Suitability of Immobilized Systems for Microbiological Degradation of Endocrine Disrupting Compounds
Abstract
1. Introduction
2. Endocrine Disrupting Compounds (EDCs) in the Environment and Associated Risks
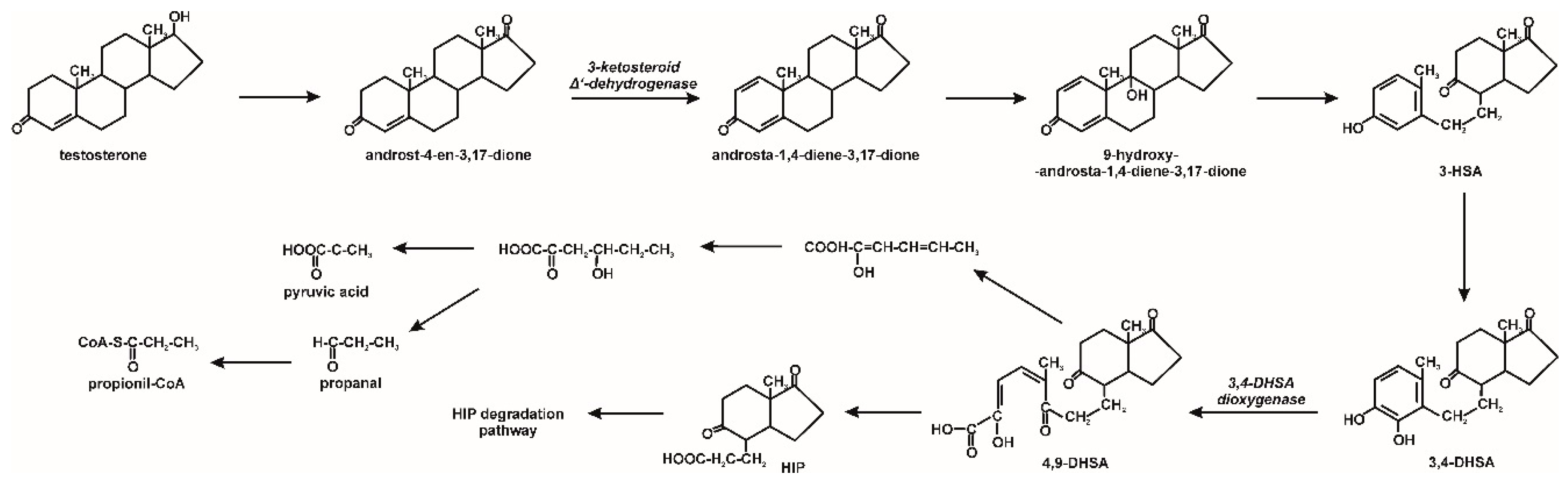
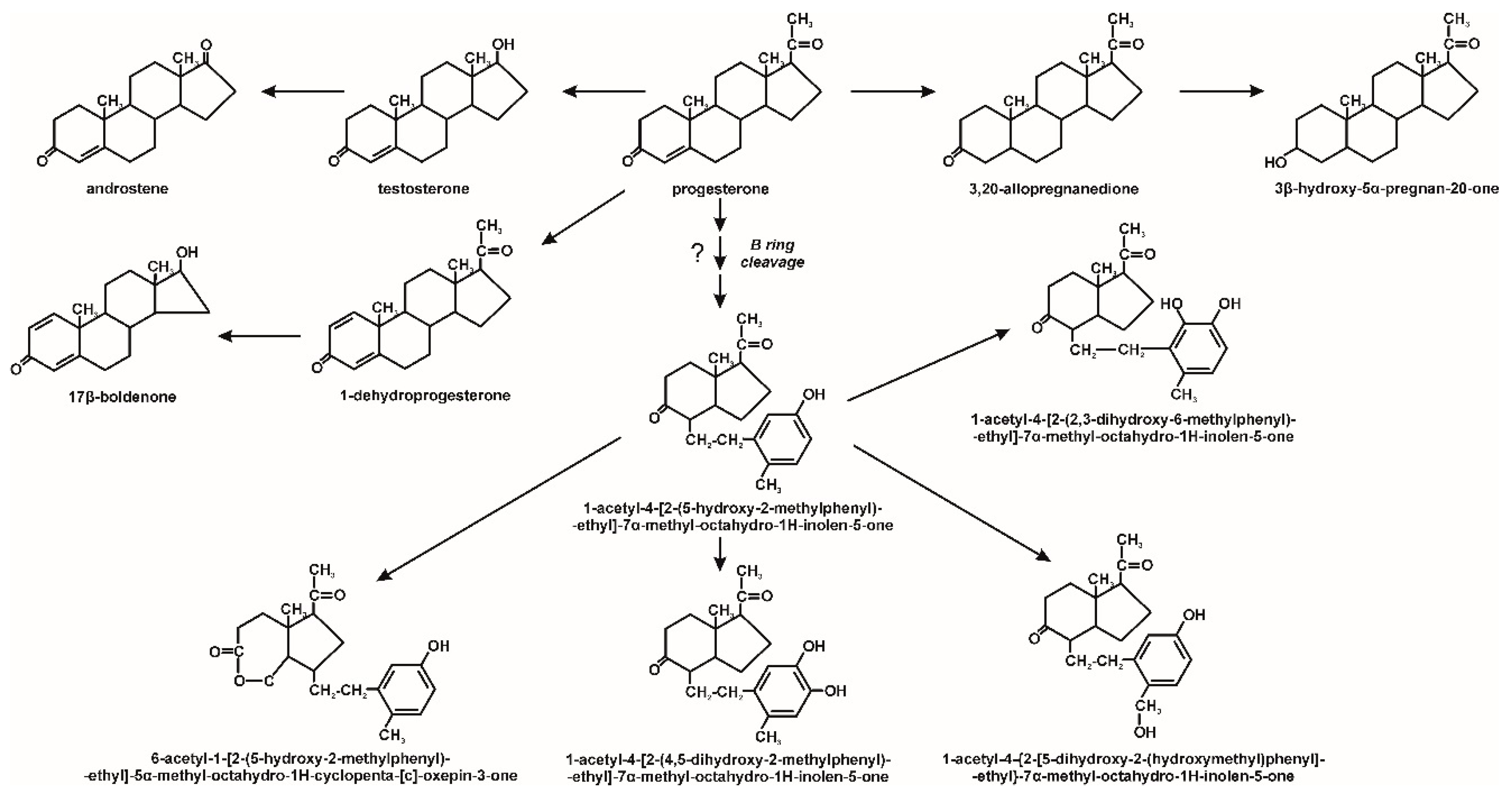
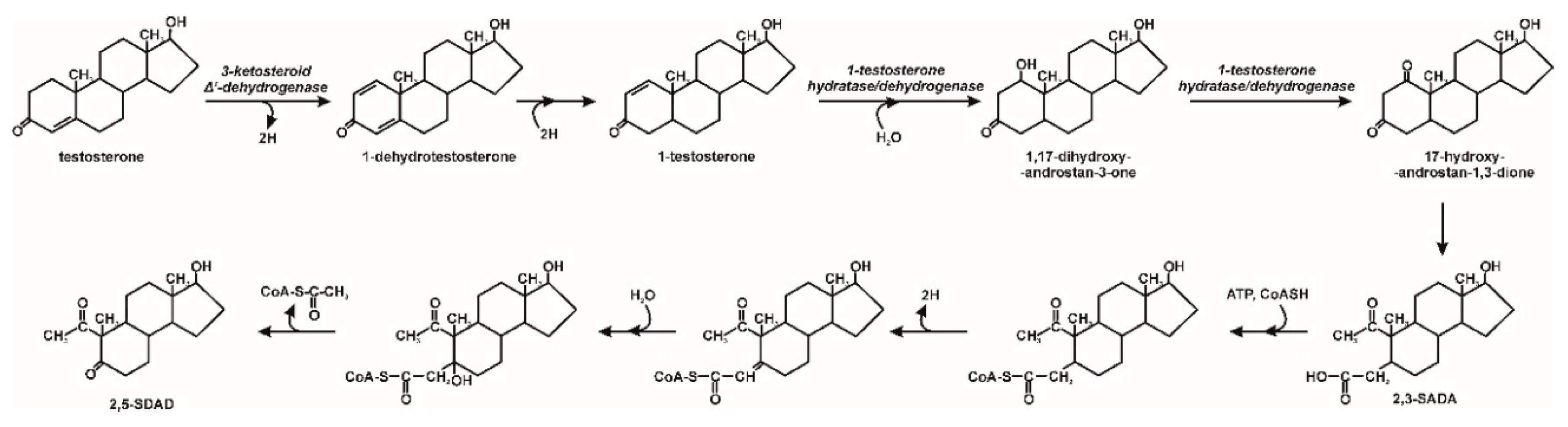
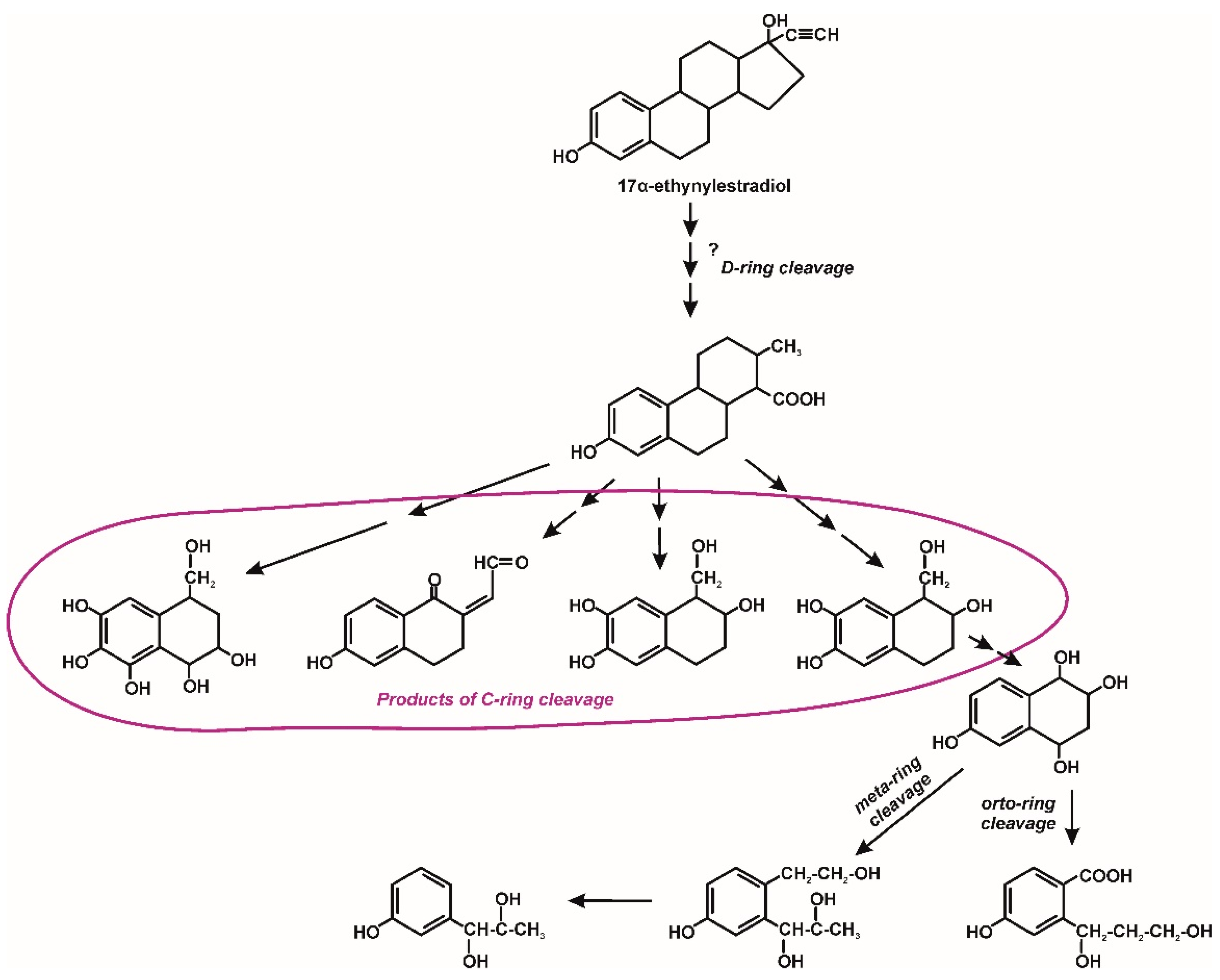
3. Microbiological Degradation of EDCs
4. Immobilized Systems in Microbiological Degradation of EDCs
5. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- AMAP. AMAP Assessment 2016: Chemicals of Emerging Arctic Concern; Arctic Monitoring and Assessment Programme (AMAP): Oslo, Norway, 2017; xvi+353pp; Available online: https://www.amap.no/documents/doc/amap-assessment-2016-chemicals-of-emerging-arctic-concern/1624 (accessed on 25 June 2020).
- Wojcieszynska, D.; Guzik, U. Naproxen in the environment: Its occurrence, toxicity to nontarget organisms and biodegradation. Appl. Microbiol. Biotechnol. 2020, 104, 1849–1857. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, L.; Louvado, A.; Esteves, V.I.; Gomes, N.C.M.; Almeida, A.; Cunha, A. Biodegradation of 17β-estradiol by bacteria isolated from deep sea sediments in aerobic and anaerobic media. J. Hazard. Mater. 2017, 323, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, S.; Li, X.; Gao, Y.; Ling, W. Removal of estrone, 17β-estradiol, and estriol from sewage and cow dung by immobilized Novosphingobium sp. ARI-1. Environ. Technol. 2018, 39, 2423–2433. [Google Scholar] [CrossRef] [PubMed]
- Michalska, J.; Piński, A.; Żur, J.; Mrozik, A. Selecting bacteria candidates for the bioaugmentation of activated sludge to improve the aerobic treatment of landfill leachate. Water 2020, 12, 140. [Google Scholar] [CrossRef]
- Liu, J.; Liu, J.; Xu, D.; Ling, W.; Li, S.; Chen, M. Isolation, immobilization, and degradation performance of the 17β-estradiol-degrading bacterium Rhodococcus sp. JX-2. Water Air Soil Pollut. 2016, 227–422. [Google Scholar] [CrossRef]
- Menashe, O.; Raizner, Y.; Kuc, M.E.; Cohen-Yaniv, V.; Kaplan, A.; Mamane, H.; Avisar, D.; Kurzbaum, E. Biodegradation of the endocrine-disrupting chemical 17α-ethynylestradiol (EE2) by Rhodococcus zopfii and Pseudomonas putida encapsulated in small bioreactor platform (SBP) capsules. Appl. Sci. 2020, 10, 336. [Google Scholar] [CrossRef]
- Dzionek, A.; Wojcieszyńska, D.; Adamczyk-Habrajska, M.; Guzik, U. Enhanced degradation of naproxen by immobilization of Bacillus thuringiensis B1(2015b) on loofah sponge. Molecules 2020, 25, 872. [Google Scholar] [CrossRef]
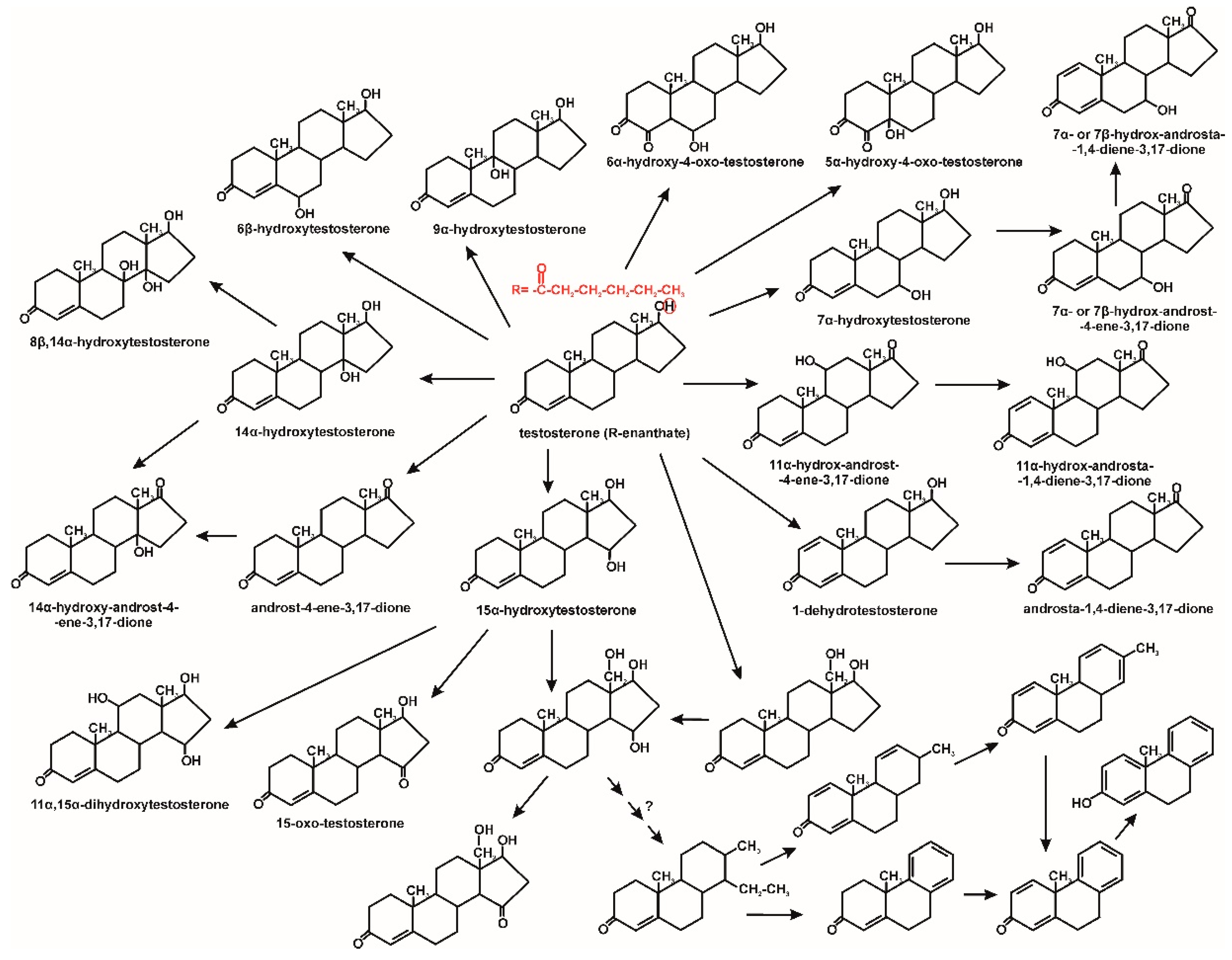
- Ibero, J.; Galán, B.; Díaz, E.; García, J.L. Testosterone degradative pathway of Novosphingobium tardaugens. Genes 2019, 10, 871. [Google Scholar] [CrossRef]
- Qiu, Q.; Wang, P.; Kang, H.; Wang, Y.; Tian, K.; Huo, H. Genomic analysis of new estrogen-degrading bacterial strain, Acinetobacter sp. DSSKY-A-001. Int. J. Genom. 2019, 2804134. [Google Scholar] [CrossRef]
- Liu, W.; Chen, Q.; He, N.; Sun, K.; Sun, D.; Wu, X.; Duan, S. Removal and biodegradation of 17β-estradiol and diethylstilbestrol by freshwater microalgae Raphidocelis subcapitata. Int. J. Environ. Res. Public Health 2018, 15, 452–466. [Google Scholar] [CrossRef]
- Chen, Y.-L.; Fu, H.-Y.; Lee, T.-H.; Shih, C.-J.; Huang, L.; Wang, Y.-S.; Ismail, W.; Chiang, Y.-R. Estrogen degraders and estrogen degradation pathway identified in an activated sludge. Appl. Environ. Microbiol. 2018, 84, e00001-18. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Q.; Hu, L.; Lu, G.; Li, Y. Occurrence of estrogens in water, sediment and biota and their ecological risk in Northern Taihu Lake in China. Environ. Geochem. Health 2015, 37, 147–156. [Google Scholar] [CrossRef]
- Avar, P.; Zrinyi, Z.; Maasz, G.; Takatsy, A.; Lovas, S.; Toth, L.G.; Pirger, Z. β-Estradiol and ethinyl-estradiol contamination in the rivers of the Carpathian Basin. Environ. Sci. Pollut. Res. 2016, 23, 11630–11638. [Google Scholar] [CrossRef] [PubMed]
