Top-Down Polyelectrolytes for Membrane-Based Post-Combustion CO2 Capture
Abstract
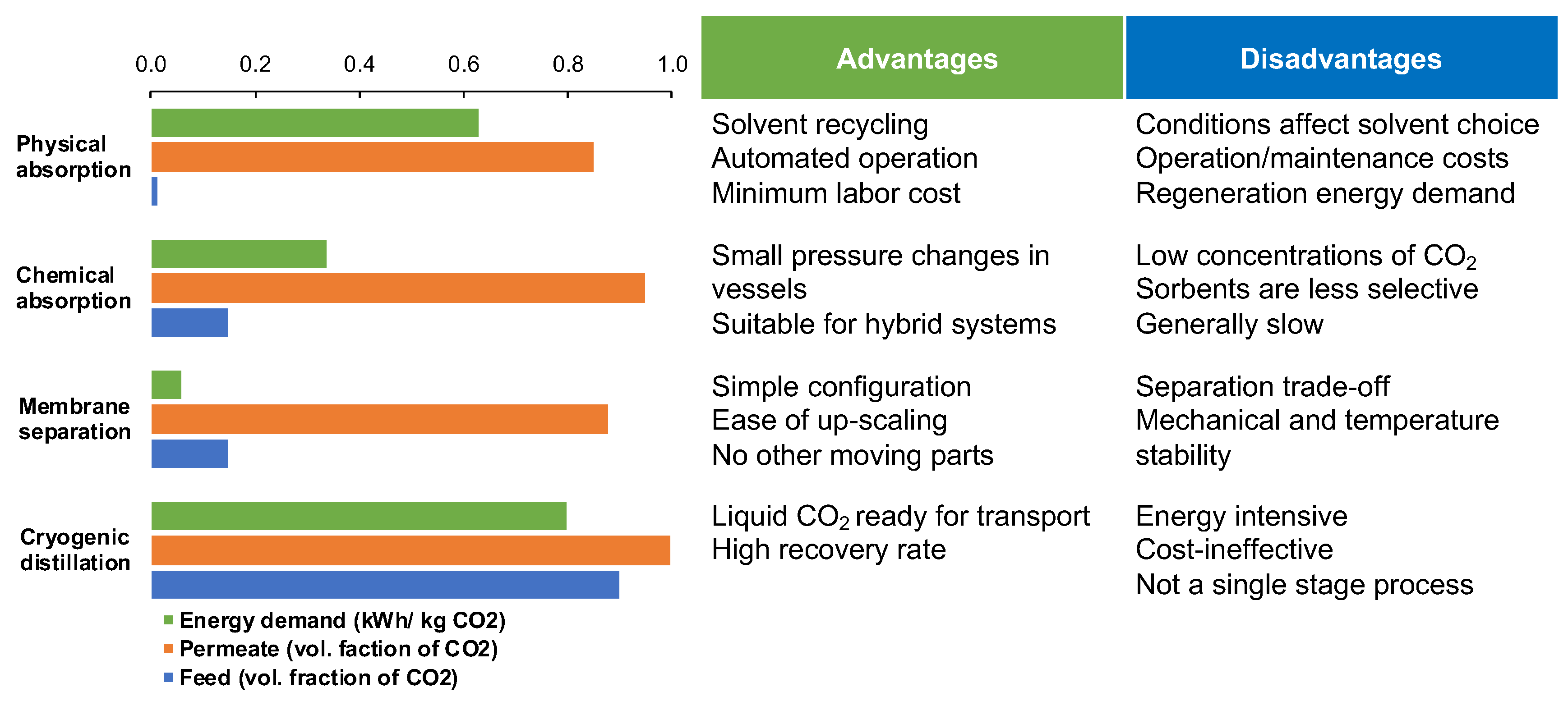
1. Introduction
| Material | Advantages | Disadvantages | Commercial Product | Supplier | [-] | [GPU] | Ref. |
|---|---|---|---|---|---|---|---|
| Polymers | Endurance | Low permeance | Cellulose acetate (CA) | Sigma Aldrich | 32 | 80 | [43] |
| Processing simplicity | Plasticszation | Matrimid® 5218 (polyimide (PI)) | Huntsman | 33 | 0.14 | [44] | |
| High selectivity | Torlon® (polyamide-imide (PAI)) | Solvay | 34 a–25 b | 0.47 a–0.84 b | [45] | ||
| Pebax® MH1657 (polyamide-polyether blocks) | Arkema | 53 | 0.79 | [46] | |||
| Polyactive™ (polyethylene oxide terephthalate/polybutylene terephthalate) | PolyVation | 53 a–56 b | 1.81 a–3.90 b | [46,47,48] | |||
| Polymer of intrinsic | Rigid structure | Moderate selectivity | PIM 1 | N/A | 25 | >12,000 c | [49] |
| microporosity (PIMs) | Low polymer chain packing density | Production cost | |||||
| High gas permeance | Physical ageing and plasticisation | ||||||
| Thermally rearranged polymers (TRP) | Processability into hollow-fibres | mechanical strength after heat treatment | Poly(benzoxazoles) | N/A | ⩽30 | ⩽1300 Barrer d | [20,21,22] |
| Mixed-matrix membranes (MMM) | Excellent separation performance | Membrane processing | Various | N/A | >50 | ⩽2000 Barrer d | [14,23,24,26,50,51,52,53] |
| Facilitated transport membranes | High selectivity | Poor chemical stability of the carriers | Polaris™ (based on Pebax® | MTR Inc. | 50 | 1000–2200 | [54,55] |
| High permeability | evaporation and degradation | ||||||
| short lifetime |
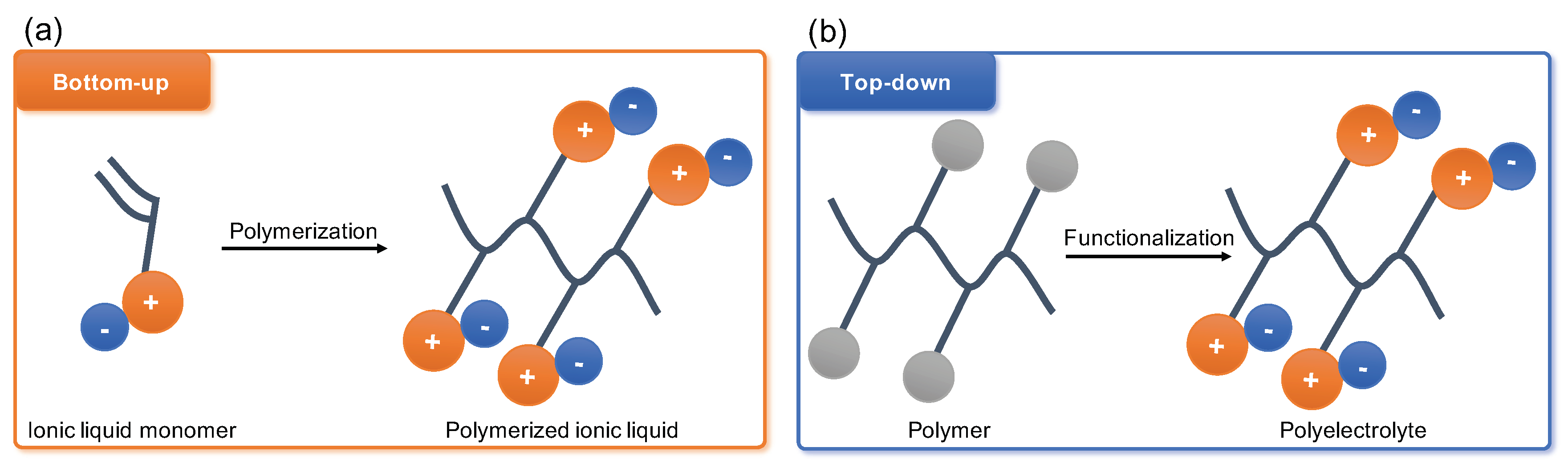
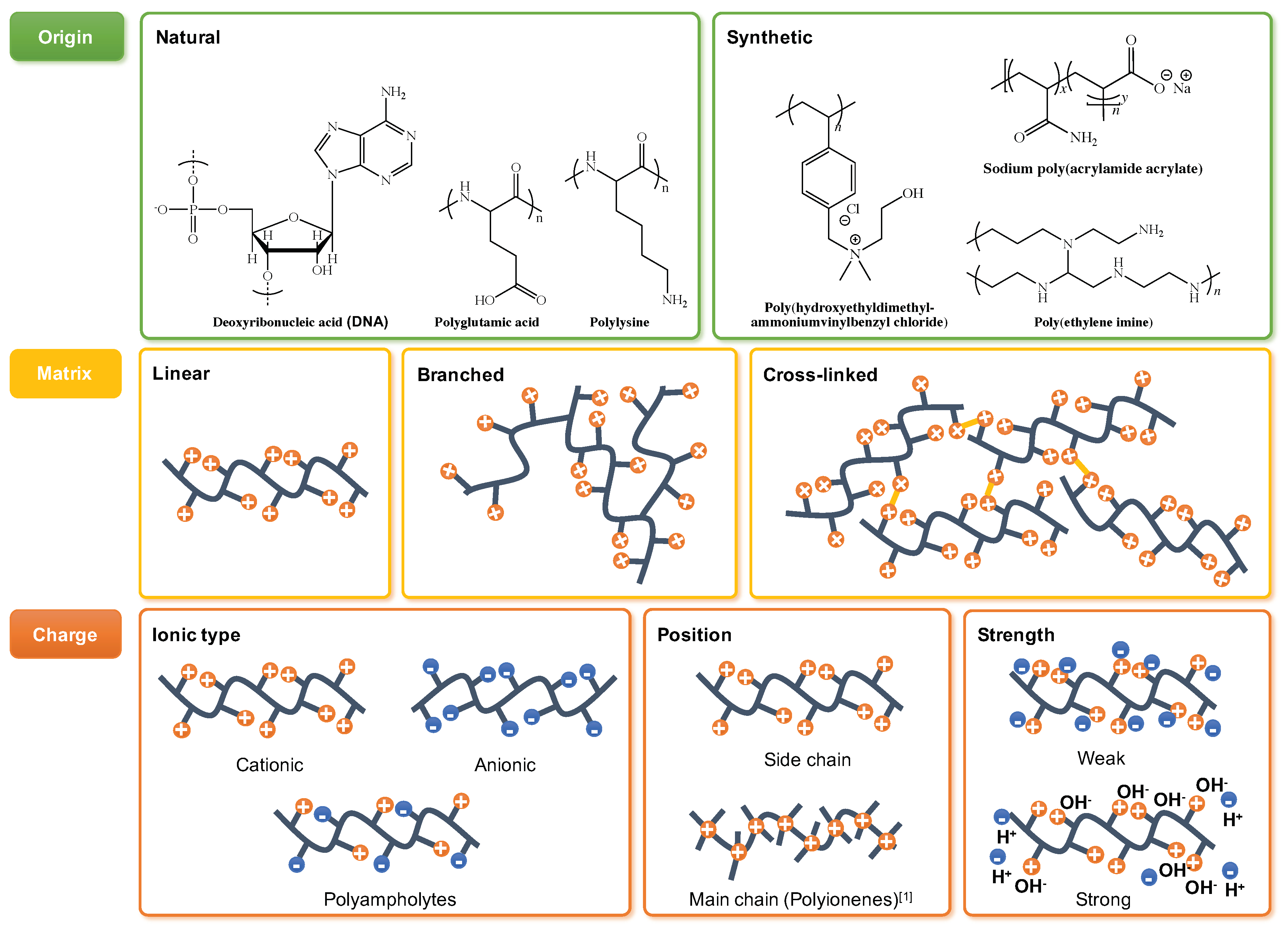
2. Polyelectrolytes: Definition
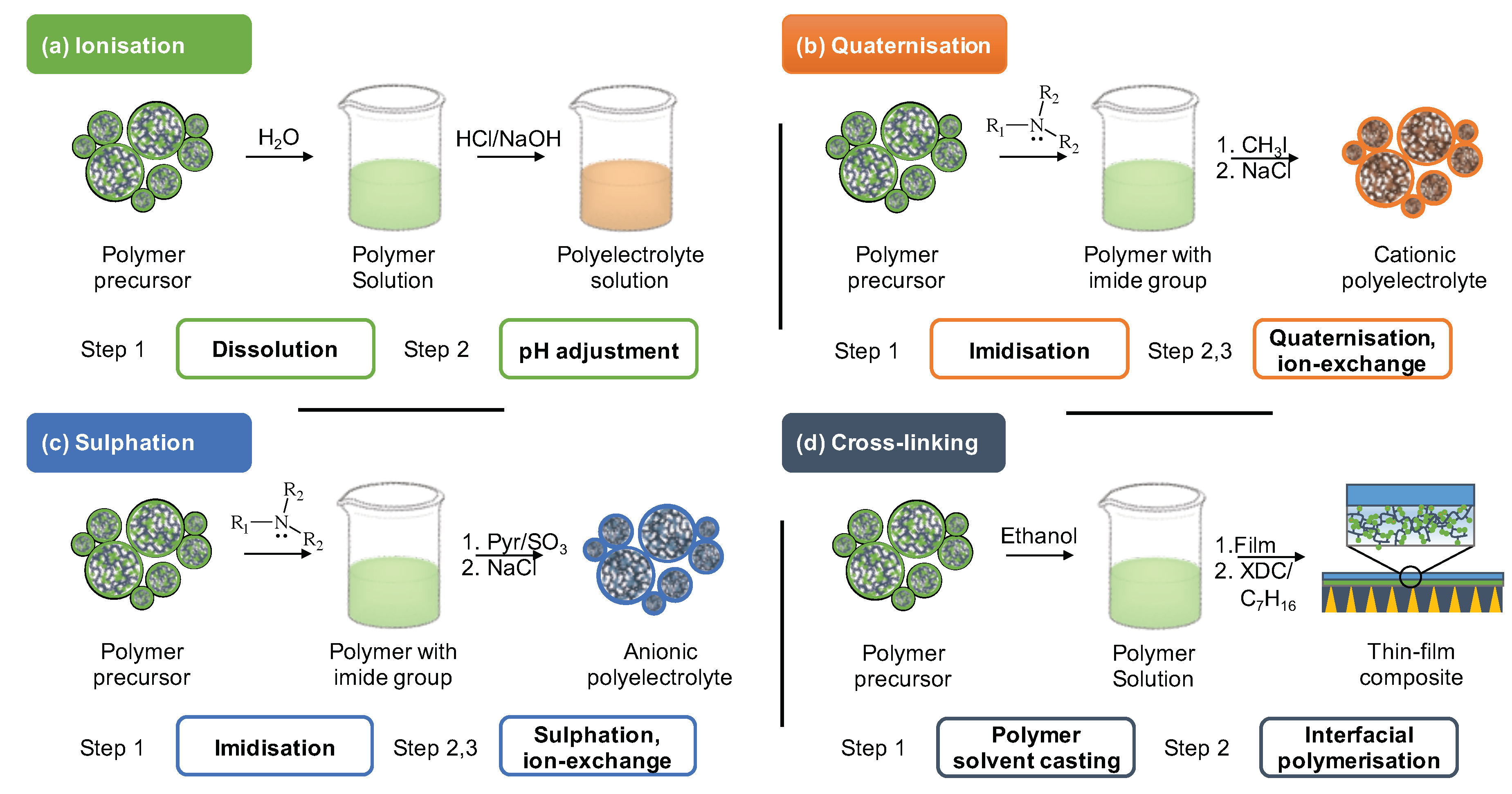
3. Post-Synthetic Modification of Polyelectrolytes
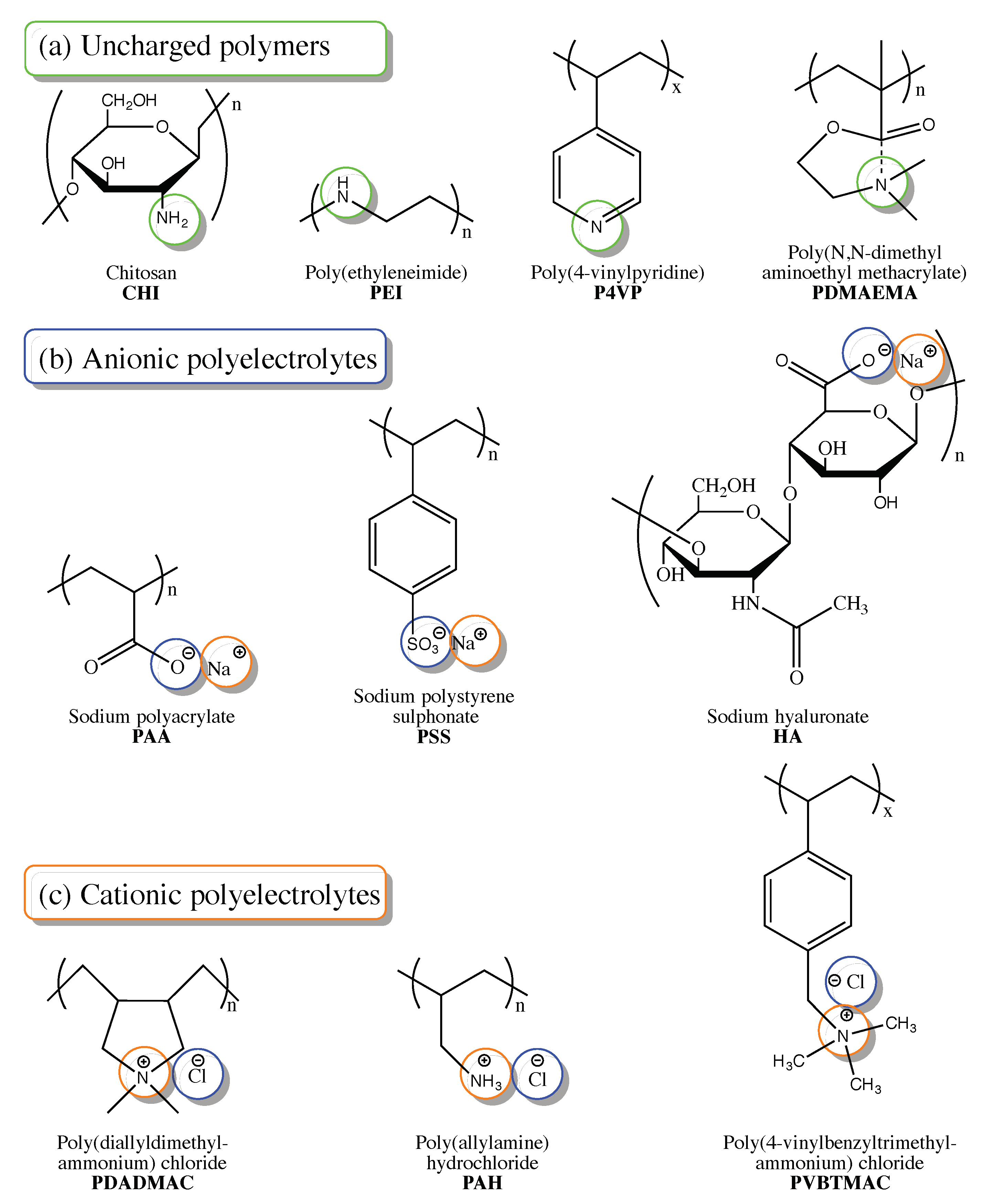
3.1. Ionisation
3.2. Cation-Functionalised Polyelectrolytes
3.3. Anion-Functionalised Polyelectrolytes
3.4. Cross-Linked Methacrylate-Based Polyelectrolytes
4. Membrane Preparation
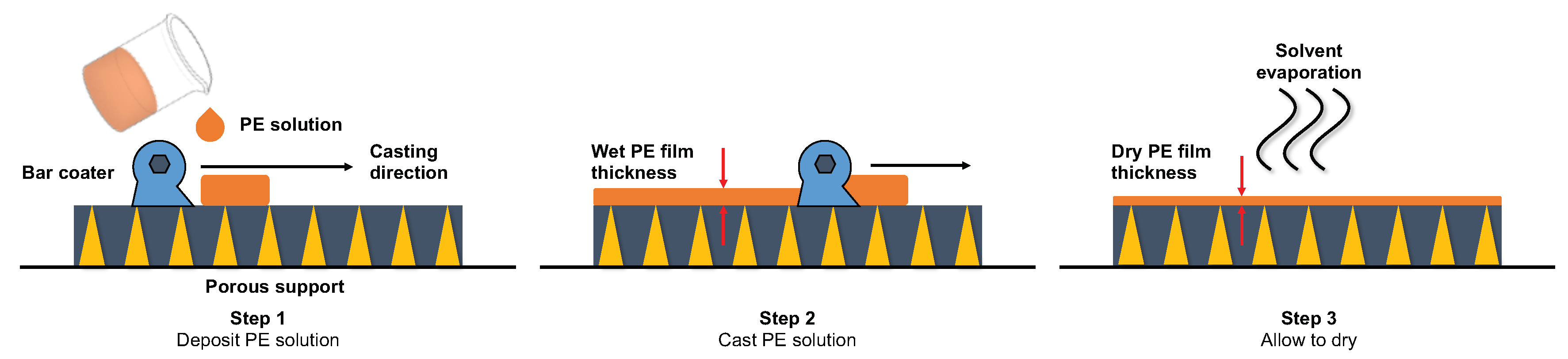
4.1. Solvent-Casting
4.2. Langmuir-Blodgett Films
4.3. Layer-by-Layer Assembly
4.4. Chemical Grafting
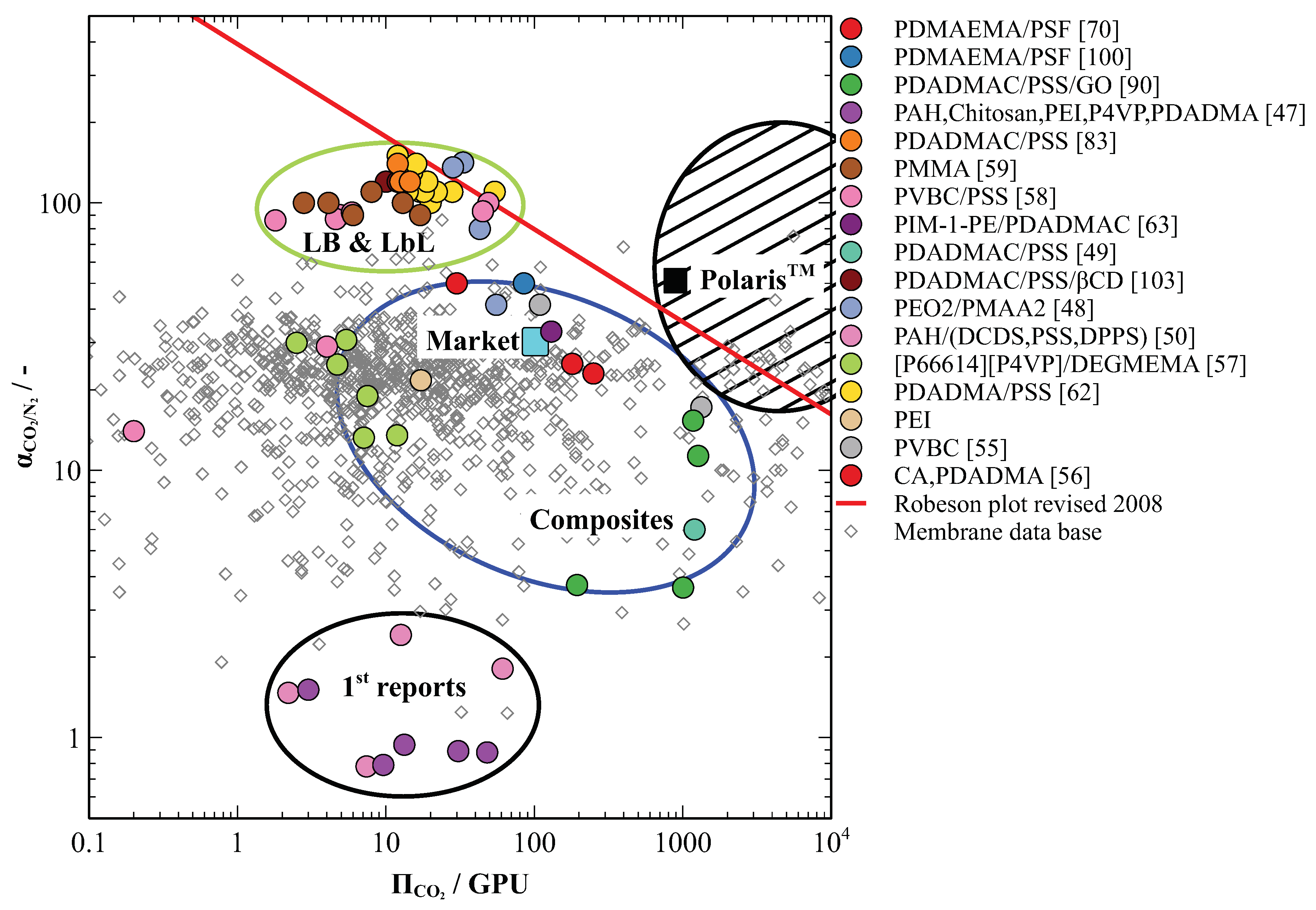
5. PE-Based Membranes Show High Selectivities for CO2 in Flue Gas Separation
6. Commercialisation Potential and Feasibility Testing
6.1. Production Up-Scaling
6.2. Feasibility Testing in Industrially Relevant Conditions
7. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| GHG | Greenhouse gas |
| CCSU | Carbon capture, sequestration, and utilisation |
| PE | Polyelectrolyte |
| IL | Ionic liquid |
| PIL | Polymerised ionic liquid |
| DNA | Deoxyribonucleic acid |
| PSS | Polystyrenesulphonate |
| PAH | Poly(allylamine) hydrochloride |
| PDADMAC | Poly(diallyldimethylammonium) chloride |
| XDC | p-Xylylene dichloride |
| LB | Langmuir-Blodgett method |
| LbL | Layer-by-layer assembly method |
| DMAEMA | N,N-dimethylaminoethanol methacrylate |
| PEGDMA | Poly(ethylene glycol dimethylacrylate) |
| CA | Cellulose acetate |
| CHI | Chitosan |
| PEI | Poly(ethyleneimide) |
| P4VP | Poly(4-vinylpyridine) |
| PDMAEMA | Poly(N,N-dimethyl aminoethyl methacrylate) |
| PAA | Sodium polyacrylate |
| HA | Sodium hyaluronate |
| PSF | Polysulphone |
| GO | Graphene oxide |
| PMMA | Poly(methylmethacrylate) |
| PVBC | Poly(vinylbenzyl) chloride |
| PIM-1-PE | Polymer of intrinsic microporosity |
| PEO | Poly(ethylene oxide) |
| PMAA | Poly(methacrylic acid) |
| DEGMEMA | Di(ethylene glycol)methyl ether methacrylate |
| PVBTMAC | Poly(4-vinylbenzyltrimethylammonium) chloride |
References
