Homology Modeling and Optimized Expression of Truncated IK Protein, tIK, as an Anti-Inflammatory Peptide
Abstract
1. Introduction
2. Results and Discussion
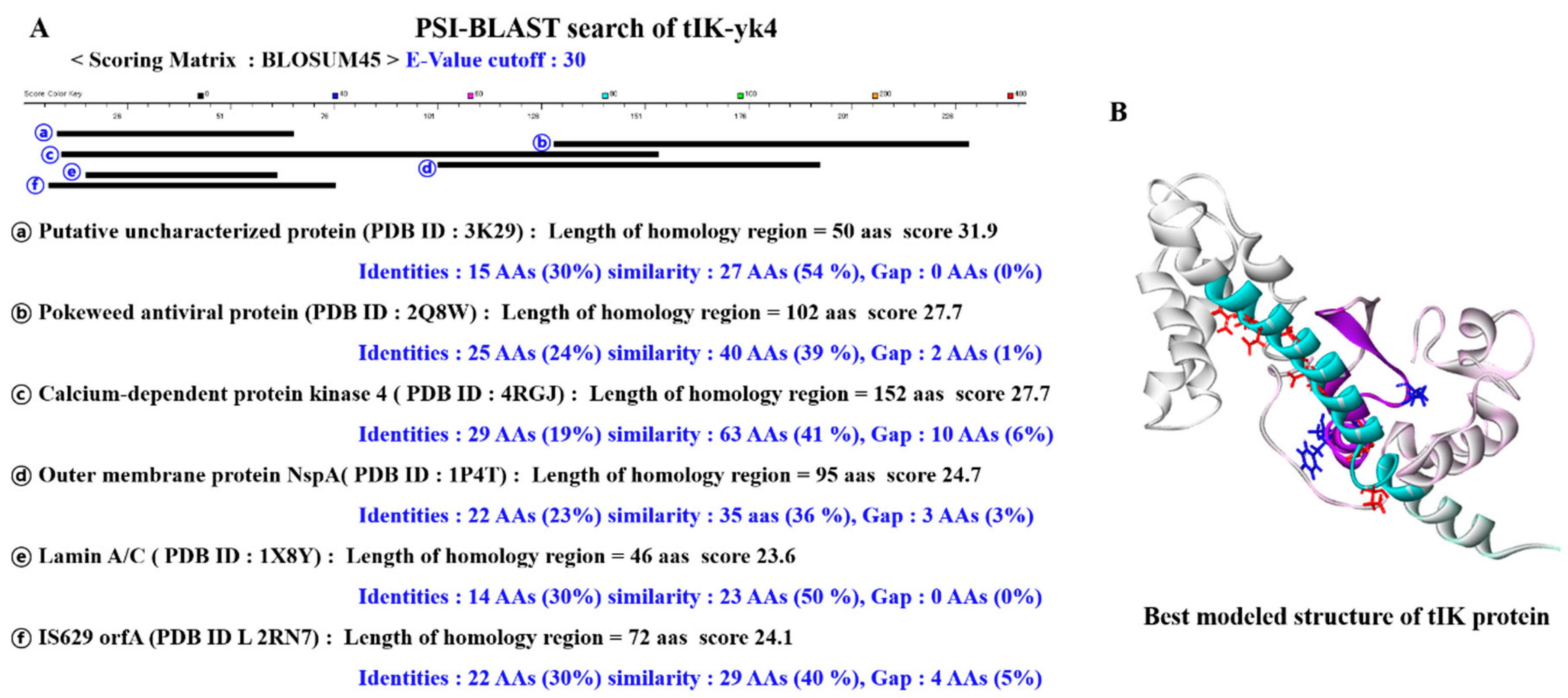
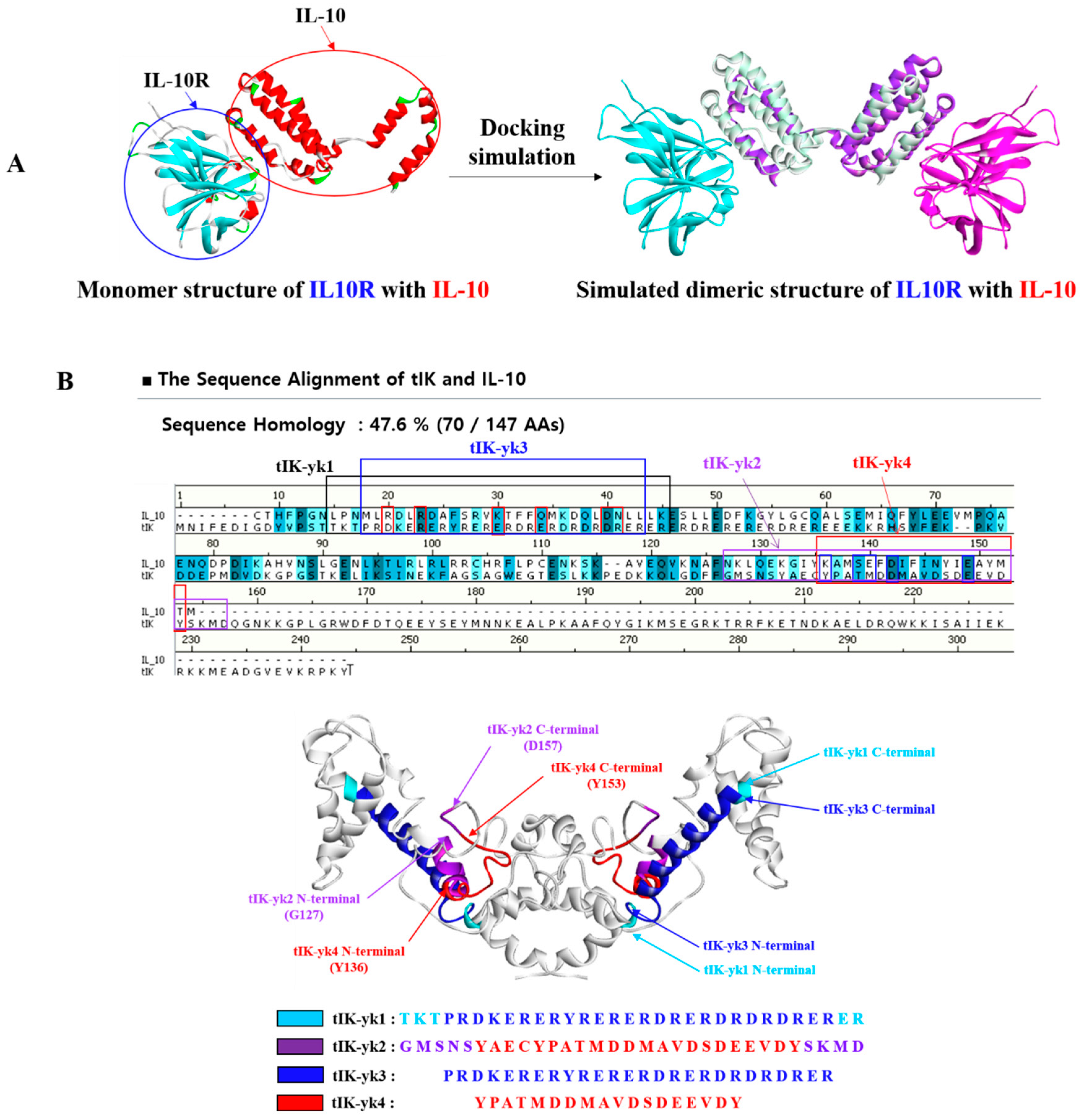
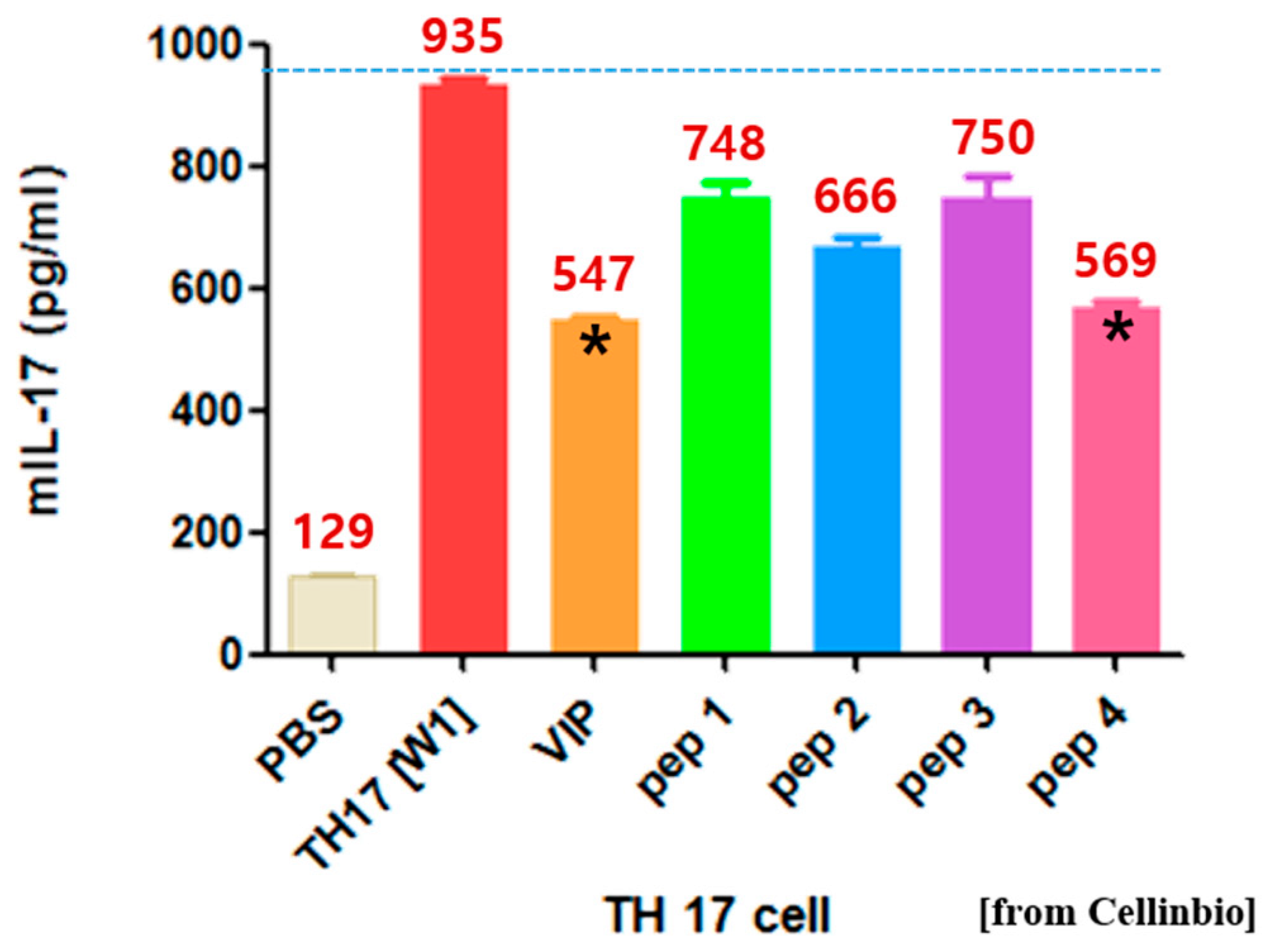
2.1. Proposal of tIK Epitope Sequence with Anti-Inflammatory Effect
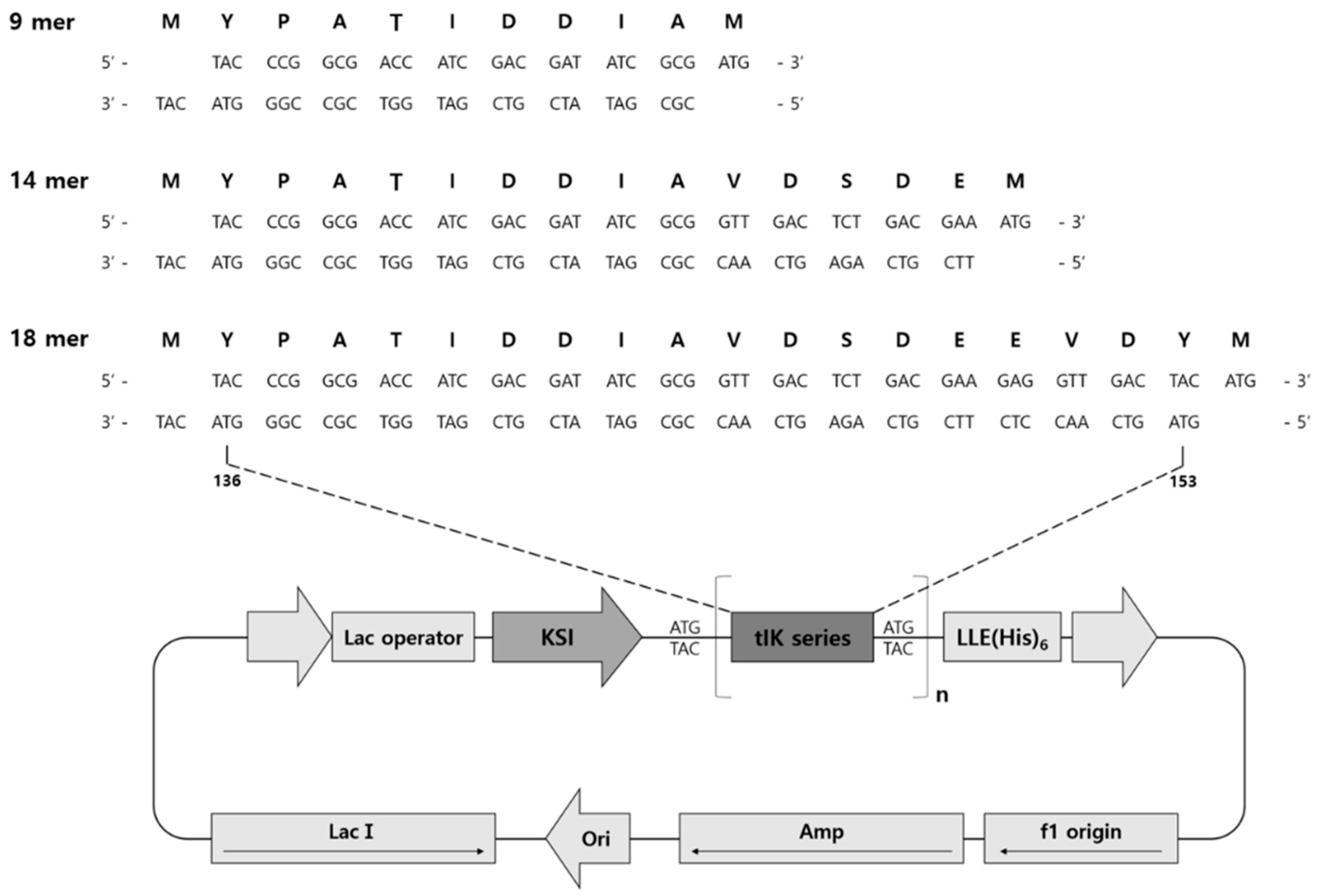
2.2. Construction of Expression Vectors
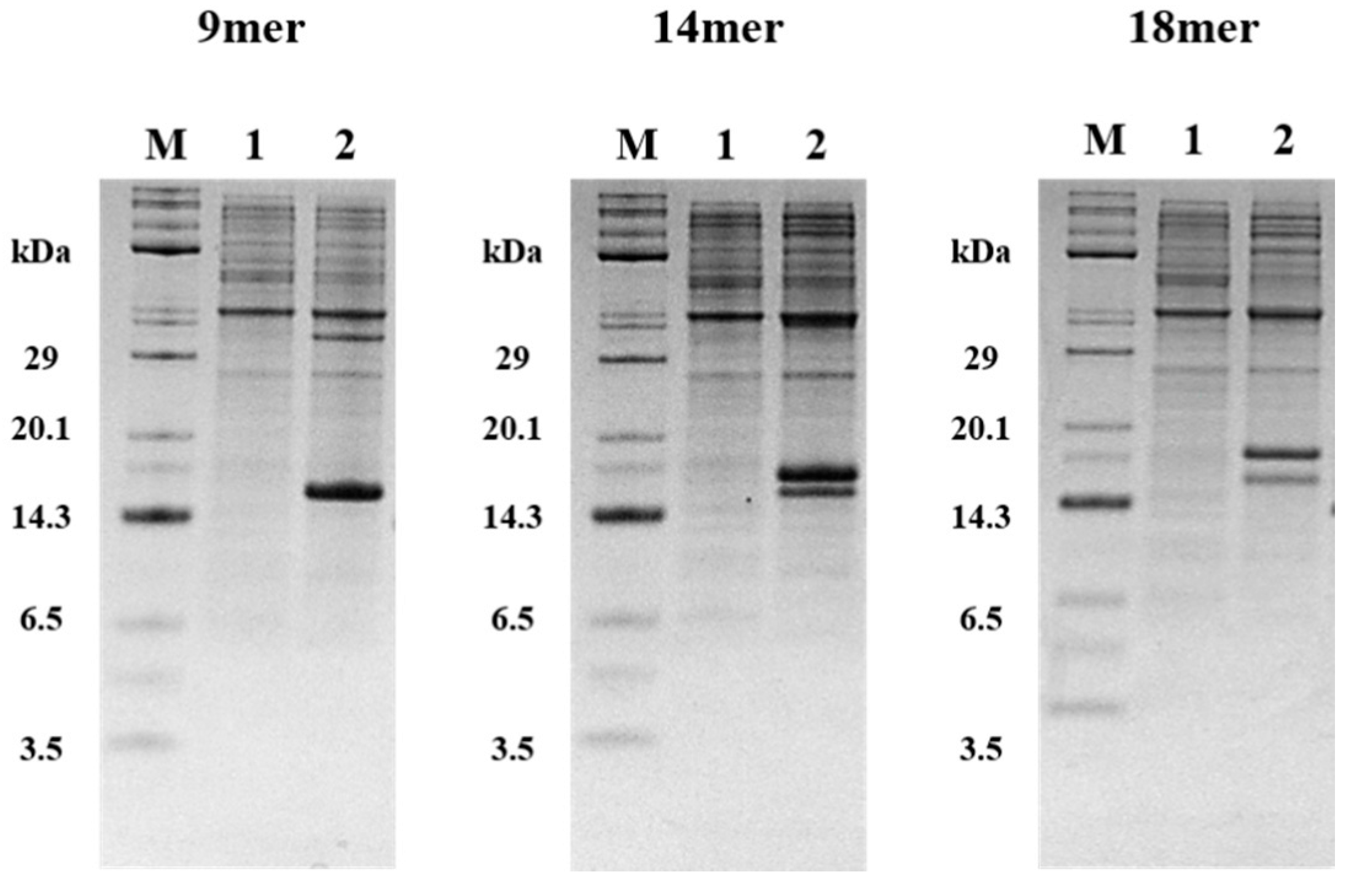
2.3. Expression of tIK-9mer, 14mer, 18mer
3. Materials and Methods
3.1. D Structure Prediction of tIK and Selection of tIK Epi Escherichia coli Tope
3.2. TH17 Cell Differentiation
3.3. Vector Construction
3.4. Expression of KSI-(tIK series)n-His6 Tag Fusion Protein
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| IK | Inhibitor K562 |
| tIK | truncated-IK |
| IL | Interleukin |
| IL-10Rα | Interleukin 10 receptor subunit alpha |
| TH17 | T helper 17 |
| MHC II | Major histocompatibility complex class II |
| IFN-γ | Interferon-γ |
| GPCR | G protein-coupled receptor |
| CVB3 | Coxsackievirus |
| JAK1 | Janus Kinase 1 |
| TYK2 | Tyrosine Kinase 2 |
| STAT3 | Signal transducer and activator of transcription 3 |