- Pratush, A.; Ye, X.; Yang, Q.; Kan, J.; Peng, T.; Wang, H.; Huang, T.; Xiong, G.; Hu, Z. Biotransformation strategies for steroid estrogen and androgen pollution. Appl. Microbiol. Biotechnol. 2020, 104, 2385–2409. [Google Scholar] [CrossRef] [PubMed]
- Kasambala, H.R.; Rwiza, M.J.; Mdegela, R.H. Levels and distribution of progesterone in receiving waters and wastewaters of a growing urban area. Water Sci. Technol. 2019, 80, 1107–1117. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, R.L.; Wilson, E.M.; Angus, R.A.; Howell, W.M.; Kirk, M. Androstenedione and progesterone in the sediment of a river receiving paper mill effluent. Toxicol. Sci. 2003, 73, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Montagner, C.C.; Jardim, W.F. Spatial and seasonal variations of pharmaceuticals and endocrine disruptors in the Atibaia River, São Paulo State (Brazil). J. Braz. Chem. Soc. 2011, 22, 1452–1462. [Google Scholar] [CrossRef]
- Barel-Cohen, K.; Shore, L.S.; Shemesh, M.; Wenzel, A.; Mueller, J.; Kronfeld-Schor, N. Monitoring of natural and synthetic hormones in a polluted river. J. Environ. Manag. 2006, 78, 16–23. [Google Scholar] [CrossRef]
- Duong, C.N.; Lee, J.H.; Lim, B.J.; Kim, S.D. Biodegradation of estrogen conjugates by bacteria isolated from river sediments. Water Sci. Technol. 2011, 64, 1750–1758. [Google Scholar] [CrossRef]
- Zheng, W.; Zou, Y.; Li, X.; Machesky, M.L. Fate of estrogen conjugate 17a-estradiol-3-sulfate in dairy wastewater: Comparison of aerobic and anaerobic degradation and metabolite formation. J. Hazard. Mater. 2013, 258, 109–115. [Google Scholar] [CrossRef]
- Chatterjee, A.; Abraham, J. Mycoremediation of 17 β-estradiol using Trichoderma citrinoviride strain AJAC3 along with enzyme studies. Environ. Prog. Sustain. Energy 2019, 38. [Google Scholar] [CrossRef]
- Duffy, T.A.; Iwanowicz, L.R.; McCormick, S.D. Comparative responses to endocrine disrupting compounds in early life stages of Atlantic salmon, Salmo salar. Aquat. Toxicol. 2014, 152, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Czarny, K.; Szczukocki, D.; Krawczyk, B.; Gadzała-Kopciuch, R.; Skrzypek, S. Toxicity of single steroid hormones and their mixtures toward the cyanobacterium Microcystis Aeruginosa. J. Appl. Phycol. 2019, 31, 3537–3544. [Google Scholar] [CrossRef]
- Chen, Y.-L.; Yu, C.-P.; Lee, T.-H.; Goh, K.-S.; Chu, K.-H.; Wang, P.-H.; Ismail, W.; Shih, C.-J.; Chiang, Y.-R. Biochemical mechanisms and catabolic enzymes involved in bacterial estrogen degradation pathways. Cell Chem. Biol. 2017, 24, 712–724.e7. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Deng, B.; Lü, H.; Yao, W.; Su, S.; Wang, D. The bioaccumulation and biodegradation of testosterone by Chlorella vulgaris. Int. J. Environ. Res. Public Health 2019, 16, 1253. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-L.; Wang, C.-H.; Yang, F.-H.; Ismail, W.; Wang, P.-H.; Shih, C.-J.; Wu, Y.-C.; Chiang, Y.-R. Identification of Comamonas testosteroni as an androgen degrader in sewage. Sci. Rep. 2016, 6, 35386. [Google Scholar] [CrossRef] [PubMed]
- Shih, C.J.; Chen, Y.L.; Wang, C.H.H.; Wei, S.T.S.; Lin, I.T.; Ismail, W.A.; Chiang, Y.R. Biochemical mechanisms and microorganisms involved in anaerobic testosterone metabolism in estuarine sediments. Front. Microbiol. 2017, 8, 1520. [Google Scholar] [CrossRef]
- Garcia-Morales, R.; Rodríguez-Delgado, M.; Gomez-Mariscal, K.; Orona-Navar, C.; Hernandez-Luna, C.; Torres, E.; Parra, R.; Cárdenas-Chávez, D.; Mahlknecht, J.; Ornelas-Soto, N. Biotransformation of endocrine-disrupting compounds in groundwater: Bisphenol A, nonylphenol, ethynylestradiol and triclosan by a laccase cocktail from Pycnoporus sanguineus CS43. Water Air Soil Pollut. 2015, 226–251. [Google Scholar] [CrossRef]
- Pezzella, C.; Macellaro, G.; Sannia, G.; Raganati, F.; Olivieri, G.; Marzocchella, A.; Schlosser, D.; Piscitelli, A. Exploitation of Trametes versicolor for bioremediation of endocrine disrupting chemicals in bioreactors. PLoS ONE 2017, 12, e0178758. [Google Scholar] [CrossRef]