- Climate Change 2014: Mitigation of Climate Change. Contribution of Working Group III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Edenhofer, O., Pichs-Madruga, R., Sokona, Y., Farahani, E., Kadner, S., Seyboth, K., Adler, A., Baum, I., Brunner, S., Eickemeier, P., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2014; pp. 418–510. [Google Scholar]
- Figueroa, J.D.; Fout, T.; Plasynski, S.; McIlvried, H.; Srivastava, R.D. Advances in CO2 capture technology—The U.S. Department of Energy’s Carbon Sequestration Program. Int. J. Greenh. Gas Control 2008, 2, 9–20. [Google Scholar] [CrossRef]
- Luis, P.; Van Gerven, T.; Van der Bruggen, B. Recent developments in membrane-based technologies for CO2 capture. Prog. Energy Combust. Sci. 2012, 38, 419–448. [Google Scholar] [CrossRef]
- Audus, H. Greenhouse gas mitigation technology: An overview of the CO2 capture and sequestration studies and further activities of the IEA greenhouse gas R and D programme. Energy 1997, 22, 217–221. [Google Scholar] [CrossRef]
- Göttlicher, G.; Pruschek, R. Comparison of CO2 removal systems for fossil-fuelled power plant processes. Energy Convers. Manag. 1997, 38, 173–178. [Google Scholar] [CrossRef]
- Wong, S.; Bioletti, R. Carbon Dioxide Separation Technologies. Carb. Energy Manag. 2002, 1–14. [Google Scholar]
- Brunetti, A.; Drioli, E.; Lee, Y.M.; Barbieri, G. Engineering evaluation of CO2 separation by membrane gas separation systems. J. Memb. Sci. 2014, 454, 305–315. [Google Scholar] [CrossRef]
- Gibson, J.A.A.; Mangano, E.; Shiko, E.; Greenaway, A.G.; Gromov, A.V.; Lozinska, M.M.; Friedrich, D.; Campbell, E.E.B.; Wright, P.A.; Brandani, S. Adsorption Materials and Processes for Carbon Capture from Gas-Fired Power Plants: AMPGas. Ind. Eng. Chem. Res. 2016, 55, 3840–3851. [Google Scholar] [CrossRef]
- Hart, A.; Gnanendran, N. Cryogenic CO2 capture in natural gas. Energy Procedia 2009, 1, 697–706. [Google Scholar] [CrossRef]
- Aaron, D.; Tsouris, C. Separation of CO2 from flue gas: A review. Sep. Sci. Technol. 2005, 40, 321–348. [Google Scholar] [CrossRef]
- Leung, D.Y.C.; Caramanna, G.; Maroto-Valer, M.M. An overview of current status of carbon dioxide capture and storage technologies. Renew. Sustain. Energy Rev. 2014, 39, 426–443. [Google Scholar] [CrossRef]
- Powell, C.E.; Qiao, G.G. Polymeric CO2/N2 gas separation membranes for the capture of carbon dioxide from power plant flue gases. J. Memb. Sci. 2006, 279, 1–49. [Google Scholar] [CrossRef]
- Wind, J.D.; Paul, D.R.; Koros, W.J. Natural gas permeation in polyimide membranes. J. Memb. Sci. 2004, 228, 227–236. [Google Scholar] [CrossRef]
- Kentish, S.E.; Scholes, C.A.; Stevens, G.W. Carbon Dioxide Separation through Polymeric Membrane Systems for Flue Gas Applications. Recent Patents Chem. Eng. 2010, 1, 52–66. [Google Scholar] [CrossRef]
- Sanders, D.F.; Smith, Z.P.; Guo, R.; Robeson, L.M.; McGrath, J.E.; Paul, D.R.; Freeman, B.D. Energy-efficient polymeric gas separation membranes for a sustainable future: A review. Polymer 2013, 54, 4729–4761. [Google Scholar] [CrossRef]
- Budd, P.M.; Ghanem, B.S.; Makhseed, S.; McKeown, N.B.; Msayib, K.J.; Tattershall, C.E. Polymers of intrinsic microporosity (PIMs): Robust, solution-processable, organic nanoporous materials. Chem. Commun. 2004, 4, 230–231. [Google Scholar] [CrossRef]
- McKeown, N.B.; Budd, P.M. Polymers of intrinsic microporosity (PIMs): Organic materials for membrane separations, heterogeneous catalysis and hydrogen storage. Chem. Soc. Rev. 2006, 35, 675–683. [Google Scholar] [CrossRef]
- Lasseuguette, E.; Carta, M.; Brandani, S.; Ferrari, M.C. Effect of humidity and flue gas impurities on CO2 permeation of a polymer of intrinsic microporosity for post-combustion capture. Int. J. Greenh. Gas Control 2016, 50, 93–99. [Google Scholar] [CrossRef]