| TNF-α | Tumor Necrosis Factor-α |
| PSI BLAST | Position-Specific Iterative Basic Local Alignment Search Tool |
| MSA | Multiple Sequence Alignment |
| E-value | Expect-value |
| IPTG | Isopropyl β-d-1-thiogalactopyranoside |
| OD600 | Optical density 600 nm |
| SDS-PAGE | sodium dodecyl sulfate- Polyacrylamide gel electrophoresis |
| PSSM | Position-Specific Scoring Matrix |
| LB | Luria-Bertani |
| KSI | Ketosteroid isomerase |
References
- Miliani, L.F.; Nielsen, O.H.; Andersen, P.S.; Girardin, S.E. Chronic inflammation: Importance of NOD2 and NALP3 in interleukin-1β generation. J. Transl. Immunol. 2007, 147, 227–235. [Google Scholar] [CrossRef]
- Rajendran, P.; Chen, Y.F.; Chen, Y.F.; Chung, L.C.; Tamilselvi, S.; Shen, C.Y.; Day, C.H.; Chen, R.J.; Viswanadha, V.P.; Kuo, W.W.; et al. The multifaceted link between inflammation and human diseases. J. Cell. Physiol. 2018, 233, 6458–6471. [Google Scholar] [CrossRef] [PubMed]
- Jaturapatporn, D.; Isaac, M.G.; McCleery, J.; Tabet, N. Aspirin, steroidal and non-steroidal anti-inflammatory drugs for the treatment of Alzheimer’s disease. Cochrane Syst. Rev. Interv. 2012, 2, 1–15. [Google Scholar] [CrossRef]
