Abstract
It has been found that both eugenol and isoeugenol derivatives reacted with 2-pyridinesulfenyl and 2-pyridineselenenyl halides in a regioselective mode affording products with opposite regiochemistry. Synthesis of new families of 2H,3H-[1,3]thia- and -selenazolo[3,2-a]pyridin-4-ium heterocycles has been developed by annulation reactions of 2-pyridinechalcogenyl halides with natural compounds (eugenol, isoeugenol, methyl eugenol, methyl isoeugenol, acetyl eugenol, trans-anethole) and their structural analogs. The influence of the substrate structure and the nature of halogen on the product yields are studied. The 2-pyridinesulfenyl and 2-pyridineselenenyl chlorides are more efficient reagents compared to corresponding bromides. The obtained condensed heterocycles are novel water-soluble functionalized compounds with promising biological activity.
1. Introduction
Sulfur-containing rings condensed with nitrogen heterocycles are important scaffolds for medicinal chemistry [1]. Condensed sulfur/nitrogen heterocycles have always been a vital part of new drug discovery. For example, penicillin and cephalosporin antibiotics contain sulfur-containing ring condensed with a nitrogen heterocycle as the key scaffold [1]. Thiazole ring presents in many natural and biologically active compounds including ritonavir (anti-HIV), sulfathiazole (antimicrobial), and tiazofurin (anticancer) drugs [1,2,3]. The combination of the thiazole ring with the pyridine heterocycle in one condensed molecule gives a very promising scaffold for medicinal chemistry. The thiazolopyridine derivatives are associated with a wide range of pharmacological activities. Among them, [1,3]thiazolo[3,2-a]pyridinium derivatives and structurally related compounds exhibit antitumor, antiviral, antibacterial, pilicide activity and properties of selective inhibitor of RNA polymerase I [4,5,6,7,8] (Figure 1). Selenium analogs, [1,3]selenazolo[3,2-a]pyridinium derivatives are less studied. Selenium-containing heterocycles [9,10,11,12,13] represent an important family of compounds exhibiting various types of biological activity including anti-inflammatory, antitumor, antifungal, and glutathione peroxidase-like action [12,13,14,15,16,17,18,19,20]. The selenium-containing heterocyclic drug ebselen is applied for the prevention and treatment of human ischemic stroke [21]. The development of effective approaches to novel selenium heterocycles by regioselective cyclization and annulation reactions is one of the main directions of our research [22,23,24,25,26,27,28,29,30,31].
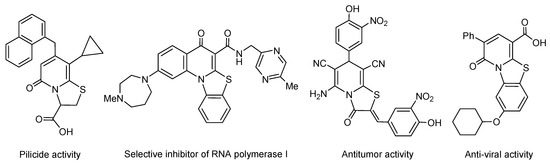
Figure 1.
Known biologically active compounds structurally related to the [1,3]thiazolo[3,2-a]pyridinium scaffold.
The reactions of 2-pyridinesulfenyl and 2-pyridineselenenyl chlorides with some alkenes have been described [32,33,34,35,36,37,38,39,40,41]. The reactions of these reagents with styrene have been reported to afford 3-phenyl-2H,3H-[1,3]thiazolo- and -selenazolo[3,2-а]pyridin-4-ium chlorides in high yields [32,35]. In spite of some progress in synthetic method for preparations of [1,3]thiazolo[3,2-a]- and [1,3]selenazolo[3,2-a]pyridin-4-ium derivatives [32,33,34,35,36,37,38,39,40,41], reactions of 2-pyridinesulfenyl and 2-pyridineselenenyl halides with many alkenes are hitherto unknown. For example, the reactions of 2-pyridinesulfenyl and 2-pyridineselenenyl chlorides with simple 1-alkenes and natural compounds containing a double bond such as eugenol, isoeugenol, anethole and its derivatives have not been described. Besides, the influence of the substrate structure and the nature of halogen on the product yields in the reaction of 2-pyridinechalcogenyl halides with alkenes is hitherto unknown.
The developments of synthetic approaches to novel derivatives of [1,3]thiazolo[3,2-a]- and [1,3]selenazolo[3,2-a]pyridin-4-ium scaffolds based on functionalized alkenes including natural products and studies of their properties remains an important task.
Chemistry of natural products is very important for providing knowledge about medicines to derive active components as lead compounds for drug discovery. It is known that the majority of new drugs have been developed from natural products and synthesis of novel compounds based on natural products is prospective for searching biologically active substances.
The goal of the present research is the development of regio- and stereoselective synthesis of novel condensed heterocycles with promising biological activity based on the annulation reactions of 2-pyridinesulfenyl and 2-pyridineselenenyl halides with natural compounds (eugenol, isoeugenol, anethole) and their derivatives and structural analogs as well as studies on the influence of the substrate structure and the nature of halogen and chalcogen on the product yields.
2. Results and Discussion
Recently we attempted two representative of phenylpropanoids, eugenol (4-allyl-2-methoxyphenol) and isoeugenol (2-methoxy-4-propenylphenol), as substrates in the annulation reactions with 2-pyridinesulfenyl and -selenenyl chlorides. The reactions of 2-pyridinesulfenyl chloride with isoeugenol leading to 3-(3,4-dimethoxyphenyl)-2-methyl-2H,3H-thiazolo[3,2-а]pyridin-4-ium chloride (1) in 70% yield was reported by us as a short letter [41]. In the present research, we increased the yield of compound 1 to 85% and proved that compound 1 has trans-configuration regarding the positions of methyl substituent and benzene ring with respect to the condensed thiazolo[3,2-а]pyridin-4-ium bicycle (Scheme 1).
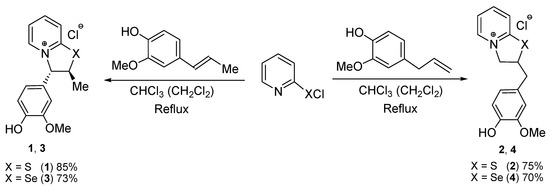
Scheme 1.
Synthesis of compounds 1–4 based on the reactions of 2-pyridinesulfenyl and 2-pyridineselenenyl chlorides eugenol and isoeugenol.
The reaction of 2-pyridinesulfenyl chloride with eugenol (an equimolar ratio of the reagents) after overnight stirring (20 h) at room temperature in methylene chloride led to 2-[(4-hydroxy-3-methoxyphenyl)methyl]-2H,3H-[1,3]thiazolo[3,2-а]pyridin-4-ium chloride (2) (34% yield), which was precipitated from the reaction mixture as a powder. Similar result was obtained using chloroform as a solvent instead of methylene chloride. The yield was increased to 75% by refluxing the mixture of 2-pyridinesulfenyl chloride with eugenol in chloroform (Scheme 1).
Compounds 1 and 2 can be regarded as the products with the opposite regiochemistry with respect to the location of aryl-containing substituents: compound 1 has aryl on the position 3 of the dihydrothiazole ring whereas aryl-containing substituent is situated on the position 2 in compound 2. Thus, the reactions of 2-pyridinesulfenyl chloride with both eugenol and isoeugenol proceeded under similar conditions in a regioselective mode giving products 1 and 2 with the opposite regiochemistry (Scheme 1).
This interesting result inspired us to study carefully the reactions of 2-pyridinesulfenyl and 2-pyridineselenenyl chlorides with eugenol and isoeugenol derivatives. Some other natural products (trans-anethole, (1S)-(−)-beta-pinene) were also investigated in reactions with 2-pyridinechalcogenyl chlorides.
The reactions of 2-pyridineselenenyl chloride with isoeugenol and eugenol under the same conditions as the synthesis of compound 1 afforded trans-3-(3,4-dimethoxyphenyl)-2-methyl-2H,3H-[1,3]selenazolo[3,2-а]pyridin-4-ium chloride (3) and 2-[(4-hydroxy-3-methoxyphenyl)methyl]-2H,3H-[1,3]selenazolo[3,2-а]pyridin-4-ium chloride (4) in 73% and 70% yields, respectively (Scheme 1).
Like the synthesis of compounds 1 and 2, the reactions of 2-pyridineselenenyl chloride with eugenol and isoeugenol led to two structural isomers of the opposite regiochemistry, compounds 3 and 4, selenium analogs of heterocycles 1 and 2.
The reactions of 2-pyridinesulfenyl chloride with methyl eugenol and methyl isoeugenol were found to be more efficient compared to those with eugenol and isoeugenol. The reaction of 2-pyridinesulfenyl chloride with methyl eugenol (an equimolar ratio of the reagents) proceeded smoothly at room temperature in methylene chloride giving 2-[(3,4-dimethoxyphenyl)methyl]-2H,3H-[1,3]thiazolo[3,2-а]pyridin-4-ium chloride (5) in quantitative yield (Scheme 2).
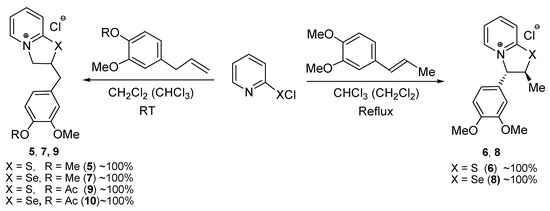
Scheme 2.
Synthesis of compounds 5–10 from eugenol and isoeugenol derivatives.
The reaction of 2-pyridinesulfenyl chloride with methyl isoeugenol occurred at reflux temperature in chloroform affording trans-3-(3,4-dimethoxyphenyl)-2-methyl-2H,3H-[1,3]thiazolo[3,2-а]pyridin-4-ium chloride (6) in quantitative yield (Scheme 2). Unlike the reactions with eugenol and isoeugenol (Scheme 1), the precipitation of the products 5 and 6 from the reaction mixture was not observed. The compounds 5 and 6 are two structural isomers with the opposite regiochemistry.
