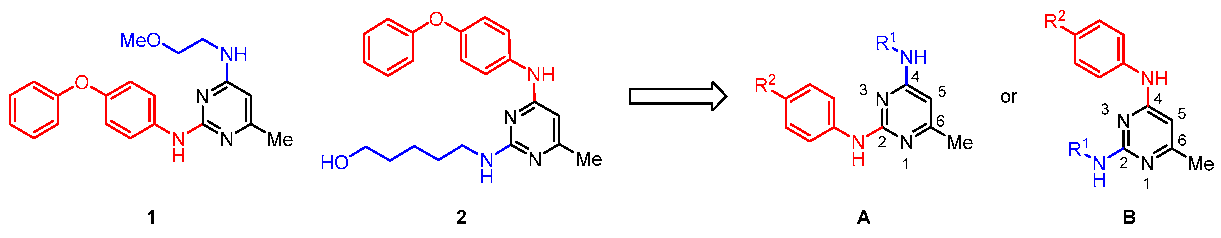
3.3. Representative Synthetic Procedures for Di-Substituted Pyrimidines (Aa1–Ae6 and Ba1–Be6)
To a stirred solution of Aa (100 mg, 0.496 mmol) and 4-aminodiphenylamine (183 mg, 0.992 mmol, 2.0 equiv.) in n-BuOH (4 mL) was added chlrotrimethylsilane (5 drops) at room temperature. The reaction mixture was heated to reflux and stirred overnight. The resulting mixture was cooled to room temperature and the solvent (n-BuOH) was removed under reduced pressure. The crude product was purified by column chromatography on silica gel (DCM/MeOH = 20:1 to 10:1) to afford Aa3 (280 mg, 81%).
N2-(4-isopropoxyphenyl)-N4-(2-methoxyethyl)-6-methylpyrimidine-2,4-diamine (Aa1). Yield: 75%; pale brown oil; 1H-NMR (500 MHz, DMSO-d6) δ 8.68 (s, 1H), 7.59 (d, J = 8.6 Hz, 2H), 6.70 (s, 1H), 6.73 (dt, J = 8.6, 2.6 Hz, 2H), 5.74 (s, 1H), 4.44 (quint., J = 6.0 Hz, 1H), 3.42 (brs, 4H), 3.23 (s, 3H), 2.05 (s, 3H), 1.19 (d, J = 6.3 Hz, 6H); 13C-NMR (125 MHz, DMSO-d6) δ 163.7, 160.0, 151.9, 135.2, 120.3, 116.2, 95.7, 71.1, 69.9, 58.5, 40.3, 23.8, 22.4; HRMS (ESI+) found 317.1978 (calculated for C17H25N4O2 ([M + H]+): 317.1968).
N4-(2-methoxyethyl)-6-methyl-N2-(4-morpholinophenyl)pyrimidine-2,4-diamine (Aa2). Yield: 54%; dark brown solid; m.p. 195–197 °C; 1H-NMR (500 MHz, DMSO-d6) δ 8.65 (s, 1H), 7.61 (d, J = 9.2 Hz, 2H), 6.96 (brs, 1H), 6.78 (d, J = 9.2 Hz, 2H), 5.75 (s, 1H), 3.67 (t, J = 4.6 Hz, 4H), 3.43 (m, 4H), 3.23 (d, J = 11.4 Hz, 3H), 2.94 (t, J = 4.6 Hz, 2H), 2.06 (s, 3H); 13C-NMR (125 MHz, DMSO-d6) δ 163.7, 160.1, 145.8, 134.7, 120.0, 116.1, 95.7, 71.2, 66.7, 58.5, 50.0, 40.3, 23.9; HRMS (ESI+) found 344.2087 (calculated for C18H26N5O2 ([M + H]+): 344.2084).
N4-(2-methoxyethyl)-6-methyl-N2-(4-(phenylamino)phenyl)pyrimidine-2,4-diamine (Aa3). Yield: 81%; blue solid; m.p. 143–145 °C; 1H-NMR (500 MHz, CDCl3) δ 7.50 (d, J = 8.6 Hz, 2H), 7.21 (t, J = 7.7 Hz, 2H), 7.04–7.03 (m, 3H), 6.96 (d, J = 7.5 Hz, 2H), 6.83 (t, J = 7.5 Hz, 1H), 5.70 (s, 1H), 5.64 (s, 1H), 5.16 (brs, 1H), 3.56-3.51 (m, 4H), 3.37 (s, 3H), 2.23 (s, 3H); 13C-NMR (125 MHz, CDCl3) δ 165.4, 163.5, 159.7, 144.6, 137.0, 134.9, 129.3, 120.5, 120.4, 119.8, 116.2, 94.4, 71.1, 58.9, 40.9, 23.8; HRMS (ESI+) found 350.1981 (calculated for C20H24N5O ([M + H]+): 350.1971).
N4-(2-methoxyethyl)-6-methyl-N2-(4-phenoxyphenyl)pyrimidine-2,4-diamine (Aa4). Yield: 81%; white solid; m.p. 159–160 °C; 1H-NMR (500 MHz, DMSO-d6) δ 8.93 (s, 1H), 7.78 (d, J = 9.2 Hz, 2H), 7.28 (dd, J = 8.6, 7.5 Hz, 2H), 7.02-6.99 (m, 2H), 6.96-6.83 (m, 4H), 5.80 (s, 1H), 3.43 (brs, 4H), 3.22 (s, 3H), 2.08 (s, 3H); 13C-NMR (125 MHz, DMSO-d6) δ 163.7, 160.0, 158.6, 149.7, 138.4, 130.3, 122.9, 120.2, 120.0, 117.8, 96.0, 71.1, 58.5, 40.3, 23.9; HRMS (ESI+) found 351.1821 (calculated for C20H23N4O2 ([M + H]+): 351.1811).
