Skin Damages—Structure Activity Relationship of Benzimidazole Derivatives Bearing a 5-Membered Ring System
Abstract
1. Introduction
2. Results and Discussion
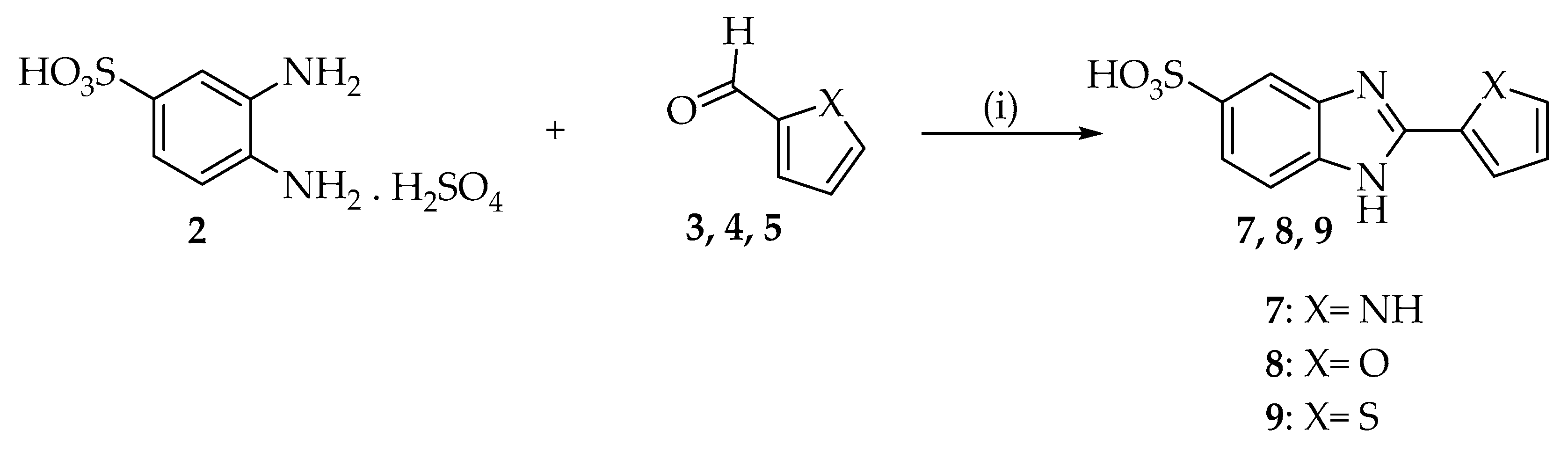
2.1. Chemistry
2.2. Evaluation of Filtering Parameters
2.2.1. In Vitro Photoprotective Activity of Benzimidazole Derivatives Solutions
2.2.2. In Vitro Photoprotective Activity of Cosmetic Formulation Containing Benzimidazole Derivatives
2.2.3. Photostability Study by Spectral Analysis
2.3. Antioxidant Activity
2.4. Antifungal Activity
2.5. Antiproliferative Activity
2.6. Antiviral Activity
3. Materials and Methods
3.1. General Information
3.2. Chemistry
3.2.1. Synthesis of 3,4-diamino-benzensulfonic acid, Sulfate Salt (2)
3.2.2. Synthesis of 2-substituted benzimidazole-5-sulfonic Acids
2-(1H-pyrrol-2-yl)-1H-benzimidazole-5-sulfonic Acid (7)
2-(furan-2-yl)-1H-benzimidazole-5-sulfonic Acid (8)
2-(thiophen-2-yl)-1H-benzimidazole-5-sulfonic Acid (9)
3.2.3. General Method for the Synthesis of 2-Substituted Benzimidazoles
2-(1H-pyrrol-2-yl)-1H-benzimidazole (10)
2-(furan-2-yl)-1H-benzimidazole (11)
2-(thiophen-2-yl)-1H-benzimidazole (12)
3.2.4. General Method for the Synthesis of 2-Substituted Benzimidazole-5-Carboxylic Acids
2-(1H-pyrrol-2-yl)-1H-benzimidazole -5-carboxylic Acid (13)
2-(furan-2-yl)-1H-benzimidazole -5-carboxylic Acid (14)
2-(thiophen-2-yl)-1H-benzimidazole -5-carboxylic Acid (15)
3.3. Biological Assays
3.3.1. In Vitro Photoprotection Activity
Filtering Parameters Evaluation of Benzimidazole Derivatives in Solution
Sunscreens Formulation
In Vitro Evaluation of Filtering Parameters
3.3.2. Antioxidant Activity
DPPH Assay
FRAP Assay
3.3.3. Antifungal Activity
Antidermatophyte Activity
Anti-Candida albicans Activity
3.3.4. Anti-Proliferative Activity
Cell Lines
Cell Proliferation
IC50 Determination
3.3.5. Anti-Viral Activity
3.4. Stability Studies
Photostability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Afaq, F.; Adhami, V.M.; Mukhtar, H. Photochemoprevention of ultraviolet B signalling and photocarcinogenesis. Mutat. Res. 2005, 571, 153–173. [Google Scholar] [CrossRef] [PubMed]
- Wondrak, G.T.; Jacobson, M.K.; Jacobson, E.L. Endogenous UVA photosensitizers: Mediators of skin photodamage and novel targets for skin photoprotection. Photochem. Photobiol. Sci. 2006, 5, 215–237. [Google Scholar] [CrossRef] [PubMed]
- Saewan, N.; Jimtaisong, A. Photoprotection of natural flavonoids. J. Appl. Pharm. Sci. 2013, 3, 129–141. [Google Scholar]