Similar results were obtained using 2-pyridineselenenyl chloride. This reagent reacted with methyl eugenol and methyl isoeugenol very smoothly under the same conditions as the synthesis of heterocycles 5 and 6 leading to the selenium analogs of these compounds in quantitative yields (Scheme 2). The obtained condensed selenium heterocycles 7 and 8 are also two structural isomers with the opposite regiochemistry.
Acetyleugenol was involved in the reactions with 2-pyridinesulfenyl and 2-pyridineselenenyl chlorides. The reactions occurred at room temperature in methylene chloride giving 2-[[4-(acetyloxy)-3-methoxyphenyl]methyl]-2H,3H-[1,3]thiazolo- and -selenazolo[3,2-а]pyridin-4-ium chlorides 9 and 10 (acetyl analogs of products 2 and 4) in quantitative yields (Scheme 2).
A complex mixture of products was obtained in the reactions of 2-pyridinesulfenyl and 2-pyridineselenenyl chlorides with (1S)-(−)-beta-pinene.
Structural analogs of eugenol and isoeugenol were involved in the reactions of 2-pyridinesulfenyl and 2-pyridineselenenyl halides. trans-Anethole, trans-1-methoxy-4-(1-propenyl)benzene, is the structural analog of isoeugenol. This compound was found to react with 2-pyridinesulfenyl and 2-pyridineselenenyl chlorides giving trans-3-(4-methoxyphenyl)-2-methyl-2H,3H-[1,3]thiazolo- and selenazolo[3,2-а]pyridin-4-ium chlorides 11 and 12 in quantitative yields (Scheme 3).
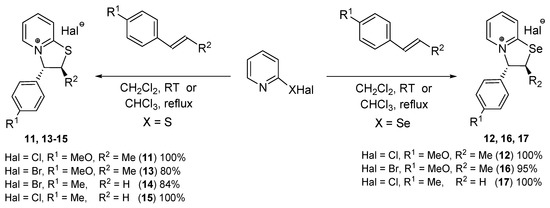
Scheme 3.
Synthesis of compounds 11–17 from structural analogs of isoeugenol.
2-Pyridinesulfenyl bromide and 2-pyridineselenenyl bromide were rarely used in organic synthesis and in the preparation of condensed heterocycles [37,38,39]. Usually organylsulfenyl and organylselenenyl bromides are less electrophilic than corresponding organylsulfenyl and organylselenenyl chlorides. On the other hand, the bromine atom is more reactive in the nucleophilic substitution reactions compared to the chlorine atom and can be easily substituted with nitrogen atom of the pyridine ring forming condensed heterocycles.
We studied the reactions of 2-pyridinesulfenyl and 2-pyridineselenenyl bromides with a series of substrates and found that, in general, these reagents are less efficient in the annulation reactions compared to corresponding chlorides and the yields of target products are higher in the case of 2-pyridinesulfenyl or 2-pyridineselenenyl chlorides.
The reactions of 2-pyridinesulfenyl bromide with anethole and 4-methylstyrene afforded compounds 13 and 14 in 80 and 84% yields, whereas quantitative yields of chloro analogs 11 and 15 were achieved with these substrates using 2-pyridinesulfenyl chloride (Scheme 3).
The selenium analog of heterocycle 15 was obtained in quantitative yield from 2-pyridineselenenenyl chloride and 4-methylstyrene (compound 17, Scheme 3).
The reactions of 2-pyridineselenenyl bromide with trans-anethole and styrene led to heterocycles 16 and 18 in 95% and 78% yields, respectively; however, analogous reactions of 2-pyridineselenenyl chloride with these substrates afforded target products 12 and 19 in quantitative yields (Scheme 3 and Scheme 4). In the case of 2-pyridinesulfenyl bromide, the reaction with styrene gave product 21 in 90% yield, whereas the formation of heterocycle 20 quantitative yield was observed in the reaction of 2-pyridinesulfenyl chloride with styrene (Scheme 4).
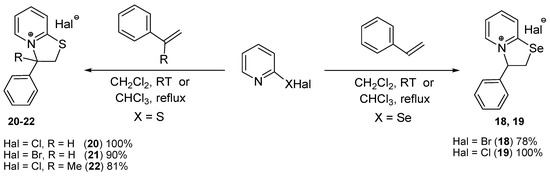
Scheme 4.
Synthesis of compounds 18–22 from styrene and its derivative.
These results indicate that, in general, 2-pyridinesulfenyl and 2-pyridineselenenyl chlorides are more efficient compared to corresponding bromides and the annulation reactions of 2-pyridinechalcogenyl chlorides afforded the desired products in higher (mostly quantitative) yields.
The introduction of methyl substituent at β-position of the double bond of styrene as well as to the position 4 of the benzene ring has little influence on the yields of products in annulation reactions. However, the introduction of methyl substituent at α-position of the double bond of styrene seems to have negative effect on the annulation process. The reaction of 2-pyridinesulfenyl chloride with α-methylstyrene led to heterocycle 22 only in 81% yield (Scheme 4). A mixture of products was obtained in the reaction of 2-pyridineselenenyl chloride with α-methylstyrene, whereas product 19 was formed in quantitative yield in the reaction of 2-pyridineselenenyl chloride with styrene (Scheme 4). Some decrease in efficiency of the annulation reaction in the case of α-methylstyrene can be attributed to steric factor: the introduction of the methyl group diminishes the rate of nucleophilic substitution by the nitrogen atom of the pyridine ring in the last stage.
Allylbenzene reacted with 2-pyridinesulfenyl chloride at room temperature in a regioselective mode affording heterocycle 23 (quantitative yield) derived from anti-Markovnikov addition to the double bond (Scheme 5). Thus, allylbenzene derivatives (eugenol, methyl eugenol, acetyleugenol) and styrene derivatives (isoeugenol, methyl isoeugenol, trans-anethole) reacted with 2-pyridinesulfenyl and 2-pyridineselenenyl halides in a regioselective mode affording products with the opposite regiochemistry with respect to the location of aryl-containing substituents.
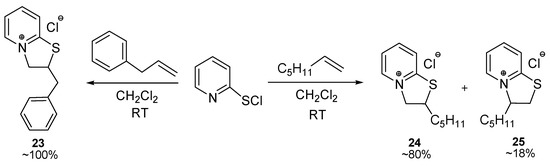
Scheme 5.
The reactions of 2-pyridinesulfenyl chloride with allylbenzene and 1-heptene.
Suggested reactions pathways can be regarded on the examples of reactions of 2-pyridinechalcogenyl halides with styrene and allylbenzene (Scheme 6). The reactions of 2-pyridinesulfenyl and selenenyl halides with compounds containing a double bond conjugated with the benzene ring (isoeugenol, methyl isoeugenol, trans-anethole, styrene, 4-methylstyrene, α-methylstyrene) proceed regioselectively via electrophilic addition of the chalcogen atom at β-carbon atom of the double bond. The regioselectivity is due to the formation of intermediate carbocation A, which is stabilized by the benzene ring (the relatively stable benzyl cation) (Scheme 6, B is also possible intermediate). Noteworthy, the known addition reactions of arylsulfenyl and arylselenenyl halides to styrene and its derivatives also afforded Markovnikov adducts [42,43].
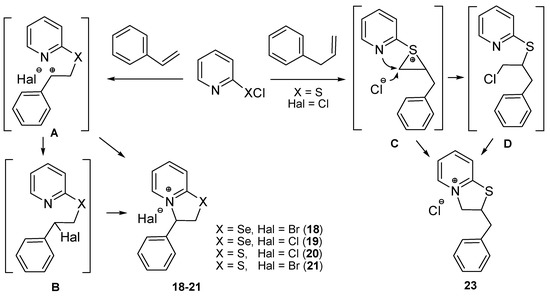
Scheme 6.
The suggested reactions pathways on the example of reactions of 2-pyridinesulfenyl and selenenyl halides with styrene and allylbenzene.
In the case of eugenol, its derivatives and structural analogs, 2-pyridinesulfenyl and selenenyl halides react with allyl group as with linear 1-alkene and electrophilic addition of the chalcogen atom occurs at α-carbon atom of the double bond (C and D are possible intermediates in the reaction of 2-pyridinesulfenyl chloride with allylbenzene) followed by intramolecular nucleophilic substitution in the formed anti-Markovnikov adduct (Scheme 6). It is known that electrophilic addition of sulfenyl halides to linear 1-alkenes afforded predominantly anti-Markovnikov products [44,45,46,47]. Thiiranium [45,46,47,48] and seleniranium [47,48,49,50,51,52,53,54] cations are often regarded as intermediates in electrophilic addition of chalcogenyl halides to the double bond, and attack of the halide anion occurs at unsubstituted carbon atom of thiiranium or seleniranium cations leading to anti-Markovnikov products. Besides, the formation of thiiranium and seleniranium species determines the reaction course as anti-addition in reactions of sulfenyl and selenenyl halides with alkenes. For example, the known reactions of arylsulfenyl and arylselenenyl chlorides with cycloalkenes proceeded as anti-addition affording adducts with trans-configuration [55,56,57,58]. The anti-addition was also observed in the reactions of 2-pyridinesulfenyl and selenenyl halides with isoeugenol, methyl isoeugenol, and trans-anethole affording products 6, 8, 11–13, 16 with trans-configuration.
