Androgen Receptor in Breast Cancer—Clinical and Preclinical Research Insights
Abstract
:1. Introduction
2. Physiology of Androgen Receptors (AR)
3. Prognostic Value of AR by Different Breast Cancer Subtypes
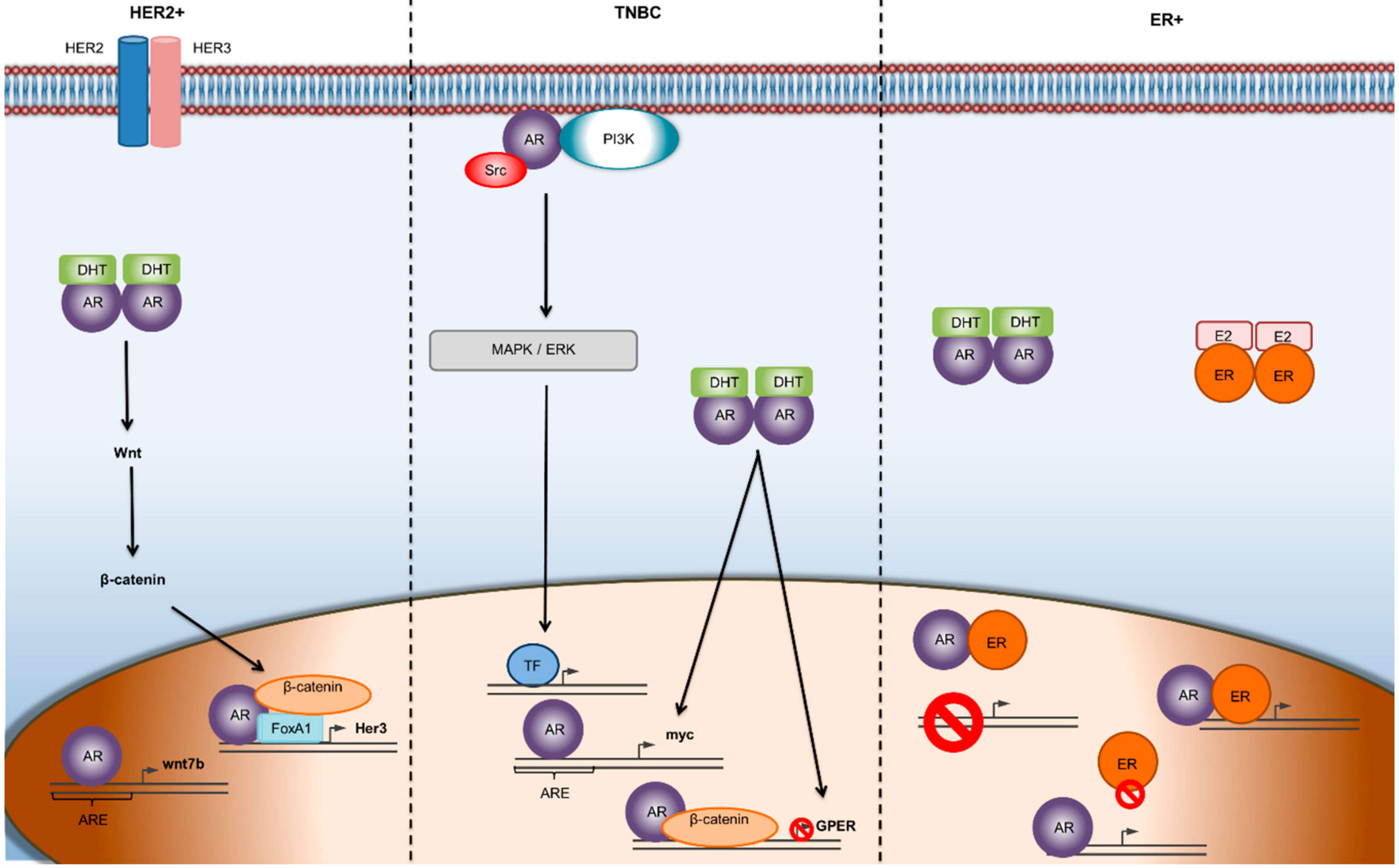
4. Predictive Role of AR
5. The Therapeutic Potential of AR: Clinical Overview
6. Future Prospects in Prognosis and Therapy
7. Conclusions
Funding
Conflicts of Interest
Abbreviations
| AR | Androgen Receptor |
| ARE | Androgen Responsive Element |
| BC | Breast Cancer |
| CTC | Circulating Tumor Cell |
| DCIS | Ductal Carcinoma in situ |
| DHT | Dihydrotestosterone |
| E2 | 17β-estradiol |
| EGFR | Epidermal Growth Factor Receptor |
| F-2 | Epidermal Growth receptor-2 |
| ERα | Estrogen Receptor alpha |
| ER-β | Estrogen Receptor beta |
| FOXO1 | Forkhead Box Protein O1 |
| HSP | Heat Shock Proteins |
| HR | Hormone Receptor |
| LAR | Luminal Androgen Receptor |
| PD-L | Programmed Death-Ligand |
| PgR | Progesterone Receptor |
| SARM | Selective AR Modulators |
| TNBC | Triple Negative Breast Cancer |
| TTP | Time to Progression |
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar]
- Curtis, C.; Shah, S.P.; Chin, S.-F.; Turashvili, G.; Rueda, O.M.; Dunning, M.J.; Speed, D.; Lynch, A.G.; Samarajiwa, S.; Yuan, Y.; et al. The genomic and transcriptomic architecture of 2000 breast tumours reveals novel subgroups. Nature 2012, 486, 346–352. [Google Scholar]
- Koboldt, D.C.; Fulton, R.S.; McLellan, M.D.; Schmidt, H.; Kalicki-Veizer, J.; McMichael, J.F.; Fulton, L.L.; Dooling, D.J.; Ding, L.; Mardis, E.R.; et al. Comprehensive molecular portraits of human breast tumours. Nature 2012, 490, 61–70. [Google Scholar]
- Rakha, E.A.; Green, A.R. Molecular classification of breast cancer: What the pathologist needs to know. Pathology 2017, 49, 111–119. [Google Scholar]
- Bergin, A.R.T.; Loi, S. Triple-negative breast cancer: recent treatment advances. F1000Research 2019, 8, 1342. [Google Scholar]
- Collins, L.C.; Cole, K.S.; Marotti, J.D.; Hu, R.; Schnitt, S.J.; Tamimi, R.M. Androgen receptor expression in breast cancer in relation to molecular phenotype: results from the Nurses’ Health Study. Mod. Pathol. 2011, 24, 924–931. [Google Scholar]
- Hickey, T.E.; Robinson, J.L.L.; Carroll, J.S.; Tilley, W.D. Minireview: The Androgen Receptor in Breast Tissues: Growth Inhibitor, Tumor Suppressor, Oncogene? Mol. Endocrinol. 2012, 26, 1252–1267. [Google Scholar]
