Performance of Graphite-Dispersed Li2CO3-K2CO3 Molten Salt Nanofluid for a Direct Absorption Solar Collector System
Abstract
1. Introduction
- The nanoparticles dominate the optical properties of the nanofluid, which allows us to tune the performance of the receiver by altering the size, shape, concentration, and material type of the particles [22].
- As the nanofluid directly absorbs the solar radiation, DACs do not require a surface absorption plate [23].
- Optically selective nanofluids allow for high absorption in the solar range and low emittance in the infrared. Therefore, a volumetric receiver can be employed in place of a selective surface receiver of higher emissive losses resulting from a poorer temperature profile [27].
2. Computational Model Development and Solution Procedure
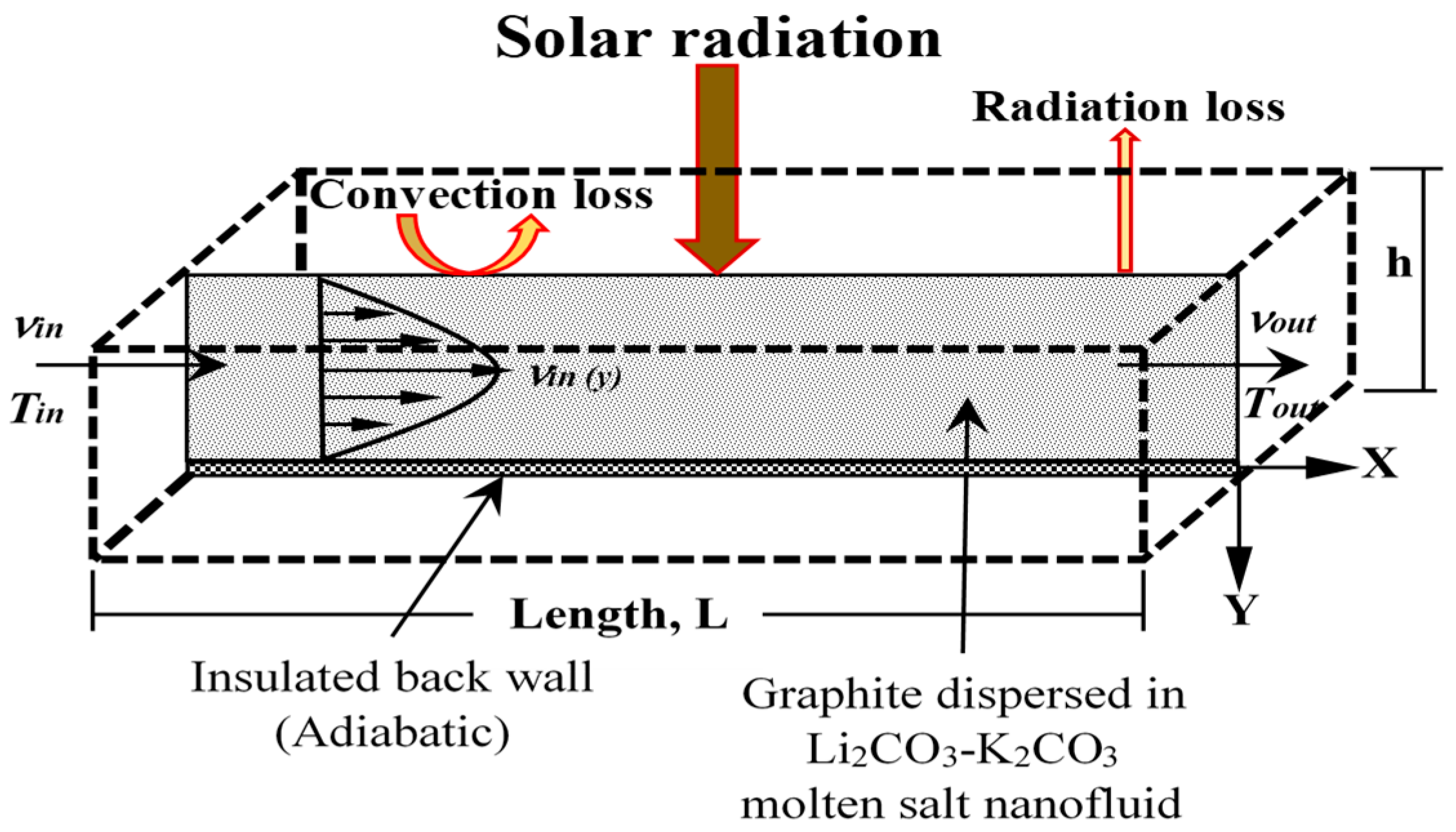
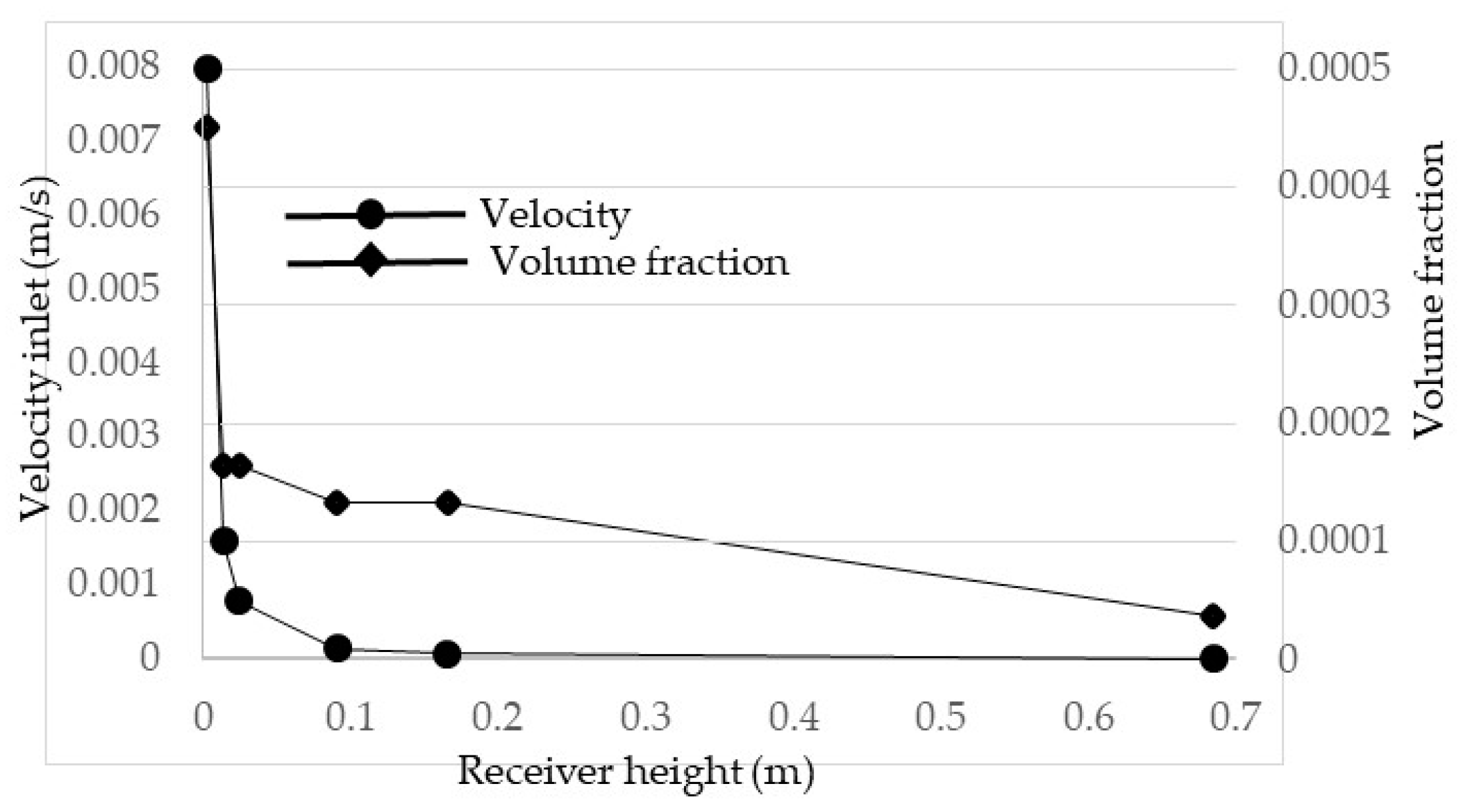
2.1. Model Setup and Boundary Conditions
2.2. Thermo-Physical and Rheological Properties of the Nanofluid
