The Uptake of Ivermectin and Its Effects in Roots, Leaves and Seeds of Soybean (Glycine max)
Abstract
1. Introduction
2. Results and Discussion
2.1. Effect of IVM on Soybean Growth and Yield
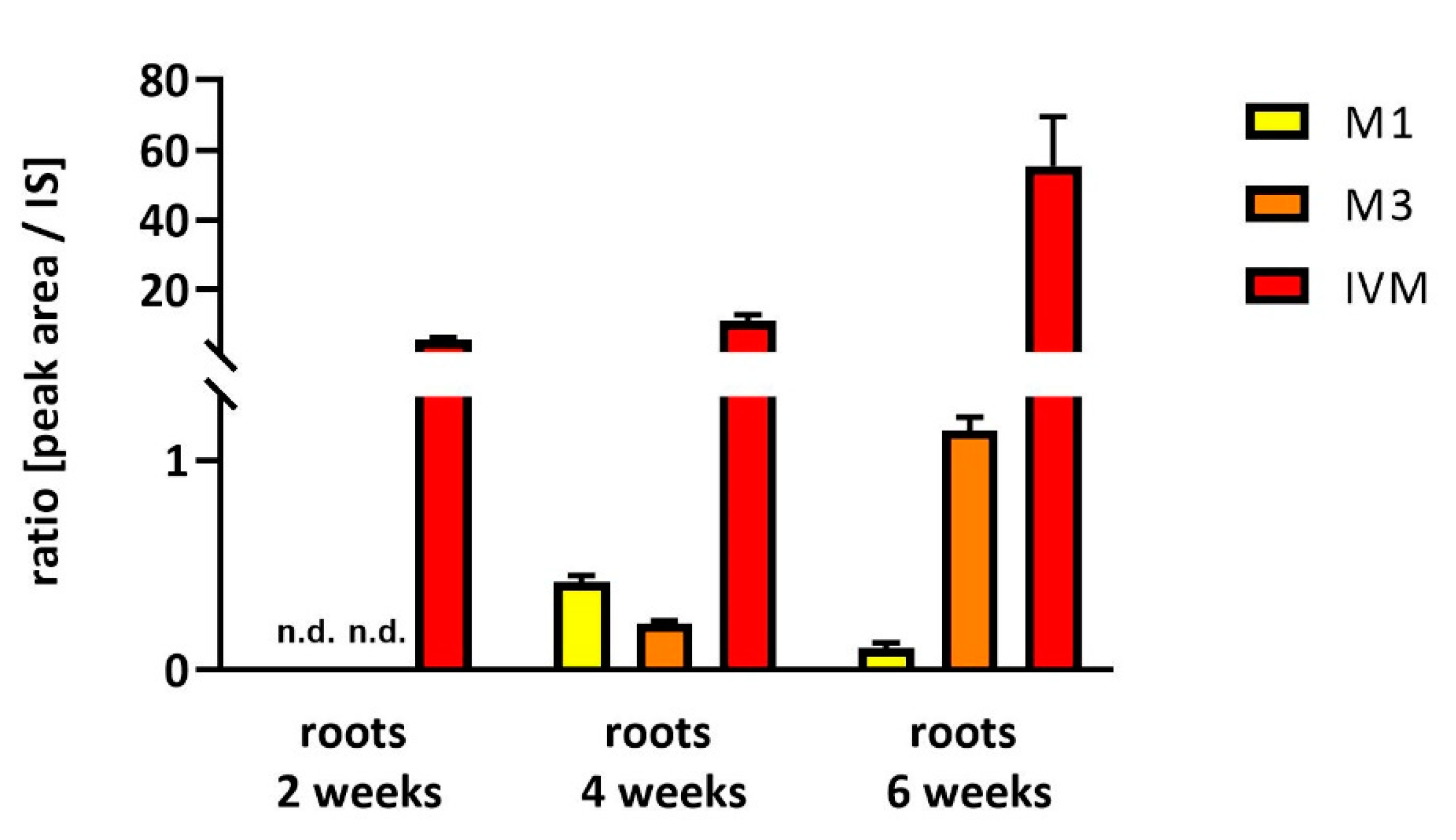
2.2. Uptake and Metabolism of IVM in Soybeans
2.3. The Effect of IVM on Antioxidant Enzyme Activities in Soybeans
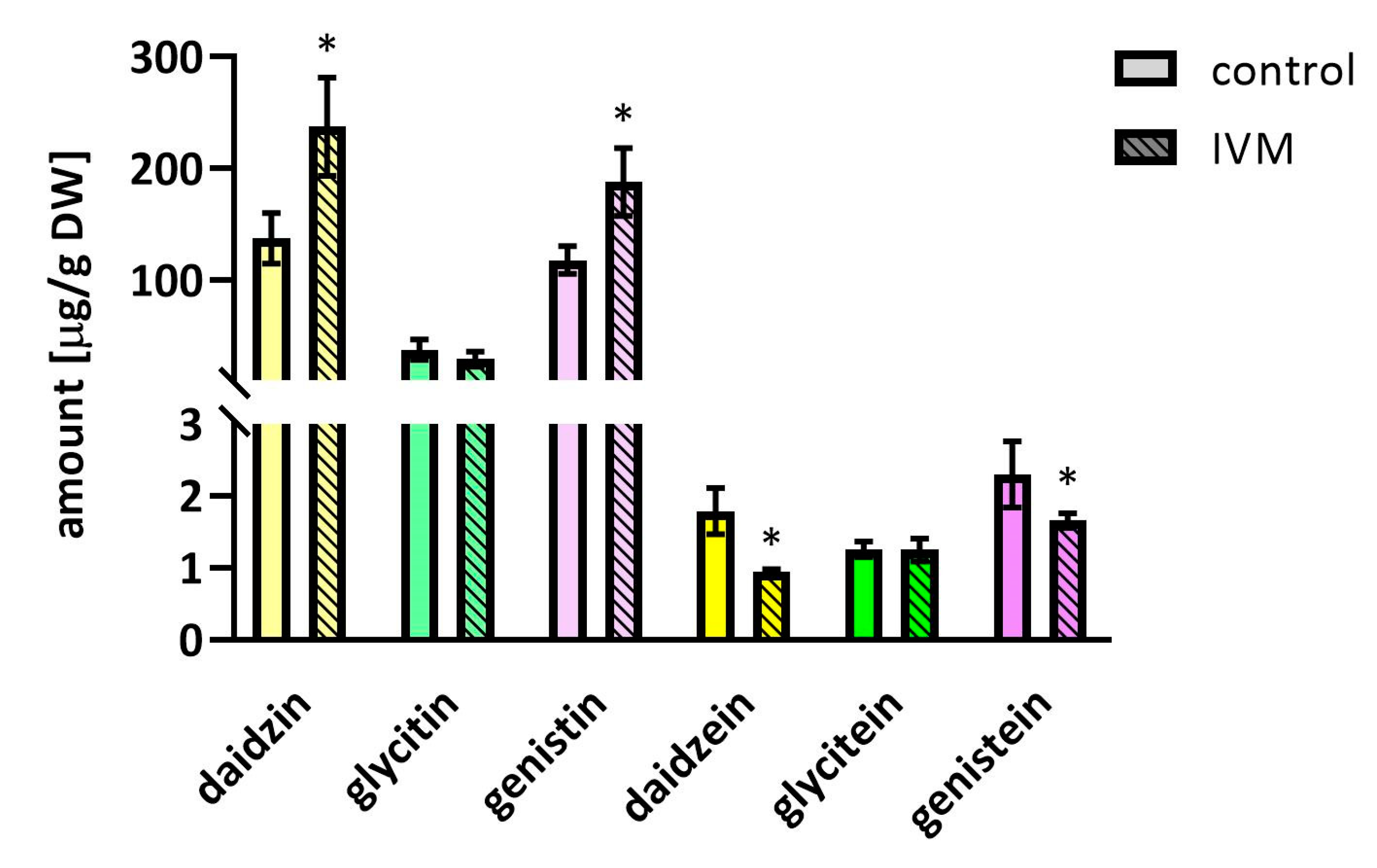
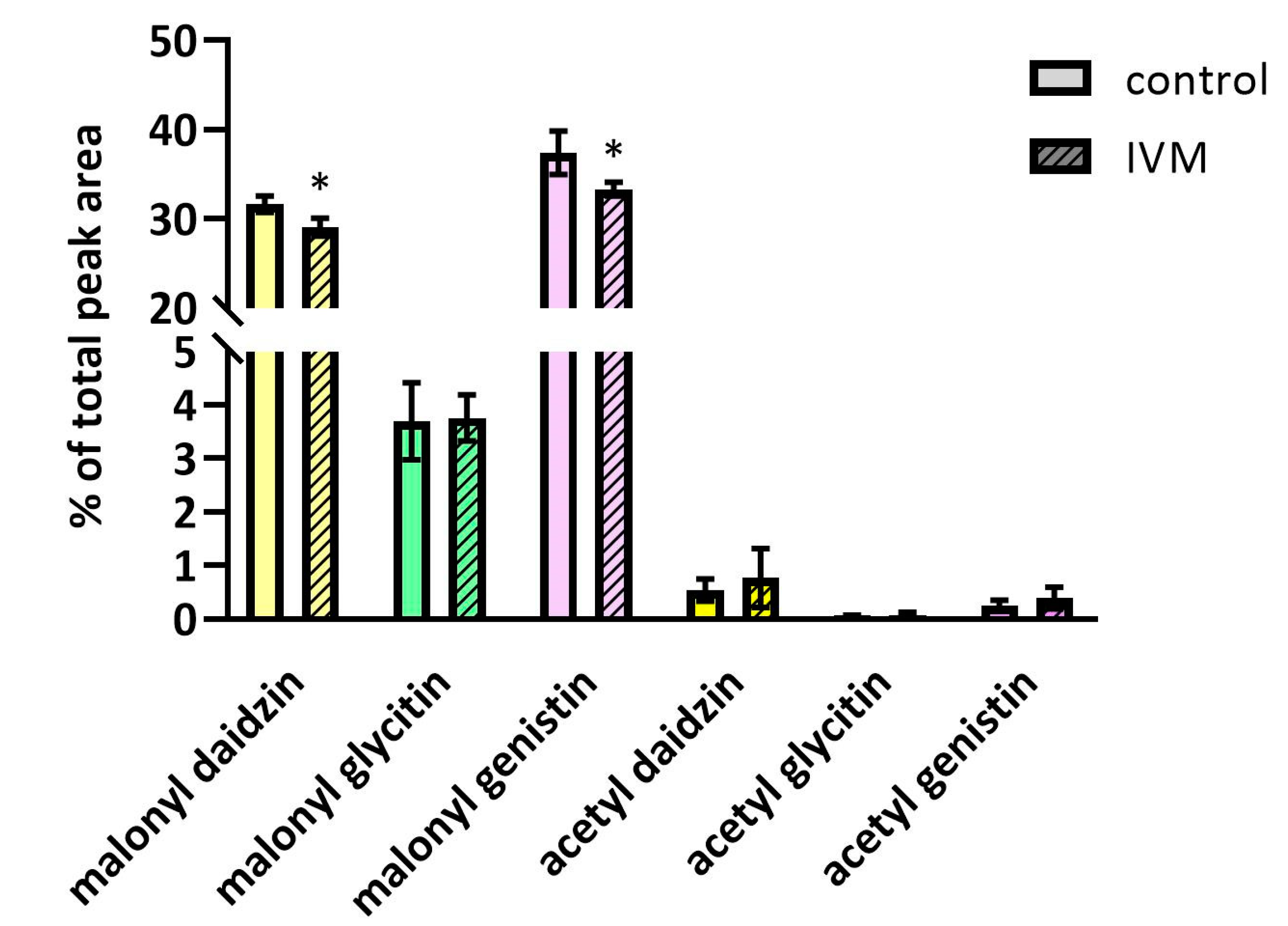
2.4. The Effect of IVM on Isoflavones Content in Soybeans
3. Materials and Methods
3.1. Cultivation of Soybean Plants in a Greenhouse
3.2. In Vitro Cultivation of Soybean Plants
3.3. HPLC Analysis of Isoflavones
3.4. UHPLC-MS/MS Analysis
3.5. Antioxidant Enzymes Activity Assay
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| UHPLC–MS/MS | Ultra-high performance liquid chromatography tandem mass spectrometry |
| SOD | superoxide dismutase |
| CAT | catalase |
| GST | glutathione S-transferase |
| APX | ascorbate peroxidase |
| POX | guaiacol-specific peroxidase |
| ESI | electrospray ionization |
| DMSO | dimethyl sulfoxide |
| MRM | multiple reaction monitoring |
| GSH | glutathione |
| GSSG | glutathione disulfide |
| NADPH | nicotinamide adenine dinucleotide phosphate |
| ANOVA | Analysis of variance |
References
- Bártíková, H.; Podlipna, R.; Skálová, L. Veterinary drugs in the environment and their toxicity to plants. Chemosphere 2016, 144, 2290–2301. [Google Scholar] [CrossRef]
- Low Greenhouse Gas Agriculture. Available online: https://orgprints.org/15690/1/niggli-etal-2009-lowgreenhouse.pdf (accessed on 10 August 2020).