- Kriszt, R.; Krifaton, C.; Szoboszlay, S.; Cserhati, M.; Kriszt, B.; Kukolya, J.; Czeh, A.; Feher-Toth, S.; Torok, L.; Szoke, Z.; et al. A new zearalenone biodegradation strategy using non-pathogenic Rhodococcus pyridinivorans K408 strain. PLoS ONE 2012, 7, e43608. [Google Scholar] [CrossRef]
- Pluemsab, W.; Fukazawa, Y.; Furuike, T.; Nodasaka, Y.; Sakairi, N. Cyclodextrin-linked alginate beads as supporting materials for Sphingomonas cloacae, a nonylphenol degrading bacteria. Bioresour. Technol. 2007, 98, 2076–2081. [Google Scholar] [CrossRef] [PubMed]
- Khattab, R.A.; Elnwishy, N.; Hannora, A.; Mattiasson, B.; Omran, H.; Alharbi, O.M.L.; Ali, I. Biodegradation of 17-β-estradiol in water. Int. J. Environ. Sci. Technol. 2019, 16, 4935–4944. [Google Scholar] [CrossRef]
- Li, M.; Zhao, X.; Zhang, X.; Wu, D.; Leng, S. Biodegradation of 17β-estradiol by bacterial co-culture isolated from manure. Sci. Rep. 2018, 8, 3787. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Kong, X.; Lan, L.; Tadda, M.A.; Liu, D. Effects of carbon sources on 17 beta-estradiol degradation by Sphingomonas sp. and the involved intracellular metabolomics analysis. Environ. Sci. Process. Impacts 2020, 22, 197–206. [Google Scholar] [CrossRef]
- Liu, C.; Liu, K.; Zhao, C.; Gong, P.; Yu, Y. The characterization of a short chain dehydrogenase/reductase (SDRx) in Comamonas testosterone. Toxicol. Rep. 2020, 7, 460–467. [Google Scholar] [CrossRef]
- Wang, Y.; Shao, H.; Zhu, S.; Tian, K.; Qiu, Q.; Huo, H. Degradation of 17 ß-estradiol and products by a mixed culture of Rhodococcus equi DSSKP-R-001 and Comamonas testosteroni QYY20150409. Biotechnol. Biotechnol. Equip. 2019, 33, 268–277. [Google Scholar] [CrossRef]
- Xiong, W.; Peng, W.; Liang, R. Identification and genome analysis of Deinococcus actinosclerus SJTR1, a novel 17β-estradiol degradation bacterium. 3 Biotech 2018, 8, 433. [Google Scholar] [CrossRef]
- Xiong, W.; Yin, C.; Peng, W.; Deng, Z.; Lin, S.; Liang, R. Characterization of an 17β-estradiol-degrading bacterium Stenotrophomonas maltophilia SJTL3 tolerant to adverse environmental factors. Appl. Microbiol. Biotechnol. 2020, 104, 1291–1305. [Google Scholar] [CrossRef]
- Shi, W.; Wang, L.; Rousseau, D.P.L.; Lens, P.N.L. Removal of estrone, 17α-ethinylestradiol, and 17ß-estradiol in algae and duckweed-based wastewater treatment systems. Environ. Sci. Pollut. Res. 2010, 17, 824–833. [Google Scholar] [CrossRef]
- Gao, Q.T.; Wong, Y.S.; Tam, N.F.Y. Removal and biodegradation of nonylphenol by immobilized Chlorella vulgaris. Bioresour. Technol. 2011, 102, 10230–10238. [Google Scholar] [CrossRef]
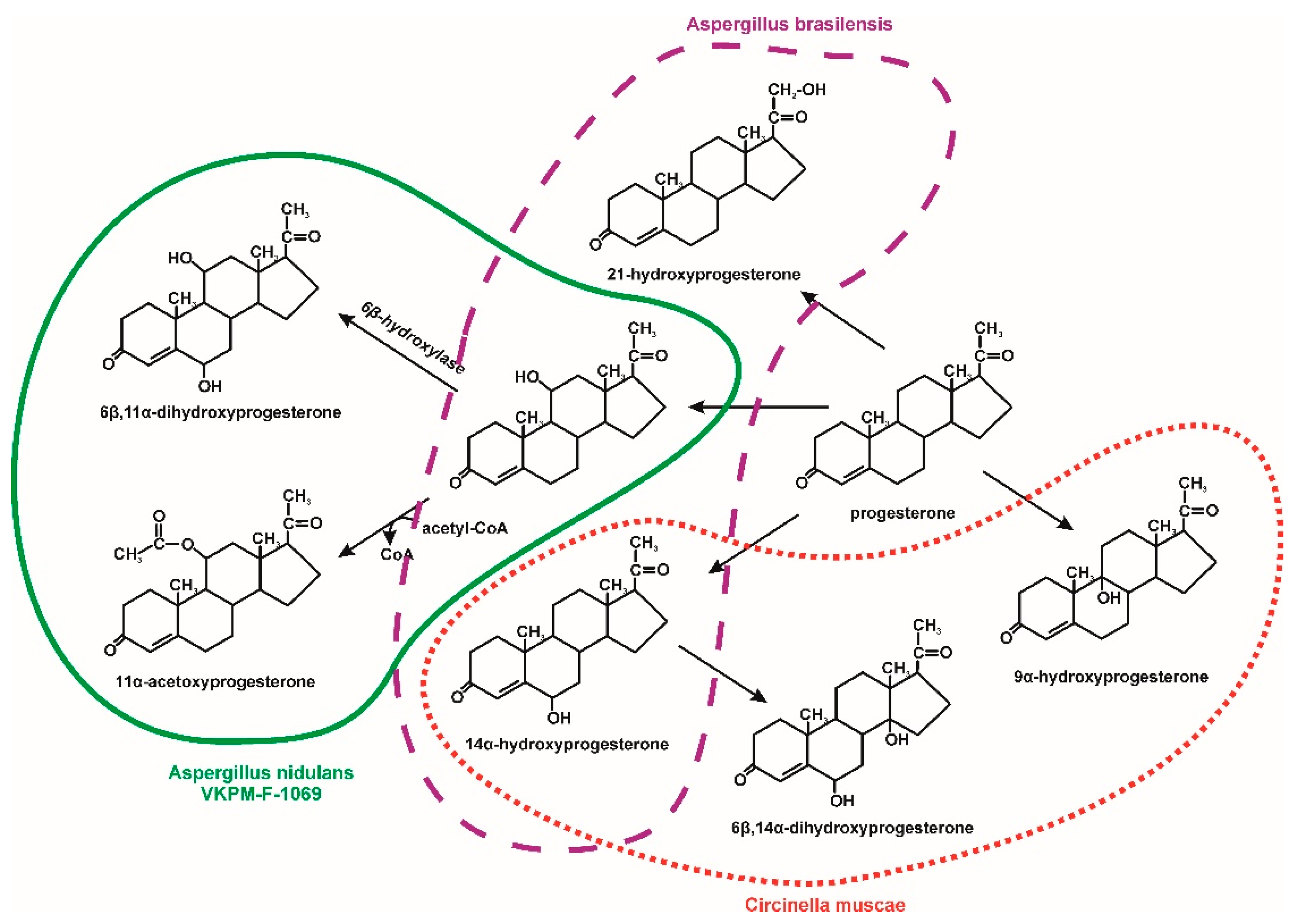
- Hosseinabadi, T.; Vahidi, H.; Nickavar, B.; Kobarfard, F. Biotransformation of progesterone by whole cells of filamentous fungi Aspergillus brasiliensis. Iran J. Pharm. Res. 2015, 14, 919–924. [Google Scholar] [CrossRef] [PubMed]
- Kollerov, V.; Shutov, A.; Kazantsev, A.; Donova, M. Biotransformation of androstenedione and androstadienedione by selected Ascomycota and Zygomycota fungal strains. Phytochemistry 2020, 169, 112160. [Google Scholar] [CrossRef] [PubMed]
- Muszyńska, B.; Żmudzki, P.; Lazur, J.; Kała, K.; Sułkowska-Ziaja, K.; Opoka, W. Analysis of the biodegradation of synthetic testosterone and 17α-ethynylestradiol using the edible mushroom Lentinula edodes. 3 Biotech 2018, 8, 424. [Google Scholar] [CrossRef] [PubMed]
- Savinova, O.S.; Solyev, P.N.; Vasina, D.V.; Tyazhelova, T.V.; Fedorova, T.V.; Savinova, T.S. Biotransformation of progesterone by Aspergillus nidulans VKPM F-1069 (wild type). Steroids 2019, 149, 108421. [Google Scholar] [CrossRef]
- Zoghi, M.; Gandomkar, S.; Habibi, Z. Biotransformation of progesterone and testosterone enanthate by Circinella muscae. Steroids 2019, 151, 108446. [Google Scholar] [CrossRef]
- Brasil Bernardelli, J.K.; Liz, M.V.; Belli, T.J.; Lobo-Recio, M.A.; Lapolli, F.R. Removal of estrogens by activated sludge under different conditions using batch experiments. Braz. J. Chem. Eng. 2015, 32, 421–432. [Google Scholar] [CrossRef]