- Park, H.B.; Jung, C.H.; Lee, Y.M.; Hill, A.J.; Pas, S.J.; Mudie, S.T.; Van Wagner, E.; Freeman, B.D.; Cookson, D.J. Polymers with cavities tuned for fast selective transport of small molecules and ions. Science 2007, 318, 254–258. [Google Scholar] [CrossRef]
- Shamsipur, H.; Dawood, B.A.; Budd, P.M.; Bernardo, P.; Clarizia, G.; Jansen, J.C. Thermally rearrangeable PIM-polyimides for gas separation membranes. Macromolecules 2014, 47, 5595–5606. [Google Scholar] [CrossRef]
- Cersosimo, M.; Brunetti, A.; Drioli, E.; Fiorino, F.; Dong, G.; Woo, K.T.; Lee, J.; Lee, Y.M.; Barbieri, G. Separation of CO2 from humidified ternary gas mixtures using thermally rearranged polymeric membranes. J. Memb. Sci. 2015, 492, 257–262. [Google Scholar] [CrossRef]
- Woo, K.T.; Dong, G.; Lee, J.; Kim, J.S.; Do, Y.S.; Lee, W.H.; Lee, H.S.; Lee, Y.M. Ternary mixed-gas separation for flue gas CO2 capture using high performance thermally rearranged (TR) hollow fiber membranes. J. Memb. Sci. 2016, 510, 472–480. [Google Scholar] [CrossRef]
- Dai, Y.; Johnson, J.R.; Karvan, O.; Sholl, D.S.; Koros, W.J. Ultem®/ZIF-8 mixed matrix hollow fiber membranes for CO2/N2 separations. J. Memb. Sci. 2012, 401–402, 76–82. [Google Scholar] [CrossRef]
- Burmann, P.; Zornoza, B.; Téllez, C.; Coronas, J. Mixed matrix membranes comprising MOFs and porous silicate fillers prepared via spin coating for gas separation. Chem. Eng. Sci. 2014, 107, 66–75. [Google Scholar] [CrossRef]
- Li, Y.; Xin, Q.; Wu, H.; Guo, R.; Tian, Z.; Liu, Y.; Wang, S.; He, G.; Pan, F.; Jiang, Z. Efficient CO2 capture by humidified polymer electrolyte membranes with tunable water state. Energy Environ. Sci. 2014, 7, 1489–1499. [Google Scholar] [CrossRef]
- Fernández-Barquín, A.; Casado-Coterillo, C.; Palomino, M.; Valencia, S.; Irabien, A. Permselectivity improvement in membranes for CO2/N2 separation. Sep. Purif. Technol. 2016, 157, 102–111. [Google Scholar] [CrossRef]
- Ward, W.J.; Robb, W.L. Carbon dioxide-oxygen separation: Facilitated transport of carbon dioxide across a liquid film. Science 1967, 156, 1481–1484. [Google Scholar] [CrossRef]
- Way, J.D.; Noble, R.D. Facilitated transport. In Membrane Handbook; Springer: New York, NY, USA, 1992; pp. 833–866. [Google Scholar]
- Deng, L.; Kim, T.J.; Hägg, M.B. Facilitated transport of CO2 in novel PVAm/PVA blend membrane. J. Memb. Sci. 2009, 340, 154–163. [Google Scholar] [CrossRef]
- Zhang, H.; Tian, H.; Zhang, J.; Guo, R.; Li, X. Facilitated transport membranes with an amino acid salt for highly efficient CO2 separation. Int. J. Greenh. Gas Control 2018, 78, 85–93. [Google Scholar] [CrossRef]
- Qu, Z.; Wu, H.; Zhou, Y.; Yang, L.; Wu, X.; Wu, Y.; Ren, Y.; Zhang, N.; Liu, Y.; Jiang, Z. Constructing interconnected ionic cluster network in polyelectrolyte membranes for enhanced CO2 permeation. Chem. Eng. Sci. 2019, 199, 275–284. [Google Scholar] [CrossRef]
- Lilleby Helberg, R.M.; Dai, Z.; Ansaloni, L.; Deng, L. PVA/PVP blend polymer matrix for hosting carriers in facilitated transport membranes: Synergistic enhancement of CO2 separation performance. Green Energy Environ. 2019. [Google Scholar] [CrossRef]
- Morozova, S.M.; Shaplov, A.S.; Lozinskaya, E.I.; Vlasov, P.S.; Sardon, H.; Mecerreyes, D.; Vygodskii, Y.S. Poly(ionic liquid)-based polyurethanes having imidazolium, ammonium, morpholinium or pyrrolidinium cations. High Perform. Polym. 2017, 29, 691–703. [Google Scholar] [CrossRef]