- Neilsen, W.E.; Kaye, A.D. Steroids: Pharmacology, complication, and practice delivery issues. Ochsner J. 2014, 14, 203–207. [Google Scholar]
- Fujita, T.; Kutsumi, H.; Sanuki, T.; Hayakumo, T.; Azuma, T. Adherence to the preventive strategies for nonsteroidal anti-inflammatory drug- or low- dose aspirin-induced gastrointestinal injuries. J. Gastroenterol. 2013, 48, 559–573. [Google Scholar] [CrossRef] [PubMed]
- Fosgerau, K.; Hoffmann, T. Peptide therapeutics: Current status and future direction. Drug Discov. Today 2015, 20, 122–128. [Google Scholar] [CrossRef]
- Otvos, L.; Wade, J. Current challenges in peptide-based drug discovery. Front. Chem. 2014, 2, 62. [Google Scholar] [CrossRef]
- McGonagle, D.; Gibbon, W.; Emery, P. Classification of inflammatory arthritis by enthesitis. Lancet 1998, 352, 1137–1140. [Google Scholar] [CrossRef]
- Boissier, M.C.; Semerano, L.; Challal, S.; Kermanach, N.S.; Falgarone, G. Rheumatoid arthritis: From autoimmunity to synovitis and joint destruction. J. Autoimmun. 2012, 39, 222–228. [Google Scholar] [CrossRef]