- Venema, C.M.; Bense, R.D.; Steenbruggen, T.G.; Nienhuis, H.H.; Qiu, S.-Q.; van Kruchten, M.; Brown, M.; Tamimi, R.M.; Hospers, G.A.P.; Schröder, C.P.; et al. Consideration of breast cancer subtype in targeting the androgen receptor. Pharmacol. Ther. 2019, 200, 135–147. [Google Scholar]
- Sarker, D.; Reid, A.H.M.; Yap, T.A.; de Bono, J.S. Targeting the PI3K/AKT Pathway for the Treatment of Prostate Cancer. Clin. Cancer Res. 2009, 15, 4799–4805. [Google Scholar]
- Ni, M.; Chen, Y.; Lim, E.; Wimberly, H.; Bailey, S.T.; Imai, Y.; Rimm, D.L.; Liu, X.S.; Brown, M. Targeting androgen receptor in estrogen receptor-negative breast cancer. Cancer Cell. 2011, 20, 119–131. [Google Scholar]
- Zhou, X. Roles of Androgen Receptor in Male and Female Reproduction: Lessons From Global and Cell-Specific Androgen Receptor Knockout (ARKO) Mice. J. Androl. 2010, 31, 235–243. [Google Scholar]
- Walters, K.A.; Simanainen, U.; Handelsman, D.J. Molecular insights into androgen actions in male and female reproductive function from androgen receptor knockout models. Hum. Reprod. Update 2010, 16, 543–558. [Google Scholar]
- Zhou, J.; Ng, S.; Adesanya-Famuiya, O.; Anderson, K.; Bondy, C.A. Testosterone inhibits estrogen-induced mammary epithelial proliferation and suppresses estrogen receptor expression. FASEB J. 2000, 14, 1725–1730. [Google Scholar]
- Dimitrakakis, C.; Zhou, J.; Wang, J.; Belanger, A.; LaBrie, F.; Cheng, C.; Powell, D.; Bondy, C. A physiologic role for testosterone in limiting estrogenic stimulation of the breast. Menopause 2003, 10, 292–298. [Google Scholar]
- Yeh, S.; Hu, Y.-C.; Wang, P.-H.; Xie, C.; Xu, Q.; Tsai, M.-Y.; Dong, Z.; Wang, R.-S.; Lee, T.-H.; Chang, C. Abnormal Mammary Gland Development and Growth Retardation in Female Mice and MCF7 Breast Cancer Cells Lacking Androgen Receptor. J. Exp. Med. 2003, 198, 1899–1908. [Google Scholar]
- Tiefenbacher, K.; Daxenbichler, G. The Role of Androgens in Normal and Malignant Breast Tissue. Breast Care 2008, 3, 325–331. [Google Scholar]
- Vera-Badillo, F.E.; Templeton, A.J.; De Gouveia, P.; Diaz-Padilla, I.; Bedard, P.L.; Al-Mubarak, M.; Seruga, B.; Tannock, I.F.; Ocana, A.; Amir, E. Androgen receptor expression and outcomes in early breast cancer: A systematic review and meta-analysis. J. Natl. Cancer Inst. 2014, 106, djt319. [Google Scholar]
- Park, S.; Koo, J.S.; Kim, M.S.; Park, H.S.; Lee, J.S.; Lee, J.S.; Kim, S.I.; Park, B.W.; Lee, K.S. Androgen receptor expression is significantly associated with better outcomes in estrogen receptor-positive breast cancers. Ann. Oncol. 2011, 22, 1755–1762. [Google Scholar]