- Laleh Farajia, S.S. Synthesis of novel benzimidazole and benzothiazole derivatives bearing a 1,2,3-triazole ring system and their acetylcholinesterase inhibitory activity. J. Chem. Res. 2017, 41, 30–35. [Google Scholar] [CrossRef]
- Joule, J.A.; Mills, K. Heterocyclic Chemistry, 5th ed.; Wiley India Pvt. Ltd.: New Delhi, India, 2010; pp. 503–506. [Google Scholar]
- Gil, M.; Tomás-Barberán, F.A.; Hesse-Pierce, B.; Kader, A.A. Antioxidant Capacities, Phenolic Compounds, Carotenoids and Vitamin C Contents of Nectarine, Peach and Plum Cultivars from California. J. Agric. Food Chem. 2002, 50, 4976–4982. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.M. A Review: Biological Importance of Heterocyclic Compounds. Pharma Chem. 2017, 9, 141–147. [Google Scholar]
- Gong, B.; Hong, F.; Kohm, C.; Bonham, L.; Klein, P. Synthesis and SAR of 2-arylbenzoxazoles, Benzothiazoles and Benzimidazoles as Inhibitors of Lysophosphatidic Acid Acyltransferase-Beta. Bioorg. Med. Chem. Lett. 2004, 14, 1455–1459. [Google Scholar] [CrossRef]
- Alpan, A.S.; Zencir, S.; Zupkó, I.; Coban, G.; Réthy, B.; Gunes, H.S.; Topcu, Z. Biological activity of bis-benzimidazole derivatives on DNA topoisomerase I and HeLa, MCF7 and A431 cells. J. Enzyme Inhib. Med. Chem. 2009, 24, 844–849. [Google Scholar] [CrossRef]
- Youssef, A.M.; Malki, A.; Badr, M.H.; Elbayaa, R.Y.; Sultan, A.S. Synthesis and anticancer activity of novel benzimidazole and benzothiazole derivatives against HepG2 liver cancer cells. Med. Chem. 2012, 8, 151–162. [Google Scholar] [CrossRef]
- Coban, G.; Zencir, S.; Zupkó, I.; Réthy, B.; Gunes, H.S.; Topcu, Z. Synthesis and biological activity evaluation of 1H-benzimidazoles via mammalian DNA topoisomerase I and cytostaticity assays. Eur. J. Med. Chem. 2009, 44, 2280–2285. [Google Scholar] [CrossRef]
- Błaszczak-Świątkiewicz, K.; Almeida, D.C.; Perry, M.D.J.; Mikiciuk-Olasik, E. Synthesis, anticancer activity and UPLC analysis of the stability of some new benzimidazole-4,7-dione derivatives. Molecules 2014, 19, 400–413. [Google Scholar] [CrossRef] [PubMed]
- Ansari, K.F.; Lal, C. Synthesis physicochemical properties and antimicrobial activity of some new benzimidazole derivatives. Eur. J. Med. Chem. 2009, 44, 4028–4033. [Google Scholar] [CrossRef]
- Bansal, Y.; Silakari, O. The therapeutic journey of benzimidazoles: A review. Bioorg. Med. Chem. 2012, 20, 6208–6236. [Google Scholar] [CrossRef] [PubMed]
- Vasantha, K.; Basavaraja Swamy, G.; Rai, M.V.; Boja, P.; Pai, V.R.; Shruthi, N.; Bhat, M. Rapid ‘one-pot’ synthesis of a novel benzimidazole-5-carboxylate and its hydrazone derivatives as potential anti-inflammatory and antimicrobial agents. Bioorg. Med. Chem. Lett. 2015, 25, 1420–1426. [Google Scholar] [CrossRef] [PubMed]