- Pratush, A.; Yang, Q.; Peng, T.; Huang, T.; Hu, Z. Identification of non-accumulating intermediate compounds during estrone (E1) metabolism by a newly isolated microbial strain BH2-1 from mangrove sediments of the South China Sea. Environ. Sci. Pollut. Res. 2020, 27, 5097–5107. [Google Scholar] [CrossRef]
- Sami, N.; Fatma, T. Studies on estrone biodegradation potential of cyanobacterial species. Biocatal. Agric. Biotechnol. 2019, 17, 576–582. [Google Scholar] [CrossRef]
- Wu, K.; Lee, T.-H.; Chen, Y.-L.; Wang, Y.-S.; Wang, P.-H.; Yu, C.-P.; Chu, K.-H.; Chiang, Y.-R. Metabolites involved in aerobic degradation of the A and B rings of estrogen. Appl. Environ. Microbiol. 2019, 85, e02223-18. [Google Scholar] [CrossRef]
- Wang, P.-H.; Chen, Y.-L.; Wei, S.T.-S.; Wu, K.; Lee, T.-H.; Wu, T.-W.; Chiang, Y.-R. Retroconversion of estrogens into androgens by bacteria via a cobalamin-mediated methylation. Proc. Natl. Acad. Sci. USA 2020, 117, 1395–1403. [Google Scholar] [CrossRef]
- Elías-Arnanz, M. Anaerobic bacteria need their vitamin B12 to digest estrogen. Proc. Natl. Acad. Sci. USA 2020, 117, 1833–1835. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.-C.; Chen, Y.-L.; Tang, S.-L.; Yu, C.-P.; Wang, P.-H.; Ismail, W.; Wang, C.-H.; Ding, J.-Y.; Yang, C.-Y.; Chiang, Y.-R. Integrated multi-omics analyses reveal the biochemical mechanisms and phylogenetic relevance of anaerobic androgen biodegradation in the environment. ISME J. 2016, 10, 1967–1983. [Google Scholar] [CrossRef]
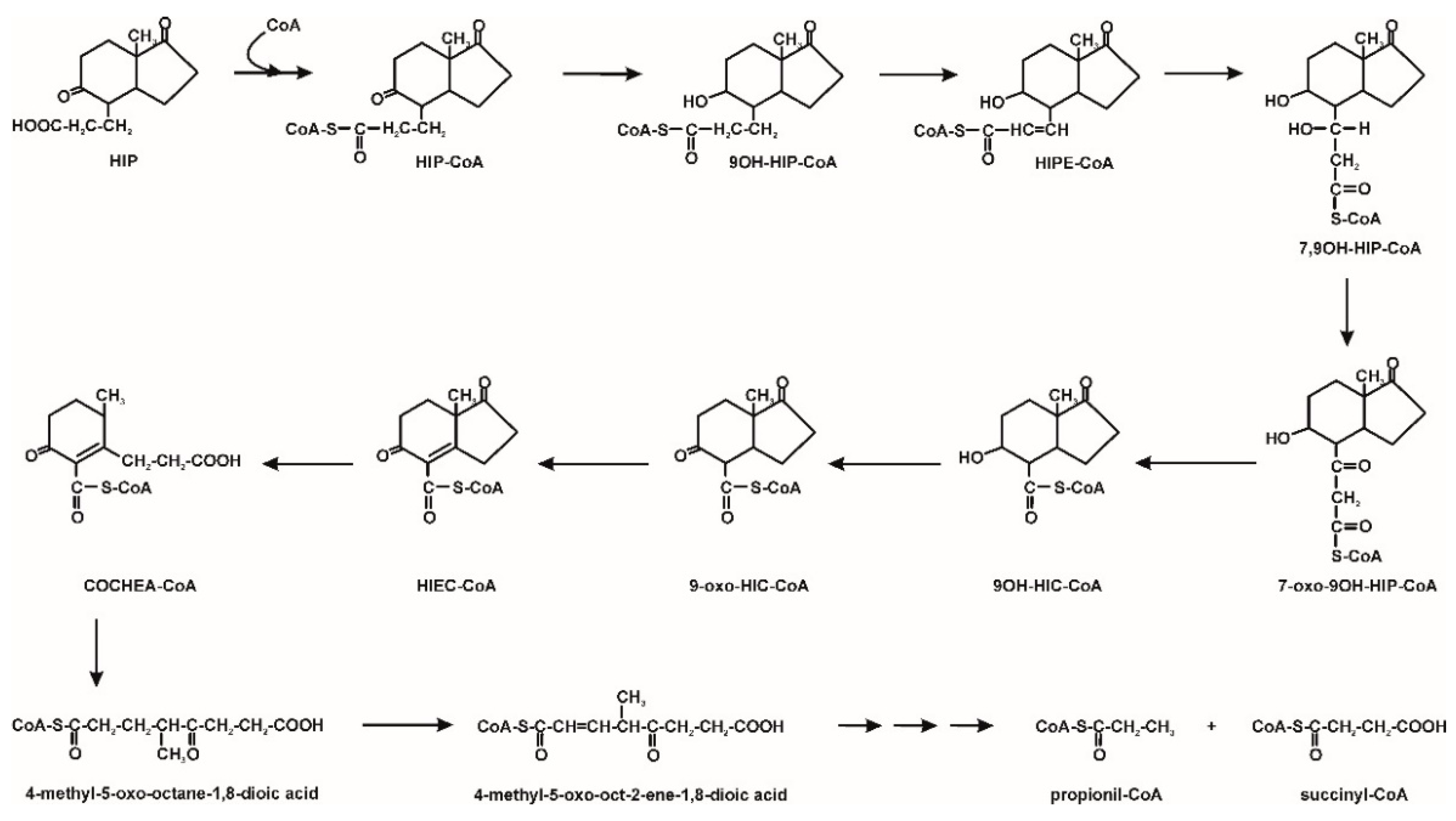
- Horinouchi, M.; Koshino, H.; Malon, M.; Hirota, H.; Hayashi, T. Steroid degradation in Comamonas testosteroni TA441: Identification of the entire β-Oxidation cycle of the cleaved B ring. Appl. Environ. Microbiol. 2019, 85, e011204-19. [Google Scholar] [CrossRef] [PubMed]
- Ojoghoro, J.O.; Chaudhary, A.J.; Campo, P.; Sumpter, J.P.; Scrimshaw, M.D. Progesterone potentially degrades to potent androgens in surface waters. Sci. Total Environ. 2017, 579, 1876–1884. [Google Scholar] [CrossRef] [PubMed]
- Chiang, Y.-R.; Fang, J.-Y.; Ismail, W.; Wang, P.-H. Initial steps in anoxic testosterone degradation by Steroidobacter denitrificans. Microbiology 2010, 156, 2253–2259. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.-H.; Yu, C.-P.; Lee, T.-H.; Lin, C.-W.; Ismail, W.; Wey, S.-P.; Kuo, A.-T.; Chiang, Y.-R. Anoxic androgen degradation by the denitrifying bacterium Sterolibacterium denitrificans via the 2,3-seco pathway. Appl. Environ. Microbiol. 2014, 80, 3442–3452. [Google Scholar] [CrossRef]
- Shreve, M.J.; Brockman, A.; Hartleb, M.; Prebihalo, S.; Dorman, F.L.; Brennan, R.A. The white-rot fungus Trametes versicolor reduces the estrogenic activity of a mixture of emerging contaminants in wastewater treatment plant effluent. Int. Biodeterior. Biodegrad. 2016, 109, 132–140. [Google Scholar] [CrossRef]
- Takeo, M.; Maeda, Y.; Maeda, J.; Nishiyama, N.; Kitamura, C.; Kato, D.-I.; Negoro, S. Two identical nonylphenol monooxygenase genes linked to IS6100 and some putative insertion sequence elements in Sphingomonas sp. NP5. Microbiology 2012, 158, 1796–1807. [Google Scholar] [CrossRef]
- Aris, A.Z.; Hir, Z.A.M.; Razak, M.R. Metal-organic frameworks (MOFs) for the adsorptive removal of selected endocrine disrupting compounds (EDCs) from aqueous solution: A review. Appl. Mater. Today 2020, 100796. [Google Scholar] [CrossRef]
- Qureshi, U.A.; Hameed, B.H.; Ahmed, M.J. Adsorption of endocrine disrupting compounds and other emerging contaminants using lignocellulosic biomass-derived porous carbons: A review. J. Water Process Eng. 2020, 101380. [Google Scholar] [CrossRef]
- Dzionek, A.; Wojcieszyńska, D.; Hupert-Kocurek, K.; Adamczyk-Habrajska, M.; Guzik, U. Immobilization of Planococcus sp. S5 strain on the Loofah sponge and its application in naproxen removal. Catalysts 2018, 8, 176. [Google Scholar] [CrossRef]
- Asada, M.; Shuler, M.L. Stimulation of ajmalicine production and excretion from Catharanthus roseus: Effects of adsorption in situ, elicitors and alginate immobilization. Appl. Microbiol. Biotechnol. 1989, 30, 475–481. [Google Scholar] [CrossRef]