We attempted the reaction of 2-pyridinesulfenyl chloride with one representative of linear 1-alkenes: 1-heptene and observed the formation of two regioisomers 24 and 25 in a 9:2 ratio (Scheme 5). The major product 24 was derived from anti-Markovnikov addition to the double bond. Thus, like 1-alkenes, allylbenzene reacted with 2-pyridinesulfenyl chloride affording heterocycle 23 derived from anti-Markovnikov addition to the double bond (Scheme 5).
The structural assignments of the synthesized compounds were made using 1H and 13C-NMR spectroscopy including two-dimensional and NOESY experiments and confirmed by elemental analysis. The products of opposite regiochemistry have characteristic signals of the carbon atoms bonded with charged nitrogen (N+) in 13C-NMR spectra: CH2N+ (63–67 ppm) and (Ar)CHN+ (75–84 ppm). The values of proton spin-spin coupling constant (3JH-H) in the (Me)CH-CH(Ar)N fragment of the dihydrothiazole cycle correspond to trans-configuration of these protons.
3. Experimental Section
3.1. General Information
1H (400.1 MHz) and 13C (100.6 MHz) NMR spectra were recorded on a Bruker DPX-400 spectrometer (Bruker BioSpin GmbH, Rheinstetten, Germany) in 5–10% solution in D2O or DMSO-d6 or CDCl3. 1H and 13C chemical shifts (δ) are reported in parts per million (ppm), relative to tetramethylsilane (external) or to the residual solvent peaks of DMSO-d6 (δ = 2.50 and 39.52 ppm in 1H- and 13C-NMR, respectively) or CDCl3 (δ = 7.26 and 77.16 ppm in 1H- and 13C-NMR, respectively). Spectral characteristics of compounds 19 and 20 are described elsewhere [32,35]. Elemental analysis was performed on a Thermo Scientific FLASH 2000 Organic Elemental Analyzer (Thermo Fisher Scientific Inc., Milan, Italy). Melting points were determined on a Kofler Hot-Stage Microscope PolyTherm A apparatus (Wagner & Munz GmbH, München, Germany). Absolute solvents were used in the reactions.
3.2. Synthesis of Compounds 1–18, 21–24
trans-3-(4-Hydroxy-3-methoxyphenyl)-2-methyl-2H,3H-[1,3]thiazolo[3,2-а]pyridin-4-ium chloride (1). A solution of sulfuryl chloride (0.135 g, 1 mmol) in chloroform (10 mL) was added dropwise to a solution of di(2-pyridine) disulfide (0.218 g, 1 mmol) in chloroform (10 mL) and the mixture was stirred for 10 min at room temperature. A solution of isoeugenol (0.328 g, 2 mmol) in chloroform (10 mL) was added dropwise and the reaction mixture stirred for 4 h at room temperature and 8 h at reflux temperature. On cooling the formed precipitate was filtered off and dried in vacuum giving the product (0.527 g, 85% yield) as a white powder, mp 235–237 °C. 1H-NMR (400 MHz, D2O): δ 1.56 (d, J = 6.7 Hz, 3H, CH3), 3.86 (s, 3H, OCH3), 4.58 (dq, J = 11.3, 6.7 Hz, 1H, SCH), 5.81 (d, J = 11.3 Hz, 1H, NCH), 6.99–7.04 (m, 2H, Ar), 7.14 (s, 1H, Ar), 7.55–7.59 (m, 1H, Py), 7.97–7.99 (m, 1H, Py), 8.10–8.12 (m, 1H, Py), 8.25–8.29 (m, 1H, Py). 13C-NMR (101 MHz, D2O): δ 17.58 (CH3), 51.37 (SCH), 57.94 (OCH3), 83.56 (NCH), 114.01 (Ar), 118.12 (Ar), 124.64 (Py), 125.39 (Py), 126.52(Ar), 143.22 (Py), 146.75 (Py), 149.11 (CO, Ar), 150.45 (CO, Ar), 161.81 (Py). Anal. Calcd for C15H16NClO2S: C 58.15, H 5.21, Cl 11.44, N 4.52, S 10.35. Found: C 57.91, H 5.07, Cl 11.63, N 4.34, S 10.13.
2-[(4-Hydroxy-3-methoxyphenyl)methyl]-2H,3H-thiazolo[3,2-а]pyridin-4-ium chloride (2) was obtained in 74% yield as a yellowish powder, mp 208–210 °C, from 2-pyridinesulfenyl chloride and eugenol under similar conditions as synthesis of compound 1. 1H-NMR (400 MHz, DMSO-d6): δ 3.03 (qd, J = 13.9, 7.2 Hz, 2H, CH2), 3.75 (s, 3H, ОCH3), 4.60–4.76 (m, 1H, SCH,), 5.10 (qd, J = 13.6, 6.5 Hz, 2H, NCH2), 6.68 (d, J = 8.0 Hz, 1H, Ar), 6.74 (d, J = 8.0 Hz, 1H, Ar), 6.89 (s, 1H, Ar), 7.69–7.73 (m, 1H, Py), 8.09–8.11 (m, 1H, Py), 8.28–8.31 (m, 1H, Py), 8.98–8.99 (m, 1H, Py). 13C-NMR (101 MHz, DMSO-d6): δ 38.51 (CH2), 48.32 (SCH), 55.65 (ОCH3), 63.31 (NCH2), 113.33 (Ar), 115.35 (Ar), 121.44 (Py), 122.40 (Py), 123.10 (Py), 127.52 (Ar), 142.91 (Ar), 144.40 (Py), 145.63 (СОH, Ar), 147.48 (СОСH3, Ar), 158.61 (NCS, Py). Anal. Calcd for C15H16NClO2S: С 58.15, H 5.21, Cl 11.44, N 4.52, S 10.35. Found: С 57.96, H 5.40, Cl 11.67, N 4.72, S 10.57.
trans-3-(4-Hydroxy-3-methoxyphenyl)-2-methyl-2H,3H-selenazolo[3,2-а]pyridine-4-ium chloride (3) was obtained in 73% yield from 2-pyridineselenenyl chloride and isoeugenol as a yellowish powder, mp 230–232 °C under similar conditions as synthesis of compound 1. 1H-NMR (400 MHz, D2O): δ 1.62 (d, J = 6.7 Hz, 3H, CH3), 3.85 (s, 3H, OCH3), 4.70 – 4.62 (m, 1H, SeCH), 5.83 (d, J = 10.5 Hz, 1H, NCH), 6.93 (d, J = 8.2 Hz, 1H, Ar), 7.00 (d, J = 8.2 Hz, 1H, Ar), 7.10 (s, 1H, Ar), 7.57-7.61 (m, 1H, Py), 8.10 – 8.20 (m, 3H, Py). 13C-NMR (101 MHz, D2O): δ 16.26 (CH3), 44.81 (SeCH), 55.58 (OCH3), 83.73 (NCH), 111.53 (Ar), 115.74 (Ar), 121.97 (Py), 122.95 (Py), 125.04 (Py), 127.13 (Ar), 142.39 (Ar), 143.59 (Py), 146.60 (СОCH3, Ar), 148.10 (СОH, Ar), 158.03 (NCSe, Py). Anal. Calcd for C15H16NClO2Se: С 50.51; H 4.52; N 3.93, Cl 9.94, Se 22.14. Found: С 50.62; H 4.41; N 3.81, Cl 9.71, Se 22.37.
2-[(4-Hydroxy-3-methoxyphenyl)methyl]-2H,3H-selenazolo[3,2-а]pyridin-4-ium chloride (4) was obtained in 70% yield from 2-pyridineselenenyl chloride and eugenol as a yellowish powder, mp 206–208 °C under similar conditions as synthesis of compound 1. 1H-NMR (400 MHz, D2O): δ 3.00-3.03 (m, 2H, CH2), 3.69 (s, 3H, ОCH3), 4.56 (s, 1H, SeCH,), 4.99 (s, 2H, NCH2), 6.61 (s, 2H, Ar), 6.78 (s, 1H, Ar), 7.46–7.48 (m, 1H, Py), 7.79–7.81 (m, 1H, Py), 7.91–7.95 (m, 1H, Py), 8.44–8.45 (m, 1H, Py). 13C-NMR (101 MHz, D2O): δ 38.72 (CH2), 45.19 (SeCH), 55.68 (ОСH3), 66.48 (NCH2), 112.96 (Ar), 115.11 (Ar), 121.88 (Py), 122.90 (Py), 126.94 (Py), 131.50 (Ar), 142.42 (Ar), 143.07 (Py), 144.23 (СОH, Ar), 145.46 (СОСH3, Ar), 158.72 (NCSe, Py). Anal. Calcd for C15H16NClO2Se: С 50.51; H 4.52; N 3.93, Cl 9.94, Se 22.14. Found: С 50.78; H 4.35; N 4.12, Cl 10.13, Se 21.93.