- Teodoro, R.M.; Tomé, L.C.; Mantione, D.; Mecerreyes, D.; Marrucho, I.M. Mixing poly(ionic liquid)s and ionic liquids with different cyano anions: Membrane forming ability and CO2/N2 separation properties. J. Memb. Sci. 2018, 552, 341–348. [Google Scholar] [CrossRef]
- Whitley, J.W.; Jeffrey Horne, W.; Shannon, M.S.; Andrews, M.A.; Terrell, K.L.; Hayward, S.S.; Yue, S.; Mittenthal, M.S.; O’Harra, K.E.; Bara, J.E. Systematic Investigation of the Photopolymerization of Imidazolium-Based Ionic Liquid Styrene and Vinyl Monomers. J. Polym. Sci. Part A Polym. Chem. 2018, 56, 2364–2375. [Google Scholar] [CrossRef]
- Nellepalli, P.; Tomé, L.C.; Vijayakrishna, K.; Marrucho, I.M. Imidazolium-Based Copoly(Ionic Liquid) Membranes for CO2/N2 Separation. Ind. Eng. Chem. Res. 2019, 58, 2017–2026. [Google Scholar] [CrossRef]
- Zulfiqar, S.; Mantione, D.; El Tall, O.; Ruipérez, F.; Sarwar, M.I.; Rothenberger, A.; Mecerreyes, D. Pyridinium Containing Amide Based Polymeric Ionic Liquids for CO2/CH4 Separation. ACS Sustain. Chem. Eng. 2019, 7, 10241–10247. [Google Scholar] [CrossRef]
- Yuan, J.; Mecerreyes, D.; Antonietti, M. Poly(ionic liquid)s: An update. Prog. Polym. Sci. 2013, 38, 1009–1036. [Google Scholar] [CrossRef]
- Nishimura, N.; Ohno, H. 15Th Anniversary of Polymerised Ionic Liquids. Polymer 2014, 3289–3297. [Google Scholar] [CrossRef]
- Xu, W.; Ledin, P.A.; Shevchenko, V.V.; Tsukruk, V.V. Architecture, Assembly, and Emerging Applications of Branched Functional Polyelectrolytes and Poly(ionic liquid)s. ACS Appl. Mater. Interfaces 2015, 7, 12570–12596. [Google Scholar] [CrossRef]
- Shaplov, A.S.; Ponkratov, D.O.; Vygodskii, Y.S. Poly(ionic liquid)s: Synthesis, properties, and application. Polym. Sci. Ser. B 2016, 58, 73–142. [Google Scholar] [CrossRef]
- Tomé, L.C.; Marrucho, I.M. Ionic liquid-based materials: A platform to design engineered CO2 separation membranes. Chem. Soc. Rev. 2016, 45, 2785–2824. [Google Scholar] [CrossRef]
- Nikolaeva, D.; Azcune, I.; Tanczyk, M.; Warmuzinski, K.; Jaschik, M.; Sandru, M.; Dahl, P.I.; Genua, A.; Lois, S.; Sheridan, E.; et al. The performance of affordable and stable cellulose-based poly-ionic membranes in CO2/N2 and CO2/CH4 gas separation. J. Memb. Sci. 2018, 564, 552–561. [Google Scholar] [CrossRef]
- Li, X.; Wang, M.; Wang, S.; Li, Y.; Jiang, Z.; Guo, R.; Wu, H.; Cao, X.; Yang, J.; Wang, B. Constructing CO2 transport passageways in Matrimid® membranes using nanohydrogels for efficient carbon capture. J. Memb. Sci. 2015, 474, 156–166. [Google Scholar] [CrossRef]
- Kosuri, M.R.; Koros, W.J. Defect-free asymmetric hollow fiber membranes from Torlon®, a polyamide-imide polymer, for high-pressure CO2 separations. J. Memb. Sci. 2008, 320, 65–72. [Google Scholar] [CrossRef]
- Lillepärg, J.; Georgopanos, P.; Shishatskiy, S. Stability of blended polymeric materials for CO2 separation. J. Memb. Sci. 2014, 467, 269–278. [Google Scholar] [CrossRef]
- Maas, P.; Nauels, N.; Zhao, L.; Markewitz, P.; Scherer, V.; Modigell, M.; Stolten, D.; Hake, J.F. Energetic and economic evaluation of membrane-based carbon capture routes for power plant processes. Int. J. Greenh. Gas Control 2016, 44, 124–139. [Google Scholar] [CrossRef]
- Sabetghadam, A.; Liu, X.; Gottmer, S.; Chu, L.; Gascon, J.; Kapteijn, F. Thin mixed matrix and dual layer membranes containing metal-organic framework nanosheets and Polyactive™ for CO2 capture. J. Memb. Sci. 2019, 570–571, 226–235. [Google Scholar] [CrossRef]
- Budd, P.M.; McKeown, N.B.; Ghanem, B.S.; Msayib, K.J.; Fritsch, D.; Starannikova, L.; Belov, N.; Sanfirova, O.; Yampolskii, Y.; Shantarovich, V. Gas permeation parameters and other physicochemical properties of a polymer of intrinsic microporosity: Polybenzodioxane PIM-1. J. Memb. Sci. 2008, 325, 851–860. [Google Scholar] [CrossRef]