2.3. Modeling Optical Properties of the Nanofluid
2.4. Governing Equations
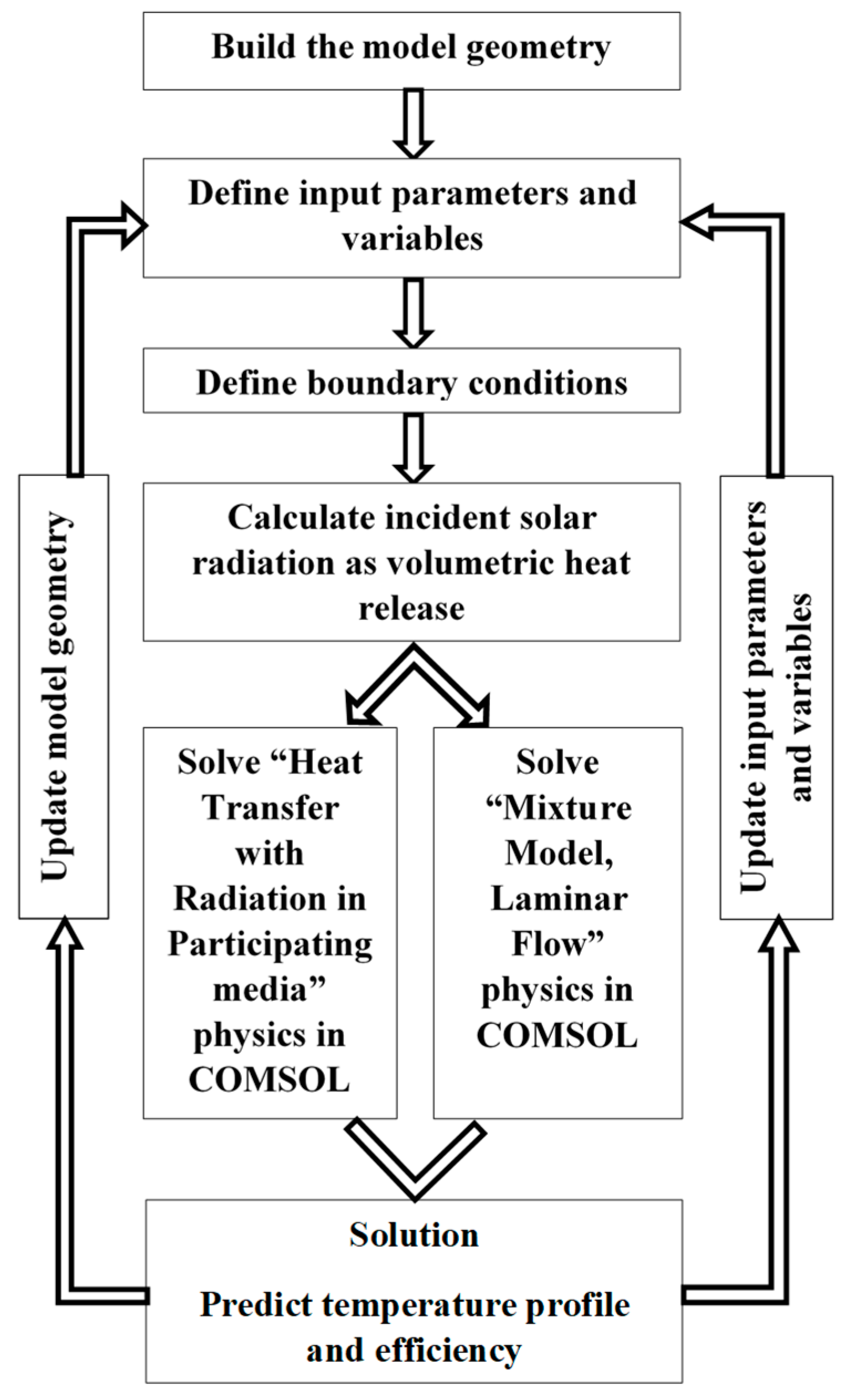
2.5. Solution Procedure
2.6. Model Parameters and Variables
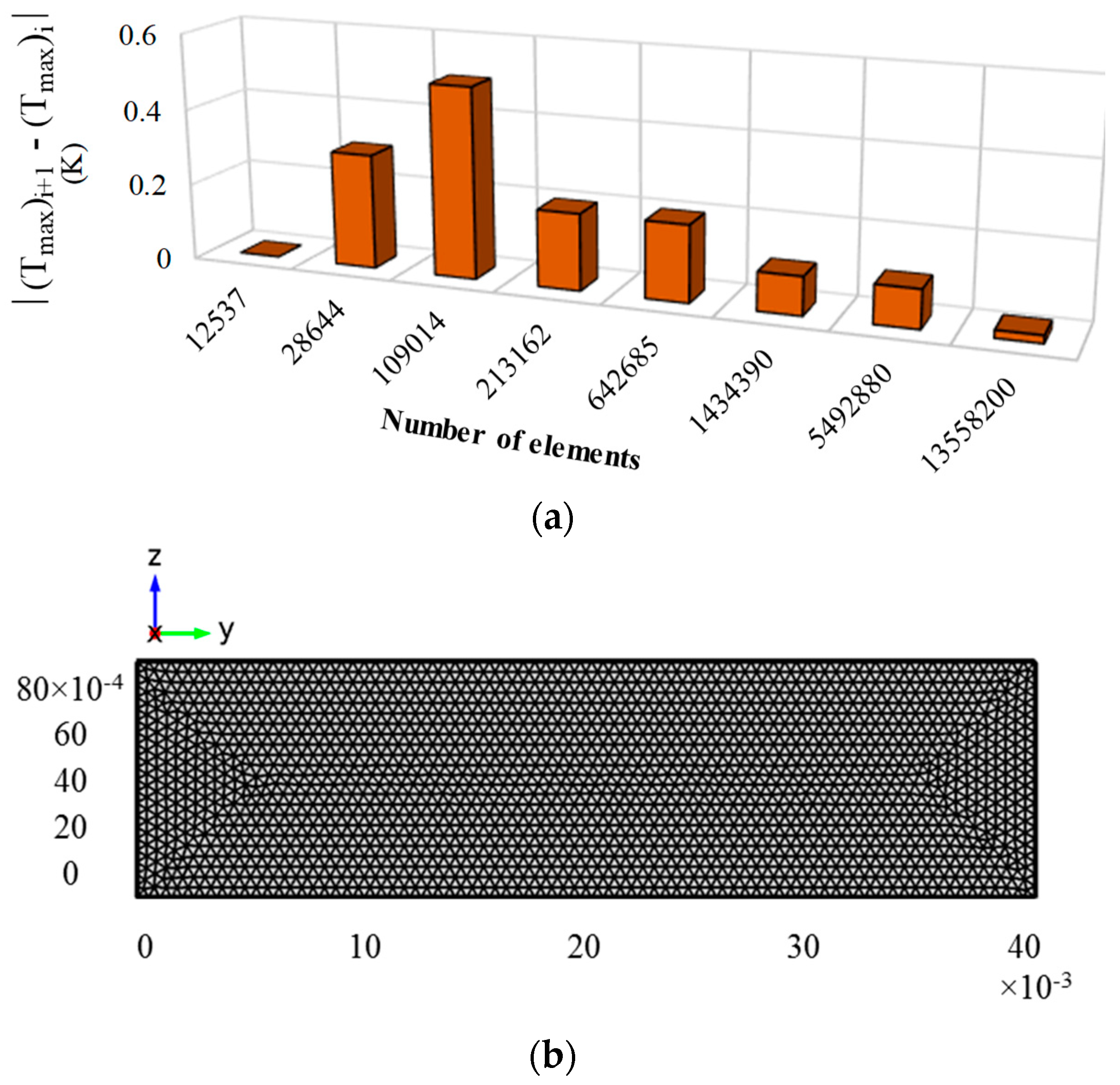
2.7. Grid Generation
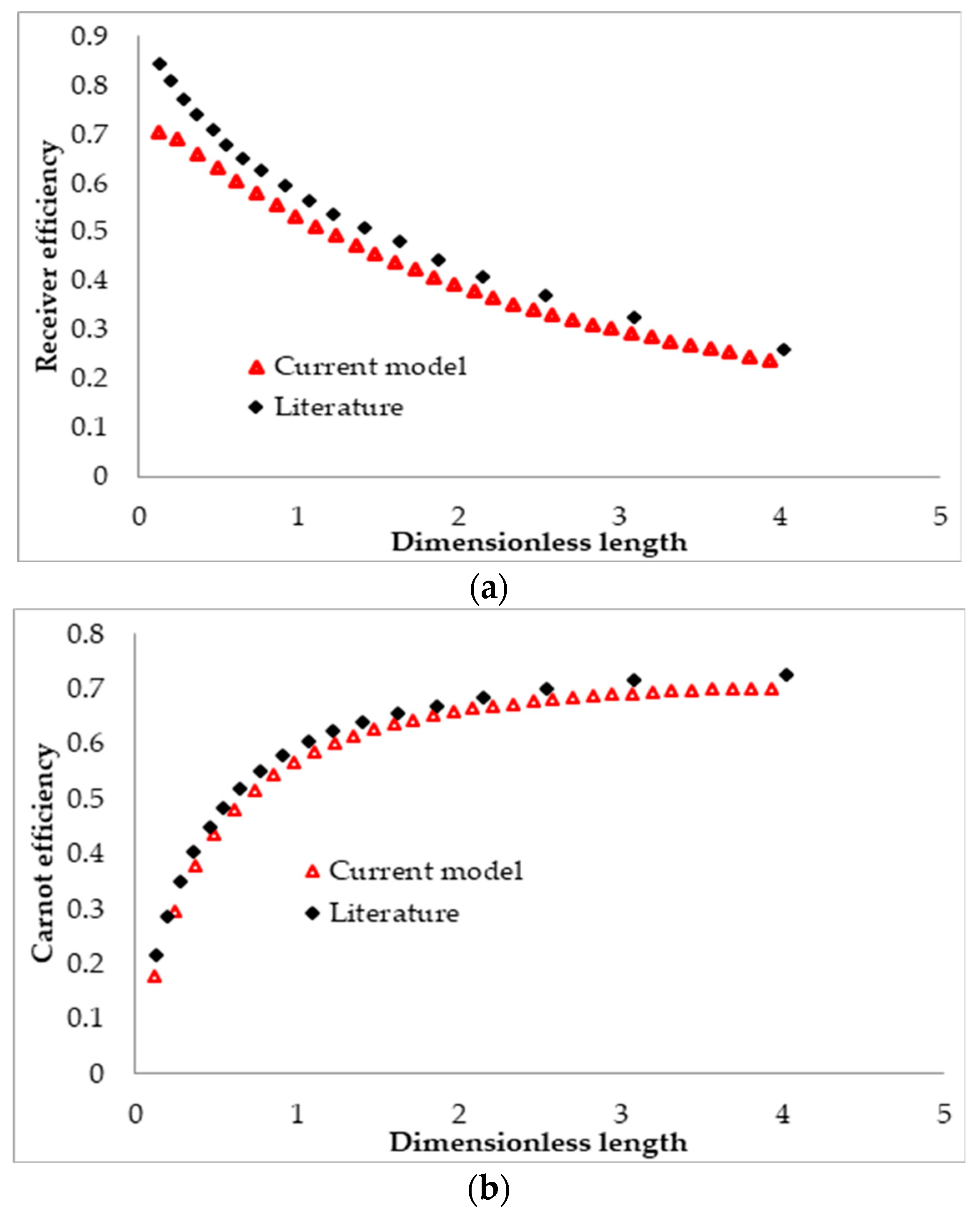
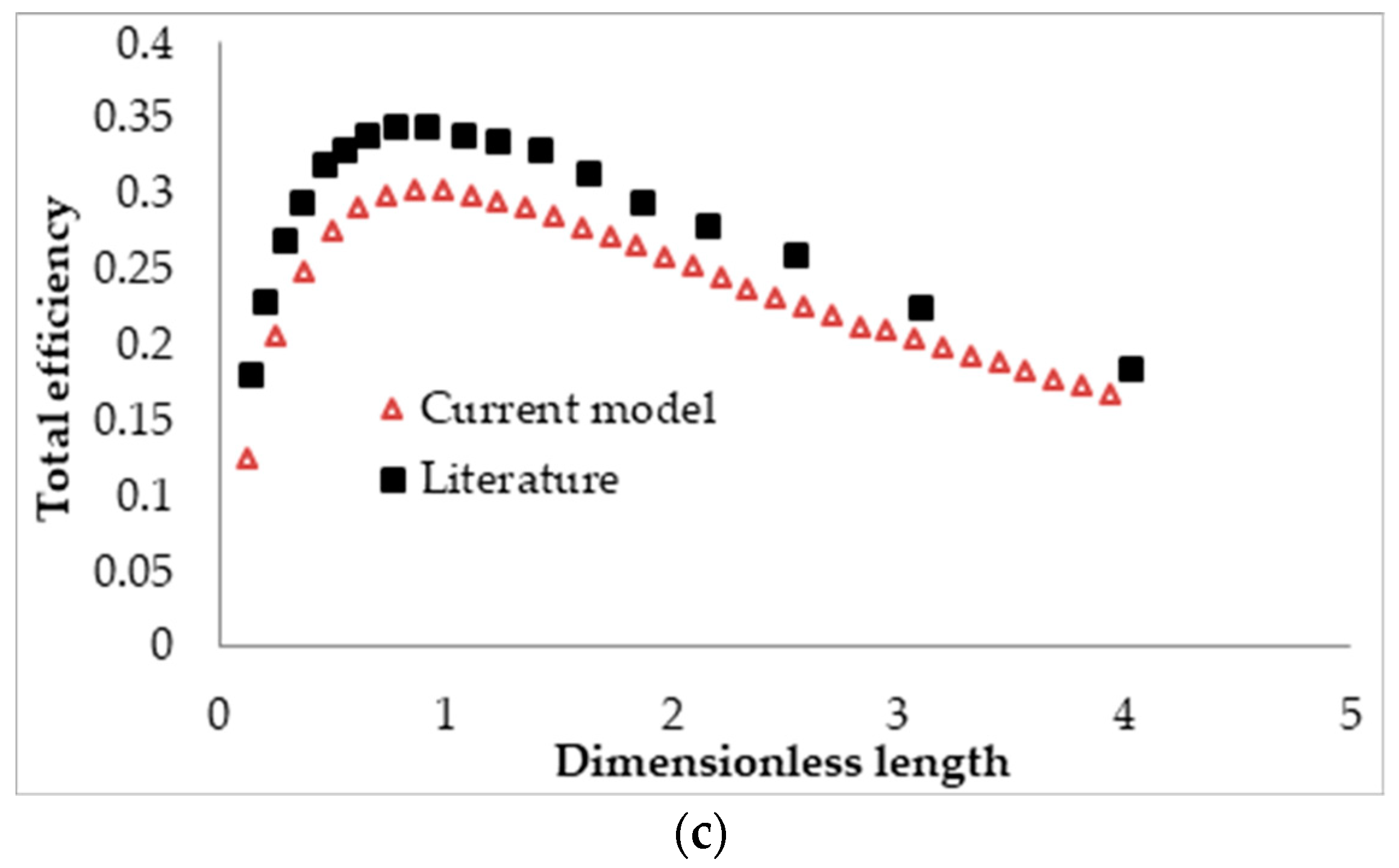
2.8. Model Validation
3. Results and Discussion
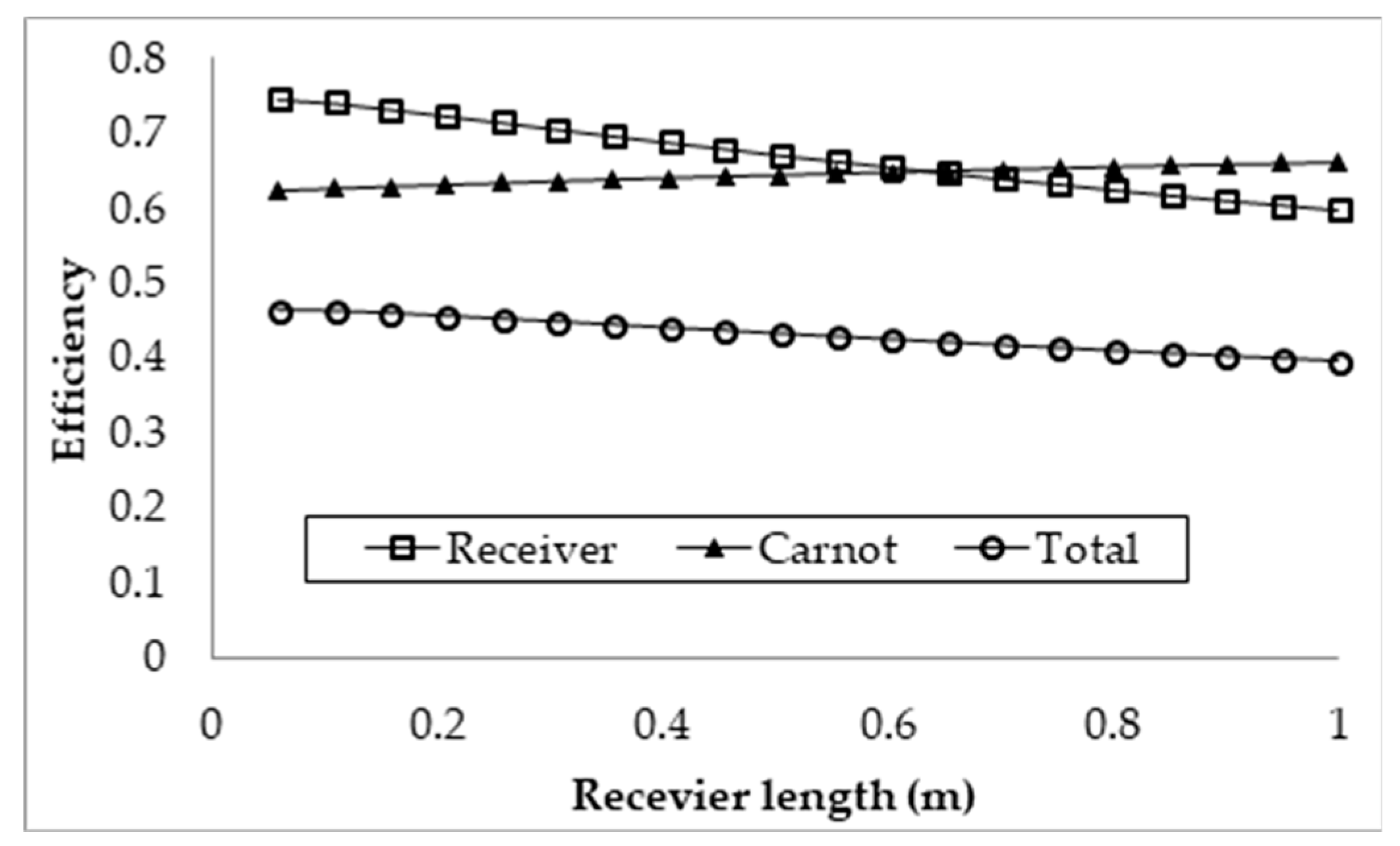
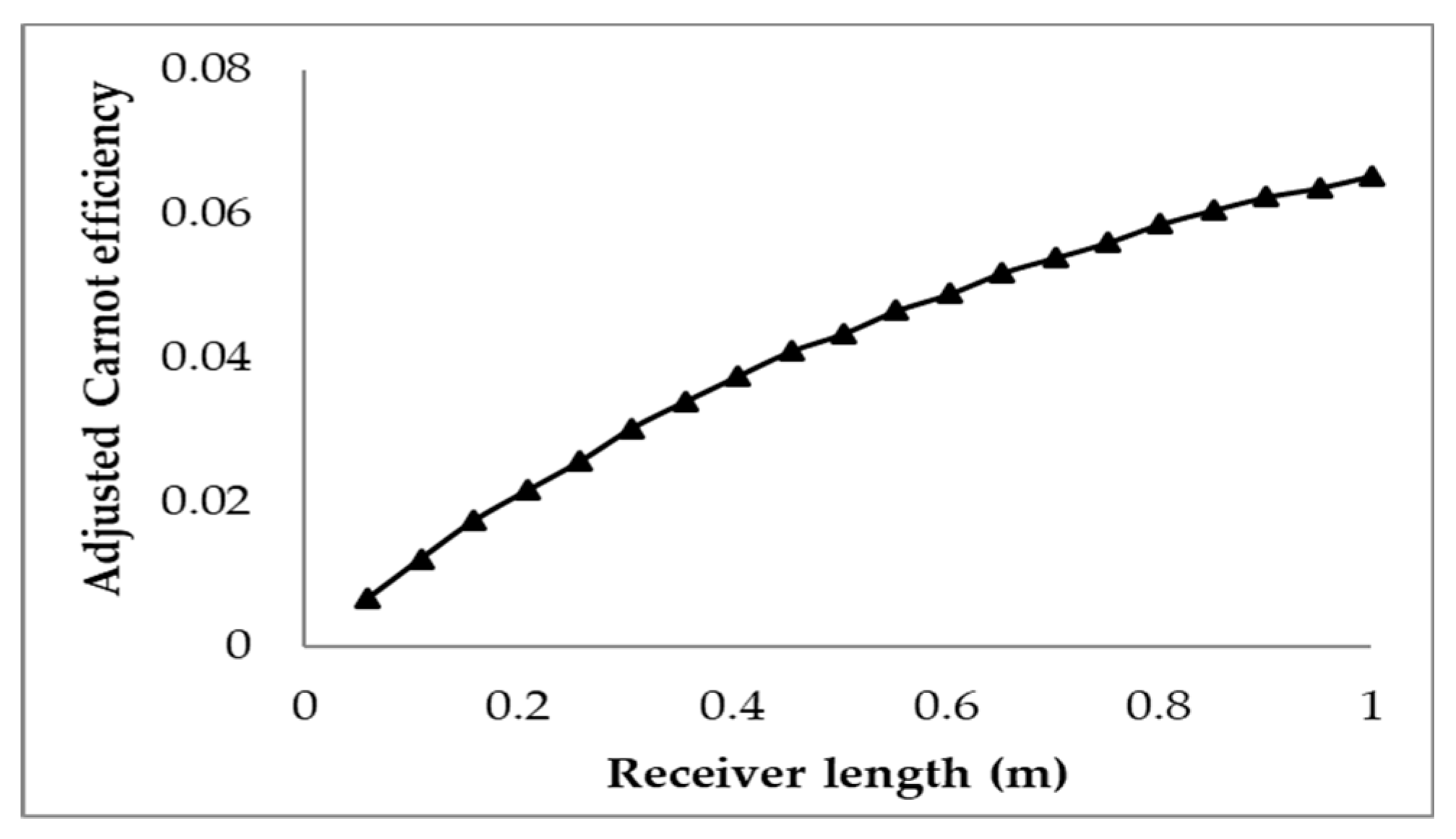
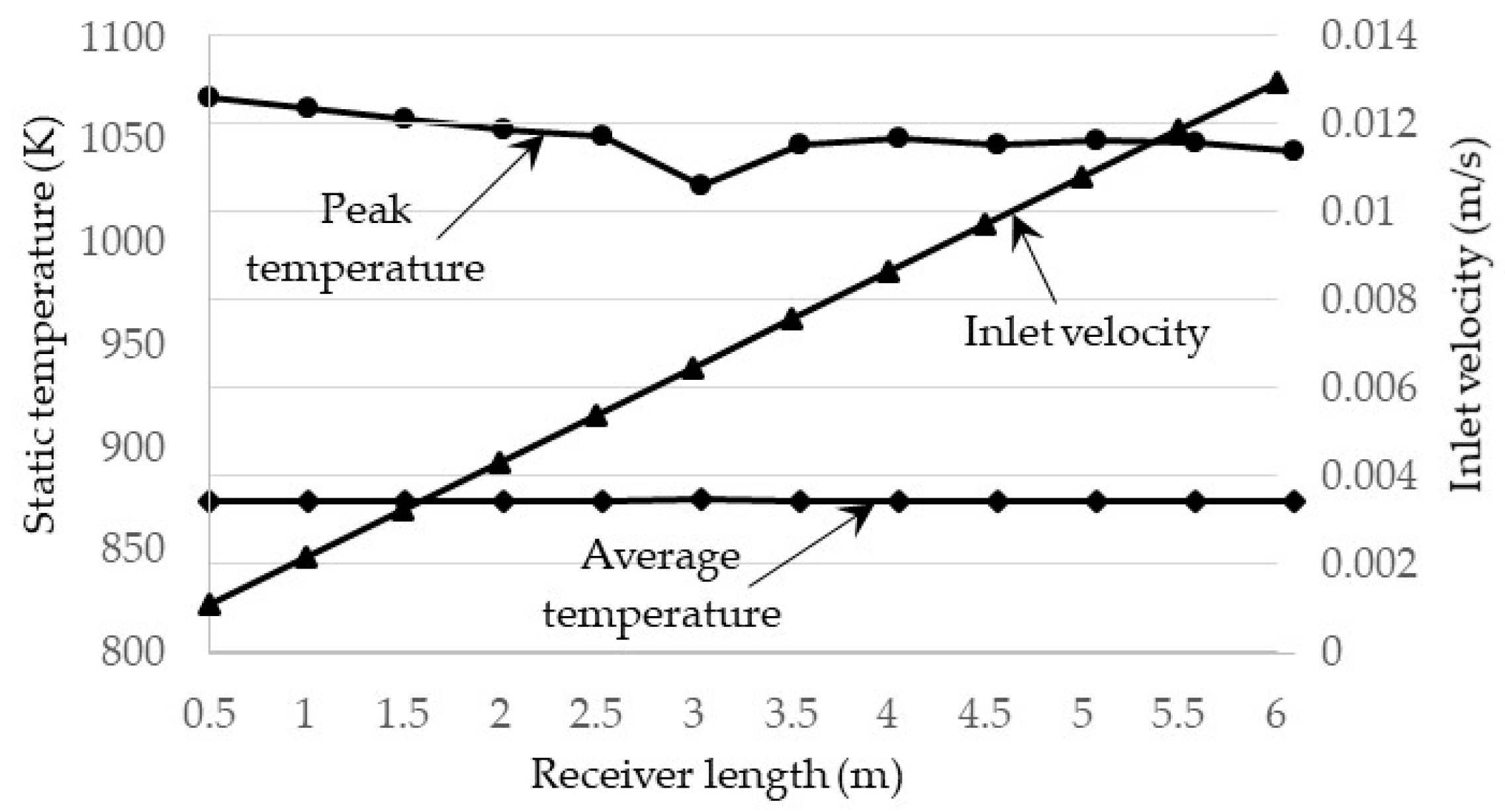