- Hamscher, G.; Bachour, G. Veterinary Drugs in the Environment: Current Knowledge and Challenges for the Future. J. Agric. Food Chem. 2018, 66, 751–752. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, P.N.; Basto, M.C.P.; Almeida, C.M.R.; Brix, H. A review of plant–pharmaceutical interactions: From uptake and effects in crop plants to phytoremediation in constructed wetlands. Environ. Sci. Pollut. Res. 2014, 21, 11729–11763. [Google Scholar] [CrossRef] [PubMed]
- Madikizela, L.M.; Ncube, S.; Chimuka, L. Uptake of pharmaceuticals by plants grown under hydroponic conditions and natural occurring plant species: A review. Sci. Total Environ. 2018, 636, 477–486. [Google Scholar] [CrossRef]
- Marsik, P.; Podlipna, R.; Vaněk, T. Study of praziquantel phytoremediation and transformation and its removal in constructed wetland. J. Hazard. Mater. 2017, 323, 394–399. [Google Scholar] [CrossRef]
- Raisová, L.S.; Podlipná, R.; Szotáková, B.; Syslová, E.; Skálová, L. Evaluation of drug uptake and deactivation in plant: Fate of albendazole in ribwort plantain (Plantago laceolata) cells and regenerants. Ecotoxicol. Environ. Saf. 2017, 141, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Chitescu, C.L.; Nicolau, A.I.; Römkens, P.; Van Der Fels-Klerx, H.J. Quantitative modelling to estimate the transfer of pharmaceuticals through the food production system. J. Environ. Sci. Heal. Part B 2014, 49, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, L.E.; Saumell, C.; Sagüés, F.; Sallovitz, J.M.; Lifschitz, A. Ivermectin dissipation and movement from feces to soil under field conditions. J. Environ. Sci. Heal. Part B 2017, 53, 42–48. [Google Scholar] [CrossRef]
- Vokral, I.; Michaela, Š.; Radka, P.; Jiří, L.; Lukáš, P.; Dominika, S.; Kateřina, L.; Barbora, S.; Lenka, S. Ivermectin environmental impact: Excretion profile in sheep and phytotoxic effect in Sinapis alba. Ecotoxicol. Environ. Saf. 2019, 169, 944–949. [Google Scholar] [CrossRef] [PubMed]
- Syslová, E.; Landa, P.; Navrátilová, M.; Stuchlíková, L.R.; Matoušková, P.; Skálová, L.; Szotáková, B.; Vaněk, T.; Podlipná, R. Ivermectin biotransformation and impact on transcriptome in Arabidopsis thaliana. Chemosphere 2019, 234, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Horvat, A.; Babic, S.; Pavlović, D.M.; Ašperger, D.; Pelko, S.; Kaštelan-Macan, M.; Petrovic, M.; Mance, A. Analysis, occurrence and fate of anthelmintics and their transformation products in the environment. TrAC Trends Anal. Chem. 2012, 31, 61–84. [Google Scholar] [CrossRef]
- Jaeger, L.H.; Carvalho-Costa, F.A. Status of benzimidazole resistance in intestinal nematode populations of livestock in Brazil: A systematic review. BMC Veter Res. 2017, 13, 358. [Google Scholar] [CrossRef] [PubMed]