2-[(3,4-dimethoxyphenyl)methyl]-2H,3H-[1,3]thiazolo[3,2-а]pyridin-4-ium chloride (5). A solution of sulfuryl chloride (0.068 g, 0.5 mmol) in methylene chloride (7 mL) was added dropwise to a solution of di(2-pyridine) disulfide (0.109 g, 0.5 mmol) in methylene chloride (7 mL) and the mixture was stirred for 10 min at room temperature. A solution of methyl eugenol (0.178 g, 1 mmol) in methylene chloride (7 mL) was added dropwise and the reaction mixture was stirred for 20 h at room temperature. The solvent was removed by rotary evaporator (RE-52AA, Xi’an Heb Biotechnology Co., Xi’an, China) and the residue was dried in vacuum giving the product (0.324 g, quantitative yield) as a light yellow oil. 1H-NMR (400 MHz, D2O): δ 3.00–3.10 (m, 2H, CH2), 3.73 (s, 3H, ОCH3), 3.77 (s, 3H, ОCH3), 4.58 (t, J = 5.1 Hz, 1H, SCH), 5.03 (qd, J = 13.6, 5.7 Hz, 2H, NCH2), 6.80 (s, 2H, Ar), 6.87 (s, 1H, Ar), 7.47–7.50 (m, 1H, Py), 8.72–8.74 (m, 1H, Py), 8.06–8.10 (m, 1H, Py), 8.47–8.48 (m, 1H, Py). 13C-NMR (101 MHz, D2O): δ 38.63 (CH2), 48.26 (SCH), 55.65 (ОСH3), 63.83 (NCH2), 111.75 (Ar), 112.66 (Ar), 122.15 (Py), 122.47 (Py), 123.14 (Py), 128.87 (Ar), 141.51 (Ar), 144.24 (Py), 147.34 (СОСH3, Ar), 147.77 (СОСH3, Ar), 159.44 (NCS, C5H4N). Anal. Calcd for C16H18NClO2S: С 59.34, H 5.60, Cl 10.95, N 4.33, S 9.90. Found: С 59.09, H 5.78, Cl 11.14, N 4.52, S 10.05.
trans-3-(3,4-dimethoxyphenyl)-2-methyl-2H,3H-[1,3]thiazolo[3,2-а]pyridin-4-ium chloride (6). A solution of sulfuryl chloride (0.068 g, 0.5 mmol) in chloroform (7 mL) was added dropwise to a solution of di(2-pyridine) disulfide (0.109 g, 0.5 mmol) in chloroform (7 mL) and the mixture was stirred for 10 min at room temperature. A solution of methyl isoeugenol (0.178 g, 1 mmol) in chloroform (7 mL) was added dropwise and the reaction mixture stirred for 1 h at room temperature and 5 h at reflux temperature. The solvent was removed by rotary evaporator and the residue was dried in vacuum giving the product (0.324 g, quantitative yield) as a light yellow oil. 1H-NMR (400 MHz, D2O): δ 1.55 (d, J = 6.7 Hz, 3H, CH3), 3.82 (s, 3H, ОCH3), 3.87 (s, 3H, ОCH3), 4.53 (dq, J = 11.3, 6.7 Hz, 1H, SCH), 5.82 (d, J = 11.3 Hz, 1H, NCH), 7.08–7.14 (m, 3H, Ar), 7.54–7.58 (m, 1H, Py), 7.96–7.98 (m, 1H, Py), 8.09–8.11 (m, 1H, Py), 8.24–8.28 (m, 1H, Py). 13C-NMR (101 MHz, D2O): δ 15.60 (CH3), 49.37 (SCH), 55.70 (ОСH3), 81.47 (NCH), 111.00 (Ar), 112.15 (Ar), 122.72 (Py), 123.41 (Py), 125.02 (Ar), 141.24 (Ar), 144.78 (Py), 149.17 (СОCH3, Ar), 150.02 (СОСH3, Ar), 159.88 (NCS, Py), Anal. Calcd for C16H18NClO2S: С 59.34, H 5.60, Cl 10.95, N 4.33, S 9.90. Found: С 59.62, H 5.81, Cl 11.16, N 4.51; S 10.12.
2-[(3,4-Dimethoxyphenyl)methyl]-2H,3H-[1,3]selenazolo[3,2-а]pyridin-4-ium chloride (7) was obtained as a light yellow oil in quantitative yield from 2-pyridineselenenyl chloride and methyl eugenol under similar conditions as synthesis of compound 5. 1H-NMR (400 MHz, D2O): δ 3.15 (s, 2H, CH2), 3.75 (d, J = 12.3 Hz, 6H, ОCH3), 4.66 (s, 1H, SeCH), 5.06–5.15 (m, 2H, NCH2), 6.82 (s, 2H, Ar), 6.90 (s, 1H, Ar), 7.50 (s, 1H, Py), 7.87–7.89 (m, 1H, Py), 7.96–7.98 (m, 1H, Py), 8.50 (s, 1H, Py). 13C-NMR (101 MHz, D2O): δ 38.85 (CH2), 44.94 (SeCH), 55.50 (ОСH3), 66.58 (NCH2), 111.66 (Ar), 112.49 (Ar), 121.93 (Py), 122.89 (Py), 126.96 (Ar), 142.46 (Ar), 143.07 (Py), 146.09 (СОСH3, С6H3), 147.12 (СОСH3, Ar), 157.90 (NCSe, Py). Anal. Calcd for C16H18NClO2Se: С 51.84; H 4.89; N 3.78, Cl 9.56, Se 21.30. Found: С 52.56; H 5.16; N 4.25, Cl 9.34, Se 21.57.
trans-3-(3,4-Dimethoxyphenyl)-2-methyl-2H,3H-[1,3]selenazolo[3,2-а]pyridin-4-ium chloride (8) was obtained as a light yellow oil in quantitative yield from 2-pyridineselenenyl chloride and methyl isoeugenol under similar conditions as synthesis of compound 6. 1H-NMR (400 MHz, D2O): δ 1.55 (d, J = 6.8 Hz, 3H, CH3), 3.84 (s, 3H, ОCH3), 3.89 (s, 3H, ОCH3), 4.67–4.71 (m, 1H, SeCH), 5.87 (d, J = 10.4 Hz, 1H, NCH), 7.04–7.06 m (1H, Ar), 7.12 (dd, J = 14.4, 5.1 Hz, 2H, Ar), 7.58–7.62 (m, 1H, Py), 8.11–8.21 (m, 3H, Py). 13C-NMR (101 MHz, D2O): δ 16.81 (CH3), 45.26 (SeCH), 55.84 (ОСH3), 84.07 (NCH), 111.04 (Ar), 112.30 (Ar), 122.44 (Py), 123.45 (Py), 126.03 (Ar), 127.61 (Ar), 142.24 (Py), 144.09 (Py), 149.29 (СОH, Ar), 150.00 (СОСH3, Ar), 158.56 (NCS, Py). Anal. Calcd for C16H18NClO2Se: С 51.84, H 4.89, N 3.78, Cl 9.56, Se 21.30. Found: С 52.00, H 5.02, N 3.91, Cl 9.73, Se 21.03.
2-[[4-(Acethyloxy)-3-methoxybenzyl]-2H,3H-[1,3]thiazolo[3,2-а]pyridin-4-ium chloride (9) was obtained as a light yellow oil in quantitative yield from 2-pyridinesulfenyl chloride and acetyleugenol under similar conditions as synthesis of compound 5. 1H-NMR (400 MHz, D2O): δ 2.32 (s, 3H, CH3), 3.14–3.23 (m, 2H, CH2), 3.81 (s, 3H, ОCH3), 4.64–4.70 (m, 1H, SCH), 5.06–5.16 (m, 2H, NCH2,), 6.95 (dd, J = 8.1, 1.8 Hz, 1H, Ar), 7.00 (d, J = 8.1 Hz, 1H, Ar), 7.09 (d, J = 1.8 Hz, 1H, Ar), 7.53–7.55 (m, 1H, Py), 7.80–7.82 (m, 1H, Py), 8.11–8.16 (m, 1H, Py), 8.51–8.53 (m, 1H, Py). 13C-NMR (101 MHz, D2O): δ 19.59 (СH3), 38.57 (CH2), 47.37 (SCH), 55.71 (ОСH3), 63.47 (NCH2), 113.74 (Ar), 121.89 (Ar), 122.26 (Py), 122.33 (Py), 122.84 (Py), 135.26 (Ar), 137.84 (Ar), 141.11 (Ar), 144.06 (Py), 149.73 (СОCH3, Ar), 159.00 (NCS, Py), 172.30 (СОOCH3, Ar). Anal. Calcd for C17H18NClO3S: С 58.03, H 5.16, N 3.98, Cl 10.08, S 9.11. Found: С 58.25, H 5.34, N 4.15, Cl 10.36, S 9.38.
2-[[4-(Acetyloxy)-3-methoxybenzyl]-2H,3H-[1,3]selenazolo[3,2-а]pyridin-4-ium chloride (10) was obtained as a light yellow oil in quantitative yield from 2-pyridineselenenyl chloride and acetyleugenol under similar conditions as synthesis of compound 6. 1H-NMR (400 MHz, D2O): δ 2.31 (s, 3H, CH3), 3.26 (s, 2H, CH2), 3.80 (s, 3H, ОCH3), 4.70 (s, 1H, SeCH), 5.09–5.21 (m, 2H, NCH2,), 6.94-6.99 (m, 2H, Ar), 7.08 (s, 1H, Ar), 7.56–7.59 (m, 1H, Py), 7.93–7.95 (m, 1H, Py), 8.02–8.04 (m, 1H, Py), 8.58–8.59 (m, 1H, Py). 13C-NMR (101 MHz, D2O): δ 19.84 (СH3), 39.15 (CH2), 44.46 (SeCH), 55.96 (ОСH3), 66.62 (NCH2), 113.86 (Ar), 122.00 (Ar), 122.54 (Py), 123.16 (Py), 127.09 (Py), 136.38 (Ar), 138.02 (Ar), 142.48 (Ar), 143.37 (Py), 149.92 (СОCH3, Ar), 157.76 (NCSe, Py), 172.59 (СОOCH3). Anal. Calcd for C17H18NClO3Se: С 51.21, H 4.55, N 3.51, Cl 8.89, Se 19.80. Found: С 51.42, H 4.72, N 3.68, Cl 9.05, Se 20.07.