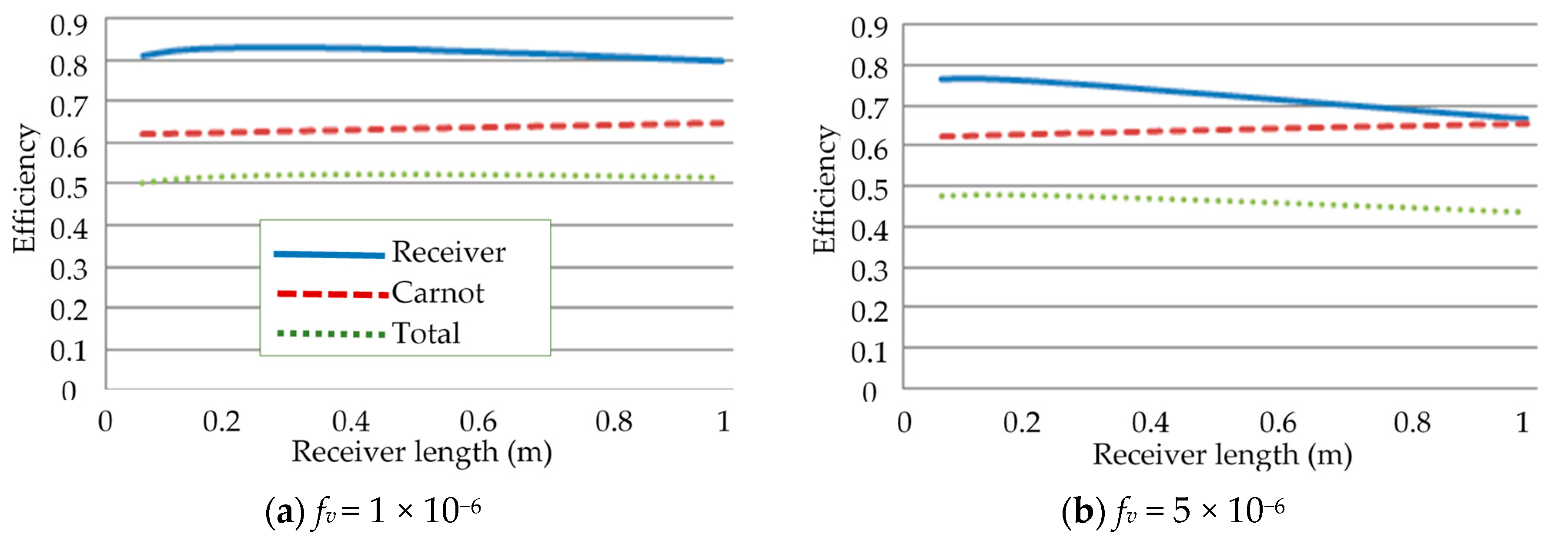
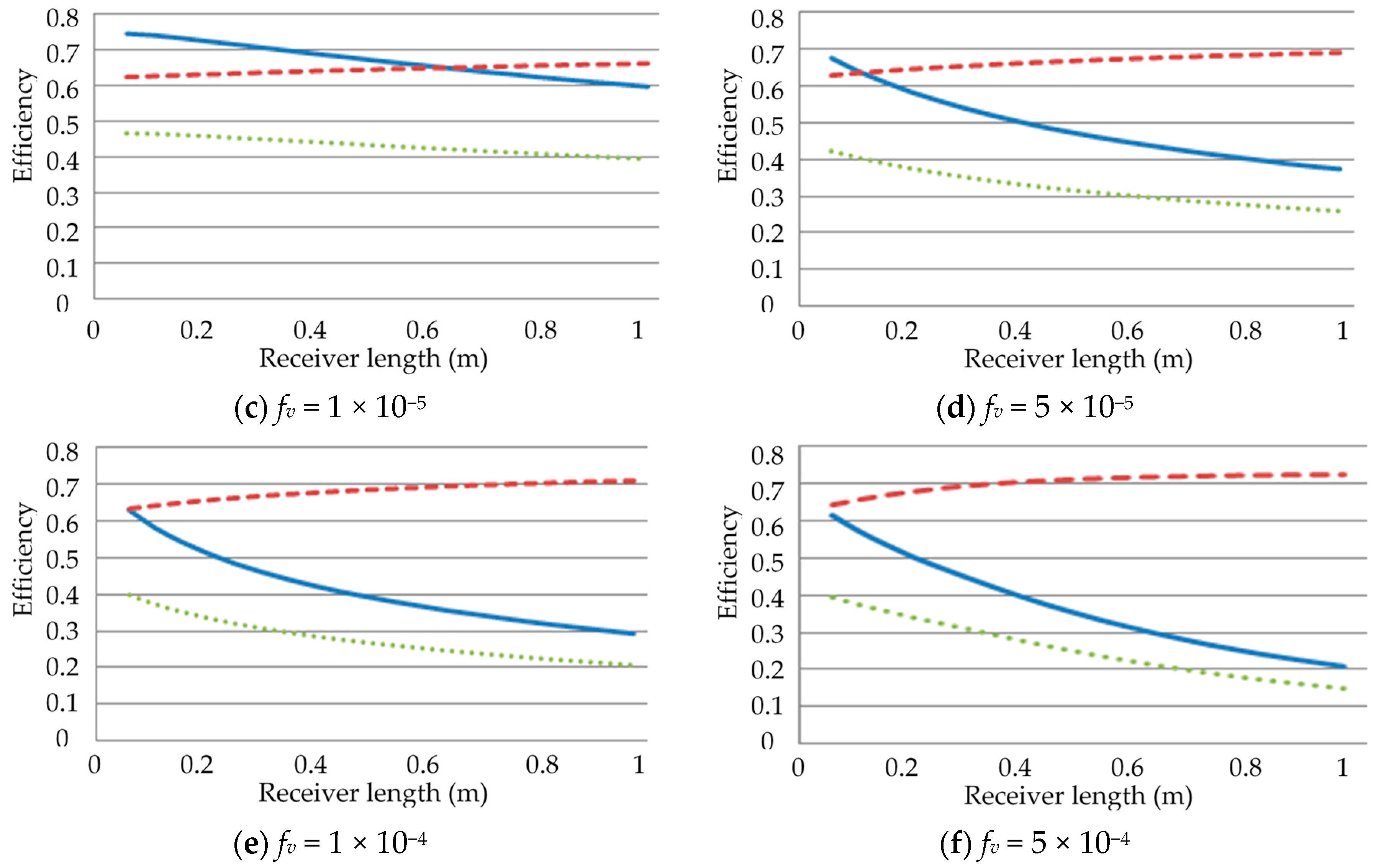
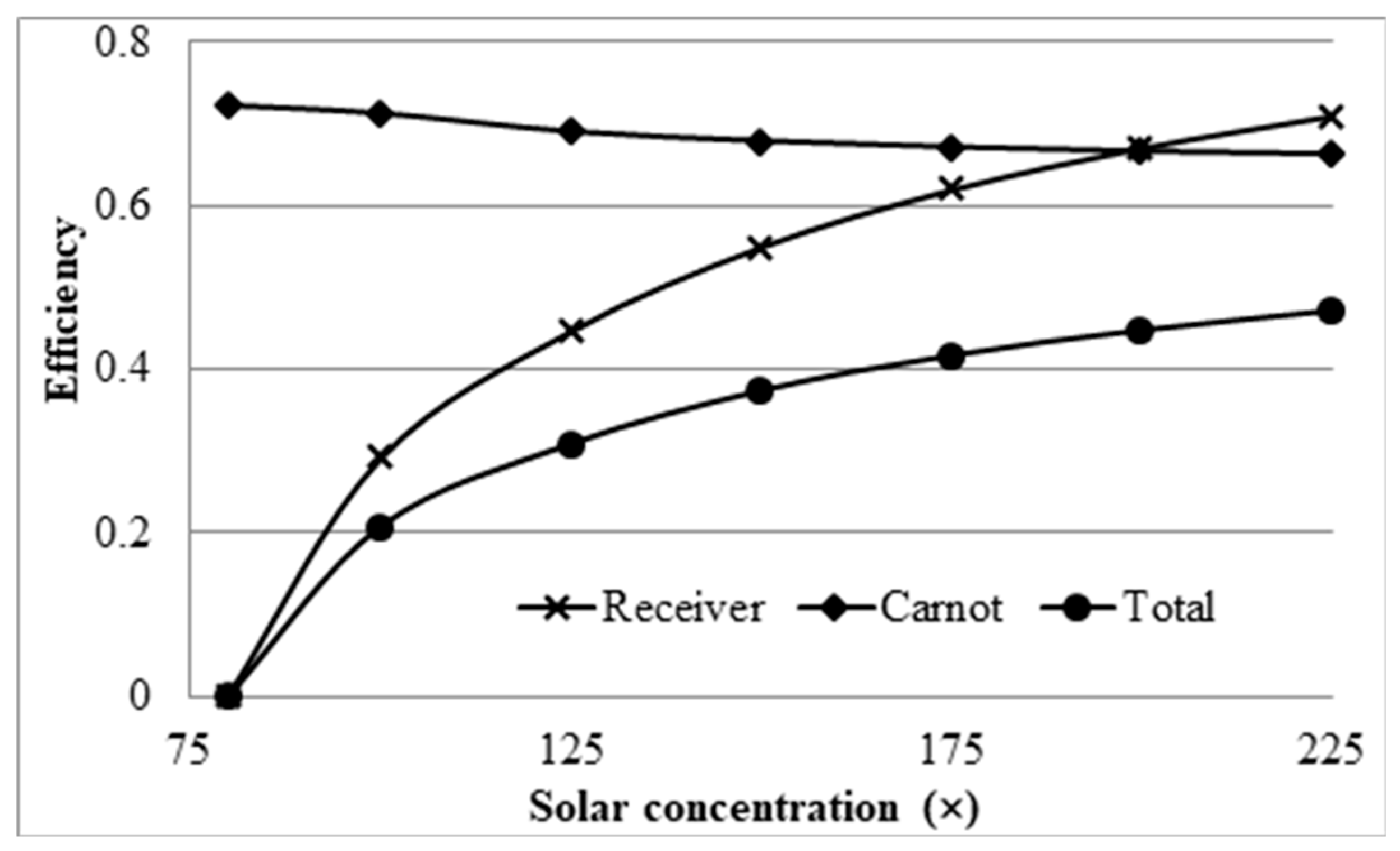
3.1. Effect of Receiver Length on the Efficiencies
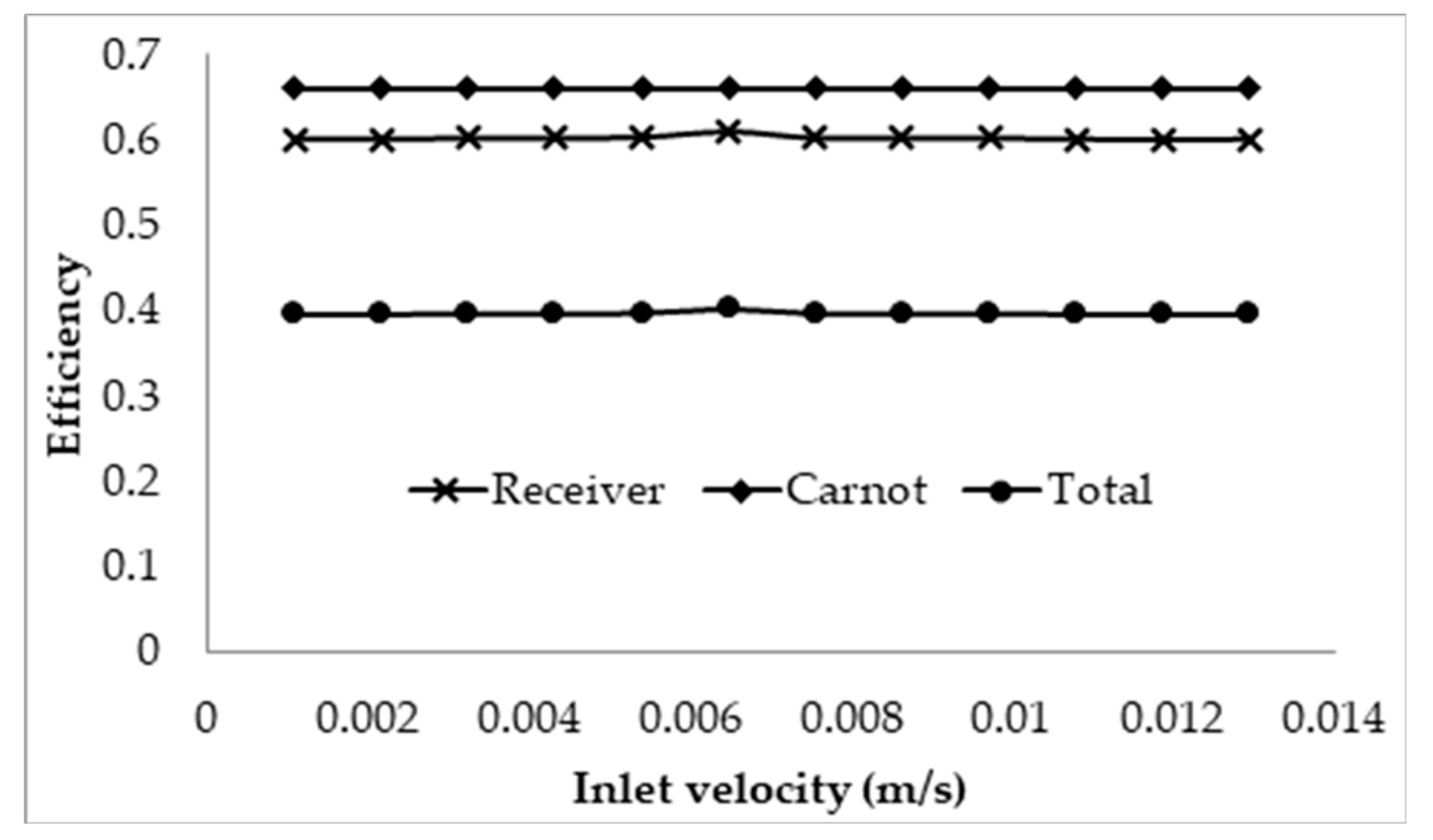
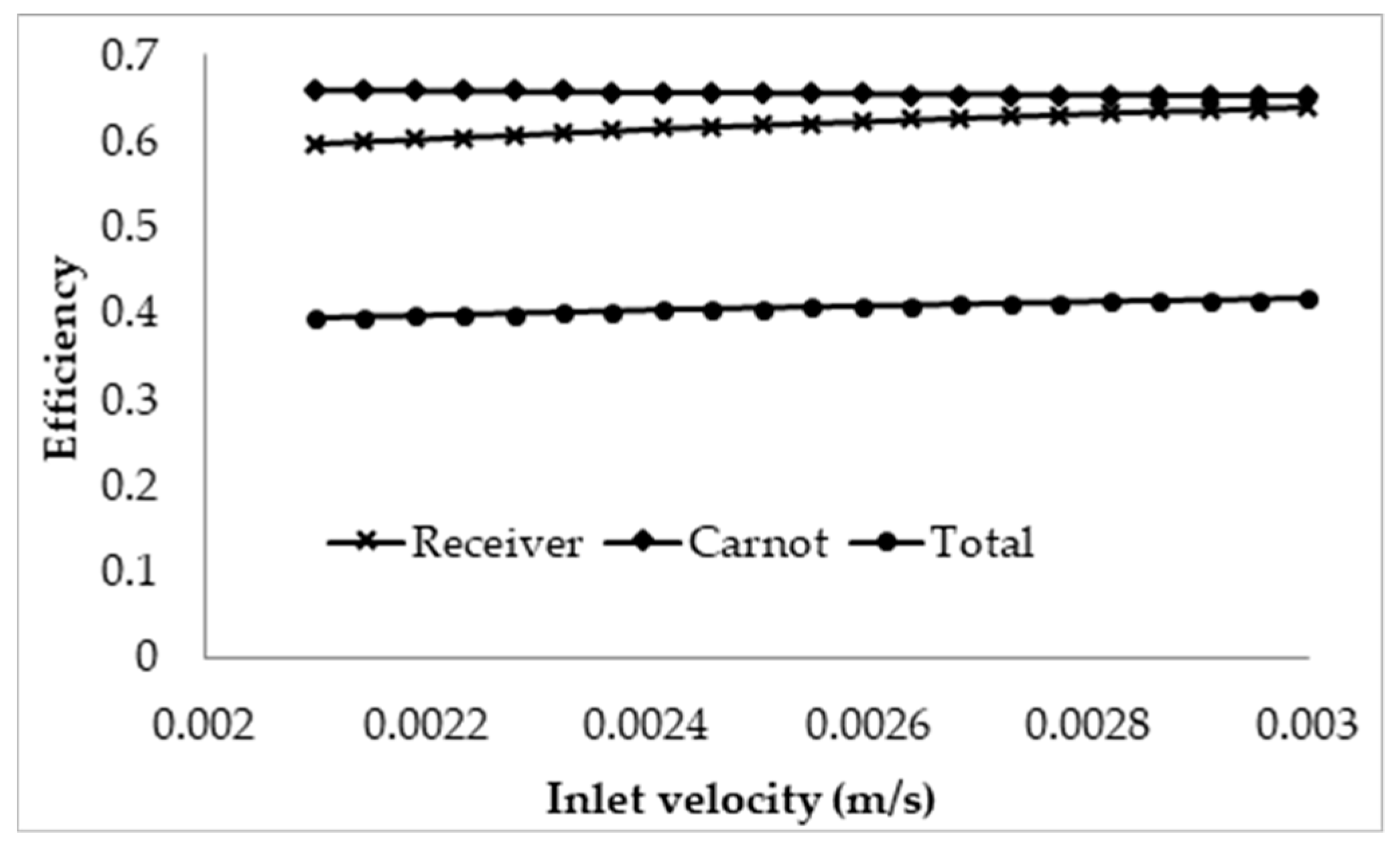
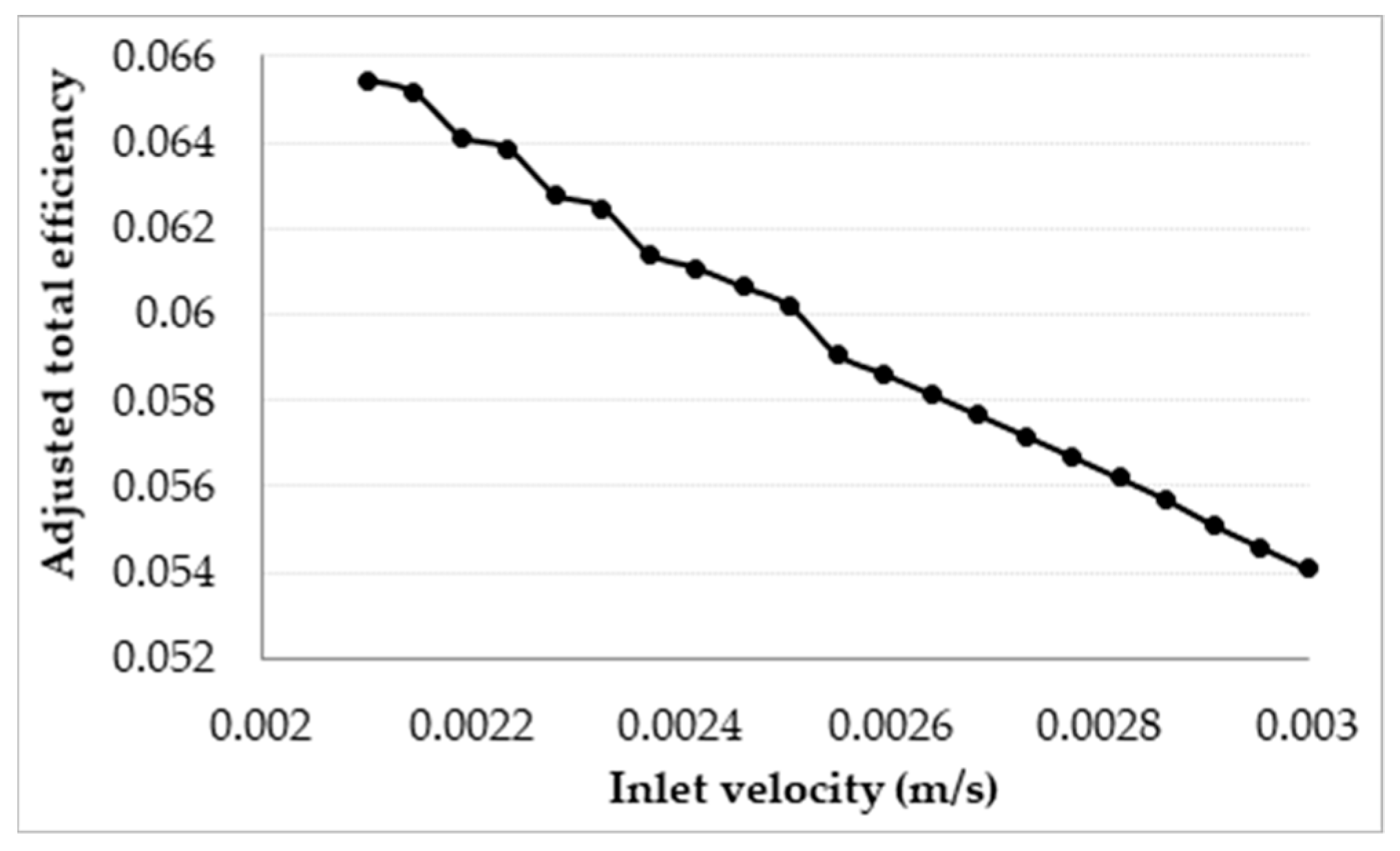
3.2. Effect of Inlet Velocity on Collector Efficiency
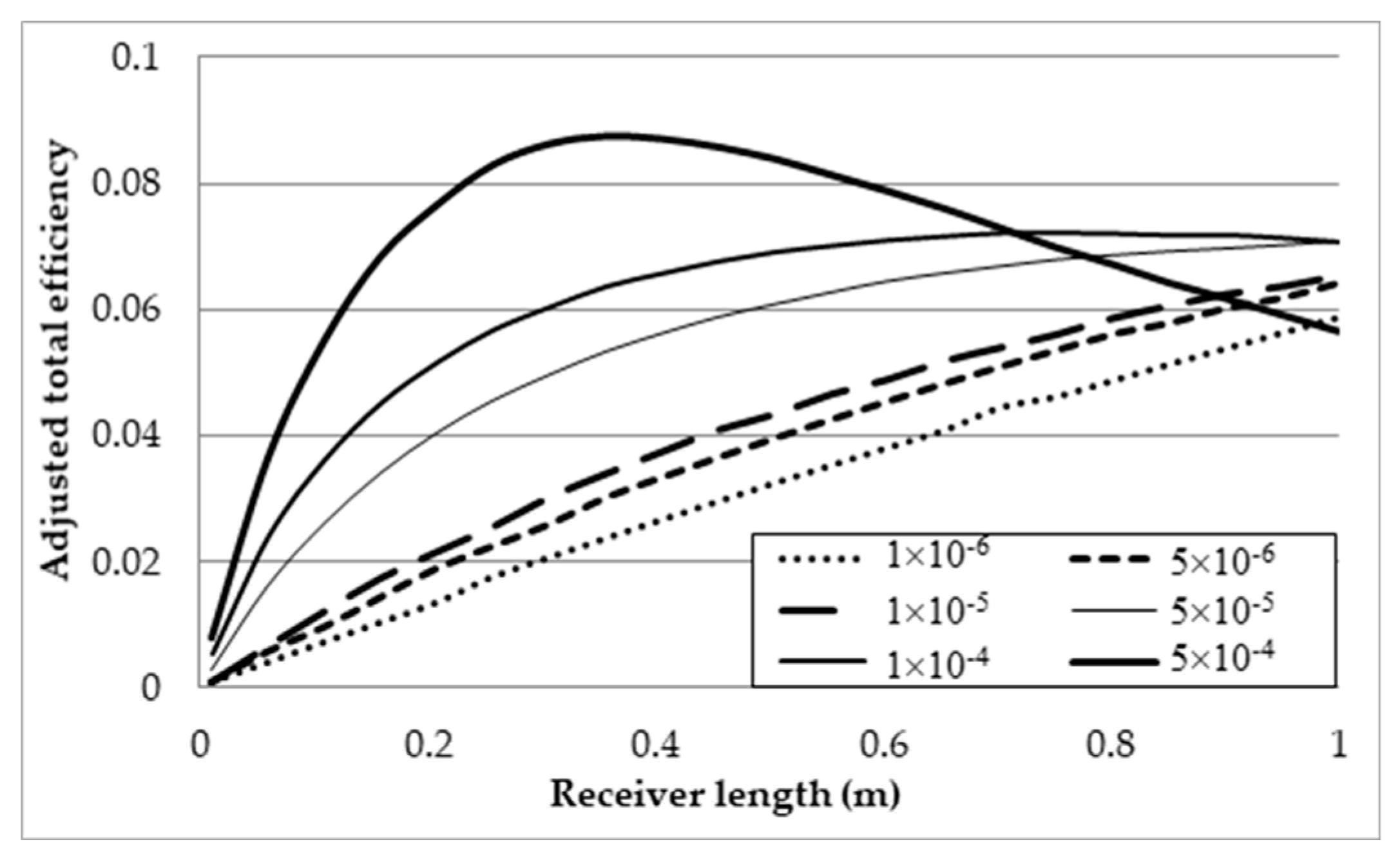
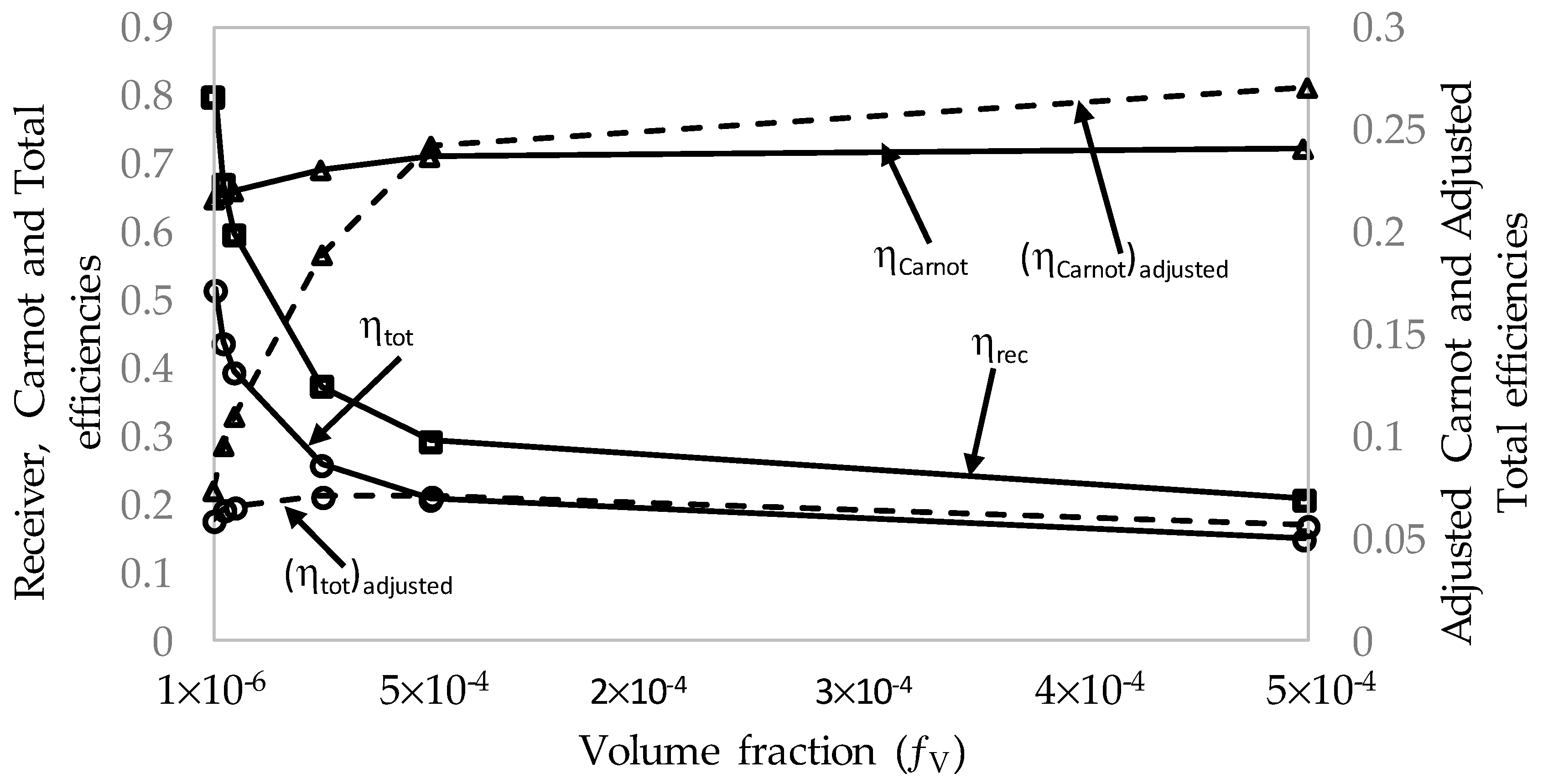
3.3. Effect of Volume Fractions, fv, on the Efficiencies