- Sondhi, S.M.; Rajvanshi, S.; Johar, M.; Bharti, N.; Azam, A. Anti-inflammatory, analgesic and antiamoebic activity evaluation of pyrimido [1,6-a]benzimidazole derivatives synthesized by the reaction of ketoisothiocyanates with mono and diamines. Eur. J. Med. Chem. 2002, 37, 835–843. [Google Scholar] [CrossRef]
- Devivar, R.V.; Kawashima, E.; Revankar, G.R.; Breitenbach, J.M.; Kreske, E.D.; Drach, J.C.; Townsend, L.B. Benzimidazole ribonucleosides: Design, synthesis and antiviral activity of certain 2-(alkylthio)-and 2-(benzylthio)-5,6-dichloro-1-(d-ribofuranosyl) benzimidazoles. J. Med. Chem. 1994, 37, 2942–2949. [Google Scholar] [CrossRef]
- Naraboli, B.S.; Biradar, J.S. Synthesis, characterization and biological evaluation of indole derivatives bearing benzimidazole/benzothiazole moiety. Int. J. Pharm. Pharm. Sci. 2017, 9, 128–138. [Google Scholar] [CrossRef]
- Subudhi, B.B.; Panda, P.K.; Kundu, T.; Sahoo, S.; Pradhn, D. Synthesis and biological evaluation of some benzimidazole and thiazolidinone derivatives. J. Pharm. Res. 2007, 6, 114–118. [Google Scholar]
- Ateş-Alagoz, Z.; Kus, C.; Çoban, T. Synthesis and antioxidant properties of novel benzimidazoles containing substituted indoles or 1,1,4,4-tetramethyl-1,2,3,4-tetrahydro-naphthalene fragments. J. Enzym. Inhib. Med. Chem. 2005, 20, 325–331. [Google Scholar] [CrossRef]
- Kubo, K.; Oda, K.; Kaneko, T.; Satoh, H.; Nohara, A. Synthesis of 2-[[(4-fluoroalkoxy-2-pyridyl)methyl]sulfinyl]-1H- benzimidazoles as antiulcer agents. Chem. Pharm. Bull. (Tokyo) 1990, 38, 2853–2858. [Google Scholar] [CrossRef]
- Schimke, K.; Davis, T.M.E. Drug evaluation: Rivoglitazone, a new oral therapy for the treatment of type-2 diabetes. Curr. Opin. Investig. Drugs 2007, 8, 338–344. [Google Scholar] [PubMed]
- Djuidje, E.N.; Dissette, V.; Bino, A.; Benetti, S.; Balzarini, J.; Liekens, S.; Manfredini, S.; Vertuani, S.; Baldisserotto, A. A Multitarget approach toward the development of 8-Substituted purines for photoprotection and prevention of UV-Related Damage. Chem. Med. Chem. 2017, 12, 760–769. [Google Scholar] [CrossRef] [PubMed]
- Anand, K.; Wakode, S. Development of drugs based on benzimidazole heterocycle: Recent advancement and insights. Int. J. Chem. Stud. 2017, 5, 350–362. [Google Scholar]
- Aljamali, N.M.; Jwad, S.M. Survey in pyrrole compounds and biological activity. ITIRJ 2015, 1, 1–8. [Google Scholar]
