nuclear factor kappaB signaling in intestinal epithelial cells, and ameliorates experimental colitis and colitis-associated colon cancer in mice. Eur. J. Nutr. 2019, 58, 1603–1613. [Google Scholar] [CrossRef] [PubMed]
- Frutas Exóticas. Available online: http://www.procolombia.co/node/1256 (accessed on 5 December 2019).
- Rotta, E.M.; Rodrigues, C.A.; Jardim, I.C.S.F.; Maldaner, L.; Visentainer, J.V. Determination of phenolic compounds and antioxidant activity in passion fruit pulp (Passiflora spp.) using a modified QuEChERS method and UHPLC-MS/MS. LWT 2019, 100, 397–403. [Google Scholar] [CrossRef]
- Gomes, S.V.F.; Portugal, L.A.; dos Anjos, J.P.; de Jesus, O.N.; de Oliveira, E.J.; David, J.P.; David, J.M. Accelerated solvent extraction of phenolic compounds exploiting a Box-Behnken design and quantification of five flavonoids by HPLC-DAD in Passiflora species. Microchem. J. 2017, 132, 28–35. [Google Scholar] [CrossRef]
- Medina, S.; Collado-González, J.; Ferreres, F.; Londoño-Londoño, J.; Jiménez-Cartagena, C.; Guy, A.; Durand, T.; Galana, J.M.; Gil-Izquierdo, A. Quantification of phytoprostanes—bioactive oxylipins—and phenolic compounds of Passiflora edulis Sims shell using UHPLC-QqQ-MS/MS and LC-IT-DAD-MS/MS. Food Chem. 2017, 229, 1–8. [Google Scholar] [CrossRef]
- Shanmugam, S.; Gomes, I.A.; Denadai, M.; Dos Santos Lima, B.; de Souza Araújo, A.A.; Narain, N.; Neta, M.T.S.L.; Serafini, M.R.; Quintans-Júnior, L.J.; Thangaraj, P. UHPLC-QqQ-MS/MS identification, quantification of polyphenols from Passiflora subpeltata fruit pulp and determination of nutritional, antioxidant, α-amylase and α-glucosidase key enzymes inhibition properties. Food Res. Int. 2018, 108, 611–620. [Google Scholar] [CrossRef]