- Arend, W.P. Cytokine imbalance in the pathogenesis of rheumatoid arthritis: The role of interleukin-1 receptor antagonist. Semin. Arthritis Rheum. 2001, 30, 1–6. [Google Scholar] [CrossRef]
- Dinarello, C.A. Anti-inflammatory agents: Present and future. Cell 2010, 140, 935–950. [Google Scholar] [CrossRef] [PubMed]
- Krief, P.; Augery-Bourget, Y.; Plaisance, S.; Merck, M.F.; Assier, E.; Tanchou, V.; Billard, M.; Bouchix, C.; Jasmin, C.; Azzarone, B. A new cytokine (IK) down-regulating HLA class II: Monoclonal antibodies, cloning and chromosome localization. Oncogene 1994, 9, 3449–3456. [Google Scholar]
- Choi, S.; Park, H.L.; Jung, S.Y.; Kim, E.K.; Cho, M.L.; Min, J.K.; Moon, S.J.; Lee, S.M.; Cho, J.H.; Lee, D.H.; et al. Therapeutic effect of exogenous truncated IK protein in inflammatory arthritis. Int. J. Mol. Sci. 2007, 18, 1976. [Google Scholar] [CrossRef] [PubMed]
- Lyer, S.S.; Cheng, G. Role of interleukin 10 transcriptional regulation in inflammation and autoimmune disease. Crit. Rev. Immunol. 2012, 32, 23–63. [Google Scholar] [CrossRef]
- Nordt, R.M.; Riley, J.K.; Greenlund, A.C.; Moore, K.W.; Darnell, J.E.; Schreiber, R.D. Stat3 recruitment by two distinct ligand-induced, tyrosine-phosphorylate docking sites in the interleukin-10 receptor intracellular domain. J. Biol. Chem. 1996, 271, 27954–27961. [Google Scholar] [CrossRef]