trans-3-(4-Methoxyphenyl)-2-methyl-2H,3H-thiazolo[3,2-а]pyridin-4-ium chloride (11) was obtained in quantitative yield from 2-pyridinesulfenyl chloride and trans-anethole as a yellowish powder, mp 140–142 °C under similar conditions as the synthesis of compound 5. 1H-NMR (400 MHz, D2O): δ 1.54–1.56 (m, 3H, CH3), 3.86 (s, 3H, ОCH3), 4.52 (dd, J = 10.6, 6.6 Hz, 1H, SCH), 5.88 (dd, J = 10.6, 2.9 Hz, 1H, NCH), 7.10–7.13 (m, 2H, Ar), 7.44–7.47 (m, 2H, Ar), 7.55–7.58 (m, 1H, Py), 7.98–7.99 (m, 1H, Py), 8.11–8.12 (m, 1H, Py), 8.25–8.28 (m, 1H, Py). 13C-NMR (101 MHz, D2O): δ 16.06 (СH3), 49.58 (SCH), 55.60 (ОСH3), 81.25 (NCH), 115.34 (Ar), 122.85 (Py), 123.55 (Py), 125.11 (Ar), 130.36 (Ar), 141.37 (Py), 144.89 (Py), 159.90 (NCS, Py), 160.76 (СОСH3, Ar). Anal. Calcd for C15H16NclOS: С 61.32, H 5.49, N 4.77, Cl 12.07, S 10.91. Found: С 61.53, H 5.58, N 4.98, Cl 11.85, S 11.04.
trans-3-(4-Methoxyphenyl)-2-methyl-2H,3H-selenazolo[3,2-а]pyridin-4-ium chloride (12). A solution of sulfuryl chloride (0.122 g, 0.9 mmol) in chloroform (10 mL) was added dropwise to a solution of di(2-pyridine) diselenide (0.28 g, 0.9 mmol) in chloroform (20 mL) and the mixture was stirred for 20 min at room temperature. A solution of trans-anethole (0.266 g, 1.8 mmol) in chloroform (10 mL) was added dropwise and the reaction mixture stirred for 1 h at room temperature and 4 h at reflux temperature. The solvent was removed by rotary evaporator and the residue was dried in vacuum giving the product (0.613 g) in quantitative yield as a yellowish powder, mp 141–143 °C. 1H-NMR (400 MHz, D2O): δ 1.67 (d, J = 6.6 Hz, 3H, CH3,), 3.83 (s, 3H, ОCH3), 4.60 (qd, J = 13.4, 6.6 Hz, 1H, SeCH), 5.97 (d, 1H, J = 9.4 Hz, NCH), 7.12 (dd, J = 9.0, 2.5 Hz, 2H, Ar), 7.41 (dd, J = 9.0, 2.5 Hz, 2H, Ar), 7.63–7.66 (m, 1H, Py), 8.16–8.26 (m, 3H, Py). 13C-NMR (101 MHz, D2O): δ 17.54 (СH3), 45.62 (SeCH), 55.69 (ОСH3), 83.78 (NCH), 115.37 (Ar), 123.60 (Py), 126.10 (Ar), 127.76 (Py), 130.05 (Ar), 142.96 (Py), 144.21 (Py), 158.58 (NCSe, Py), 160.61 (СОСH3, Ar). Anal. Calcd for C15H16NclOSe: С 52.88, H 4.73, N 4.11, Cl 10.41, Se 23.18. Found: С 53.11, H 4.93, N 4.36, Cl 10.60, Se 23.40.
trans-3-(4-Methoxyphenyl)-2-methyl-2H,3H-thiazolo[3,2-а]pyridin-4-ium bromide (13). A solution of bromine (0.122 g, 0.76 mmol) in methylene chloride (8 mL) was added dropwise to a solution of di(2-pyridine) disulfide (0.168 g, 0.76 mmol) in methylene chloride (8 mL) and the mixture was stirred for 30 min at room temperature. A solution of trans-anethole (0.225 g, 1.52 mmol) in methylene chloride (8 mL) was added dropwise and the reaction mixture stirred for 24 h at room temperature. The mixture was filtered and the solvent was removed by rotary evaporator. The residue was dried in vacuum giving the product (0.411 g, 80% yield) as a light yellow oil. 1H-NMR (400 MHz, D2O): δ 1.57–1.58 (m, 3H, CH3), 3.88 (s, 3H, ОCH3), 4.51–4.58 (m, 1H, SCH), 5.91 (d, J = 10.6 Hz, 1H, NCH), 7.13–7.15 (m, 2H, Ar), 7.47–7.49 (m, 2H, Ar), 7.58–7.60 (m, 1H, Py), 7.99–8.01 (m, 1H, Py), 8.12–8.14 (m, 1H, Py), 8.26–8.30 (m, 1H, Py). 13C-NMR (101 MHz, D2O): δ 15.98 (СH3), 49.50 (SCH), 55.54 (ОСH3), 81.15 (N+CH), 115.26 (С6H4), 122.77 (Py), 123.48 (Py), 125.00 (С6H4), 130.31 (С6H4), 141.29 (Py), 144.79 (Py), 159.80 (NCS, Py), 160.66 (СОСH3, С6H4). Anal. Calcd for C15H16NBrOS: С 53.26, H 4.77, N 4.14, Br 23.62, S 9.48. Found: С 53.58, H 4.90, N 4.32, Br 23.92, S 9.71.
3-(4-Methylphenyl)-2H,3H-thiazolo[3,2-а]pyridin-4-ium bromide (14) was obtained in 84% yield from 2-pyridinesulfenyl bromide and 4-methylstyrene as a yellowish powder, mp 199–201 °C under similar conditions as synthesis of compound 13. 1H-NMR (400 MHz, D2O): δ 2.33 (s, 3H, CH3), 3.86 (dd, J = 11.8, 9.9 Hz, 1H, SCH2), 4.21 (dd, J = 11.8, 8.7 Hz, 1H, SCH2), 6.37 (t, J = 8.7 Hz, 1H, NCH), 7.32–7.38 (m, 4H, Ar), 7.56–7.59 (m, 1H, Py), 8.04–8.06 (m, 1H, Py), 8.15–8.17 (m, 1H, Py), 8.27–8.31 (m, 1H, Py). 13C-NMR (101 MHz, D2O): δ 21.38 (CH3), 37.63 (SCH2), 76.16 (NCH), 123.68 (Py), 124.31 (Py), 128.85 (Ar), 131.26 (Ar), 132.06 (Ar), 141.91 (Py), 142.02 (Ar), 145.54 (Py), 160.95 (SCN+, Py). Anal. Calcd for C14H14NBrS: С 54.55, H 4.58, N 4.54, Br 25.92, S 10.40. Found: С 54.68, H 4.62, N 4.78, Br 26.23, S 10.69.
3-(4-Methylphenyl)-2H,3H-thiazolo[3,2-а]pyridin-4-ium chloride (15) was obtained in quantitative yield from 2-pyridinesulfenyl chloride and 4-methylstyrene as a yellowish oil under similar conditions as synthesis of compound 5. 1H-NMR (400 MHz, D2O): δ 2.30 (s, 3H, CH3), 3.78 – 3.88 (m, 1H), 4.14 (dd, J = 11.9, 8.0 Hz, 1H, SCH2), 6.29 (t, J = 8.9 Hz, 1H, NCH), 7.27–7.33 (m, 4H, Ar), 7.50–7.53 (m, 1H, Py), 7.97–7.99 (m, 1H, Py), 8.10–8.12 (m, 1H, Py), 8.21–8.25 (m, 1H, Py). 13C-NMR (101 MHz, D2O): δ 20.50 (CH3), 36.70 (SCH2), 75.49 (NCH), 122.88 (Py), 123.48 (Py), 128.05 (Ar), 130.47 (Ar), 131.32 (Ar), 141.16 (Py), 141.34 (Ar), 144.76 (Py), 160.26 (NCS, Py).Anal. Calcd for C14H14NClS: С 63.74, H 5.35, N 5.31, Cl 13.44. S 12.16. Found: С 63.94, H 5.49, N 5.51, Cl 13.17. S 11.97.
trans-3-(4-Methoxyphenyl)-2-methyl-2H,3H-[1,3]selenazolo[3,2-а]pyridin-4-ium bromide (16). A solution of bromine (0.051 g, 0.32 mmol) in chloroform (10 mL) was added dropwise to a solution of di(2-pyridine) diselenide (0.1 g, 0.32 mmol) in chloroform (10 mL) and the mixture was stirred for 20 min at room temperature. A solution of trans-anethole (0.095 g, 0.64 mmol) in chloroform (10 mL) was added dropwise and the reaction mixture stirred for 1 h at room temperature and 5 h at reflux temperature. The mixture was filtered and the solvent was removed by rotary evaporator. The residue was dried in vacuum giving the product (0.234 g, 95% yield) as a light orange oil. 1H-NMR (400 MHz, D2O): δ 1.62 (d, J = 6.5 Hz, 3H, CH3,), 3.85 (s, 3H, ОCH3), 4.57–4.61 (m, 1H, SeCH), 5.91 (d, 1H, J = 9.7 Hz, NCH), 7.10 (d, J = 8.3 Hz, 2H, Ar), 7.39 (d, J = 8.3 Hz, 2H, Ar), 7.59–7.60 (m, 1H, Py), 8.11–8.20 (m, 3H, Py). 13C-NMR (101 MHz, D2O): δ 17.19 (СH3), 45.48 (SeCH), 55.56 (ОСH3), 83.68 (NCH), 115.25 (Ar), 123.41 (Py), 125.84 (Ar), 127.61 (Py), 130.05 (Ar), 142.85 (Py), 144.02 (Py), 158.47 (NCSe, Py), 160.52 (СОСH3, Ar). Anal. Calcd for C15H16NbrOSe: С 46.78, H 4.19, N 3.64, Br 20.75, Se 20.50. Found: С 48.02, H 4.48, N 3.98, Br 21.03, Se 20.21.