- Wahbi, H.I.; Ishak, C.Y.; Khalid, A.; Adlan, T. Inverse virtuals of some new pyrazolo-[1,5-a]pyrimidine; 4,6-dihetarylpyrimidin-2-amine and ethyl 2-oxo-4,6-di(hetar-2-yl)cyclohex-3-encarboxylate heterocyclic compounds from 1,3-dihetaryl-2-propen-1-one. Int. J. Pharm. Phytopharmacol. Res. 2014, 4, 13–19. [Google Scholar]
- Idhayadhulla, A.; Kumar, R.S.; Nasser, A.J.A.; Manilal, A. Synthesis of some new pyrrole and pyridine derivatives and their antimicrobial, anticancer activities. Int. J. Biol. Chem. 2013, 7, 15–26. [Google Scholar] [CrossRef]
- Bino, A.; Baldisserotto, A.; Scalambra, E.; Dissette, V.; Vedaldi, D.E.; Salvador, A.; Durini, E.; Manfredini, S.; Vertuani, S. Design, synthesis and biological evaluation of novel hydroxy-phenyl-1H-benzimidazoles as radical scavengers and UV-protective agents. J. Enzym. Inhib. Med. Chem. 2017, 32, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Diffey, B.L.; Robson, J. A new substrate to measure sunscreen protection factors throughout the ultraviolet spectrum. J. Soc. Cosmet. Chem. 1989, 40, 127–133. [Google Scholar]
- SPF Calculator Software, Version 2.1; Shimadzu: Milan, Italy.
- US Food and Drug Administration. 21 CFR Parts 347 and 352, Sunscreen Drug Products for Over-the-Counter Human Use. Proposed Amendment of Final Monograph; Silver Spring, MD, USA, 2007. Available online: https://www.fda.gov/OHRMS/DOCKETS/98fr/cd031.pdf (accessed on 25 April 2017).
- Garoli, D.; Pelizzo, M.G.; Bernardini, B.; Nicolosi, P.; Alaibac, M. Sunscreen tests: Correspondence between in vitro data and values reported by the manufacturers. J. Dermatol. Sci. 2008, 52, 193–204. [Google Scholar] [CrossRef]
- Hojerová, J.; Medovcíková, A.; Mikula, M. Photoprotective efficacy and photostability of fifteen sunscreen products having the same label SPF subjected to natural sunlight. Int. J. Pharm. 2011, 408, 27–38. [Google Scholar] [CrossRef]
- Varni, A.J.; Fortney, A.; Baker, M.A.; Worch, J.C.; Qiu, Y.; Yaron, D.; Bernhard, S.; Noonan, K.J.T.; Kowalewski, T. Photostable Helical Polyfurans. J. Am. Chem. Soc. 2019, 141, 8858–8867. [Google Scholar] [CrossRef] [PubMed]
- Patani, G.A.; LaVoie, E.J. Bioisosterism: A Rational Approach in Drug Design. Chem. Rev. 1996, 96, 3147–3176. [Google Scholar] [CrossRef] [PubMed]
- Kühbacher, A.; Burger-Kentischer, A.; Steffen, R. Interaction of Candida species with the skin. Microorganisms 2017, 5, 32. [Google Scholar] [CrossRef] [PubMed]