- Srinivasan, B.; Kolli, A.R.; Esch, M.B.; Abaci, H.E.; Shuler, M.L.; Hickman, J.J. TEER Measurement techniques for In Vitro barrier model systems. J. Lab. Autom. 2015, 20, 107–126. [Google Scholar] [CrossRef] [Green Version]
- John, L.J.; Fromm, M.; Schulzke, J.-D. Epithelial barriers in intestinal inflammation. Antioxid Redox Sign. 2011, 15, 1255–1270. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Moon, K.M.; Kim, C.Y. Tight junction in the intestinal epithelium: Its association with diseases and regulation by phytochemicals. J. Immunol. Res. 2018, 2018, 2645465. Available online: https://www.hindawi.com/journals/jir/2018/2645465/ (accessed on 5 December 2019). [CrossRef] [PubMed] [Green Version]
- Hossen, I.; Hua, W.; Ting, L.; Mehmood, A.; Jingyi, S.; Duoxia, X.; Yanping, C.; Hongqing, W.; Zhipeng, G.; Kaiqi, Z. Phytochemicals and inflammatory bowel disease: A review. Crit Rev. Food Sci Nutr. 2019. [Google Scholar] [CrossRef] [PubMed]
- Woodhouse, C.A.; Patel, V.C.; Singanayagam, A.; Shawcross, D.L. Review article: The gut microbiome as a therapeutic target in the pathogenesis and treatment of chronic liver disease. Aliment. Pharmacol. Ther. 2018, 47, 192–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rhayat, L.; Maresca, M.; Nicoletti, C.; Perrier, J.; Brinch, K.S.; Christian, S.; Devillard, E.; Eckhardt, E. Effect of bacillus subtilis strains on intestinal barrier function and inflammatory response. Front. Immunol. 2019. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6449611/ (accessed on 5 December 2019). [CrossRef]