3-(4-Methylphenyl)-2H,3H-selenazolo[3,2-а]pyridin-4-ium chloride (17) was obtained as a light yellow oil in quantitative yield from 2-pyridineselenenyl chloride and 4-methylstyrene under similar conditions as the synthesis of compound 16. 1H-NMR (400 MHz, D2O) δ 2.30 (s, 1H, CH3), 3.81–3.86 (m, 1H, SeCH2), 4.09–4.14 (m, 1H, SeCH2), 6.28 (t, J = 8.4 Hz, 1H, NCH), 7.26–7.29 (m, 4H, Ar), 7.54 (ddd, J = 6.5, 2.4, 1.0 Hz, 1H, Py), 8.10–8.17 (m, 3H, Py). 13C-NMR (101 MHz, D2O) δ 19.82 (CH3), 30.21 (SeCH2), 77.27 (NCH), 122.79 (Py), 126.71 (Py), 127.28 (Ar), 129.78 (Ar), 131.31 (Ar), 140.49 (Ar), 141.93 (Py), 143.23 (Py), 157.93 (NCSe, Py). Anal. Calcd for C14H14NclSe: С 54.12, H 4.54, N 4.51, Cl 11.41, Se 25.42. Found: С 53.87, H 4.68, N 4.39, Cl 11.56, Se 25.69.
3-Phenyl-2H,3H-selenazolo[3,2-a]pyridin-4-ium bromide (18) was obtained as a light orange oil in 78% yield from 2-pyridineselenenyl bromide and styrene under similar conditions as the synthesis of compound 16. 1H-NMR (400 MHz, DMSO-d6): δ 3.78 (dd, J = 10.9, 7.4 Hz, 1H, SCH2), 4.26 (dd, J = 10.9, 7.4 Hz, 1H, SCH2), 6.58 (t, J = 7.4 Hz, 1H, NCH), 7.38–7.40 (m, 2H, Ar), 7.48–7.50 (m, 3H, Ar), 7.71–7.75 (m, 1H, Py), 8.30–8.34 (m, 1H, Py), 8.42–8.44 (m, 1H, Py), 8.49–8.51 (m, 1H, Py). 13C-NMR (101 MHz, DMSO-d6): δ 32.13 (SCH2), 76.37 (NCH), 123.55 (Py), 127.44 (Py), 127.44 (Ar), 129.38 (Ar), 129.71 (Ar), 136.08 (Ar), 143.30 (Py), 143.95 (Py), 159.32 (NCS, Py). Anal. Calcd for C13H12BrNSe: С 45.77, H 3.55, N 4.11, Br 23.43, Se 23.15. Found: С 45.98, H 3.69, N 4.23, Br 23.65, Se 22.96.
3-Phenyl-2H,3H-selenazolo[3,2-a]pyridin-4-ium chloride (19) was obtained as a yellowish powder (mp 205–207 °C) in quantitative yield from 2-pyridineselenenyl chloride and styrene under similar conditions as synthesis of compound 5. Spectral characteristics of compound 19 are described [32].
3-Phenyl-2H,3H-[1,3]thiazolo[3,2-a]pyridin-4-ium chloride (20) was obtained as a yellowish powder (mp 209–211 °C) in quantitative yield from 2-pyridinesulfenyl chloride and styrene under similar conditions as synthesis of compound 5. Spectral characteristics of compound 20 are described [35].
3-Phenyl-2H,3H-[1,3]thiazolo[3,2-a]pyridin-4-ium bromide (21) was obtained as a light yellow oil in 90% yield from 2-pyridinesulfenyl bromide and styrene under similar conditions as the synthesis of compound 16. 1H-NMR (400 MHz, D2O): δ 3.95 (dt, J = 23.7, 11.8 Hz, 1H, SCH2), 4.23 (dt, J = 16.4, 8.2 Hz, 1H, SCH2), 6.37–6.42 (m, 1H, NCH), 7.50–7.52 (m, 2H, Ar, Py), 7.56–7.59 (m, 4H, Ar), 8.02–8.04 (m, 1H, Py), 8.20–8.22 (m, 1H, Py), 8.26–8.30 (m, 1H, Py). 13C-NMR (101 MHz, D2O): δ 36.77 (SCH2), 75.75 (NCH), 122.87 (Py), 123.49 (Py), 128.22 (Ar), 129.89 (Ar), 130.76 (Ar), 134.27 (Ar), 141.35 (Py), 144.76 (Py), 156.45 (NCS, Py). Anal. Calcd for C13H12BrNS: С 53.07, H 4.11, N 4.76, Br 27.16, S 10.90. Found: С 53.48, H 4.29, N 5.01, Br 27.36, S 11.12.
3-Methyl-3-phenyl-2H,3H-thiazolo[3,2-a]pyridin-4-ium chloride (22) was obtained as a light yellowish oil in 81% yield from 2-pyridinesulfenyl chloride and α-methylstyrene under similar conditions as synthesis of compound 16. 1H-NMR (400 MHz, D2O): δ 2.20 (s, 3H, CH3), 4.00 (d, J = 12.2 Hz, 1H, SCH2), 4.13 (d, J = 12.2 Hz, 1H, SCH2), 7.36–7.39 (m, 2H, Ar), 7.51–7.52 (m, 3H, Ar), 7.64–7.67 (m, 1H, Py), 8.03–8.05 (m, 1H, Py), 8.29–8.32 (m, 1H, Py). 13C-NMR (101 MHz, D2O): δ 23.41 (CH3), 43.38 (SCH2), 81.28 (NC), 123.00 (Py), 123.33 (Py), 125.98 (Ar), 129.04 (Ar), 129.51 (Ar), 138.13 (Ar), 140.04 (Py), 144.27 (Py), 159.57 (NCS, Py). Anal. Calcd for C14H14NClS: С 63.74, H 5.35, N 5.31, Cl 13.44. S 12.16. Found: С 63.52, H 5.13, N 5.53, Cl 13.64. S 11.92.
2-(Phenylmethyl)-2H,3H-thiazolo[3,2-а]pyridine-4-ium chloride (23) was obtained in quantitative yield as a light yellow oil from 2-pyridinesulfenyl bromide and allylbenzene under similar conditions as the synthesis of compound 13 (but 60 h stirring at room temperature). 1H-NMR (400 MHz, D2O): δ 3.17 (qd, J = 14.2, 6.7 Hz, 2H, CH2), 4.60–4.66 (m, 1H, SCH,), 5.06 (qd, J = 13.5, 5.9 Hz, 2H, NCH2), 7.25-7.31 (m, 5H, Ar), 7.49–7.52 (m, 1H, Py), 7.77–7.79 (m, 1H, Py), 8.08–8.12 (m, 1H, Py), 8.50–8.52 (m, 1H, Py).13C-NMR (101 MHz, D2O): δ 38.57 (CH2), 47.55 (SCH), 63.39 (NCH2), 122.07 (Py), 122.83 (Py), 123.47 (Py), 127.09 (Ar), 127.83 (Ar),128.26 (Ar), 128.83 (Ar), 143.90 (Py), 158.93 (NCS, Py). Anal. Calcd for C14H14NClS: С 63.74, H 5.35, N 5.31, Cl 13.44. S 12.16. Found: С 63.98, H 5.53, N 5.17, Cl 13.21. S 12.37.
2-Pentyl-2H,3H-thiazolo[3,2-а]pyridin-4-ium chloride (24) was obtained in 80% yield (in the mixture with compound 25, a ratio 24/25 = 9:2) from 2-pyridinesulfenyl chloride and 1-heptene under similar conditions as synthesis of compound 13. Compound 24 was characterized in the mixture with compound 25 (see Supplementary Materials for the 1H and 13C-NMR spectra pictures). 1H-NMR (400 MHz, CDCl3): δ 0.66–0.70 (m, 3H, CH3), 1.11–1.12 (m, 4H, CH2), 1.25–1.26 (m, 2H, CH2), 1.38 (dt, J = 16.2, 8.7 Hz, 1H, CH2), 1.77–1.86 (m, 1H, CH2), 4.23–4.30 (m, 1H, SCH), 5.14 (dd, J = 13.7, 7.2 Hz, 1H, CH2N), 5.27 (dd, J = 13.7, 7.5 Hz, 1H, CH2N), 7.52–7.56 (m, 1H, Py), 7.81–7.84 (m, 1H, Py), 8.16–8.20 (m, 1H, Py), 9.64–9.66 (m, 1H, Py). 13C-NMR (101 MHz, CDCl3): δ 13.70 (CH3), 22.07 (CH2), 27.02 (CH2), 30.87 (CH2), 33.42 (CH2), 48.80 (SCH), 64.79 (NCH2), 122.71 (Py), 123.03 (Py), 143.95 (Py), 144.65 (Py), 159.03 (NCS, Py).
4. Conclusions
Both eugenol and isoeugenol derivatives reacted with 2-pyridinesulfenyl and 2-pyridineselenenyl halides in a regioselective mode affording products with the opposite regiochemistry with respect to the location of aryl-containing substituents. Synthesis of new ensembles of 2H,3H-[1,3]thia- and -selenazolo[3,2-a]pyridin-4-ium heterocycles 1–18 and 21–24 in up to quantitative yields has been developed by annulation reactions of 2-pyridinechalcogenyl chlorides with natural compounds (eugenol, isoeugenol, methyl eugenol, methyl isoeugenol, trans-anethole) and their structural analogs. The obtained condensed heterocycles are novel water-soluble functionalized compounds with promising biological activity.