- Bryan, N.; Lasseuguette, E.; Van Dalen, M.; Permogorov, N.; Amieiro, A.; Brandani, S.; Ferrari, M.C. Development of mixed matrix membranes containing zeolites for post-combustion carbon capture. Energy Procedia 2014, 63, 160–166. [Google Scholar] [CrossRef]
- Li, S.; Jiang, X.; Sun, H.; He, S.; Zhang, L.; Shao, L. Mesoporous dendritic fibrous nanosilica (DFNS) stimulating mix matrix membranes towards superior CO2 capture. J. Memb. Sci. 2019, 586, 185–191. [Google Scholar] [CrossRef]
- Jiang, X.; He, S.; Li, S.; Bai, Y.; Shao, L. Penetrating chains mimicking plant root branching to build mechanically robust, ultra-stable CO2-philic membranes for superior carbon capture. J. Mater. Chem. A 2019, 7, 16704–16711. [Google Scholar] [CrossRef]
- Sen, D.; Kalipcilar, H.; Yilmaz, L. Development of zeolite filled polycarbonate mixed matrix gas separation membranes. Desalination 2006, 200, 222–224. [Google Scholar] [CrossRef]
- Merkel, T.C.; Lin, H.; Wei, X.; Baker, R. Power plant post-combustion carbon dioxide capture: An opportunity for membranes. J. Memb. Sci. 2010, 359, 126–139. [Google Scholar] [CrossRef]
- Roussanaly, S.; Anantharaman, R.; Lindqvist, K.; Zhai, H.; Rubin, E. Membrane properties required for post-combustion CO2 capture at coal-fired power plants. J. Memb. Sci. 2016, 511, 250–264. [Google Scholar] [CrossRef]
- Staudinger, H. Über Isopren und Kautchuk 36. Mitteilung. Über die Konstitution des Kautchuks. Angew. Chem. 1932, 15, 276–280. [Google Scholar] [CrossRef]
- Morawetz, H. Polyelectrolyte Solutions: Phenomena and Interpretation. In Polyelectrolytes and Polyzwitterions; Lowe, A., McCormick, C.L., Eds.; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2006; pp. 1–18. [Google Scholar]
- Visakh, P.M. Polyelectrolyte: Thermodynamics and Rheology. In Polyelectrolytes Thermodynamics and Rheology; Visakh, P.M., Picó, G.A., Bayraktar, O., Eds.; Springer: Berlin, Germany, 2014; pp. 1–18. [Google Scholar]
- Rembaum, A. Introduction. In Polyelectrolytes and Their Application; Rembaum, A., Sélégny, E., Eds.; D. Reidel Publishing Company: Dordrecht, Holland, 1975; pp. vii–viii. [Google Scholar]
- Mortimer, D.A. Synthetic polyelectrolytes—A review. Polym. Int. 1991, 25, 29–41. [Google Scholar] [CrossRef]
- Laschewsky, A. Recent trends in the synthesis of polyelectrolytes. Curr. Opin. Colloid Interface Sci. 2012, 17, 56–63. [Google Scholar] [CrossRef]
- Krasemann, L.; Tieke, B. Composite membranes with ultrathin separation layer prepared by self-assembly of polyelectrolytes. Mater. Sci. Eng. C 1999, 8–9, 513–518. [Google Scholar] [CrossRef]
- Song, Y.; Lugo, E.L.; Powell, S.; Tzeng, P.; Wilhite, B.A.; Grunlan, J.C. Highly selective multilayer polymer thin films for CO2/N2 separation. J. Polym. Sci. Part B Polym. Phys. 2017, 55, 1730–1737. [Google Scholar] [CrossRef]
- Pramanik, N.B.; Regen, S.L. Layer-by-layer assembly of a polymer of intrinsic microporosity: Targeting the CO2/N2 separation problem. Chem. Commun. 2019, 55, 4347–4350. [Google Scholar] [CrossRef]
- Van Ackern, F.; Krasemann, L.; Tieke, B. Ultrathin membranes for gas separation and pervaporation prepared upon electrostatic self-assembly of polyelectrolytes. Thin Solid Films 1998, 327–329, 762–766. [Google Scholar] [CrossRef]
- Chen, D.; Hickner, M.A. Ion clustering in quaternary ammonium functionalized benzylmethyl containing poly(arylene ether ketone)s. Macromolecules 2013, 46, 9270–9278. [Google Scholar] [CrossRef]
- Bhavsar, R.S.; Kumbharkar, S.C.; Kharul, U.K. Polymeric ionic liquids (PILs): Effect of anion variation on their CO2 sorption. J. Memb. Sci. 2012, 389, 305–315. [Google Scholar] [CrossRef]