- Van De Walle, J.; Hendrickx, A.; Romier, B.; Larondelle, Y.; Schneider, Y.-J. Inflammatory parameters in Caco-2 cells: Effect of stimuli nature, concentration, combination and cell differentiation. Toxicol. Vitr. 2010, 24, 1441–1449. [Google Scholar] [CrossRef]
- Sonnier, D.I.; Bailey, S.R.; Schuster, R.M.; Lentsch, A.B.; Pritts, T.A. TNF-α induces vectorial secretion of IL-8 in Caco-2 cells. J. Gastrointest Surg. 2010, 14, 1592–1599. [Google Scholar] [CrossRef] [Green Version]
- Susewind, J.; de Souza Carvalho-Wodarz, C.; Repnik, U.; Collnot, E.-M.; Schneider-Daum, N.; Griffiths, G.W.; Lehr, C.M. A 3D co-culture of three human cell lines to model the inflamed intestinal mucosa for safety testing of nanomaterials. Nanotoxicology 2016, 10, 53–62. [Google Scholar] [CrossRef]
- Le Phuong Nguyen, T.; Fenyvesi, F.; Remenyik, J.; Homoki, J.R.; Gogolák, P.; Bácskay, I.; Fehér, P.; Ujhelyi, Z.; Vasvári, G.; Vecsernyés, M. Protective effect of pure sour cherry anthocyanin extract on cytokine-induced inflammatory Caco-2 monolayers. Nutrients. 2018, 10, 861. [Google Scholar] [CrossRef] [Green Version]
- Burgos-Edwards, A.; Martín-Pérez, L.; Jiménez-Aspee, F.; Theoduloz, C.; Schmeda-Hirschmann, G.; Larrosa, M. Anti-inflammatory effect of polyphenols from Chilean currants (Ribes magellanicum and R. punctatum) after in vitro gastrointestinal digestion on Caco-2 cells: Anti-inflammatory activity of in vitro digested Chilean currants. J. Funct. Foods. 2019, 59, 329–336. [Google Scholar] [CrossRef]
- Vitale, M.; Masulli, M.; Rivellese, A.A.; Bonora, E.; Cappellini, F.; Nicolucci, A.; Syuatrito, S.; Antenucci, D.; Barrea, A.; Bianchi, C.; et al. Dietary intake and major food sources of polyphenols in people with type 2 diabetes: The TOSCA.IT Study. Eur. J. Nutr. 2018, 57, 679–688. [Google Scholar] [CrossRef] [PubMed]