- Djuidje, E.N.; Sciabica, S.; Buzzi, R.; Dissette, V.; Balzarini, J.; Liekens, S.; Serra, E.; Andreotti, E.; Manfredini, S.; Vertuani, S.; et al. Design, synthesis and evaluation of benzothiazole derivatives as multifunctional agents. Bioorg. Chem. 2020, 101, 103960. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Shao, Y.; Li, J.; Rangarajan, M.; LaVoie, E.J.; Huang, T.C.; Ho, T.C. Antioxidative phenolic compounds from Sage (Salvia officinalis). J. Agric. Food. Chem. 1998, 46, 4869–4873. [Google Scholar] [CrossRef]
- Baldisserotto, A.; Demurtas, M.; Lampronti, I.; Moi, D.; Balboni, G.; Vertuani, S.; Manfredini, S.; Onnis, V. Benzofuran hydrazones as potential scaffold in the development of multifunctional drugs: Synthesis and evaluation of antioxidant, photoprotective and antiproliferative activity. Eur. J. Med. Chem. 2018, 156, 118–125. [Google Scholar] [CrossRef]
- Guihua, X.; Xing Qian, Y.; Jianchu, C.; Donghong, L. Effect of heat treatment on the phenolic compounds and antioxidant capacity of citrus peel extract. J. Agric. Food. Chem. 2007, 55, 330–335. [Google Scholar]
Sample Availability: Samples of the compounds 7, 8, 9, 12, 13, 14 and 15 are available from the authors. |




| Compound | Percentage (%) | SPF | UVAPF | UVA/UVB | λc (nm) |
|---|---|---|---|---|---|
| 1 | 3.22 | 0.94 | 0.33 | 319 | |
| PBSA | 2 | 4.63 | 0.94 | 0.22 | 321 |
| 3 | 5.09 | 1.02 | 0.25 | 324 | |
| 1 | 2.44 | 1.16 | 0.70 | 341 | |
| 10 | 2 | 2.72 | 1.32 | 0.73 | 357 |
| 3 | 3.06 | 1.54 | 0.75 | 364 | |
| 1 | 6.84 | 1.94 | 0.69 | 368 | |
| 11 | 2 | 10.39 | 1.53 | 0.13 | 366 |
| 3 | 11.15 | 1.82 | 0.18 | 371 | |
| 1 | 2.41 | 1.16 | 0.71 | 340 | |
| 12 | 2 | 2.59 | 1.22 | 0.70 | 342 |
| 3 | 2.79 | 1.26 | 0.69 | 344 |
| Compound | DPPH (% Inhibition) | DPPH IC50 (µg/mL) | FRAP (µmolTE/g) |
|---|---|---|---|
| Caffeic acid | 81.35 ± 0.35 | - | 10156.12 ± 15.5 |
| PBSA | <LOQ * | - | <LOQ * |
| 7 | 53.88 ± 2.30 | 1093.09 ± 8.65 | 239.91 ± 1.79 |
| 10 | 70.00 ± 3.25 | 64.10 ± 3.21 | 1085.57 ± 0.88 |
| 13 | 33.00 ± 1.28 | - | 108.89 ± 2.98 |
| 8 | 10.96 ± 0.56 | - | <LOQ * |
| 11 | 15.46 ± 0.98 | - | 4462.64 ± 18.29 |
| 14 | 52.06 ± 1.48 | 1416.38 ± 21.40 | 26.67 ± 2.89 |
| 9 | 12.52 ± 0.88 | - | 82.16 ± 0.22 |
| 12 | 35.05 ± 0.87 | - | 75.89 ± 5.43 |
| 15 | 30.23 ± 1.45 | - | 181.84 ± 2.66 |
| IC50 (µg/mL) | |||||
|---|---|---|---|---|---|
| Compound | M. gypseum | M. canis | T. mentagrophytes | T. tonsurans | E. floccosum |
| 10 | 1.53 ± 0.05 | 1.34 ± 0.02 | 1.38 ± 0.08 | 0.97 ± 0.06 | 1.07 ± 0.03 |
| 11 | 1.54 ± 0.02 | 1.58 ± 0.06 | 1.61 ± 0.10 | 1.89 ± 0.22 | 2.44 ± 0.05 |
| 12 | 1.55 ± 0.03 | 2.14 ± 0.09 | 1.74 ± 0.11 | 2.42 ± 0.15 | 3.80 ± 0.07 |