- Kumbharkar, S.C.; Bhavsar, R.S.; Kharul, U.K. Film forming polymeric ionic liquids (PILs) based on polybenzimidazoles for CO2 separation. RSC Adv. 2014, 4, 4500–4503. [Google Scholar] [CrossRef]
- Rewar, A.S.; Shaligram, S.V.; Kharul, U.K. Polybenzimidazole based polymeric ionic liquids possessing partial ionic character: Effects of anion exchange on their gas permeation properties. J. Memb. Sci. 2016, 497, 282–288. [Google Scholar] [CrossRef]
- Nikolaeva, D.; Azcune, I.; Sheridan, E.; Sandru, M.; Genua, A.; Tanczyk, M.; Jaschik, M.; Warmuzinski, K.; Jansen, J.C.; Vankelecom, I.F.J. Poly(vinylbenzyl chloride)-based poly(ionic liquids) as membranes for CO2 capture from flue gas. J. Mater. Chem. A 2017, 5, 19808–19818. [Google Scholar] [CrossRef]
- Hammann, M.; Castillo, D.; Anger, C.; Rieger, B. Highly selective CO2 separation membranes through tunable poly(4-vinylphenolate)-CO2 interactions. J. Mater. Chem. A 2014, 2, 16389–16396. [Google Scholar] [CrossRef]
- Lin, C.; Stedronsky, E.R.; Regen, S.L. PKa-Dependent Facilitated Transport of CO2 across Hyperthin Polyelectrolyte Multilayers. ACS Appl. Mater. Interfaces 2017, 9, 19525–19528. [Google Scholar] [CrossRef]
- Lin, C.; Yi, S.; Regen, S.L. Consequences of Tacticity on the Growth and Permeability of Hyperthin Polyelectrolyte Multilayers. Langmuir 2016, 32, 375–379. [Google Scholar] [CrossRef]
- Shishatskiy, S.; Pauls, J.R.; Nunes, S.P.; Peinemann, K.V. Quaternary ammonium membrane materials for CO2 separation. J. Memb. Sci. 2010, 359, 44–53. [Google Scholar] [CrossRef]
- Wang, M.; Janout, V.; Regen, S.L. Gas transport across hyperthin membranes. Acc. Chem. Res. 2013, 46, 2743–2754. [Google Scholar] [CrossRef]
- Pramanik, N.B.; Regen, S.L. Hyperthin Membranes for Gas Separations via Layer-by-Layer Assembly. Chem. Rec. 2019, 1–12. [Google Scholar] [CrossRef]
- Yi, S.; Leon, W.; Vezenov, D.; Regen, S.L. Tightening Polyelectrolyte Multilayers with Oligo Pendant Ions. ACS Macro Lett. 2016, 5, 915–918. [Google Scholar] [CrossRef]
- Reynolds, D.D.; Kenyon, W.O. Preparation and reactions of Sulfonic Esters. III. Reaction of Polyvinyl Sulfonates with Primary and Secondary Amines. Commun. Kodak Res. Lab. 1950, 1278, 1591–1593. [Google Scholar]
- Kaiser, E.T. Reactions of Sulfonate and Sulfate Esters. Org. Chem. Sulfur 1977, 649–679. [Google Scholar]
- Izumrudov, V.A.; Mussabayeva, B.K.; Murzagulova, K.B. Polyelectrolyte multilayers: Preparation and applications. Russ. Chem. Rev. 2018, 87, 192–200. [Google Scholar] [CrossRef]
- Matsuyama, H.; Teramoto, M.; Sakakura, H. Selective permeation of CO2 through poly2-(N, N-dimethyl) aminoethyl methacrylate membrane prepared by plasma-graft polymerization technique. J. Memb. Sci. 1996, 114, 193–200. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Fujimoto, K.; Kinugawa, H.; Kitao, T.; Ogata, N. Selective Permeation of Carbon Dioxide Through Synthetic Polymeric Membranes Having Amine Moiety. Chem. Lett. 1994, 243–246. [Google Scholar] [CrossRef]
- Du, R.; Feng, X.; Chakma, A. Poly(N,N-dimethylaminoethyl methacrylate)/polysulfone composite membranes for gas separations. J. Memb. Sci. 2006, 279, 76–85. [Google Scholar] [CrossRef]
- Loeb, S.; Sourirajan, S.; Weaver, D.E. High Flow Porous Membranes for Separating Water From Saline Solutions. US 3133137, 12 May 1964. [Google Scholar]
- Paul, D.R. Gas transport in homogeneous multicomponent polymers. J. Memb. Sci. 1984, 18, 75–86. [Google Scholar] [CrossRef]
- Robeson, L.M. The upper bound revisited. J. Memb. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Baker, R.W.; Low, B.T. Gas separation membrane materials: A perspective. Macromolecules 2014, 47, 6999–7013. [Google Scholar] [CrossRef]