- Miranda, A.M.; Steluti, J.; Fisberg, R.M.; Marchioni, D.M. Association between coffee consumption and its polyphenols with cardiovascular risk factors: A population-based study. Nutrients 2017, 9, 276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valdez, J.C.; Bolling, B.W. Anthocyanins and intestinal barrier function: A review. JFB 2019, 31, 18–30. [Google Scholar] [CrossRef] [Green Version]
- Ou, S.; Kwok, K.-C. Ferulic acid: Pharmaceutical functions, preparation and applications in foods. J. Sci. Food Agric. 2004, 84, 1261–1269. [Google Scholar] [CrossRef]
- Sergent, T.; Piront, N.; Meurice, J.; Toussaint, O.; Schneider, Y.-J. Anti-inflammatory effects of dietary phenolic compounds in an in vitro model of inflamed human intestinal epithelium. Chem-Biol. Interact. 2010, 188, 659–667. [Google Scholar] [CrossRef]
- Xie, L.; Lee, S.G.; Vance, T.M.; Wang, Y.; Kim, B.; Lee, J.-Y.; Chun, O.K.; Bolling, B.W. Bioavailability of anthocyanins and colonic polyphenol metabolites following consumption of aronia berry extract. Food Chem. 2016, 211, 860–868. [Google Scholar] [CrossRef]
- Borges, G.; van der Hooft, J.J.J.; Crozier, A. A comprehensive evaluation of the [2-14C](−)-epicatechin metabolome in rats. Free Radic. Biol. Med. 2016, 99, 128–138. [Google Scholar] [CrossRef]
- Czank, C.; Cassidy, A.; Zhang, Q.; Morrison, D.J.; Preston, T.; Kroon, P.A.; Botting, N.P.; Kay, C.D. Human metabolism and elimination of the anthocyanin, cyanidin-3-glucoside: A (13)C-tracer study. Am. J. Clin. Nutr. 2013, 97, 995–1003. [Google Scholar] [CrossRef] [Green Version]