- Gontier, E.; Sangwan, B.S.; Barbotin, J.N. Effects of calcium, alginate, and calcium-alginate immobilization on growth and tropane alkaloid levels of a stable suspension cell line of Datura innoxia Mill. Plant Cell Rep. 1994, 13, 533–536. [Google Scholar] [CrossRef] [PubMed]
- Bacerra, M.; Baroli, B.; Fadda, A.M.; Méndez, J.B.; Siso, M.I.G. Lactose bioconversion by calcium-alginate immobilization of Kluyveromyces lactis cells. Enzyme Microb. Technol. 2001, 29, 506–512. [Google Scholar] [CrossRef]
- Olajuyigbe, F.M.; Adetuyi, O.Y.; Fatokun, C.O. Characterization of free and immobilized laccase from Cyberlindnera fabianii and application in degradation of bisphenol A. Int. J. Biol. Macromol. 2019, 125, 856–864. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Jing, Z.; Zhao, Y.; Iqbal, H.M.N. Immobilization of fungal laccase on glutaraldehyde cross-linked chitosan beads and its bio-catalytic potential to degrade bisphenol A. Biocatal. Agric. Biotechnol. 2019, 19, 101174. [Google Scholar] [CrossRef]
- Ma, C.; Qim, D.; Sun, Q.; Zhang, F.; Liu, H.; Yu, C.-P. Removal of environmental estrogens by bacterial cell immobilization technique. Chemosphere 2016, 144, 607–614. [Google Scholar] [CrossRef]
- da Silva, F.B.; de Morais Júnior, W.G.; da Silva, C.V.; Vieira, A.T.; Batista, A.C.F.; de Fria, A.M.; Assunção, R.M.N. Preparation and characterization of cellulose triacetate as support for lecitase ultra immobilization. Molecules 2017, 22, 1930. [Google Scholar] [CrossRef]
- Lacerda, M.F.A.R.; Lopes, F.M.; Sartoratto, A.; Ponezi, A.N.; Thomaz, D.V.; Schimidt, F.; Santiago, M.F. Stability of immobilized laccase on Luffa Cylindrica fibers and assessment of synthetic hormone degradation. Prep. Biochem. Biotechnol. 2019, 49, 58–63. [Google Scholar] [CrossRef]
- Ji, C.; Nguyen, L.N.; Hou, J.; Hai, F.I.; Chen, V. Direct immobilization of laccase on titania nanoparticles from crude enzyme extracts of P. ostreatus culture for micro-pollutant degradation. Sep. Purif. Technol. 2017, 187, 215–223. [Google Scholar] [CrossRef]
- Brugnaria, T.; Pereira, M.G.; Bubna, G.A.; de Freitasa, E.N.; Contato, A.G.; Corrêa, R.C.G.; Castoldi, R.; de Souza, C.G.M.; Polizeli, M.L.T.M.; Bracht, A.; et al. A highly reusable MANAE-agarose-immobilized Pleurotus ostreatus laccase for degradation of bisphenol A. Sci. Total Environ. 2018, 634, 1346–1351. [Google Scholar] [CrossRef]
- Bezerra, C.S.; de Farias Lemos, C.M.G.; de Sousa, M.; Gonçalves, L.R.B. Enzyme immobilization onto renewable polymeric matrixes: Past, present, and future trends. J. Appl. Polym. Sci. 2015, 132, 42125. [Google Scholar] [CrossRef]
- Becker, D.; Rodriguez-Mozaz, S.; Insa, S.; Schoevaart, R.; Barceló, D.; de Cazes, M.; Belleville, M.-P.; Sanchez-Marcano, J.; Misovic, A.; Oehlmann, J.; et al. Removal of endocrine disrupting chemicals in wastewater by enzymatic treatment with fungal laccases. Org. Process Res. Dev. 2017, 21, 480–491. [Google Scholar] [CrossRef]
- Xiao, F.; Xiao, P.; Jiang, W.; Wang, D. Immobilization of horseradish peroxidase on Fe3O4 nanoparticles for enzymatic removal of endocrine disrupting chemicals. Environ. Sci. Pollut. Res. 2020, 27, 24357–24368. [Google Scholar] [CrossRef]
- Maryškova, M.; Schaabová, M.; Tománková, H.; Novotný, V.; Rysová, M. Wastewater treatment by novel polyamide/polyethylenimine nanofibers with immobilized laccase. Water 2020, 12, 588. [Google Scholar] [CrossRef]
- Bayramoglu, G.; Karagoz, B.; Arica, M.Y. Cyclic-carbonate functionalized polymer brushes on polymeric microspheres: Immobilized laccase for degradation of endocrine disturbing compounds. J. Ind. Eng. Chem. 2018, 60, 407–417. [Google Scholar] [CrossRef]

| EDCs | Concentration | Occurrence | Sampling Period | References |
|---|---|---|---|---|
| Estrone | 10.4 ng/L | Leca River (Portugal) | Spring to autumn | [4] |
| 17β-Estradiol | 5.9 ng/L | Leca River (Portugal) | Spring to autumn | [4] |
| Estriol | 4.4 ng/L | Leca River (Portugal) | Spring to autumn | [4] |
| Estrone | 1.27 ng/L | Wuluo River (Taiwan) | December to May | [12] |
| 17β-Estradiol | 313.60 ng/L | Wuluo River (Taiwan) | December to May | [12] |