First studies on the influence of the substrate structure and the nature of halogen and chalcogen on the product yields in the reactions of 2-pyridinesulfenyl and 2-pyridineselenenyl halides with alkenes were carried out. The introduction of methyl substituent at β-position of the double bond of styrene as well as to the position 4 of the benzene ring has little influence on the yields of products in annulation reactions. However, the introduction of methyl substituent at α-position of the double bond of styrene has negative effect on the annulation process. The 2-pyridinesulfenyl and 2-pyridineselenenyl chlorides are more efficient compared to corresponding bromides and the annulation reactions of 2-pyridinechalcogenyl chlorides usually afforded the desired products in higher (mostly quantitative) yields. Regarding the influence of the chalcogen nature, 2-pyridinesulfenyl and 2-pyridineselenenyl chlorides exhibit close reactivity.
Supplementary Materials
The following are available online https://www.mdpi.com/1420-3049/25/2/376/s1, examples of 1H- and 13C-NMR spectra of the obtained compounds.
Author Contributions
Conceptualization and the paper preparation, V.A.P.; methodology research experiments, R.S.I.; research experiments, I.V.S.; NMR experiments, S.V.Z.; supervision, S.V.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Russian Science Foundation, grant number 18-13-00372.
Acknowledgments
The authors thank Baikal Analytical Center SB RAS for providing the instrumental equipment for structural investigations.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Feng, M.; Tang, B.; Liang, S.H.; Jiang, X. Sulfur Containing Scaffolds in Drugs: Synthesis and Application in Medicinal Chemistry. Curr. Top. Med. Chem. 2016, 16, 1200–1216. [Google Scholar] [CrossRef]
- Mishra, R.; Sharma, P.K.; Verma, P.K.; Tomer, I.; Mathur, G.; Dhakad, P.K. Biological Potential of Thiazole Derivatives of Synthetic Origin. J. Heterocycl. Chem. 2017, 54, 2103–2116. [Google Scholar] [CrossRef]
- Chhabria, M.T.; Patel, S.; Modi, P.; Brahmkshatriya, P.S. Thiazole: A review on chemistry, synthesis and therapeutic importance of its derivatives. Curr. Top. Med. Chem. 2016, 16, 2841–2862. [Google Scholar] [CrossRef]
- Haddach, M.; Schwaebe, M.K.; Michaux, J.; Nagasawa, J.; O’Brien, S.E.; Whitten, J.P.; Pierre, F.; Kerdoncuff, P.; Darjania, L.; Stansfield, R.; et al. Discovery of CX-5461, the First Direct and Selective Inhibitor of RNA Polymerase I, for Cancer Therapeutics. ACS Med. Chem. Lett. 2012, 3, 602–606. [Google Scholar] [CrossRef] [PubMed]
- Good, J.A.D.; Kulen, A.M.; Almqvist, K.F.; Cairns, A.G.; Ponten, J.F. 2,3-Dihydro-Thiazolo[3,2-a] Pyridin-5-One Derivatives, Intermediates Thereof, and Their Use as Antibacterial Agents. WO Patent 2016075296, 19 May 2016. [Google Scholar]
- Shi, F.; Li, C.; Xia, M.; Miao, K.; Zhao, Y.; Tu, S.; Zheng, W.; Zhang, G.; Ma, N. Green chemoselective synthesis of thiazolo [3,2-a] pyridine derivatives and evaluation of their antioxidant and cytotoxic activities. Bioorg. Med. Chem. Lett. 2009, 19, 5565–5568. [Google Scholar] [CrossRef] [PubMed]
- Manfroni, G.; Meschini, F.; Barreca, M.L.; Leyssen, P.; Samuele, A.; Iraci, N.; Sabatini, S.; Massari, S.; Maga, G.; Neyts, J.; et al. Pyridobenzothiazole derivatives as new chemotype targeting the HCV NS5B polymerase. Bioorg. Med. Chem. 2012, 20, 866–876. [Google Scholar] [CrossRef] [PubMed]
- Walker, K.A.; Sjogren, E.B.; Matthews, T.R. Antitrichomonal activity of mesoionic thiazolo [3,2-a]pyridines. J. Med. Chem. 1985, 28, 1673–1679. [Google Scholar] [CrossRef]
- Elsherbini, M.; Hamama, W.S.; Zoorob, H.H. Recent advances in the chemistry of selenium-containing heterocycles: Five-membered ring systems. Coord. Chem. Rev. 2016, 312, 149–177. [Google Scholar] [CrossRef]
- Elsherbini, M.; Hamama, W.S.; Zoorob, H.H. Recent advances in the chemistry of selenium-containing heterocycles: Six-membered ring systems. Coord. Chem. Rev. 2017, 330, 110–126. [Google Scholar] [CrossRef]
- Wang, M.; Fan, Q.; Jiang, X. Transition-Metal-Free Diarylannulated Sulfide and Selenide Construction via Radical/Anion-Mediated Sulfur–Iodine and Selenium–Iodine Exchange. Org. Lett. 2016, 18, 5756–5759. [Google Scholar] [CrossRef]
- Banerjee, B.; Koketsu, M. Recent developments in the synthesis of biologically relevant selenium-containing scaffolds. Coord. Chem. Rev. 2017, 339, 104–127. [Google Scholar] [CrossRef]
- Rafique, J.; Canto, R.F.S.; Saba, S.; Barbosa, F.A.R.; Braga, A.L. Recent Advances in the Synthesis of Biologically Relevant Selenium-containing 5-Membered Heterocycles. Curr. Org. Chem. 2016, 20, 166–188. [Google Scholar] [CrossRef]
- Tiekink, E.R.T. Therapeutic potential of selenium and tellurium compounds: Opportunities yet unrealized. Dalton Trans. 2012, 41, 6390–6395. [Google Scholar] [CrossRef] [PubMed]
- Braga, A.L.; Rafique, J. Synthesis of biologically relevant small molecules containing selenium. Part, B. Anti-infective and anticancer compounds. In Patai’s Chemistry of Functional Groups. Organic Selenium and Tellurium Compounds; Rappoport, Z., Ed.; John Wiley and Sons: Chichester, UK, 2013; Volume 4, pp. 1053–1117. [Google Scholar]
- Gladyshev, V.N.; Hatfield, D.L. Selenocysteine-Containing Proteins in Mammals. J. Biomed. Sci. 1999, 6, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, C.W.; Zeni, G.; Rocha, J.B.T. Organoselenium and Organotellurium Compounds: Toxicology and Pharmacology. Chem. Rev. 2004, 104, 6255–6286. [Google Scholar] [CrossRef] [PubMed]
- Mugesh, G.; du Mont, W.W.; Sies, H. Chemistry of Biologically Important Synthetic Organoselenium Compounds. Chem. Rev. 2001, 101, 2125–2180. [Google Scholar] [CrossRef]
- Organoselenium Chemistry: Between Synthesis and Biochemistry; Santi, C., Ed.; Bentham Science Publishers: Sharjah, UAE, 2014; p. 563. [Google Scholar]
- Selenium and Tellurium Chemistry. From Small Molecules to Biomolecules and Materials; Woollins, J.D., Laitinen, R.S., Eds.; Springer: Heidelberg, Germany, 2011; p. 334. [Google Scholar]
- Azad, G.K.; Tomar, R.S. Ebselen, a promising antioxidant drug: Mechanisms of action and targets of biological pathways. Mol. Biol. Rep. 2014, 41, 4865–4879. [Google Scholar] [CrossRef]
- Amosova, S.V.; Filippov, A.S.; Makhaeva, N.A.; Albanov, A.I.; Potapov, V.A. New methodology of nucleophilic substitution at three different centers of a seleniranium intermediate in reactions of 2-bromomethyl-1,3-thiaselenole with mercapto benzazoles. New J. Chem. 2019, 43, 11189–11199. [Google Scholar] [CrossRef]
- Musalov, M.V.; Yakimov, V.A.; Potapov, V.A.; Amosova, S.V.; Borodina, T.N.; Zinchenko, S.V. A novel methodology for the synthesis of condensed selenium heterocycles based on the annulation and annulation–methoxylation reactions of selenium dihalides. New J. Chem. 2019, 43, 18476–18483. [Google Scholar] [CrossRef]
- Amosova, S.V.; Filippov, A.S.; Potapov, V.A.; Penzik, M.V.; Makhaeva, N.A.; Albanov, A.I. Regio-and stereoselective synthesis of a novel family of unsaturated compounds with the S–Se bond and their cyclization to 2,3-dihydro-1,4-thiaselenines. Synthesis 2019, 51, 1832–1840. [Google Scholar] [CrossRef]
- Potapov, V.A.; Musalov, M.V.; Amosova, S.V. Reactions of selenium dichloride and dibromide with unsaturated ethers. Annulation of 2,3-dihydro-1,4-oxaselenine to the benzene ring. Tetrahedron Lett. 2011, 52, 4606–4610. [Google Scholar] [CrossRef]