- Xiao, J.; Högger, P. Stability of dietary polyphenols under the cell culture conditions: Avoiding erroneous conclusions. J. Agric. Food Chem. 2015, 63, 1547–1557. [Google Scholar] [CrossRef]
- Correa-Betanzo, J.; Allen-Vercoe, E.; McDonald, J.; Schroeter, K.; Corredig, M.; Paliyath, G. Stability and biological activity of wild blueberry (Vaccinium angustifolium) polyphenols during simulated in vitro gastrointestinal digestión. Food Chem. 2014, 165, 522–531. [Google Scholar] [CrossRef]
- Garbetta, A.; Nicassio, L.; D’Antuono, I.; Cardinali, A.; Linsalata, V.; Attolico, G.; Minervini, F. Influence of in vitro digestion process on polyphenolic profile of skin grape (cv. Italia) and on antioxidant activity in basal or stressed conditions of human intestinal cell line (HT-29). Food Res. Int. 2018, 106, 878–884. [Google Scholar] [CrossRef] [PubMed]
- Torres, A. Caracterización física, química y compuestos bioactivos de pulpa madura de tomate de árbol (Cyphomandra betacea) (Cav.) Sendtn. Arch. Latinoam. Nutr. 2012, 62, 381–388. [Google Scholar]
- Moreno, E.; Ortiz, B.L.; Restrepo, L.P. Total phenolic content and antioxidant activity of pulp extracts of six tropical fruits. Rev. Colomb. Qum. 2014, 43, 41–48. [Google Scholar] [CrossRef]
- Putt, K.; Pei, R.; White, H.; Bolling, B. Yogurt inhibits intestinal barrier dysfunction in Caco-2 cells by increasing tight junctions. Food Funct. 2017, 8, 406–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Sample Availability: Samples of the raw material Colombian Passiflora edulis var. Flavicarpa (Maracuyá), Passiflora edulis var. Sims (Gulupa), and Passiflora ligularis var. Juss (Granadilla) (passion fruits) are available from the authors. |