| Estriol | 210.00 ng/L | Wuluo River (Taiwan) | December to May | [12] |
| 17β-Estradiol | 40–117 ng/L | Surface water of Taihu Lake, China | May | [13] |
| 17β-Estradiol | 9.78–151 ng/L | Sediments of Taihu Lake, China | May | [13] |
| 17β-Estradiol | 0.076–0.233 ng/L | Lake Balaton, Hungary | No data | [14] |
| 17α-Ethynyl-estradiol | 0.68 ng/L | River Zala, Hungary | No data | [14] |
| Estrogen | 3.22–20.61 ng/L | Yangtze estuary, China | Four seasons | [15] |
| Progesterone | 20.4–439 ng/L | Themi River, Tanzania | March | [16] |
| Progesterone | 16,689 ng/L | Kalansanan River, Malaysia | No data | [16] |
| Androstenedione | 40 ng/L | Fenholloway River, USA | May and December | [17] |
| Progesterone | 2060 ng/L | Fenholloway River, USA | May and December | [17] |
| 17α-Ethynyl-estradiol | 981–4390 ng/L | Atibaia River, Brazil | High rainy periods | [18] |
| 17α-Ethynyl-estradiol | 17–501 ng/L | Atibaia River, Brazil | Dry winter period | [18] |
| 17β-Estradiol | 464–6806 ng/L | Atibaia River, Brazil | High rainy periods | [18] |
| 17β-Estradiol | 106–2273 ng/L | Atibaia River, Brazil | Dry winter period | [18] |
| Estrone | 16 ng/L | Atibaia River, Brazil | High rainy periods and dry winter period | [18] |
| Progesterone | 20 ng/L | Atibaia River, Brazil | High rainy periods | [18] |
| Progesterone | 20–195 ng/L | Atibaia River, Brazil | Dry winter period | [18] |
| Levonorgestrel | 19 ng/L | Atibaia River, Brazil | High rainy periods | [18] |
| Levonorgestrel | 19–663 ng/L | Atibaia River, Brazil | Dry winter period | [18] |
| 4-Octylphenol | 21 ng/L | Atibaia River, Brazil | High rainy periods and dry winter period | [18] |
| 4-Nonylphenol | 18 ng/L | Atibaia River, Brazil | High rainy periods and dry winter period | [18] |
| Testosterone | 1.9–2.7 ng/L | Lower Jordan River | May | [19] |
| Testosterone | 1.0–3.8 ng/L | Lower Jordan River | October | [19] |
| Estrogen | 0.6–2.2 ng/L | Lower Jordan River | May | [19] |
| Estrogen | 1.2–3.4 ng/L | Lower Jordan River | October | [19] |
| Estriol | 0.9–1.8 ng/L | Lower Jordan River | May | [19] |
| Estriol | 0.9–2.9 ng/L | Lower Jordan River | October | [19] |
| Organism | EDCs | Dose | Efficiency [%] | References |
|---|---|---|---|---|
| Virgibacillus halotolerans | 17β-estradiol | 5 mg/L | 100 | [3] |
| Bacillus flexus | 17β-estradiol | 5 mg/L | 100 | [3] |
| Bacillus licheniformis | 17β-estradiol | 5 mg/L | 100 | [3] |
| Novosphingobium sp. ARI-1 | estrone | 1.75 µg/L | 80.43 | [4] |
| estriol | 1.52 µg/L | 100 | [4] | |
| 17β-estradiol | 0.71 µg/L | 94.76 | [4] | |
| Rhodococcus sp. JX-2 | 17β-estradiol | 30 mg/L | 94 | [6] |
| Rhodococcus zopfii | 17α-ethynylestradiol | 2 mg/L | 86.5 | [7] |
| Pseudomonas putida F1 | 17α-ethynylestradiol | 0.5 mg/L | 73.8 | [7] |
| Acinetobacter sp. DSSKY-A-001 | 17β-estradiol | 40 mg/L | 90 | [10] |
| Acinetobacter sp. LHJ1 | 17β-estradiol | 0.5 mg/L | 100 | [10] |
| Acinetobacter sp. BP8 | 17β-estradiol | 1.8 mg/L | 100 | [10] |
| Acinetobacter sp. BP10 | 17β-estradiol | 5 mg/L | 100 | [10] |
| Novosphingobium sp. SLCC | estrone | 1 mM | no data | [12] |
| Sphingomonas sp. KC8 | estrone | 0.05 mg/L | 100 | [25] |
| 17β-estradiol | 0.05 mg/L | 100 | [25] | |
| testosterone | 0.05 mg/L | 100 | [25] | |
| Thauera sp. GDN1 | testosterone | 1 mM | no data | [28] |
| Rhodococcus pyridinivorans K408 | zearalenone | 5 mg/L | 87.21 | [31] |
| Bacillus sp. | 17β-estradiol | 0.2 mg/L | 91.70 | [33] |
| Aeromonas punctata | 17β-estradiol | 0.2 mg/L | 94.20 | [33] |
| Klebsiella sp | 17β-estradiol | 0.2 mg/L | 100 | [33] |
| Enterobacter sp. I | 17β-estradiol | 0.2 mg/L | 77.10 | [33] |
| Enterobacter sp. II | 17β-estradiol | 0.2 mg/L | 85.40 | [33] |
| Aeromonas veronii | 17β-estradiol | 0.2 mg/L | 93.40 | [33] |
| Acinetobacter sp. LM1 | 17β-estradiol | 5 mg/L | 77.00 | [34] |
| Pseudomonas sp. LY1 | 17β-estradiol | 5 mg/L | 68.00 | [34] |
| Sphingomonas sp. MCCC 1A06484 | 17β-estradiol | 1.8 mg/L | 78.70–98.80 | [35] |
| Comamonas testosterone ATCC1199 | testosterone | 0.5 mM | 60.00 | [36] |