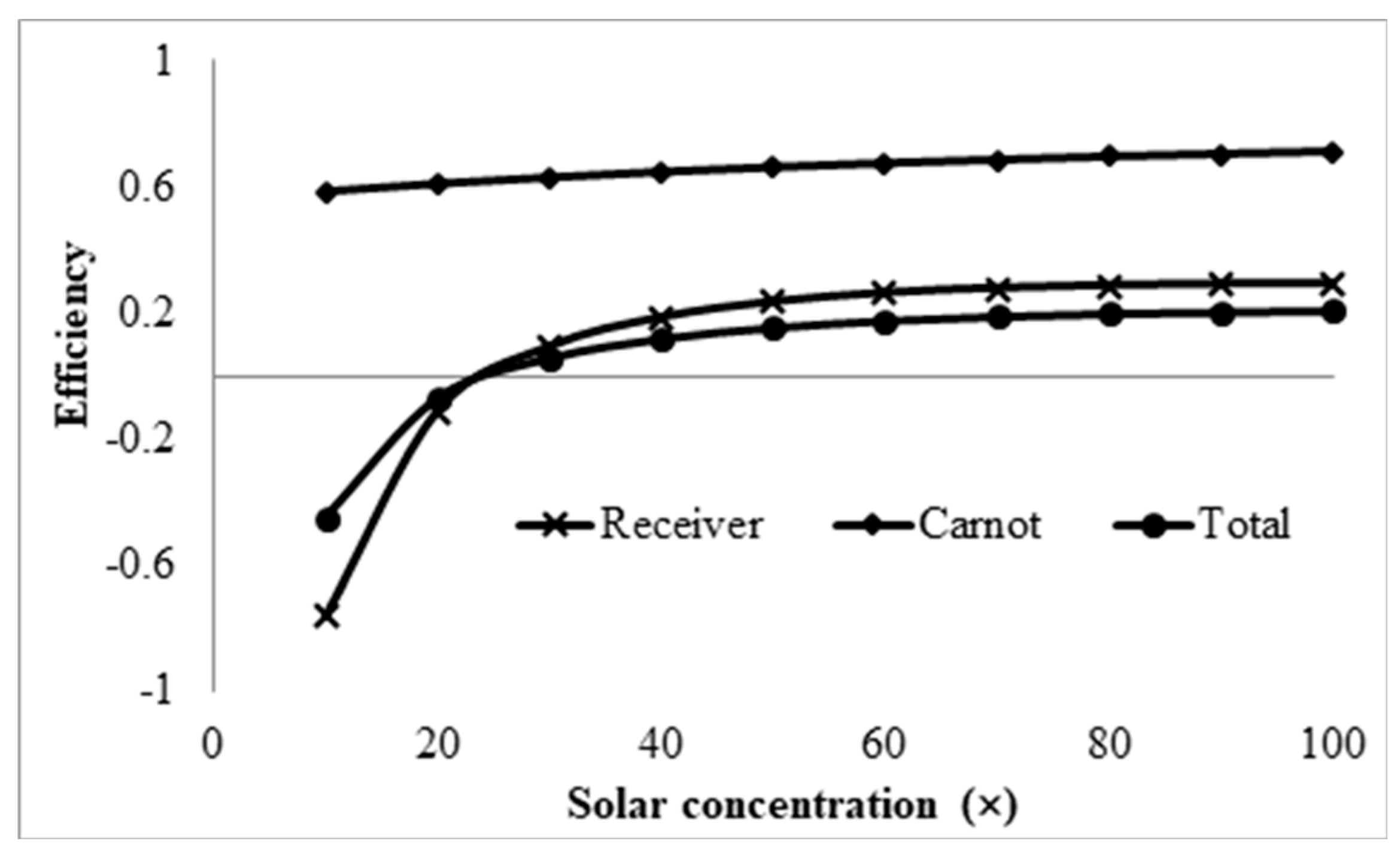
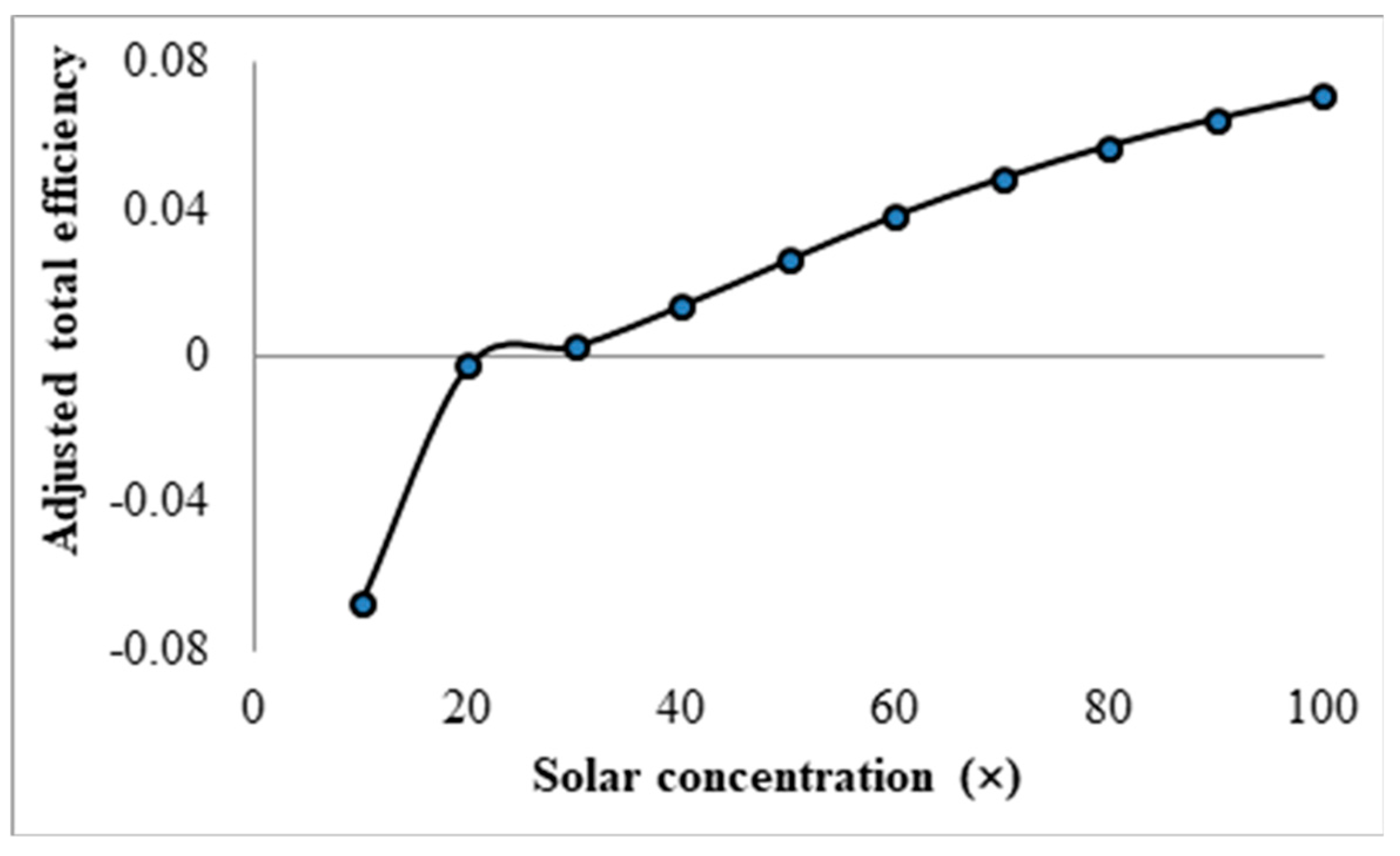
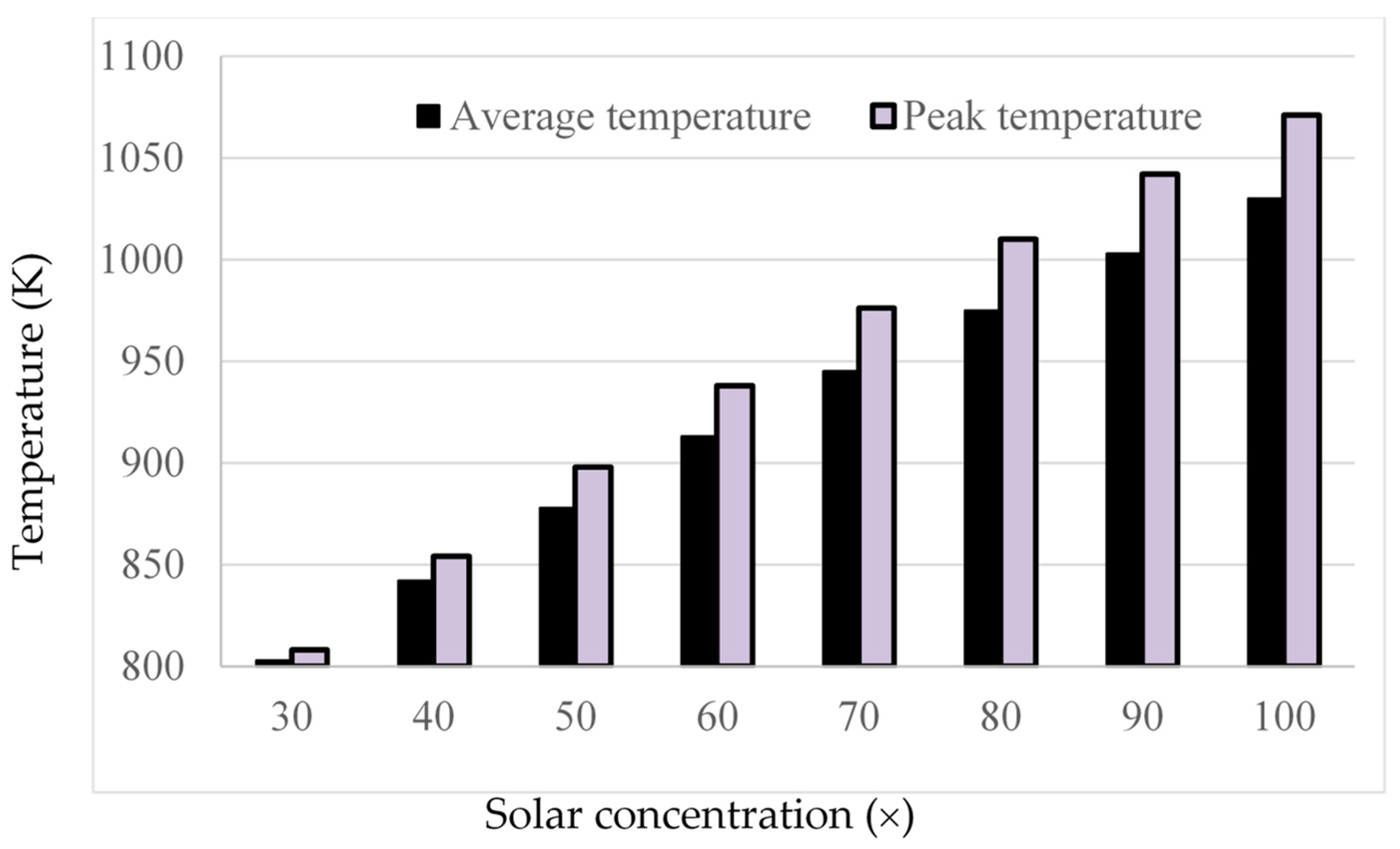
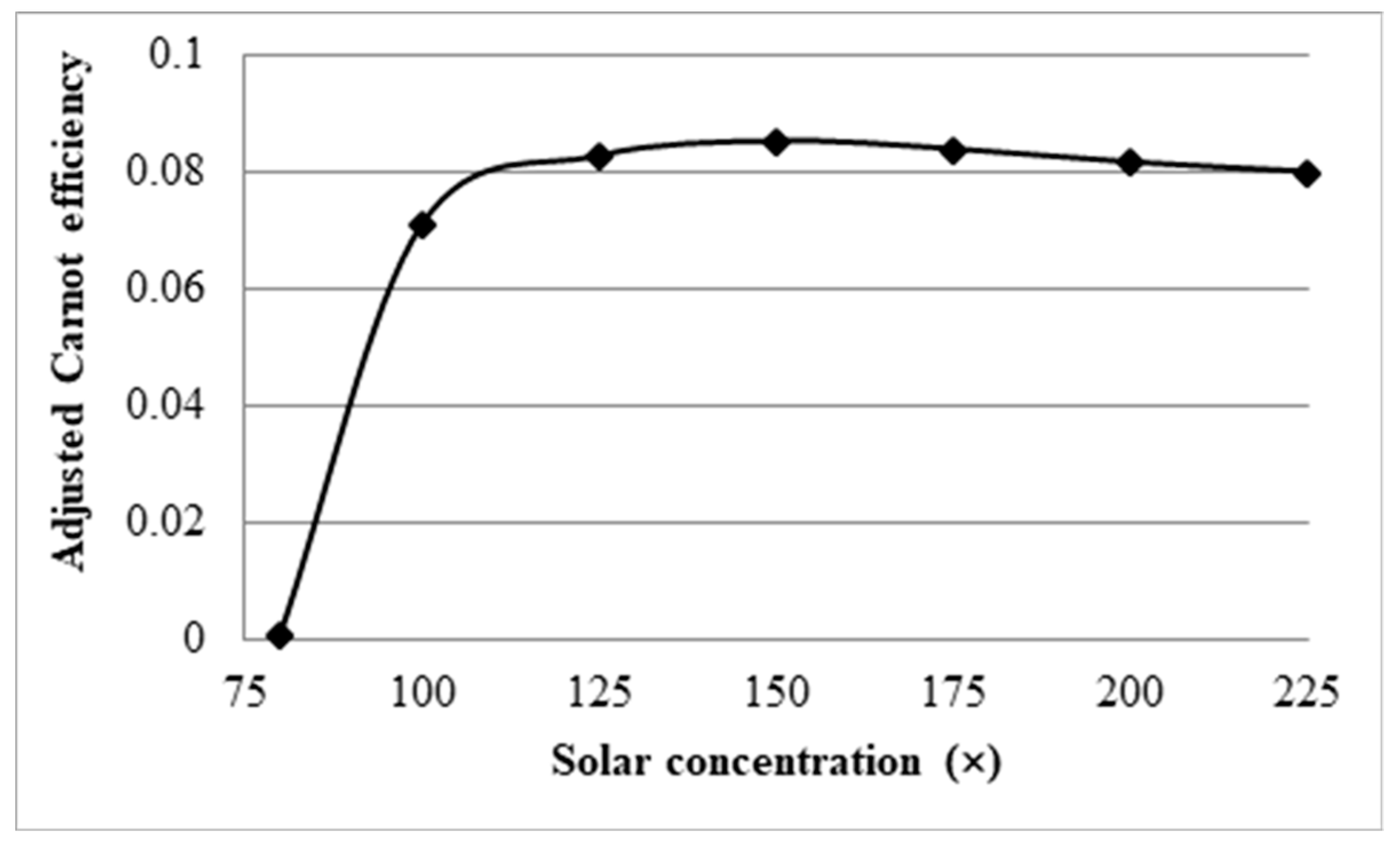
3.4. Effect Solar Concentration
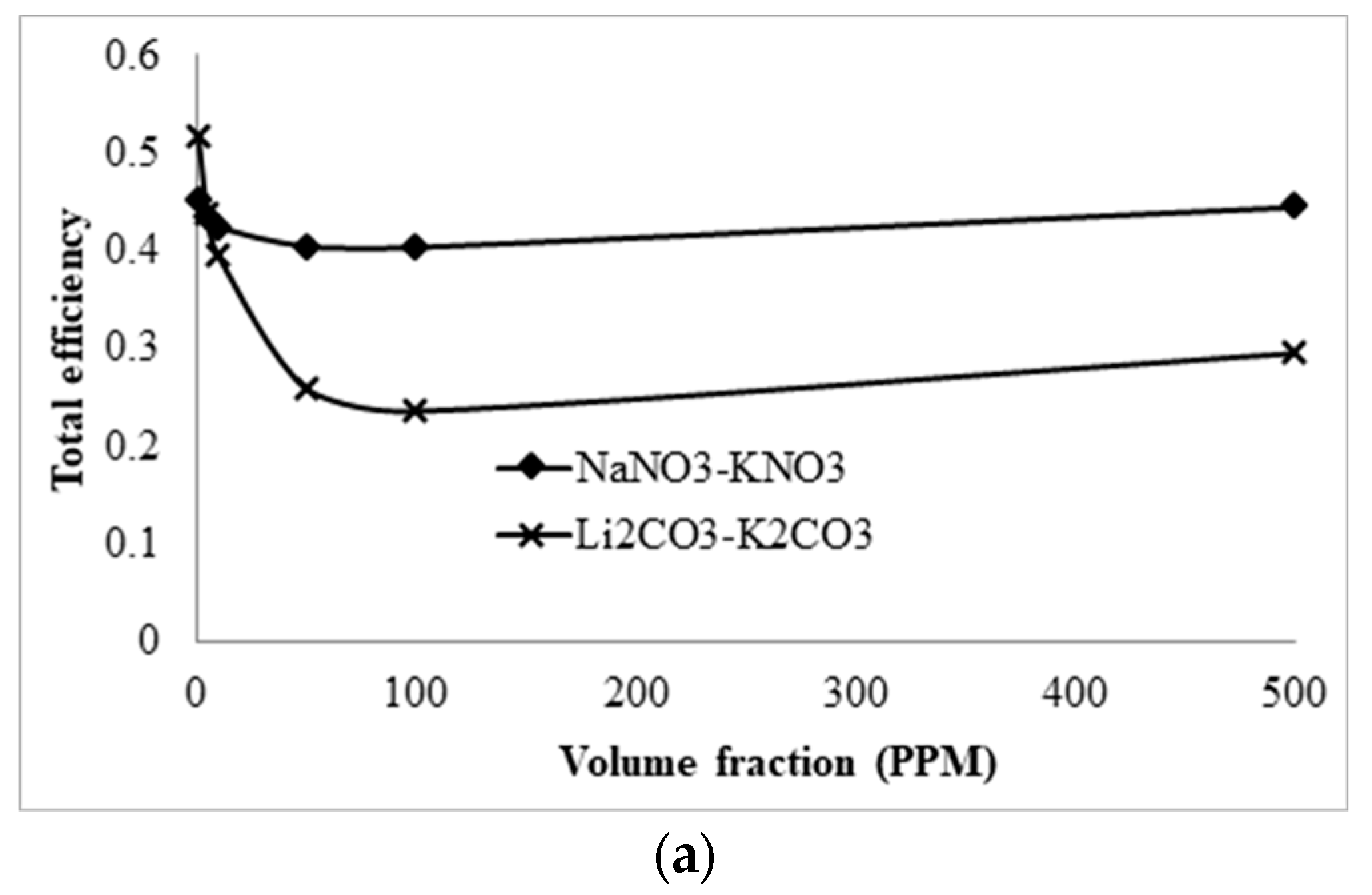
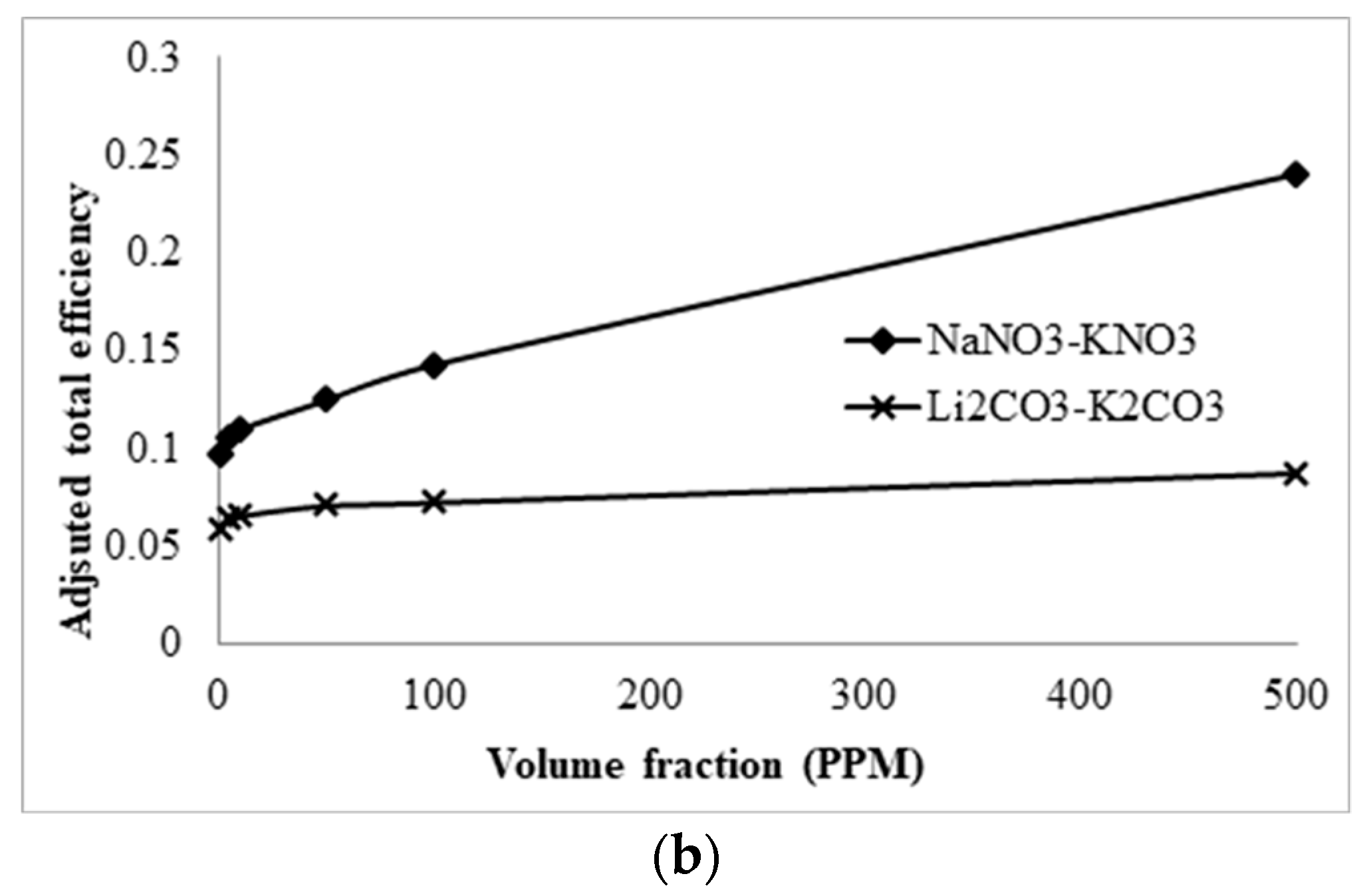
3.5. Comparison with Solar Salt Nanofluid
4. Conclusions
- ⮚
- Investigation into the receiver length and nanofluid volume concentration shows that, at high operating temperature, the receiver efficiency (from ≈0.75 to 0.6) and total efficiency (from ≈0.48 to 0.4) are decreasing, while the Carnot efficiency (from ≈0.615 to 0.68) is increasing slightly with receiver length. An adjusted Carnot efficiency (inlet temperature as the low temperature instead of the ambient temperature) shows that the rise in average temperature of the HTF is exponential with receiver length to a certain maximum receiver length, beyond which the temperature rise is not enough to offset the receiver efficiency decrease.