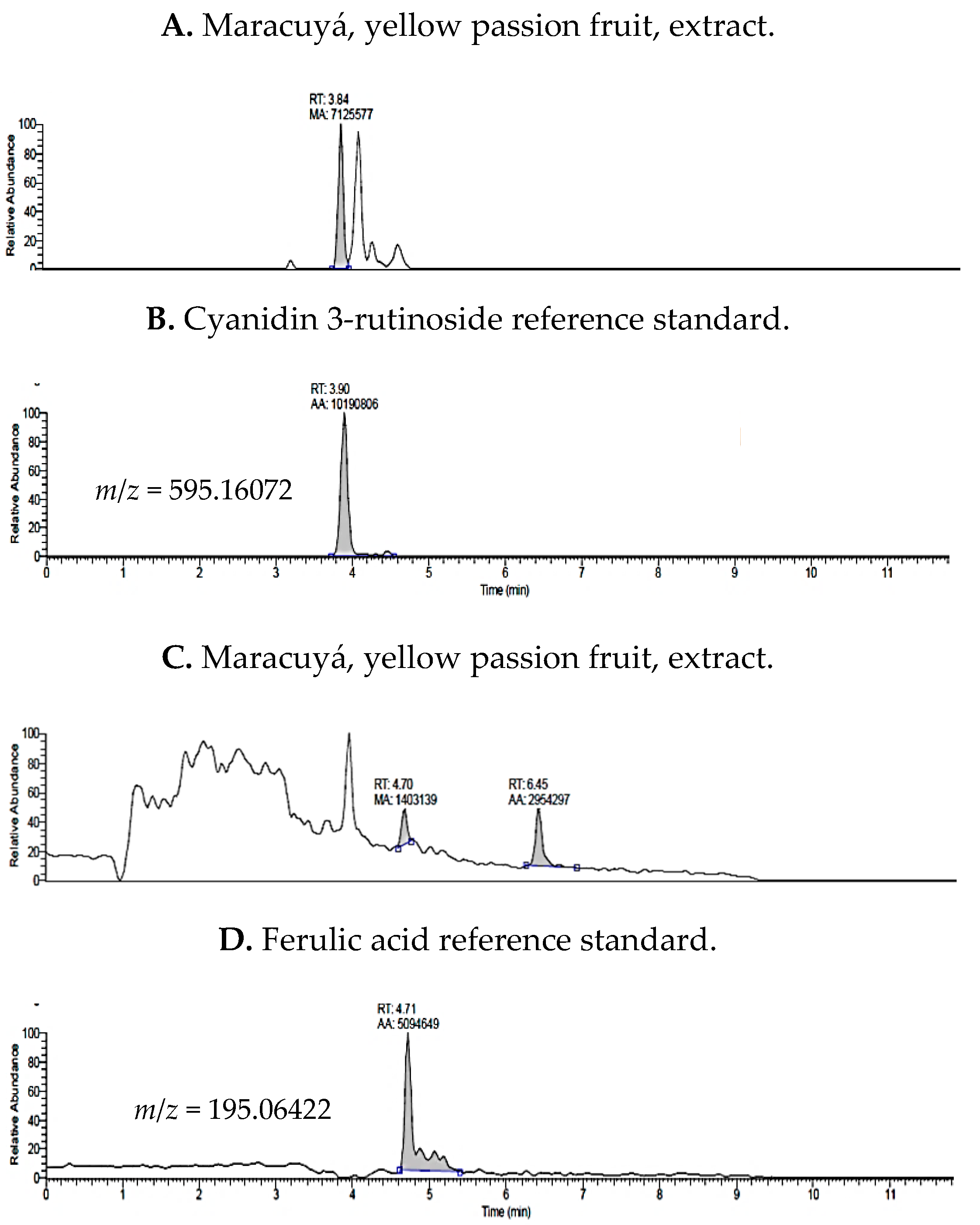
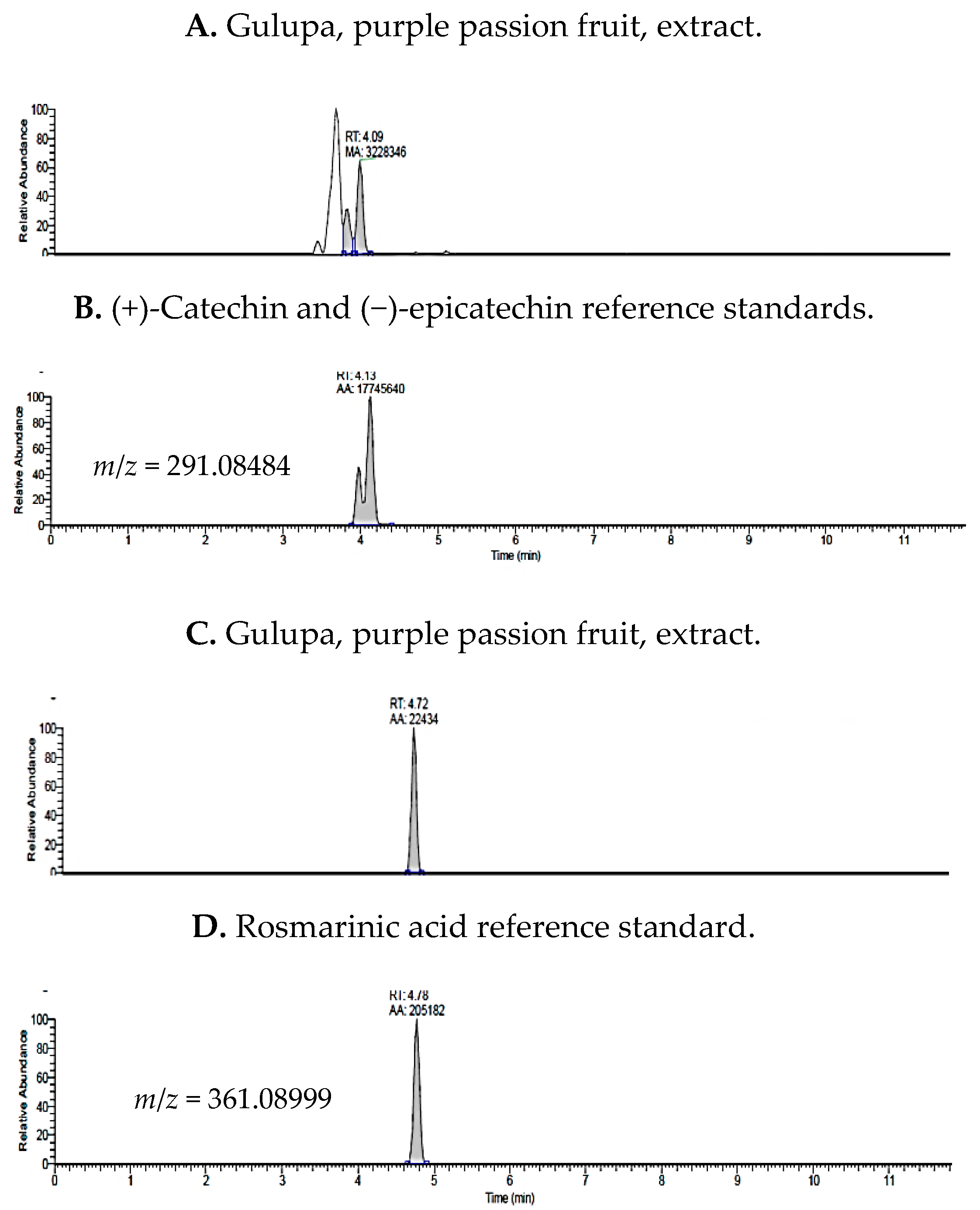
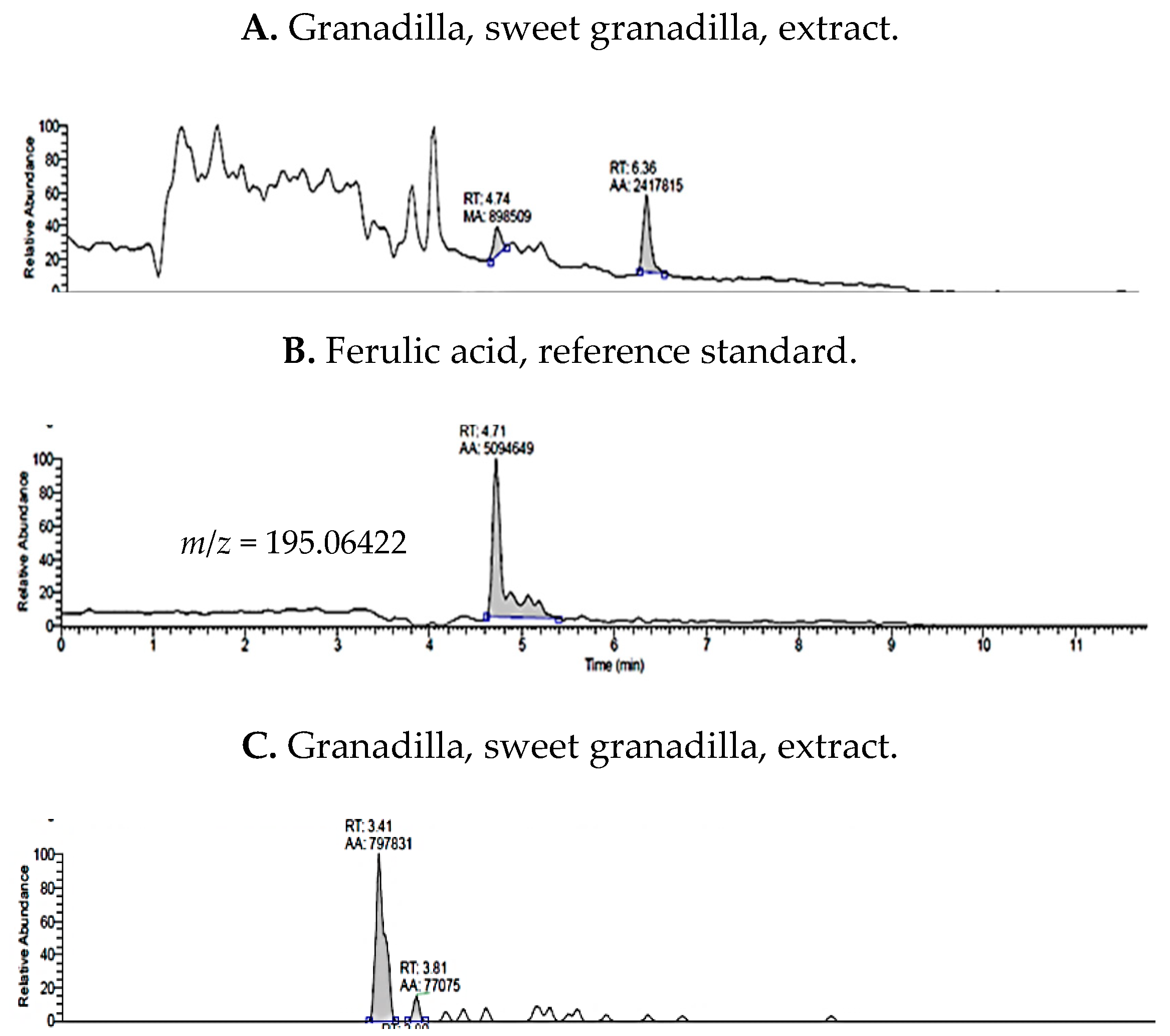
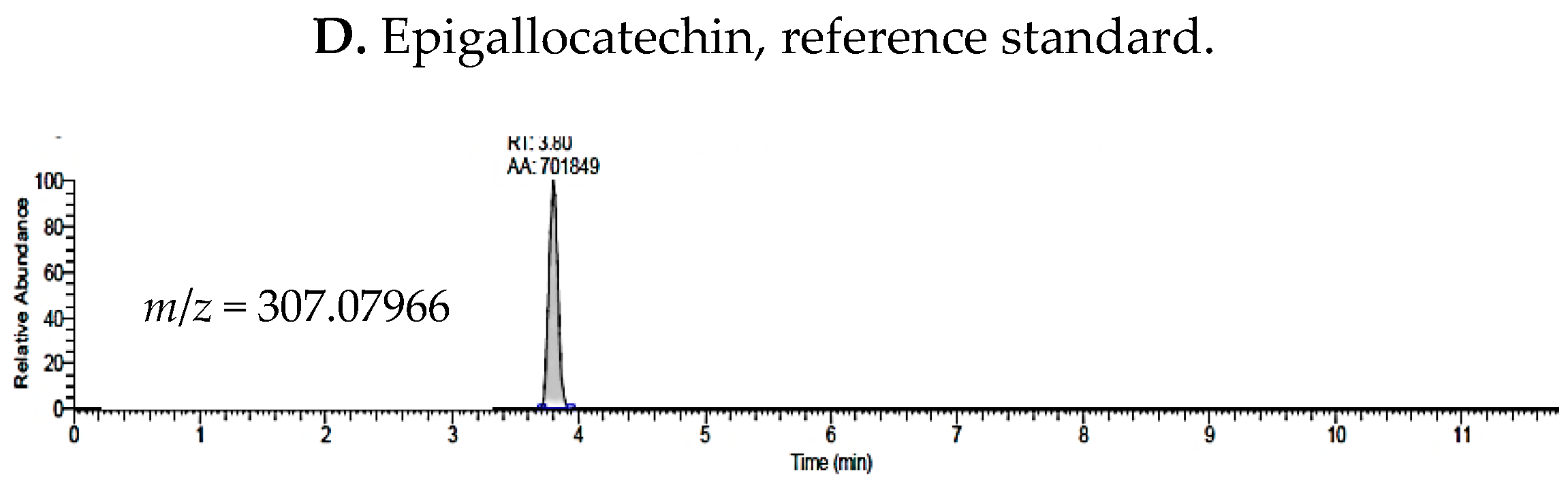
| Reference Standards | Retention Time (min) | [M + H]+ (m/z) | P. edulis var. Flavicarpa (Maracuyá) | P. edulis var. Sims (Gulupa) | P. ligularis var. Juss (Granadilla) |
|---|---|---|---|---|---|
| theobromine | 3.63 | 181.07109 | ✓ | ✓ | ✓ |
| theophylline | 3.78 | 181.07291 | ✕ | ✕ | ✕ |
| epigallocatechin | 3.80 | 307.07966 | ✓ | ✓ | ✓ |
| cyanidin 3-rutinoside | 3.90 | 595.16072 | ✓ | ✓ | ✓ |
| (+)-catechin | 3.97 | 291.08484 | ✓ | ✓ | ✓ |
| pelargonidin 3-glucoside | 4.06 | 433.11133 | ✕ | ✕ | ✕ |
| caffeine | 4.09 | 195.08672 | ✓ | ✓ | ✓ |
| caffeic acid | 4.10 | 181.04859 | ✕ | ✕ | ✕ |
| (−)-epicatechin | 4.13 | 291.08776 | ✓ | ✓ | ✓ |
| epigallocatechin gallate | 4.15 | 459.08990 | ✓ | ✓ | ✓ |
| vanillic acid | 4.16 | 169.04865 | ✕ | ✕ | ✕ |
| quercetin 3-glucoside | 4.45 | 465.09987 | ✓ | ✓ | ✓ |
| epicatechin gallate | 4.49 | 443.09508 | ✕ | ✕ | ✕ |
| cyanidin | 4.55 | 287.05416 | ✓ | ✓ | ✓ |
| kaempferol 3-glucoside | 4.59 | 449.10558 | ✕ | ✕ | ✕ |
| p-coumaric acid | 4.63 | 165.05377 | ✓ | ✓ | ✓ |
| quercetin | 4.63 | 303.04838 | ✕ | ✕ | ✕ |
| ferulic acid | 4.71 | 195.06422 | ✓ | ✓ | ✓ |
| rosmarinic acid | 4.78 | 361.08999 | ✓ | ✓ | ✓ |
| pelargonidin | 4.82 | 271.05928 | ✓ | ✓ | ✓ |
| luteolin | 5.24 | 287.05356 | ✓ | ✓ | ✓ |
| naringenin | 5.49 | 273.07433 | ✕ | ✕ | ✕ |
| apigenin | 5.53 | 271.05874 | ✓ | ✓ | ✓ |
| kaempferol | 5.57 | 287.05644 | ✕ | ✕ | ✕ |
| pinocembrin | 6.16 | 257.07951 | ✕ | ✕ | ✕ |
| carnosic acid | 7.94 | 333.20433 | ✕ | ✕ | ✕ |