- Li, W.; Zhang, F.; Yi, S.; Huang, C.; Zhang, H.; Pan, M. Effects of casting solvent on microstructure and ionic conductivity of anhydrous sulfonated poly(ether ether ketone)-ionic liquid composite membranes. Int. J. Hydrogen Energy 2012, 37, 748–754. [Google Scholar] [CrossRef]
- Robertson, G.P.; Mikhailenko, S.D.; Wang, K.; Xing, P.; Guiver, M.D.; Kaliaguine, S. Casting solvent interactions with sulfonated poly(ether ether ketone) during proton exchange membrane fabrication. J. Memb. Sci. 2003, 219, 113–121. [Google Scholar] [CrossRef]
- Bernard, F.L.; Duczinski, R.B.; Rojas, M.F.; Fialho, M.C.C.; Carreño, L.Á.; Chaban, V.V.; Vecchia, F.D.; Einloft, S. Cellulose based poly(ionic liquids): Tuning cation-anion interaction to improve carbon dioxide sorption. Fuel 2018, 211, 76–86. [Google Scholar] [CrossRef]
- Moghadam, F.; Kamio, E.; Yoshioka, T.; Matsuyama, H. New approach for the fabrication of double-network ion-gel membranes with high CO2/N2 separation performance based on facilitated transport. J. Memb. Sci. 2017, 530, 166–175. [Google Scholar] [CrossRef]
- Seon Bang, H.; Jang, S.; Soo Kang, Y.; Won, J. Dual facilitated transport of CO2 using electrospun composite membranes containing ionic liquid. J. Memb. Sci. 2015, 479, 77–84. [Google Scholar] [CrossRef]
- Du, R.; Chakma, A.; Feng, X. Interfacially formed poly(N,N-dimethylaminoethyl methacrylate)/polysulfone composite membranes for CO2/N2 separation. J. Memb. Sci. 2007, 290, 19–28. [Google Scholar] [CrossRef]
- Xu, L.; Tetreault, A.R.; Khaligh, H.H.; Goldthorpe, I.A.; Wettig, S.D.; Pope, M.A. Continuous Langmuir-Blodgett Deposition and Transfer by Controlled Edge-to-Edge Assembly of Floating 2D Materials. Langmuir 2019, 35, 51–59. [Google Scholar] [CrossRef]
- Alkire, R.C.; Kolb, D.M.; Lipkowski, J.; Ross, P.N. Chemically Modified Electrodes. Chem. Modif. Electrodes 2011, 11, 1–267. [Google Scholar]
- Ariga, K.; Yamauchi, Y.; Mori, T.; Hill, J.P. 25th Anniversary article: What can be done with the langmuir-blodgett method? Recent developments and its critical role in materials science. Adv. Mater. 2013, 25, 6477–6512. [Google Scholar] [CrossRef]
- Clark, G.L.; Leppla, P.W. X-Ray Diffraction Studies of BUilt up Films. Contrib. Chem. Labo. Univ. Ill. 1936, 32, 2199–2201. [Google Scholar]
- Gaines, G.L., Jr. From monolayer to multilayer: Some unanswered questions. Thin Solid Films 1980, 68, 1–5. [Google Scholar] [CrossRef]
- Bruening, M.L.; Dotzauer, D.M.; Jain, P.; Ouyang, L.; Baker, G.L. Creation of functional membranes using polyelectrolyte multilayers and polymer brushes. Langmuir 2008, 24, 7663–7673. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Janout, V.; Regen, S.L. Creating poly(ethylene oxide)-based polyelectrolytes for thin film construction using an ionic linker strategy. Chem. Mater. 2010, 22, 1285–1287. [Google Scholar] [CrossRef]
- Wang, M.; Janout, V.; Regen, S.L. Unexpected barrier properties of structurally matched and unmatched polyelectrolyte multilayers. Chem. Commun. 2013, 49, 3576–3578. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Chen, Q.; Yi, S.; Wang, M.; Regen, S.L. Polyelectrolyte multilayers on PTMSP as asymmetric membranes for gas separations: Langmuir-blodgett versus self-assembly methods of anchoring. Langmuir 2014, 30, 687–691. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.X.; Pagliaro, M.; Xu, Y.J.; Liu, B. Layer-by-layer assembly of versatile nanoarchitectures with diverse dimensionality: A new perspective for rational construction of multilayer assemblies. Chem. Soc. Rev. 2016, 45, 3088–3121. [Google Scholar] [CrossRef]