- ⮚
- Using the normal Carnot efficiency, it was shown that an increase in heat transfer fluid (HTF) velocity resulted in no apparent effect on the overall efficiency ( = 0.66, at a fixed peak temperature (1071 K) receiver, and resulted in a slight increase in the overall efficiency of a fixed length (1 m) receiver as outlet temperature dropped. However, the adjusted total efficiency of the fixed length receiver was found to be decreased with the increase of inlet velocity. This implied that the most efficient receiver was one that had very low HTF velocity, as that resulted in a higher temperature, which is true to a certain extent, until the upper temperature limit of the nanofluid was reached.
- ⮚
- A range of volume fraction (from 1 × 10−6 to 5 × 10−4) of nanoparticles was investigated over a range of different receiver lengths with initial results indicating that the most efficient receiver () was that with the lowest volume fraction and at the shortest receiver length. That again was not an accurate representation as when considering the adjusted total efficiency. It was found that the efficiency of the receiver increased with volume fraction and receiver length to an extent, as an optimal receiver length became evident. It was discovered that the higher the volume fraction was, the shorter the optimal length became. A higher volume fraction resulted in a higher average outlet temperature and greater efficiency, but a greater susceptibility to heat loss to the ambient.
- ⮚
- Initial investigations into the effects of solar concentration revealed that both the normal Carnot efficiency (from 0.6 to 0.7) and the total efficiency (from −0.55 to 0.2), and the adjusted Carnot efficiency and the adjusted total efficiency, are gradually increased with an increase in the solar concentration (from 10× to 100×). An interesting trend was observed, however, where the difference between the average and peak temperatures of the receiver actually increased by 5–41 K by increasing solar concentration from 30× to 100×. This discovery implies that, if the peak temperature were kept constant by balancing the receiver length and inlet velocity, the average temperature would actually decrease at some point with increasing solar concentration, resulting in a decrease in the Carnot efficiency and a drop in the overall efficiency of the receiver, which contradicts the initial results. By keeping the peak temperature constant, an optimal solar concentration was indeed discovered when considering the adjusted Carnot efficiency. For a receiver with a volume fraction of 1 × 10−4 and a length of 1 m, the optimal solar concentration is approximately 150×.
- ⮚
- A comparative study shows that solar salt is superior to the lithium carbonate salt because of the higher total efficiency of the collector. However, LiCO3–K2CO3 will be more desirable for thermal storage, as a greater amount of energy can be stored for a smaller volume of liquid at higher operating temperature.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| Collector area (m2) | |
| ao and a1 | Constant parameters |
| Concentration factor | |
| Speed of light (m/s) | |
| Specific heat (W/mK) | |
| Particle diameter (m) | |
| Volume fraction of nanoparticles | |
| Planck’s constant | |
| Nanofluid heat transfer coefficient (W/mK) | |
| Overall heat transfer coefficient (W/mK) | |
| Incident radiation (W/m2) | |
| Scattered irradiation (W/m2) | |
| Thermal conductivity (W/m2K) | |
| Boltzmann constant | |
| Thermal conductivity of nanofluid (W/m2K) | |
| Thermal conductivity of top plate (W/m2K) | |
| Characteristic length taken as receiver height (m) | |
| Length of receiver (m) | |
| Relative complex refractive index of the nanofluid | |
| Mass flow rate (kg/s) | |
| Number of scattering particles in the beam path | |
| Refractive index | |
| Nusselt number | |
| Pe | Peclet number |
| Solar irradiance integrated over solar wavelength range (W/m2) | |
| Prandtl number | |
| Heat source (W) | |
| Reynolds number | |
| Attenuation constant, which accounts for the average attenuation through Earth’s atmosphere | |
| Complex refractive index of the base fluid | |
| Strength of the resonant vibration mode | |
| Complex refractive index of the nanoparticles | |
| Temperature (K) | |
| Thickness of top plate (m) | |
| Ambient temperature (K) | |
| Inlet temperature (K) | |
| Average outlet temperature (K) | |
| Temperature of the sun (K) | |
| Fully developed velocity (m/s) | |
| Inlet nanofluid velocity (m/s) |
Greek Symbols
| Absorptive index | |
| Scattering angle | |
| Solid angle of the sun as seen from Earth (steradian) | |
| Damping parameter of the resonant vibration mode | |
| Maximum packing concentration | |
| Carnot efficiency (%) | |
| Receiver efficiency (%) | |
| Total efficiency (%) | |
| Radiation wavelength (m) | |
| Characteristic wavelength of the resonant vibration mode (m) | |
| Incident angle (rad) | |
| Density (kg/m3) |
References
- Karim, M.A.; Perez, E.; Amin, Z.M. Mathematical modeling of counter flow v-grove solar air collector. Renew. Energy 2014, 67, 192–201. [Google Scholar] [CrossRef]
- Islam, M.; Karim, A.; Saha, S.C.; Yarlagadda, P.K.; Miller, S.; Ullah, I. Visualization of thermal characteristics around the absorber tube of a standard parabolic trough thermal collector by 3D simulation. In Proceedings of the 4th International Conference on Computational Methods (ICCM2012), Crowne Plaza, Gold Coast, Australia, 25–27 November 2012. [Google Scholar]
- Islam, M.; Karim, M.A.; Saha, S.C.; Miller, S.; Yarlagadda, P.K.D.V. Development of Empirical Equations for Irradiance Profile of a Standard Parabolic Trough Collector Using Monte Carlo Ray Tracing Technique. Adv. Mater. Res. 2014, 860–863, 180–190. [Google Scholar] [CrossRef]
- Islam, M.; Miller, S.; Yarlagadda, P.K.; Karim, A. Investigation of the effect of physical and optical factors on the optical performance of a parabolic trough collector. Energies 2017, 10, 1907. [Google Scholar] [CrossRef]
- Islam, M.; Yarlagadda, P.; Karim, A. Effect of the Orientation Schemes of the Energy Collection Element on the Optical Performance of a Parabolic Trough Concentrating Collector. Energies 2018, 12, 128. [Google Scholar] [CrossRef]
- Karim, M.; Arthur, O.; Yarlagadda, P.K.; Islam, M.; Mahiuddin, M. Performance investigation of high temperature application of molten solar salt nanofluid in a direct absorption solar collector. Molecules 2019, 24, 285. [Google Scholar] [CrossRef]
- Islam, M.; Karim, A.; Saha, S.C.; Miller, S.; Yarlagadda, P.K. Three dimensional simulation of a parabolic trough concentrator thermal collector. In Proceedings of the 50th Annual Conference, Australian Solar Energy Society (AuSES), Swinburne University of Technology, Melbourne, Australia, 6–7 December 2012. [Google Scholar]
- Tiznobaik, H.; Shin, D. Enhanced specific heat capacity of high-temperature molten salt-based nanofluids. Int. J. Heat Mass Transf. 2013, 57, 542–548. [Google Scholar] [CrossRef]
- Romero, M.; Buck, R.; Pacheco, J.E. An update on solar central receiver systems, projects, and technologies. J. Sol. Energy Eng. 2002, 124, 98–108. [Google Scholar] [CrossRef]
- Starace, A.K.; Gomez, J.C.; Glatzmaier, G.C. Can particle-stabilized inorganic dispersions be high-temperature heat-transfer and thermal energy storage fluids? J. Mater. Sci. 2013, 48, 4023–4031. [Google Scholar] [CrossRef]
- Heat Transfer Fluids. Available online: http://www.dynalene.com/Heat-transfer-fluids-s/1477.htm (accessed on 14 August 2019).