- Potapov, V.A.; Amosova, S.V.; Kashik, A.S. Reactions of selenium and tellurium metals with phenylacetylene in 3-phase catalytical systems. Tetrahedron Lett. 1989, 30, 613–616. [Google Scholar] [CrossRef]
- Potapov, V.A.; Volkova, K.A.; Penzik, M.V.; Albanov, A.I.; Amosova, S.V. Reaction of selenium dichloride with divinyl selenide. Russ. J. Org. Chem. 2008, 44, 1556–1557. [Google Scholar] [CrossRef]
- Musalov, M.V.; Potapov, V.A. Selenium dihalides: New possibilities for the synthesis of selenium-containing heterocycles. Chem. Heterocycl. Compd. 2017, 53, 150–152. [Google Scholar] [CrossRef]
- Accurso, A.A.; Cho, S.-H.; Amin, A.; Potapov, V.A.; Amosova, S.V.; Finn, M.G. Thia-, Aza-, and Selena[3.3.1]bicyclononane Dichlorides: Rates vs Internal Nucleophile in Anchimeric Assistance. J. Org. Chem. 2011, 76, 4392–4395. [Google Scholar] [CrossRef] [PubMed]
- Potapov, V.A.; Amosova, S.V.; Abramova, E.V.; Lyssenko, K.A.; Musalov, M.V.; Finn, M.G. Transannular Addition of Selenium Dichloride and Dibromide to 1,5-Cyclooctadiene: Synthesis of 2,6-Dihalo-9-selenabicyclo[3.3.1]nonanes and Their Complexes with Selenium Dihalides. New J. Chem. 2015, 39, 8055–8059. [Google Scholar] [CrossRef]
- Potapov, V.A.; Musalov, M.V.; Musalova, M.V.; Amosova, S.V. Recent Advances in Organochalcogen Synthesis Based on Reactions of Chalcogen Halides with Alkynes and Alkene. Curr. Org. Chem. 2016, 20, 136–145. [Google Scholar] [CrossRef]
- Borisov, A.V.; Matsulevich, Z.V.; Osmanov, V.K.; Borisova, T.N.; Savikhina, E.V. Heterocyclization in the reaction of pyridine-2-selanyl chloride with styrene. Chem. Heterocycl. Comp. 2007, 43, 525–526. [Google Scholar] [CrossRef]
- Borisov, A.V.; Osmanov, V.K.; Borisova, G.N.; Matsulevich, Z.V.; Fukin, G.K. Synthesis of condensed sulfur- and nitrogen-containing heterocycles via polar cycloaddition of hetarene sulfenyl chlorides to a C–C multiple bond. Mendeleev Commun. 2009, 19, 49–51. [Google Scholar] [CrossRef]
- Borisov, A.V.; Matsulevich, Z.V.; Osmanov, V.K.; Borisova, G.N.; Mammadova, G.Z.; Maharramov, A.M.; Khrustalev, V.N. Cycloaddition of di (2-pyridyl) diselenide to styrene activated with antimony pentachloride. Russ. Chem. Bull. 2011, 60, 2057–2062. [Google Scholar] [CrossRef]
- Borisov, A.V.; Matsulevich, Z.V.; Osmanov, V.K.; Borisova, G.N.; Mammadova, G.Z.; Maharramov, A.M.; Khrustalev, V.N. Sulfenyl halides in the synthesis of heterocycles. 4*. Heterocyclization in reactions of alkenes with sulfenylating reagents based on di (2-pyridyl) disulfide. Chem. Heterocycl. Comp. 2012, 48, 1098–1104. [Google Scholar] [CrossRef]
- Borisov, A.V.; Matsulevich, Z.V.; Osmanov, V.K.; Borisova, G.N. Synthesis of 2,3-dihydroselenazolo[3,2-a]pyridinium salts based on reactions of pyridine-2-selanyl chloride with alkenes and dienes. Chem. Heterocycl. Comp. 2012, 48, 492–496. [Google Scholar] [CrossRef]
- Potapov, V.A.; Malinovich, D.A.; Amosova, S.V.; Rusakov, Y.Y.; Bhasin, K.K. Reaction of 2-pyridylselenenyl bromide with divinyl selenide. Chem. Heterocycl. Comp. 2012, 48, 1129–1131. [Google Scholar] [CrossRef]
- Potapov, V.A.; Musalova, M.V.; Ishigeev, R.S.; Musalov, M.V.; Panov, V.A.; Khabibulina, A.G.; Amosova, S.V.; Bhasin, K.K. Efficient and selective syntheses of novel unsaturated chalcogen-containing pyridine derivatives. Tetrahedron Lett. 2016, 57, 5341–5343. [Google Scholar] [CrossRef]
- Potapov, V.A.; Ishigeev, R.S.; Amosova, S.V.; Borodina, T.N. Synthesis of a novel family of water-soluble 2H,3H-[1,3]thia- and -selenazolo[3,2-a]pyridin-4-ium heterocycles by annulation reactions. Tetrahedron Lett. 2019, 60, 475–479. [Google Scholar] [CrossRef]
- Potapov, V.A.; Ishigeev, R.S.; Amosova, S.V. Synthesis of 3-(2-oxopyrrolidin-1-yl)-2H,3H-[1,3]selenazolo[3,2-a]pyridin-4-ium chloride. Russ. J. Org. Chem. 2017, 53, 1604–1605. [Google Scholar] [CrossRef]
- Potapov, V.A.; Ishigeev, R.S.; Amosova, S.V. Regioselective Reaction of Pyridine-2-Sulfenyl Chloride with Isoeugenole. Russ. J. Org. Chem. 2018, 54, 1262–1263. [Google Scholar]
- Samuilov, Y.D.; Gainullin, V.I.; Solov’eva, S.E.; Konovalov, A.I. Reactivity of styrenes toward electrophilic addition of phenylsulfenyl chloride. Zhurnal Organicheskoi Khimii 1988, 24, 795–803. (In Russian) [Google Scholar]
- Liotta, D.; Zima, G. An examination of the synthetic utility of phenylselenenyl chloride additions to olefins. Tetrahedron Lett. 1978, 50, 4977–4980. [Google Scholar] [CrossRef]
- Rasteikiene, L.; Greiciute, D.; Lin’kova, M.G.; Knunyants, I.L. The Addition of Sulphenyl Chlorides to Unsaturated Compounds. Russ. Chem. Rev. 1977, 46, 548–564. [Google Scholar] [CrossRef]
- Smit, V.A.; Zefirov, N.S.; Bodrikov, I.V.; Krimer, M.Z. Episulfonium ions: Myth and reality. Acc. Chem. Res. 1979, 12, 282–288. [Google Scholar] [CrossRef]
- Abu-yousef, I.A.; Harpp, D.N. New Sulfenyl Chloride Chemistry: Synthesis, Reactions and Mechanisms toward Carbon-Carbon Double Bonds. Sulfur Rep. 2003, 24, 255–282. [Google Scholar] [CrossRef]
- Denmark, S.E.; Vogler, T. Synthesis and Reactivity of Enantiomerically Enriched Thiiranium Ions. Chem. Eur. J. 2009, 15, 11737–11745. [Google Scholar] [CrossRef]
- Denmark, S.E.; Collins, W.R.; Cullen, M.D. Observation of Direct Sulfenium and Selenenium Group Transfer from Thiiranium and Seleniranium Ions to Alkenes. J. Am. Chem. Soc. 2009, 131, 3490–3492. [Google Scholar] [CrossRef]
- Denmark, S.E.; Edwards, M.G. On the Mechanism of the Selenolactonization Reaction with Selenenyl Halides. J. Org. Chem. 2006, 71, 7293–7306. [Google Scholar] [CrossRef] [PubMed]
- Denmark, S.E.; Kalyani, D.; Collins, W.R. Preparative and mechanistic studies toward the rational development of catalytic, enantioselective selenoetherification reactions. J. Am. Chem. Soc. 2010, 132, 15752–15765. [Google Scholar] [CrossRef] [PubMed]
- Musalov, M.V.; Potapov, V.A.; Kurkutov, E.O.; Musalova, M.V.; Khabibulina, A.G.; Amosova, S.V. Regioselective syntheses of bis(2-haloalkyl) selenides and dihalo[bis(2-haloalkyl)]-λ4-selanes from selenium dihalides and 1-alkenes, and the methoxyselenenylation reaction. Arkivoc 2017, iii, 365–376. [Google Scholar] [CrossRef]
- Kurkutov, E.O.; Musalov, M.V.; Potapov, V.A.; Larina, L.I.; Amosova, S.V. Rearrangements in methanolysis of bis(2-bromoalkyl) selenides. Russ. J. Org. Chem. 2016, 52, 186–191. [Google Scholar] [CrossRef]
- Fiorito, S.; Epifano, F.; Preziuso, F.; Taddeo, V.A.; Santi, C.; Genovese, S. New insights into the seleniranium ion promoted cyclization of prenyl and propenylbenzene aryl ethers. Tetrahedron Lett. 2017, 58, 371–374. [Google Scholar] [CrossRef]
- Poleschner, H.; Seppelt, K. Seleniranium and Telluriranium Salts. Chem. Eur. J. 2018, 24, 17155–17161. [Google Scholar] [CrossRef]
- Sharpless, K.B.; Lauer, R.F. Electrophilic organoselenium reagents. New route to allylic acetates and ethers. J. Org. Chem. 1974, 39, 429–430. [Google Scholar] [CrossRef]
- Antipin, R.L.; Klak, V.N.; Beloglazkina, E.K.; Zyk, N.V. Reactions of areneselenenamides with alkenes in the presence of phosphorus (V) and sulfur (IV) oxyhalides. New synthesis of β-haloalkyl selenides. Russ. J. Org. Chem. 2009, 45, 842–847. [Google Scholar] [CrossRef]
- Soloshonok, V.A.; Nelson, D.J. Alkene selenenylation: A comprehensive analysis of relative reactivities, stereochemistry and asymmetric induction, and their comparisons with sulfenylation. Beilstein J. Org. Chem. 2011, 7, 744–758. [Google Scholar] [CrossRef] [PubMed]
- Cresswell, A.J.; Eey, S.T.-C.; Denmark, S.E. Catalytic, stereospecific syn-dichlorination of alkenes. Nat. Chem. 2015, 7, 146–152. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).