- Hu, R.; Dawood, S.; Holmes, M.D.; Collins, L.C.; Schnitt, S.J.; Cole, K.; Marotti, J.D.; Hankinson, S.E.; Colditz, G.A.; Tamimi, R.M. Androgen receptor expression and breast cancer survival in postmenopausal women. Clin. Cancer Res. 2011, 17, 1867–1874. [Google Scholar]
- Elebro, K.; Borgquist, S.; Simonsson, M.; Markkula, A.; Jirström, K.; Ingvar, C.; Rose, C.; Jernström, H. Combined androgen and estrogen receptor status in breast cancer: Treatment prediction and prognosis in a population-based prospective cohort. Clin. Cancer Res. 2015, 21, 3640–3650. [Google Scholar]
- Ravaioli, S.; Tumedei, M.M.; Foca, F.; Maltoni, R.; Rocca, A.; Massa, I.; Pietri, E.; Bravaccini, S. Androgen and oestrogen receptors as potential prognostic markers for patients with ductal carcinoma in situ treated with surgery and radiotherapy. Int. J. Exp. Pathol. 2017, 98, 289–295. [Google Scholar]
- Tumedei, M.M.; Silvestrini, R.; Ravaioli, S.; Massa, I.; Maltoni, R.; Rocca, A.; Folli, S.; Buggi, F.; Curcio, A.; Serra, L.; et al. Role of androgen and estrogen receptors as prognostic and potential predictive markers of ductal carcinoma in situ of the breast. Int. J. Biol. Markers 2015, 30, e425–e428. [Google Scholar]
- Cochrane, D.R.; Bernales, S.; Jacobsen, B.M.; Cittelly, D.M.; Howe, E.N.; D’Amato, N.C.; Spoelstra, N.S.; Edgerton, S.M.; Jean, A.; Guerrero, J.; et al. Role of the androgen receptor in breast cancer and preclinical analysis of enzalutamide. Breast Cancer Res. 2014, 16, R7. [Google Scholar]
- Kraby, M.R.; Valla, M.; Opdahl, S.; Haugen, O.A.; Sawicka, J.E.; Engstrøm, M.J.; Bofin, A.M. The prognostic value of androgen receptors in breast cancer subtypes. Breast Cancer Res. Treat. 2018, 172, 283–296. [Google Scholar]
- Gucalp, A.; Tolaney, S.; Isakoff, S.J.; Ingle, J.N.; Liu, M.C.; Carey, L.A.; Blackwell, K.; Rugo, H.; Nabell, L.; Forero, A.; et al. Phase II trial of bicalutamide in patients with androgen receptor-positive, estrogen receptor-negative metastatic breast cancer. Clin. Cancer Res. 2013, 19, 5505–5512. [Google Scholar]
- Safarpour, D.; Pakneshan, S.; Tavassoli, F.A. Androgen receptor (AR) expression in 400 breast carcinomas: is routine AR assessment justified? Am. J. Cancer Res. 2014, 4, 353–368. [Google Scholar]
- Thike, A.A.; Chong, L.Y.Z.; Cheok, P.Y.; Li, H.H.; Yip, G.W.C.; Bay, B.H.; Tse, G.M.K.; Iqbal, J.; Tan, P.H. Loss of androgen receptor expression predicts early recurrence in triple-negative and basal-like breast cancer. Mod. Pathol. 2014, 27, 352–360. [Google Scholar]