| 17β-estradiol | 0.5 mM | 35.00 | [36] | |
| estrone | 0.5 mM | 45.00 | [36] | |
| estriol | 0.5 mM | 25.00 | [36] | |
| Comamonas testosteroniQYY20150409(CT) | 17β-estradiol | 1 mg/L | 76.00 | [37] |
| Rhodococcus equi DSSKP-R-001(RS) | 17β-estradiol | 1 mg/L | 86.00 | [37] |
| Deinococcus actinosclerus SJTR1 | 17β-estradiol | 10 mg/L | 90.00 | [38] |
| Stenotrophomonas maltophilia SJTL3 | 17β-estradiol | 10 mg/L | 90.00 | [39] |
| Raphidocelis subcapitata | 17β-estradiol | 1.5 mg/L | 74.60 | [11] |
| diethylstilbestrol | 1.5 mg/L | 54.10 | [11] | |
| Chlorella vulgaris | testosterone | 0.2 mg/L | 69.64 | [26] |
| Chlorella vulgaris | nonylphenol | 1 mg/L | 93.00 | [41] |
| Trichoderma citrinoviride AJAC3 | 17β-estradiol | 200 mg/L | 99.60 | [22] |
| Trametes versicolo | dimethyl phthalate | 100 µM | 60.00 | [30] |
| methyl paraben | 100 µM | 100.00 | [30] | |
| butyl paraben | 100 µM | 100.00 | [30] | |
| nonylphenol | 100 µM | 85.00 | [30] | |
| bisphenol A | 100 µM | 100.00 | [30] | |
| Pleurotus ostreatus | dimethyl phthalate | 100 µM | 50.00 | [30] |
| methyl paraben | 100 µM | 100.00 | [30] | |
| butyl paraben | 100 µM | 98.00 | [30] | |
| nonylphenol | 100 µM | 68.00 | [30] | |
| bisphenol A | 100 µM | 60.00 | [30] | |
| Phanerochaete chrysosporium | dimethyl phthalate | 100 µM | 65.00 | [30] |
| methyl paraben | 100 µM | 69.00 | [30] | |
| butyl paraben | 100 µM | 58.00 | [30] | |
| nonylphenol | 100 µM | 69.00 | [30] | |
| bisphenol A | 100 µM | 66.00 | [30] | |
| Aspergillus brasiliensis | progesterone | 10 mg/L | no data | [42] |
| Absidia coerulea VKM F-833 | androst-4-ene-3,17-dione | 1 g/L | 29.00 | [43] |
| Beauveria bassiana VKM F-2533 | androst-4-ene-3,17-dione | 1 g/L | 65.00 | [43] |
| androsta-1,4-diene-3,17-dion | 1 g/L | 30.00 | [43] | |
| Drechslera sp. Ph F-34 | androst-4-ene-3,17-dione | 1 g/L | 4.00 | [43] |
| androsta-1,4-diene-3,17-dion | 1 g/L | 8.00 | [43] | |
| testosterone | 1 g/L | 100.00 | [43] | |
| Gibberella zeae VKMF-2600 | androsta-1,4-diene-3,17-dion | 1 g/L | 7.00 | [43] |
| Cunninghamella echinulata VKM F-439 | androst-4-ene-3,17-dione | 1 g/L | 6.00 | |
| Lentinula edodes | testosterone | 200 mg/L | no data | [44] |
| 17α-ethynylestradiol | 0.8 mg/L | no data | [44] | |
| Aspergillus nidulans VKPM F-1069 | progesterone | 1 g/L | no data | [45] |
| Circinella muscae | progesterone | 7 g/L | no data | [46] |
| testosterone enanthate | 7 g/L | no data | [46] |
| Immobilization Matrix/Technology | Pros and Cons of Matrix/Technology | Microorganism/Enzyme | EDCs | References |
|---|---|---|---|---|
| Alginate/CaCl2 | + increased degradation efficiency and/or enzyme activity + better drug tolerance + improved thermal stability of enzymes + low cost + non-toxic carrier + simple technology + favourable mass transfer characteristics +/− high mechanical stability − poor recycling and reuse properties − risk of pore occupation and diffusion limitation during system reuse − unstable in the presence of phosphate − possibility of compound sorption on/in carrier | Novosphingobium sp. ARI-1 Rhodococcus sp. JX-2 Chlorella vulgaris Cyberlindnera fabianii (laccase) | estrone estriol 17β-estradiol 17β-estradiol nonylphenol bisphenol A | [4] [6] [41] [66] |
| Alginate/CaCl2 + α-cyclodextrin (α-CD) | + better degradation efficiency compared to non-α-CD alginate beads + better drug tolerance + low cost + simple technology + favourable mass transfer characteristics + high mechanical stability +/− strong affinity for nonylphenol − use of toxic compounds during process (e.g., cyanogen bromide) − poor recycling and reuse properties − degradation slower than freely-suspended cells − risk of pore occupation and diffusion limitation during system reuse | Sphingomonas cloacae | nonylphenol | [32] |
| possibility of compound sorption on/in carrier | ||||
| Encapsulation in small bioreactor platform (SBP) | + better biodegradation capabilities + high physically separates + high permeability cellulose acetate microfiltration membrane + non-toxic carrier + relatively simple technology + low carrier absorption level | Rhodococcus zopfii Pseudomonas putida F1 | 17α-ethynylestradiol 17α-ethynylestradiol | [7] |