- Geerts, S.; Gryseels, B. Drug resistance in human helminths: Current situation and lessons from livestock. Clin. Microbiol. Rev. 2000, 13, 207–222. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.; William, S.; Day, T.A.; Botros, S.; Tao, L.F.; Bennett, J.L.; Farghally, A.; Metwally, A. Resistance to praziquantel: Direct evidence from Schistosoma mansoni isolated from Egyptian villagers. Am. J. Trop. Med. Hyg. 1999, 60, 932–935. [Google Scholar] [CrossRef]
- Vamanu, E.; Gatea, F. Correlations between Microbiota Bioactivity and Bioavailability of Functional Compounds: A Mini-Review. Biomedicines 2020, 8, 39. [Google Scholar] [CrossRef]
- Svobodníková, L.; Kummerová, M.; Zezulka, Š.; Babula, P. Possible use of a Nicotiana tabacum ‘Bright Yellow 2′ cell suspension as a model to assess phytotoxicity of pharmaceuticals (diclofenac). Ecotoxicol. Environ. Saf. 2019, 182, 109369. [Google Scholar] [CrossRef]
- Singh, V.; Pandey, B.; Suthar, S. Phytotoxicity and degradation of antibiotic ofloxacin in duckweed (Spirodela polyrhiza) system. Ecotoxicol. Environ. Saf. 2019, 179, 88–95. [Google Scholar] [CrossRef]
- Singh, V.; Pandey, B.; Suthar, S. Phytotoxicity of amoxicillin to the duckweed Spirodela polyrhiza: Growth, oxidative stress, biochemical traits and antibiotic degradation. Chemosphere 2018, 201, 492–502. [Google Scholar] [CrossRef]
- Riaz, L.; Mahmood, T.; Coyne, M.; Khalid, A.; Rashid, A.; Hayat, M.T.; Gulzar, A.; Amjad, M. Physiological and antioxidant response of wheat (Triticum aestivum) seedlings to fluoroquinolone antibiotics. Chemosphere 2017, 177, 250–257. [Google Scholar] [CrossRef]
- Stuchlíková, L.R.; Skálová, L.; Szotáková, B.; Syslová, E.; Vokral, I.; Vaněk, T.; Podlipná, R. Biotransformation of flubendazole and fenbendazole and their effects in the ribwort plantain (Plantago lanceolata). Ecotoxicol. Environ. Saf. 2018, 147, 681–687. [Google Scholar] [CrossRef]
- Gutierrez-Gonzalez, J.J.; Guttikonda, S.K.; Tran, S.P.-L.; Aldrich, D.L.; Zhong, R.; Yu, O.; Nguyen, H.T.; Sleper, D.A. Differential Expression of Isoflavone Biosynthetic Genes in Soybean During Water Deficits. Plant Cell Physiol. 2010, 51, 936–948. [Google Scholar] [CrossRef] [PubMed]
- Lozovaya, V.V.; Lygin, A.V.; Ulanov, A.V.; Nelson, R.L.; Dayde, J.; Widholm, J.M. Effect of Temperature and Soil Moisture Status during Seed Development on Soybean Seed Isoflavone Concentration and Composition. Crop. Sci. 2005, 45, 1934–1940. [Google Scholar] [CrossRef]
- Syslová, E.; Landa, P.; Stuchlíková, L.R.; Matoušková, P.; Skálová, L.; Szotáková, B.; Navrátilová, M.; Vaněk, T.; Podlipná, R. Metabolism of the anthelmintic drug fenbendazole in Arabidopsis thaliana and its effect on transcriptome and proteome. Chemosphere 2019, 218, 662–669. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.J.; An, C.H.; Park, S.-C.; Pyun, J.W.; Lee, J.-Y.; Kim, S.W.; Kim, H.-S.; Kim, H.; Jeong, J.C.; Kim, C.Y. Methyl Jasmonate Increases Isoflavone Production in Soybean Cell Cultures by Activating Structural Genes Involved in Isoflavonoid Biosynthesis. J. Agric. Food Chem. 2018, 66, 4099–4105. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, Y.; Tsurugasaki, W.; Nakamura, S.; Osada, K. Comparison of regulative functions between dietary soy isoflavones aglycone and glucoside on lipid metabolism in rats fed cholesterol. J. Nutr. Biochem. 2005, 16, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Gamborg, O.; Miller, R.; Ojima, K. Nutrient requirements of suspension cultures of soybean root cells. Exp. Cell Res. 1968, 50, 151–158. [Google Scholar] [CrossRef]