| ursolic acid | 9.46 | 457.36631 | ✓ | ✓ | ✓ |
| Compounds | P. edulis var. Flavicarpa (Maracuyá) | P. edulis var. Sims (Gulupa) | P. ligularis var. Juss (Granadilla) |
|---|---|---|---|
| cyanidin 3-rutinoside | 0.60 ± 0.31 | <LOQ b | <LOQ |
| ferulic acid | 0.45 ± 0.30 | <LOQ | 0.54 ± 0.43 |
| (+)-catechin | 0.29 ± 0.02 | 0.28 ± 0.01 | <LOQ |
| (−)-epicatechin | 0.18 ± 0.07 | 0.22 ± 0.11 | 0.05 ± 0.01 |
| theobromine | 0.09 ± 0.06 | <LOQ | <LOQ |
| quercetin 3-glucoside | 0.09 ± 0.06 | <LOQ | <LOQ |
| rosmarinic acid | <LOQ | 0.13 ± 0.11 | <LOQ |
| epigallocatechin gallate | <LOQ | <LOQ | 0.07 ± 0.04 |
| epigallocatechin | <LOQ | <LOQ | 0.09 ± 0.04 |
| caffeine | <LOQ | <LOQ | <LOQ |
| p-coumaric acid | <LOQ | <LOQ | <LOQ |
| luteolin | <LOQ | <LOQ | <LOQ |
| apigenin | <LOQ | <LOQ | <LOQ |
| ursolic acid | <LOQ | <LOQ | <LOQ |
| cyanidin | <LOQ | <LOQ | <LOQ |
| pelargonidin | <LOQ | <LOQ | <LOQ |
| Passifloras/Contents | P. edulis | P. edulis | P. ligularis |
|---|---|---|---|
| var. Flavicarpa (Maracuyá) | var. Sims (Gulupa) | var. Juss (Granadilla) | |
| Total extract concentration (µg/mL) | 1.7 ± 0.82 | 0.63 ± 0.23 | 0.75 ± 0.52 |
| Total extract content (µg/g DW) | 218.41 ± 0.14 | 75.22 ± 0.08 | 96.36 ± 0.13 |
| TPC (mg GAE/g DW) | 1.18 ± 0.01 | 1.62 ± 0.09 | 1.55 ± 0.00 |
| Treatment | Time (h) | |||
|---|---|---|---|---|
| 12 | 24 | 36 | 48 | |
| Control Media (TEER, % 0 h) | 107 ± 6.9 a | 106 ± 4.2 a | 109 ± 4.0 a | 109 ± 2.9 a |
| IC (TEER, % 0 h) | 81 ± 2.6 b | 56 ± 1.3 b | 51 ± 2.1 b | 48 ± 3.5 b |
| TEER % | |||
|---|---|---|---|
| Treatments | P. edulis var. Flavicarpa (Maracuyá) | P. edulis var. Sims (Gulupa) | P. ligularis var. Juss (Granadilla) |