- Visser, A.E.; Bridges, N.J.; Garcia-Diaz, B.L.; Gray, J.R.; Fox, E.B. Al2O3-based nanoparticle-enhanced ionic liquids (NEILs) for advanced heat transfer fluids. In Ionic Liquids: Science and Applications; ACS Publications: Washington, DC, USA, 2012; pp. 259–270. [Google Scholar]
- Murshed, S.S.; de Castro, C.N.; Lourenço, M.; Lopes, M.; Santos, F. Current research and future applications of nano-and ionano-fluids. J. Phys. Conf. Ser. 2012, 395, 012117. [Google Scholar] [CrossRef]
- Puliti, G.; Paolucci, S.; Sen, M. Nanofluids and their properties. Appl. Mech. Rev. 2011, 64, 030803. [Google Scholar] [CrossRef]
- Shin, D.; Banerjee, D. Enhanced specific heat capacity of nanomaterials synthesized by dispersing silica nanoparticles in eutectic mixtures. J. Heat Transf. 2013, 135, 032801. [Google Scholar] [CrossRef]
- Bogaerts, W.F.; Lampert, C.M. Materials for photothermal solar energy conversion. J. Mater. Sci. 1983, 18, 2847–2875. [Google Scholar] [CrossRef]
- Kaluri, R.; Vijayaraghavan, S.; Ganapathisubbu, S. Model development and performance studies of a concentrating direct absorption solar collector. J. Sol. Energy Eng. 2015, 137, 021005. [Google Scholar] [CrossRef]
- Cengel, Y.; Özişik, M. Solar radiation absorption in solar ponds. Sol. Energy 1984, 33, 581–591. [Google Scholar] [CrossRef]
- Beard, J.; Iachetta, F.; Lilleleht, L.; Huckstep, F.; May, W. Design and Operational Influences on Thermal Performance of “Solaris” Solar Collector. J. Eng. Power 1978, 100, 497–502. [Google Scholar] [CrossRef]
- Arai, N.; Itaya, Y.; Hasatani, M. Development of a “volume heat-trap” type solar collector using a fine-particle semitransparent liquid suspension (FPSS) as a heat vehicle and heat storage medium Unsteady, one-dimensional heat transfer in a horizontal FPSS layer heated by thermal radiation. Sol. Energy 1984, 32, 49–56. [Google Scholar] [CrossRef]
- Viskanta, R. Direct absorption solar radiation collection systems. In Solar Energy Utilization; NATO ASI Series (Series E: Applied Sciences); Springer: Dordrecht, The Netherlands, 1987; pp. 334–360. [Google Scholar]
- Taylor, R.A.; Phelan, P.E.; Otanicar, T.P.; Walker, C.A.; Nguyen, M.; Trimble, S.; Prasher, R. Applicability of nanofluids in high flux solar collectors. J. Renew. Sustain. Energy 2011, 3, 023104. [Google Scholar] [CrossRef]
- Lee, S.-H.; Jang, S.P. Efficiency of a volumetric receiver using aqueous suspensions of multi-walled carbon nanotubes for absorbing solar thermal energy. Int. J. Heat Mass Transf. 2015, 80, 58–71. [Google Scholar] [CrossRef]
- Lee, J.-H.; Lee, S.-H.; Choi, C.J.; Jang, S.P.; Choi, S.U. A review of thermal conductivity data, mechanisms and models for nanofluids. Int. J. Micro Nano Scale Transp. 2010, 1, 269–322. [Google Scholar] [CrossRef]
- Paul, T.C.; Morshed, A.; Fox, E.B.; Visser, A.E.; Bridges, N.J.; Khan, J.A. Enhanced thermal performance of ionic liquid-Al2O3 nanofluid as heat transfer fluid for solar collector. In Proceedings of the ASME 7th International Conference on Energy Sustainability Collocated with the ASME Heat Transfer Summer Conference and the ASME 11th International Conference on Fuel Cell Science, Engineering and Technology, Minneapolis, MN, USA, 14–19 July 2013; pp. 14–19. [Google Scholar]
- Akermi, M.; Jaballah, N.; Alarifi, I.M.; Rahimi-Gorji, M.; Chaabane, R.B.; Ouada, H.B.; Majdoub, M. Synthesis and characterization of a novel hydride polymer P-DSBT/ZnO nano-composite for optoelectronic applications. J. Mol. Liq. 2019, 287, 110963. [Google Scholar] [CrossRef]
- Lenert, A.; Wang, E.N. Optimization of nanofluid volumetric receivers for solar thermal energy conversion. Sol. Energy 2012, 86, 253–265. [Google Scholar] [CrossRef]
- Tyagi, H.; Phelan, P.; Prasher, R. Predicted efficiency of a low-temperature nanofluid-based direct absorption solar collector. J. Sol. Energy Eng. 2009, 131, 041004. [Google Scholar] [CrossRef]
- Otanicar, T.P.; Phelan, P.E.; Prasher, R.S.; Rosengarten, G.; Taylor, R.A. Nanofluid-based direct absorption solar collector. J. Renew. Sustain. Energy 2010, 2, 033102. [Google Scholar] [CrossRef]
- Luo, Z.; Wang, C.; Wei, W.; Xiao, G.; Ni, M. Performance improvement of a nanofluid solar collector based on direct absorption collection (DAC) concepts. Int. J. Heat Mass Transf. 2014, 75, 262–271. [Google Scholar] [CrossRef]
- Parvin, S.; Nasrin, R.; Alim, M. Heat transfer and entropy generation through nanofluid filled direct absorption solar collector. Int. J. Heat Mass Transf. 2014, 71, 386–395. [Google Scholar] [CrossRef]
- Kameya, Y.; Hanamura, K. Enhancement of solar radiation absorption using nanoparticle suspension. Sol. Energy 2011, 85, 299–307. [Google Scholar] [CrossRef]
- Souayeh, B.; Reddy, M.G.; Sreenivasulu, P.; Poornima, T.; Rahimi-Gorji, M.; Alarifi, I.M. Comparative analysis on non-linear radiative heat transfer on MHD Casson nanofluid past a thin needle. J. Mol. Liq. 2019, 284, 163–174. [Google Scholar] [CrossRef]
- Vajjha, R.S.; Das, D.K. Specific heat measurement of three nanofluids and development of new correlations. J. Heat Transf. 2009, 131, 071601. [Google Scholar] [CrossRef]
- Zhou, S.-Q.; Ni, R. Measurement of the specific heat capacity of water-based Al2O3 nanofluid. Appl. Phys. Lett. 2008, 92, 093123. [Google Scholar] [CrossRef]
- Shin, D.; Banerjee, D. Enhanced specific heat of silica nanofluid. J. Heat Transf. 2011, 133, 024501. [Google Scholar] [CrossRef]
- Tiznobaik, H.; Shin, D. Experimental validation of enhanced heat capacity of ionic liquid-based nanomaterial. Appl. Phys. Lett. 2013, 102, 173906. [Google Scholar] [CrossRef]
- Shin, D.; Jo, B.; Kwak, H.-E.; Banerjee, D. Investigation of high temperature nanofluids for solar thermal power conversion and storage applications. In Proceedings of the 14th International Heat Transfer Conference, Omni Shoreham Hotel, Washington, DC, USA, 8–13 August 2010; pp. 583–591. [Google Scholar]
- Shin, D.; Banerjee, D. Specific heat of nanofluids synthesized by dispersing alumina nanoparticles in alkali salt eutectic. Int. J. Heat Mass Transf. 2014, 74, 210–214. [Google Scholar] [CrossRef]
- Glaser, P.E. Power from the sun: Its future. Science 1968, 162, 857–861. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.; Banerjee, D. Enhancement of specific heat capacity of high-temperature silica-nanofluids synthesized in alkali chloride salt eutectics for solar thermal-energy storage applications. Int. J. Heat Mass Transf. 2011, 54, 1064–1070. [Google Scholar] [CrossRef]
- Dudda, B.; Shin, D. Effect of nanoparticle dispersion on specific heat capacity of a binary nitrate salt eutectic for concentrated solar power applications. Int. J. Therm. Sci. 2013, 69, 37–42. [Google Scholar] [CrossRef]
- Chieruzzi, M.; Cerritelli, G.F.; Miliozzi, A.; Kenny, J.M. Effect of nanoparticles on heat capacity of nanofluids based on molten salts as PCM for thermal energy storage. Nanoscale Res. Lett. 2013, 8, 448. [Google Scholar] [CrossRef]
- Jung, S.; Banerjee, D. Enhancement of heat capacity of nitrate salts using mica nanoparticles. Dev. Strat. Mater. Comput. Ii Ceram. Eng. Sci. Proc. 2011, 32, 127–137. [Google Scholar]
- Bridges, N.J.; Visser, A.E.; Fox, E.B. Potential of nanoparticle-enhanced ionic liquids (NEILs) as advanced heat-transfer fluids. Energy Fuels 2011, 25, 4862–4864. [Google Scholar] [CrossRef]
- Paul, T.C.; Morshed, A.K.M.M.; Fox, E.B.; Khan, J.A. Enhanced thermophysical properties of NEILs as heat transfer fluids for solar thermal applications. Appl. Therm. Eng. 2017, 110, 1–9. [Google Scholar] [CrossRef]
- Paul, T.C.; Morshed, A.M.; Khan, J.A. Nanoparticle Enhanced Ionic Liquids (NEILS) as working fluid for the next generation solar collector. Procedia Eng. 2013, 56, 631–636. [Google Scholar] [CrossRef]
- De Castro, C.N.; Murshed, S.S.; Lourenço, M.; Santos, F.; Lopes, M.; França, J. Enhanced thermal conductivity and specific heat capacity of carbon nanotubes ionanofluids. Int. J. Therm. Sci. 2012, 62, 34–39. [Google Scholar] [CrossRef]
- Wang, F.; Han, L.; Zhang, Z.; Fang, X.; Shi, J.; Ma, W. Surfactant-free ionic liquid-based nanofluids with remarkable thermal conductivity enhancement at very low loading of graphene. Nanoscale Res. Lett. 2012, 7, 314. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, F.; Zhang, L.; Fang, X.; Zhang, Z. Thermodynamic properties and thermal stability of ionic liquid-based nanofluids containing graphene as advanced heat transfer fluids for medium-to-high-temperature applications. Renew. Energy 2014, 63, 519–523. [Google Scholar] [CrossRef]
- Veeraragavan, A.; Lenert, A.; Yilbas, B.; Al-Dini, S.; Wang, E.N. Analytical model for the design of volumetric solar flow receivers. Int. J. Heat Mass Transf. 2012, 55, 556–564. [Google Scholar] [CrossRef]
- Jo, B.; Banerjee, D. Viscosity measurements of multi-walled carbon nanotubes-based high temperature nanofluids. Mater. Lett. 2014, 122, 212–215. [Google Scholar] [CrossRef]
- Jo, B.; Banerjee, D. Enhanced specific heat capacity of molten salt-based nanomaterials: Effects of nanoparticle dispersion and solvent material. Acta Mater. 2014, 75, 80–91. [Google Scholar] [CrossRef]
- Jo, B.; Banerjee, D. Effect of dispersion homogeneity on specific heat capacity enhancement of molten salt nanomaterials using carbon nanotubes. J. Sol. Energy Eng. 2015, 137, 011011. [Google Scholar] [CrossRef]
- Jo, B.; Banerjee, D. Study of high temperature nanofluids using carbon nanotubes (CNT) for solar thermal storage applications. In Proceedings of the ASME 4th International Conference on Energy Sustainability, Phoenix, AZ, USA, 17–22 May 2010; pp. 741–748. [Google Scholar]
- Kumar, K.G.; Rahimi-Gorji, M.; Reddy, M.G.; Chamkha, A.J.; Alarifi, I.M. Enhancement of heat transfer in a convergent/divergent channel by using carbon nanotubes in the presence of a Darcy–Forchheimer medium. In Microsystem Technologies; Springer: Berlin/Heidelberg, Germany, 2019; pp. 1–10. [Google Scholar] [CrossRef]
- Araki, N.; Matsuura, M.; Makino, A.; Hirata, T.; Kato, Y. Measurement of thermophysical properties of molten salts: Mixtures of alkaline carbonate salts. Int. J. Thermophys. 1988, 9, 1071–1080. [Google Scholar] [CrossRef]
- Nguyen, T.; van der Meer, D.; van den Berg, A.; Eijkel, J.C. Investigation of the effects of time periodic pressure and potential gradients on viscoelastic fluid flow in circular narrow confinements. Microfluid. Nanofluidics 2017, 21, 37. [Google Scholar] [CrossRef]
- Kahshan, M.; Lu, D.; Rahimi-Gorji, M. Hydrodynamical study of flow in a permeable channel: Application to flat plate dialyzer. Int. J. Hydrogen Energy 2019, 44, 17041–17047. [Google Scholar] [CrossRef]
- Uddin, S.; Mohamad, M.; Rahimi-Gorji, M.; Roslan, R.; Alarifi, I.M. Fractional electro-magneto transport of blood modeled with magnetic particles in cylindrical tube without singular kernel. In Microsystem Technologies; Springer: Berlin/Heidelberg, Germany, 2019; pp. 1–10. [Google Scholar] [CrossRef]
- Zeitlin, M.; Tavlarides, L.L. Fluid-fluid interactions and hydrodynamics in agitated dispersions: A simulation model. Can. J. Chem. Eng. 1972, 50, 207–215. [Google Scholar] [CrossRef]
- Therminol VP-1, Heat Transfer Fluid by Solutia. In Vapor Phase/Liquid Phase Heat Transfer Fluid 54°F to 750°F; Solutia Inc.: St. Louis, MO, USA, 1999; Available online: http://therminolkorea.co.kr/data/THERMINOL%20VP-1%20technical%20bulletin.pdf (accessed on 10 April 2018).
Sample Availability: Not available. |

| Parameters | Values |
|---|---|
| Height of receiver, | 0.0142 (m) |
| Width of receiver, | 0.005 (m) |
| Length of receiver, | 1.0 (m) |
| 0.135 (W/mK) | |
| 1600 (J/kgK) | |
| 2100 (kg/m3) | |
| 2100 (kg/m3) | |
| 0.00328 (Pa.s) | |
| 780 (K) | |
| 0.0034 (m/s) | |
| Speed of light, | 299,792,458 (m/s) |
| Diameter of nanoparticles, | 50 × 10−9 (m) |
| 0.00005 | |
| 0.73 | |
| 100× | |
| 6.8 × 10−5 (steradians) | |
| Planck’s constant, | 6.62606957 × 10−34 (J.s) |
| 1.3806488 × 10−23 (J/K) | |
| 5780 (K) | |
| 297K | |
| 2.72 | |
| 0.01 (m) | |
| 1.3 (W/m.K) | |
| 1.31 | |
| 1.63 | |
| 3.86 × 10−8 | |
| 200 × 10−9 (m) | |
| 2500 × 10−9 (m) | |
| 13.5 (W/m2K) |
| Variables | Expression |
|---|---|
| Concentrated normally incident solar radiation distribution, | |
| Concentrated normally incident solar radiation, | |
| Distance from surface of receiver, | |
| Relative complex refraction index of nanofluid, | |
| Total mass of the nanofluid, | |
| Absorption efficiency, | |
| Scattering efficiency, | |
| Absorption coefficient of nanoparticles, | |
| Absorption coefficient of base fluid, | |
| Spectral flux, | |
| Divergence of the spectral flux, | |
| Volumetric heat release, | |
| Average outlet temperature, | |
| Efficiency of receiver, |
| fv | Lrec (m) | vin (m/s) | Tavg (K) | ηrec (%) | ηCarnot (%) | (ηCarnot)adjusted (%) | Tpeak (K) | ηTotal (%) | (ηTotal)adjusted (%) |
|---|---|---|---|---|---|---|---|---|---|
| 1 × 10−6 | 1 | 0.000575 | 842 | 0.798 | 0.647 | 0.074 | 1071 | 0.517 | 0.059 |
| 5 × 10−6 | 0.0021 | 863 | 0.668 | 0.656 | 0.096 | 0.438 | 0.064 | ||
| 1 × 10−5 | 0.0021 | 876 | 0.597 | 0.661 | 0.110 | 0.395 | 0.065 | ||
| 5 × 10−5 | 0.0026 | 962 | 0.374 | 0.691 | 0.189 | 0.259 | 0.071 | ||
| 1 × 10−4 | 0.0026 | 1029 | 0.293 | 0.711 | 0.242 | 0.208 | 0.071 | ||
| 5 × 10−4 | 0.0072 | 1069 | 0.209 | 0.722 | 0.270 | 0.151 | 0.057 | ||
| 1 × 10−4 | 0.7525 | 0.0026 | 994 | 0.336 | 0.701 | 0.215 | 1053 | 0.236 | 0.072 |
| 5 × 10−4 | 0.3565 | 0.0072 | 984 | 0.422 | 0.698 | 0.207 | 1001 | 0.295 | 0.0878 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karim, M.A.; Islam, M.; Arthur, O.; Yarlagadda, P.K. Performance of Graphite-Dispersed Li2CO3-K2CO3 Molten Salt Nanofluid for a Direct Absorption Solar Collector System. Molecules 2020, 25, 375. https://doi.org/10.3390/molecules25020375
Karim MA, Islam M, Arthur O, Yarlagadda PK. Performance of Graphite-Dispersed Li2CO3-K2CO3 Molten Salt Nanofluid for a Direct Absorption Solar Collector System. Molecules. 2020; 25(2):375. https://doi.org/10.3390/molecules25020375
Chicago/Turabian StyleKarim, M. A., Majedul Islam, Owen Arthur, and Prasad KDV Yarlagadda. 2020. "Performance of Graphite-Dispersed Li2CO3-K2CO3 Molten Salt Nanofluid for a Direct Absorption Solar Collector System" Molecules 25, no. 2: 375. https://doi.org/10.3390/molecules25020375
APA StyleKarim, M. A., Islam, M., Arthur, O., & Yarlagadda, P. K. (2020). Performance of Graphite-Dispersed Li2CO3-K2CO3 Molten Salt Nanofluid for a Direct Absorption Solar Collector System. Molecules, 25(2), 375. https://doi.org/10.3390/molecules25020375