- Lee, Y.-W.; Kim, J.-D.; Zheng, J.; Row, K.H. Comparisons of isoflavones from Korean and Chinese soybean and processed products. Biochem. Eng. J. 2007, 36, 49–53. [Google Scholar] [CrossRef]
- Liu, J.; Hu, B.; Liu, W.; Qin, W.; Wu, H.; Zhang, J.; Yang, C.; Deng, J.; Shu, K.; Du, J.; et al. Metabolomic tool to identify soybean [Glycine max (L.) Merrill] germplasms with a high level of shade tolerance at the seedling stage. Sci. Rep. 2017, 7, 42478. [Google Scholar] [CrossRef]
- Szymczak, G.; Wójciak, M.; Sowa, I.; Zapała, K.; Strzemski, M.; Kocjan, R. Evaluation of isoflavone content and antioxidant activity of selected soy taxa. J. Food Compos. Anal. 2017, 57, 40–48. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Carlberg, I.; Mannervik, B. Glutathione-Reductase. In Methods in Enzymology; Academic Press: New York, NY, USA, 1985; Volume 113, pp. 484–490. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |


| tR [min] | m/z Values of [M + NH4]+ ions | Molecular Formula | Description of Metabolite Formation | Product ion [M + H]+, m/z | Metabolite Designation |
|---|---|---|---|---|---|
| 11.774 | 878 | C47H72O14 | -CH2 | 307, 551 | M1 |
| 12.422 | 908 | C48H74O15 | + O | 307, 567 | M2 |
| 12.483 | 908 | C48H74O15 | + O | 307, 567 | M3 |
| 13.096 | 908 | C48H74O15 | + O | 307, 567 | M4 |
| 13.929 | 892 | C48H78O14 | - | 137, 307, 569 | IVM |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navrátilová, M.; Raisová Stuchlíková, L.; Moťková, K.; Szotáková, B.; Skálová, L.; Langhansová, L.; Podlipná, R. The Uptake of Ivermectin and Its Effects in Roots, Leaves and Seeds of Soybean (Glycine max). Molecules 2020, 25, 3655. https://doi.org/10.3390/molecules25163655
Navrátilová M, Raisová Stuchlíková L, Moťková K, Szotáková B, Skálová L, Langhansová L, Podlipná R. The Uptake of Ivermectin and Its Effects in Roots, Leaves and Seeds of Soybean (Glycine max). Molecules. 2020; 25(16):3655. https://doi.org/10.3390/molecules25163655
Chicago/Turabian StyleNavrátilová, Martina, Lucie Raisová Stuchlíková, Kateřina Moťková, Barbora Szotáková, Lenka Skálová, Lenka Langhansová, and Radka Podlipná. 2020. "The Uptake of Ivermectin and Its Effects in Roots, Leaves and Seeds of Soybean (Glycine max)" Molecules 25, no. 16: 3655. https://doi.org/10.3390/molecules25163655
APA StyleNavrátilová, M., Raisová Stuchlíková, L., Moťková, K., Szotáková, B., Skálová, L., Langhansová, L., & Podlipná, R. (2020). The Uptake of Ivermectin and Its Effects in Roots, Leaves and Seeds of Soybean (Glycine max). Molecules, 25(16), 3655. https://doi.org/10.3390/molecules25163655