- Loibl, S.; Müller, B.M.; Von Minckwitz, G.; Schwabe, M.; Roller, M.; Darb-Esfahani, S.; Ataseven, B.; Du Bois, A.; Fissler-Eckhoff, A.; Gerber, B.; et al. Androgen receptor expression in primary breast cancer and its predictive and prognostic value in patients treated with neoadjuvant chemotherapy. Breast Cancer Res. Treat. 2011, 130, 477–487. [Google Scholar]
- Masuda, H.; Baggerly, K.A.; Wang, Y.; Zhang, Y.; Gonzalez-Angulo, A.M.; Meric-Bernstam, F.; Valero, V.; Lehmann, B.D.; Pietenpol, J.A.; Hortobagyi, G.N.; et al. Differential response to neoadjuvant chemotherapy among 7 triple-negative breast cancer molecular subtypes. Clin. Cancer Res. 2013, 19, 5533–5540. [Google Scholar]
- Astvatsaturyan, K.; Yue, Y.; Walts, A.E.; Bose, S. Androgen receptor positive triple negative breast cancer: Clinicopathologic, prognostic, and predictive features. PLoS ONE 2018, 13, e0197827. [Google Scholar]
- Bronte, G.; Rocca, A.; Ravaioli, S.; Puccetti, M.; Tumedei, M.M.; Scarpi, E.; Andreis, D.; Maltoni, R.; Sarti, S.; Cecconetto, L.; et al. Androgen receptor in advanced breast cancer: Is it useful to predict the efficacy of anti-estrogen therapy? BMC Cancer 2018, 18, 348. [Google Scholar]
- Traina, T.A.; Miller, K.; Yardley, D.A.; Eakle, J.; Schwartzberg, L.S.; O’Shaughnessy, J.; Gradishar, W.; Schmid, P.; Winer, E.; Kelly, C.; et al. Enzalutamide for the treatment of androgen receptor-expressing triple-negative breast cancer. J. Clin. Oncol. 2018, 36, 884–890. [Google Scholar]
- Anestis, A.; Karamouzis, M.V.; Dalagiorgou, G.; Papavassiliou, A.G. Is androgen receptor targeting an emerging treatment strategy for triple negative breast cancer? Cancer Treat. Rev. 2015, 41, 547–553. [Google Scholar]
- Anestis, A.; Sarantis, P.; Theocharis, S.; Zoi, I.; Tryfonopoulos, D.; Korogiannos, A.; Koumarianou, A.; Xingi, E.; Thomaidou, D.; Kontos, M.; et al. Estrogen receptor beta increases sensitivity to enzalutamide in androgen receptor-positive triple-negative breast cancer. J. Cancer Res. Clin. Oncol. 2019, 145, 1221–1233. [Google Scholar]
- De Mattos Lima Lin, F.; Pincerato, K.M.; Bacchi, C.E.; Baracat, E.C.; Carvalho, F.M. Coordinated expression of oestrogen and androgen receptors in HER2-positive breast carcinomas: Impact on proliferative activity. J. Clin. Pathol. 2012, 65, 64–68. [Google Scholar]
- Need, E.F.; Selth, L.A.; Harris, T.J.; Birrell, S.N.; Tilley, W.D.; Buchanan, G. Research resource: Interplay between the genomic and transcriptional networks of androgen receptor and estrogen receptor α in luminal breast cancer cells. Mol. Endocrinol. 2012, 26, 1941–1952. [Google Scholar]