| Loofah (Luffa spp.) sponge | + natural carrier + low cost + simple technology + biodegradable carrier + high porosity − poor reuse properties − low mechanical durability | Pleurotus ostreatus (laccase) | 17α-ethinylestradiol | [70] |
| Chitosan/glutaraldehyde | + good stability + possibility to reuse system + good mechanical durability + increased enzyme stability during storage + increased enzyme thermal stability + low cost carrier + simple technology + non-toxic carrier | Trametes versicolor (laccase) | bisphenol-A | [67] |
| Cellulose triacetate | + high hydrate permeation + high mechanical durability + high porosity + biodegradability potential − slight degradation slower than freely-suspended cells | Sphingomonas sp. AHC-F Sphingobium sp. AX-B | 17β-estradiol estrone 17β-estradiol estrone | [68] |
| Titania nanoparticles | + low price + good chemical stability + good biocompatibility + chemical coordination ability + high reuse potential + great carrier modification potential + increased enzyme stability in various pH + increased enzyme stability at various temperatures | Pleurotus ostreatus (laccase) | bisphenol A carbamazepine | [71] |
| MANAE-agarose | + great reuse potential + increased degradation efficiency + increased enzyme stability during storage + increased enzyme thermal stability + simple technology + low cost + effective against surface carboxyl group particles + increased degradation efficiency | Pleurotus ostreatus (laccase) | bisphenol A | [72] |
| Acrylic beads (IB-EC-1) | + good enzyme absorption on carrier + possibility to use low enzyme amount | Trametes versicolor (laccase) Myceliophthora thermophila (laccase) | steroids and EDCs mixture 1 | [74] |
| Ceramic membrane | + good enzyme absorption on carrier − very low degradation efficiency | Trametes versicolor (laccase) | steroids and EDCs mixture 1 | [74] |
| Fe3O4 nanoparticles | + possibility to simple carrier recycling in magnetic field + high reuse possibilities + increased degradation efficiency + low toxicity + good biocompatibility + increased enzyme stability during storage + increased enzyme thermal stability | horseradish peroxidase | bisphenol-A 17α-ethinylestradiol | [75] |
| Polyamide/Polyethylenimine Nanofibers | + simple technology + low toxicity + high stability in the environment + safe and easy to handle + presence of plenty of surface amino groups + increased enzyme stability during storage | Trametes versicolor (laccase) | bisphenol A 17α-ethinylestradiol triclosan | [76] |
| Cyclic carbonate group on polymeric microspheres [PS-co-DVB-g-P(CCMA)] | + high amount of enzyme binding sites on carrier (cyclic epoxy and cyclic carbonate groups) + wider pH and temperature range of enzyme activity + increased enzyme thermal stability + increased enzyme stability during storage − advanced carrier synthesis − use of toxic compounds during process | Trametes versicolor (laccase) | bisphenol-A | [77] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wojcieszyńska, D.; Marchlewicz, A.; Guzik, U. Suitability of Immobilized Systems for Microbiological Degradation of Endocrine Disrupting Compounds. Molecules 2020, 25, 4473. https://doi.org/10.3390/molecules25194473
Wojcieszyńska D, Marchlewicz A, Guzik U. Suitability of Immobilized Systems for Microbiological Degradation of Endocrine Disrupting Compounds. Molecules. 2020; 25(19):4473. https://doi.org/10.3390/molecules25194473
Chicago/Turabian StyleWojcieszyńska, Danuta, Ariel Marchlewicz, and Urszula Guzik. 2020. "Suitability of Immobilized Systems for Microbiological Degradation of Endocrine Disrupting Compounds" Molecules 25, no. 19: 4473. https://doi.org/10.3390/molecules25194473
APA StyleWojcieszyńska, D., Marchlewicz, A., & Guzik, U. (2020). Suitability of Immobilized Systems for Microbiological Degradation of Endocrine Disrupting Compounds. Molecules, 25(19), 4473. https://doi.org/10.3390/molecules25194473