- Kirkland, J.J. Porous Thin-Layer Modified Glass Bead Supports for Gas Liquid Chromatography. Anal. Chem. 1965, 37, 1458–1461. [Google Scholar] [CrossRef]
- Iler, R.K. Multilayers of colloidal particles. J. Colloid Interface Sci. 1966, 21, 569–594. [Google Scholar] [CrossRef]
- Chen, W.; McCarthy, T.J. Layer-by-layer deposition: A tool for polymer surface modification. Macromolecules 1997, 30, 78–86. [Google Scholar] [CrossRef]
- Bellanger, H.; Casdorff, K.; Muff, L.F.; Ammann, R.; Burgert, I.; Michen, B. Layer-by-layer deposition on a heterogeneous surface: Effect of sorption kinetics on the growth of polyelectrolyte multilayers. J. Colloid Interface Sci. 2017, 500, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.; Choi, M.; Chang, J.; Ji, D.; Kang, S.W.; Hong, J. Highly Permeable Graphene Oxide/Polyelectrolytes Hybrid Thin Films for Enhanced CO2/N2 Separation Performance. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Edmondson, S.; Osborne, V.L.; Huck, W.T.S. Polymer brushes via surface-initiated polymerizations. Chem. Soc. Rev. 2004, 33, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Hensarling, R.M. Polymer Surface Engineering Via Thiol-Mediated Reactions. Bachelor’s Thesis, University of Southern Mississippi, Hattiesburg, MS, USA, 2012; pp. 1–214. [Google Scholar]
- Cho, W.K.; Kang, S.M.; Lee, J.K. Non-biofouling polymeric thin films on solid substrates. J. Nanosci. Nanotechnol. 2014, 14, 1231–1252. [Google Scholar] [CrossRef]
- Khire, V.S.; Harant, A.W.; Watkins, A.W.; Anseth, K.S.; Bowman, C.N. Ultrathin patterned polymer films on surfaces using thiol-ene polymerizations. Macromolecules 2006, 39, 5081–5086. [Google Scholar] [CrossRef]
- Robeson, L.M. Correlation of separation factor versus permeability for polymeric membranes. J. Memb. Sci. 1991, 62, 165–185. [Google Scholar] [CrossRef]
- Nikolaeva, D. Poly(ionic liquids) for CO2 capture. Bachelor’s Thesis, KU Leuven, Leuven, Belgium, 2019. [Google Scholar]
- Chen, X.Y.; Kaliaguine, S.; Rodrigue, D. A Comparison between Several Commercial Polymer Hollow Fiber Membranes for Gas Separation. J. Membr. Sep. Technol. 2017, 6, 1–15. [Google Scholar] [CrossRef]
- Sandru, M.; Dahl, P.I.; Sheridan, E.; Mollan Parnas, L.E.; Fantini, S.; Loïs, S.; Azcune, I.; Odriozola, I.; Cabañero, G. Novel CO2 capture membranes based on polymerized ionic liquids and polymeric porous supports. In Advanced Membrane Technology VII; ECI Symposium Series; Escobar, I.C., Hestekin, J., Eds.; Curran Associates Inc.: Red Hook, NY, USA, 2016. [Google Scholar]


| Component | a | Price per Unit Weight | Price per Unit Area b | ||
|---|---|---|---|---|---|
| (kg m −2) | (m) | (m) | (euro kg−1) | (euro m−2) | |
| PP/PE c | 100 | 0.07 | 3 | 0.21 | |
| PI c | 528 d | 50 | 0.0264 | 50 | 1.32 |
| CA-PE e | 1480 | 5 (0.5) d | 0.0074 | 4745 | 35.113 (3.5) f |
| PDMS c | 970 | 3 (0.3) d | 0.0029 | 5 | 0.0150 (0.0015) d |
| Total price per m2 of CA-PE TFC membrane | 37 (5) | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikolaeva, D.; Luis, P. Top-Down Polyelectrolytes for Membrane-Based Post-Combustion CO2 Capture. Molecules 2020, 25, 323. https://doi.org/10.3390/molecules25020323
Nikolaeva D, Luis P. Top-Down Polyelectrolytes for Membrane-Based Post-Combustion CO2 Capture. Molecules. 2020; 25(2):323. https://doi.org/10.3390/molecules25020323
Chicago/Turabian StyleNikolaeva, Daria, and Patricia Luis. 2020. "Top-Down Polyelectrolytes for Membrane-Based Post-Combustion CO2 Capture" Molecules 25, no. 2: 323. https://doi.org/10.3390/molecules25020323
APA StyleNikolaeva, D., & Luis, P. (2020). Top-Down Polyelectrolytes for Membrane-Based Post-Combustion CO2 Capture. Molecules, 25(2), 323. https://doi.org/10.3390/molecules25020323