- Lee, A.C.; Harris, J.L.; Khanna, K.K.; Hong, J.H. A comprehensive review on current advances in peptide drug development and design. Int. J. Mol. Sci. 2019, 20, 2983. [Google Scholar] [CrossRef]
- Bray, J.E.; Todd, A.E.; Pearl, F.M.G.; Thornton, J.M.; Orengo, C.A. The CATH Dictionary of Homologous Superfamilies (DHS): A consensus approach for identifying distant structural homologues. Protein Eng. Des. Sel. 2000, 13, 153–165. [Google Scholar] [CrossRef]
- Pernis, A.B. Th17 cells in rheumatoid arthritis and systemic lupus erythematosus. J. Int. Med. 2009, 265, 644–652. [Google Scholar] [CrossRef]
- Toussirot, E. The IL23/Th17 pathway as a therapeutic target in chronic inflammatory diseases. Inflamm. Allergy Drug Targets 2012, 11, 159–168. [Google Scholar] [CrossRef]
- Seo, S.; Miyake, H.; Alganabi, M.; Lok, M.J.; O’Connell, J.S.; Lee, C.; Li, B.; Pierro, A. Vasoactive intestinal peptide decreases inflammation and tight junction disruption in experimental necrotizing enterocolites. J. Pediatric Surg. 2019, 54, 2520–2523. [Google Scholar] [CrossRef]