| Fluconazole | 18.5 ± 1.23 | 29.6 ± 1.84 | 3.53 ± 0.26 | 19.41 ± 0.87 | 0.08 ± 0.005 |
| Econazole nitrate | 0.05 ± 0.0001 | 0.51 ± 0.02 | 0.006 ± 0.0001 | 0.13 ± 0.008 | 0.47 ± 0.015 |
| MIC (µg/mL) | ||
|---|---|---|
| Compound | 24 h | 48 h |
| 7 | 64 | - |
| 10 | - | - |
| 13 | - | - |
| 8 | - | - |
| 11 | - | - |
| 14 | 16 | - |
| 9 | 64 | - |
| 12 | - | - |
| 15 | 16 | - |
| Fluconazole | 0.5 | - |
| IC50 (µM) | SI * | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Compound | CEM | HeLa | Mia Paca-2 | SK-Mel 5 | Hek293 | CEM | HeLa | Mia Paca-2 | SK-Mel 5 |
| 7 | 31 ± 21 | >100 | 28 ± 10 | >100 | 67 ± 47 | 2.16 | - | 2.39 | - |
| 10 | 14 ± 1 | 37 ± 0 | 15 ± 9 | 9.7 ± 1.7 | 31 ± 3 | 2.21 | - | 2.07 | 3.20 |
| 13 | >100 | >100 | >100 | >100 | >100 | - | - | - | - |
| 8 | >100 | ≥100 | ≥100 | ≥100 | >100 | - | - | - | - |
| 11 | >100 | >100 | >100 | >100 | >100 | - | - | - | - |
| 14 | 75 ± 35 | >100 | >100 | >100 | >100 | 1.33 | - | - | - |
| 9 | 34 ± 2 | >100 | 43 ± 14 | >100 | >100 | 2.94 | - | 2.33 | - |
| 12 | >100 | >100 | ≥100 | 86 ± 17 | >100 | - | - | - | 1.16 |
| 15 | 50 ± 10 | >100 | 80 ± 12 | >100 | >100 | 2 | - | 1.25 | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Djuidje, E.N.; Durini, E.; Sciabica, S.; Serra, E.; Balzarini, J.; Liekens, S.; Manfredini, S.; Vertuani, S.; Baldisserotto, A. Skin Damages—Structure Activity Relationship of Benzimidazole Derivatives Bearing a 5-Membered Ring System. Molecules 2020, 25, 4324. https://doi.org/10.3390/molecules25184324
Djuidje EN, Durini E, Sciabica S, Serra E, Balzarini J, Liekens S, Manfredini S, Vertuani S, Baldisserotto A. Skin Damages—Structure Activity Relationship of Benzimidazole Derivatives Bearing a 5-Membered Ring System. Molecules. 2020; 25(18):4324. https://doi.org/10.3390/molecules25184324
Chicago/Turabian StyleDjuidje, Ernestine Nicaise, Elisa Durini, Sabrina Sciabica, Elena Serra, Jan Balzarini, Sandra Liekens, Stefano Manfredini, Silvia Vertuani, and Anna Baldisserotto. 2020. "Skin Damages—Structure Activity Relationship of Benzimidazole Derivatives Bearing a 5-Membered Ring System" Molecules 25, no. 18: 4324. https://doi.org/10.3390/molecules25184324
APA StyleDjuidje, E. N., Durini, E., Sciabica, S., Serra, E., Balzarini, J., Liekens, S., Manfredini, S., Vertuani, S., & Baldisserotto, A. (2020). Skin Damages—Structure Activity Relationship of Benzimidazole Derivatives Bearing a 5-Membered Ring System. Molecules, 25(18), 4324. https://doi.org/10.3390/molecules25184324