- Coss, C.C.; Jones, A.; Dalton, J.T. Selective androgen receptor modulators as improved androgen therapy for advanced breast cancer. Steroids 2014, 90, 94–100. [Google Scholar]
- Narayanan, R.; Ahn, S.; Cheney, M.D.; Yepuru, M.; Miller, D.D.; Steiner, M.S.; Dalton, J.T. Selective Androgen Receptor Modulators (SARMs) negatively regulate triple-negative breast cancer growth and epithelial:mesenchymal stem cell signaling. PLoS ONE 2014, 9, e103202. [Google Scholar]
- Tran, C.; Ouk, S.; Clegg, N.J.; Chen, Y.; Watson, P.A.; Arora, V.; Wongvipat, J.; Smith-Jones, P.M.; Yoo, D.; Kwon, A.; et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science 2009, 324, 787–790. [Google Scholar]
- Agarwal, N.; Di Lorenzo, G.; Sonpavde, G.; Bellmunt, J. New agents for prostate cancer. Ann. Oncol. 2014, 25, 1700–1709. [Google Scholar]
- De Kruijff, I.E.; Sieuwerts, A.M.; Onstenk, W.; Jager, A.; Hamberg, P.; de Jongh, F.E.; Smid, M.; Kraan, J.; Timmermans, M.A.; Martens, J.W.M.; et al. Androgen receptor expression in circulating tumor cells of patients with metastatic breast cancer. Int. J. Cancer 2019, 145, 1083–1089. [Google Scholar]
- De Laere, B.; van Dam, P.J.; Whitington, T.; Mayrhofer, M.; Diaz, E.H.; Van den Eynden, G.; Vandebroek, J.; Del-Favero, J.; Van Laere, S.; Dirix, L.; et al. Comprehensive Profiling of the Androgen Receptor in Liquid Biopsies from Castration-resistant Prostate Cancer Reveals Novel Intra-AR Structural Variation and Splice Variant Expression Patterns. Eur. Urol. 2017, 72, 192–200. [Google Scholar]
- Conteduca, V.; Wetterskog, D.; Sharabiani, M.T.A.; Grande, E.; Fernandez-Perez, M.P.; Jayaram, A.; Salvi, S.; Castellano, D.; Romanel, A.; Lolli, C.; et al. Androgen receptor gene status in plasma DNA associates with worse outcome on enzalutamide or abiraterone for castration-resistant prostate cancer: A multi-institution correlative biomarker study. Ann. Oncol. 2017, 28, 1508–1516. [Google Scholar]
- Aceto, N.; Bardia, A.; Wittner, B.S.; Donaldson, M.C.; O’Keefe, R.; Engstrom, A.; Bersani, F.; Zheng, Y.; Comaills, V.; Niederhoffer, K.; et al. AR expression in breast cancer CTCs associates with bone metastases. Mol. Cancer Res. 2018, 16, 720–727. [Google Scholar]
- Bishop, J.L.; Sio, A.; Angeles, A.; Roberts, M.E.; Azad, A.A.; Chi, K.N.; Zoubeidi, A. PD-L1 is highly expressed in Enzalutamide resistant prostate cancer. Oncotarget 2015, 6, 234–242. [Google Scholar]