- Foloppe, N.; MacKerell, A.D. All-atom empirical force field for nucleic acids: I. Parameter optimization based on small molecule and condensed phase macromolecular target data. J. Comput. Chem. 2000, 21, 86–104. [Google Scholar] [CrossRef]
- MacKerell, A.D.; Banavali, N.K. All-atom empirical force field for nucleic acids: II. Application to molecular dynamics simulations of DNA and RNA in solution. J. Comput. Chem. 2000, 21, 105–120. [Google Scholar] [CrossRef]
- Im, W.; Lee, M.S.; Brooks III, C.L. Generalized born model with simple smoothing function. J. Comput. Chem. 2003, 24, 1691–1702. [Google Scholar] [CrossRef] [PubMed]
- Park, T.J.; Kim, J.S.; Ahn, H.C.; Kim, Y. Solution and Solid-State NMR Structural Studies of Antimicrobial Peptides LPcin-I and LPcin-II. Biophys. J. 2011, 101, 1193–1201. [Google Scholar] [CrossRef]
- Park, T.J.; Kim, J.S.; Choi, S.S.; Kim, Y. Clonnig expression, isotope labeling, purification, and characterization of bovine antimicrobial peptide, lactophoricin in Escherichia coli. Protein Expr. Purif. 2009, 65, 23–29. [Google Scholar] [CrossRef]