| Residues after extraction | 70.65 ± 6.6 a | 83.70 ± 6.9 b | 71.06 ± 7.1 b |
| Lyophilized extracts at 2.5 mg/dL | n.d. | n.d. | 63.10 ± 3.7 a |
| Lyophilized extracts at 5.0 mg/dL | 62.74 ± 4.0 a | n.d. | 63.42 ± 3.6 a |
| Lyophilized extracts at 10.0 mg/dL | 62.88 ± 3.6 a | 59.52 ± 6.6 a | 72.87 ± 4.0 b |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carmona-Hernandez, J.C.; Taborda-Ocampo, G.; Valdez, J.C.; Bolling, B.W.; González-Correa, C.H. Polyphenol Extracts from Three Colombian Passifloras (Passion Fruits) Prevent Inflammation-Induced Barrier Dysfunction of Caco-2 Cells. Molecules 2019, 24, 4614. https://doi.org/10.3390/molecules24244614
Carmona-Hernandez JC, Taborda-Ocampo G, Valdez JC, Bolling BW, González-Correa CH. Polyphenol Extracts from Three Colombian Passifloras (Passion Fruits) Prevent Inflammation-Induced Barrier Dysfunction of Caco-2 Cells. Molecules. 2019; 24(24):4614. https://doi.org/10.3390/molecules24244614
Chicago/Turabian StyleCarmona-Hernandez, Juan Carlos, Gonzalo Taborda-Ocampo, Jonathan C. Valdez, Bradley W. Bolling, and Clara Helena González-Correa. 2019. "Polyphenol Extracts from Three Colombian Passifloras (Passion Fruits) Prevent Inflammation-Induced Barrier Dysfunction of Caco-2 Cells" Molecules 24, no. 24: 4614. https://doi.org/10.3390/molecules24244614
APA StyleCarmona-Hernandez, J. C., Taborda-Ocampo, G., Valdez, J. C., Bolling, B. W., & González-Correa, C. H. (2019). Polyphenol Extracts from Three Colombian Passifloras (Passion Fruits) Prevent Inflammation-Induced Barrier Dysfunction of Caco-2 Cells. Molecules, 24(24), 4614. https://doi.org/10.3390/molecules24244614