- Ardiani, A.; Farsaci, B.; Rogers, C.J.; Protter, A.; Guo, Z.; King, T.H.; Apelian, D.; Hodge, J.W. Combination therapy with a second-generation androgen receptor antagonist and a metastasis vaccine improves survival in a spontaneous prostate cancer model. Clin. Cancer Res. 2013, 19, 6205–6218. [Google Scholar]

| Identifier | Study Design | Class of Agents | Agents | Molecular Profile | Patients (n) | Endpoint | Status of Trial |
|---|---|---|---|---|---|---|---|
| NCT02463032 | Randomized, Open label Phase II | Selective-AR modulator | GTx-024 | ER+ AR+ BC | 88 | CBR | Ongoing |
| NCT00468715 | Open label Phase II | AR inhibitor | Bicalutamide | Metastatic TNBC | 28 | CR or PR | Ongoing |
| NCT02091960 | Open label Phase II | AR inhibitor HER 2 Inhibitor | Enzalutamide Transtuzumab | HER2+ AR+ metastatic or locally advanced BC. | 103 | CBR | Ongoing |
| NCT00755885 | Non randomized Open label Phase I/II | AR inhibitor | Abiraterone Acetate | Advanced or Metastatic AR+ BC | 77 | MTD CBR | Completed |
| NCT01842321 | Single Group Assignment Open label Phase II | AR inhibitor | Abiraterone Acetate | Advanced or Metastatic TNBC | 31 | CBR | Ongoing |
| NCT02580448 | Non randomized Open label Phase I/II | Lyase-selective CYP17 inhibitor | Seviteronel | Advanced TNBC, ER+ BC | 175 | CBR | Completed |
| NCT02689427 | Non randomized Open label Phase IIB | AR Inhibitor Microtubule stabilizer | Enzalutamide Paclitaxel | AR+ TNBC Stage I-III | 37 | pCR RCB-I | Recruiting |
| NCT00004205 | Randomized, double-blind-phase-III | Aromatase inhibitor Selective ER modulator | Letrozole Tamoxifen | ER+ PgR+ BC | 8028 | DFS | Completed |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anestis, A.; Zoi, I.; Papavassiliou, A.G.; Karamouzis, M.V. Androgen Receptor in Breast Cancer—Clinical and Preclinical Research Insights. Molecules 2020, 25, 358. https://doi.org/10.3390/molecules25020358
Anestis A, Zoi I, Papavassiliou AG, Karamouzis MV. Androgen Receptor in Breast Cancer—Clinical and Preclinical Research Insights. Molecules. 2020; 25(2):358. https://doi.org/10.3390/molecules25020358
Chicago/Turabian StyleAnestis, Aristomenis, Ilianna Zoi, Athanasios G. Papavassiliou, and Michalis V. Karamouzis. 2020. "Androgen Receptor in Breast Cancer—Clinical and Preclinical Research Insights" Molecules 25, no. 2: 358. https://doi.org/10.3390/molecules25020358
APA StyleAnestis, A., Zoi, I., Papavassiliou, A. G., & Karamouzis, M. V. (2020). Androgen Receptor in Breast Cancer—Clinical and Preclinical Research Insights. Molecules, 25(2), 358. https://doi.org/10.3390/molecules25020358