- Kim, J.S.; Jeong, J.H.; Kim, K.S.; Kim, Y. Optimized Expression and Characterization of Antimicrobial Peptides, LPcin Analogs. Bull. Korean Chem. Soc. 2015, 36, 1148–1154. [Google Scholar] [CrossRef]
Sample Availability: Samples of tIK peptides are available from the authors. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Y.; Kim, M.; Kim, Y. Homology Modeling and Optimized Expression of Truncated IK Protein, tIK, as an Anti-Inflammatory Peptide. Molecules 2020, 25, 4358. https://doi.org/10.3390/molecules25194358
Song Y, Kim M, Kim Y. Homology Modeling and Optimized Expression of Truncated IK Protein, tIK, as an Anti-Inflammatory Peptide. Molecules. 2020; 25(19):4358. https://doi.org/10.3390/molecules25194358
Chicago/Turabian StyleSong, Yuyoung, Minseon Kim, and Yongae Kim. 2020. "Homology Modeling and Optimized Expression of Truncated IK Protein, tIK, as an Anti-Inflammatory Peptide" Molecules 25, no. 19: 4358. https://doi.org/10.3390/molecules25194358
APA StyleSong, Y., Kim, M., & Kim, Y. (2020). Homology Modeling and Optimized Expression of Truncated IK Protein, tIK, as an Anti-Inflammatory Peptide. Molecules, 25(19), 4358. https://doi.org/10.3390/molecules25194358