N2-(4-(4-chlorophenoxy)phenyl)-N4-(2-methoxyethyl)-6-methylpyrimidine-2,4-diamine (Aa5). Yield: 72%; white solid; m.p. 160–161 °C; 1H-NMR (500 MHz, DMSO-d6) δ 8.97 (s, 1H), 7.50 (dt, J = 9.2, 2.3 Hz, 2H), 7.21 (dt, J = 9.2, 2.9 Hz, 2H), 7.04 (brs, 1H), 6.96-6.83 (m, 4H), 5.81 (s, 1H), 4.43 (brs, 4H), 3.22 (s, 3H), 2.08 (s, 3H); 13C-NMR (125 MHz, DMSO-d6) δ 163.7, 160.0, 157.6, 149.2, 138.7, 130.1, 126.6, 120.2, 119.2, 96.1, 71.1, 58.4, 40.3, 23.9; HRMS (ESI+) found 385.1431 (calculated for C20H22ClN4O2 ([M + H]+): 385.1422).
N4-(2-methoxyethyl)-6-methyl-N2-(4-(p-tolyloxy)phenyl)pyrimidine-2,4-diamine (Aa6). Yield: 79%; white solid; m.p. 128–129 °C; 1H-NMR (500 MHz, DMSO-d6) δ 8.90 (s, 1H), 7.75 (d, J = 9.2, 2.9 Hz, 2H), 7.09 (d, J = 8.6 Hz, 2H), 7.03 (brs, 1H), 6.84 (dt, J = 8.6, 2.6 Hz, 2H), 6.80 (dt, J = 8.5, 2.6 Hz, 2H), 5.79 (s, 1H), 3.43 (brs, 4H), 3.22 (s, 3H), 2.21 (s, 3H), 2.07 (s, 3H); 13C-NMR (125 MHz, DMSO-d6) δ 163.7, 160.0, 156.1, 150.3, 138.0, 131.9, 130.6, 120.2, 119.5, 118.0, 96.1, 71.1, 58.4, 40.2, 23.9, 20.7; HRMS (ESI+) found 365.1967 (calculated for C20H23N4O2 ([M + H]+): 365.1978).
N4-(4-isopropoxyphenyl)-N2-(2-methoxyethyl)-6-methylpyrimidine-2,4-diamine (Ba1). Yield: 71%; black solid; m.p. 140–142 °C; 1H-NMR (500 MHz, DMSO-d6) δ 8.80 (s, 1H), 7.49 (d, J = 8.6 Hz, 2H), 6.78 (dt, J = 8.6, 2.6 Hz, 2H), 6.44 (s, 1H), 5.77 (s, 1H), 4.44 (quint., J = 6.0 Hz, 1H), 3.42–3.36 (m, 4H), 3.22 (s, 3H), 2.04 (s, 3H), 1.19 (d, J = 6.3 Hz, 6H); 13C-NMR (125 MHz, DMSO-d6) δ 164.9, 162.2, 161.7, 152.8, 134.1, 121.8, 116.4, 94.6, 71.4, 69.9, 58.4, 40.7, 24.0, 22.4; HRMS (ESI+) found 317.1978 (calculated for C17H24N4O2 ([M + H]+): 317.1968).
N2-(2-methoxyethyl)-6-methyl-N4-(4-morpholinophenyl)pyrimidine-2,4-diamine (Ba2). Yield: 60%; dark blue solid; m.p. 124–126 °C; 1H-NMR (500 MHz, DMSO-d6) δ 8.80 (brs, 1H), 7.46 (d, J = 8.6 Hz, 2H), 6.82 (d, J = 9.2 Hz, 2H), 6.44 (brs, 1H), 5.76 (s, 1H), 3.68 (t, J = 4.6 Hz, 4H), 3.41-3.36 (m, 4H), 3.22 (s, 3H), 2.98 (t, J = 4.6 Hz, 4H), 2.04 (s, 3H); 13C-NMR (125 MHz, DMSO-d6) δ 164.6, 162.2, 161.8, 146.8, 133.4, 121.5, 116.1, 94.7, 71.3, 66.7, 58.4, 49.7, 40.7, 23.9; HRMS (ESI+) found 344.2087 (calculated for C18H26N5O2 ([M + H]+): 344.2077).
N2-(2-methoxyethyl)-6-methyl-N4-(4-(phenylamino)phenyl)pyrimidine-2,4-diamine (Ba3). Yield: 59%; pale purple foam; 1H-NMR (500 MHz, DMSO-d6) δ 8.79 (s, 1H), 7.90 (s, 1H), 7.49 (d, J = 8.1 Hz, 2H), 7.21 (dd, J = 8.6, 7.5 Hz, 2H), 6.97 (d, J = 8.6 Hz, 2H), 6.94 (d, J = 7.5 Hz, 2H), 6.70 (t, J = 7.2 Hz, 1H), 6.42 (brs, 1H), 5.76 (s, 1H), 3.45–3.45 (m, 4H), 3.21 (s, 3H), 2.04 (s, 3H); 13C-NMR (125 MHz, DMSO-d6) δ 164.5, 162.0, 161.7, 144.9, 138.0, 134.1, 129.6, 121.6, 119.2, 118.8, 116.0, 95.1, 71.3, 58.4, 40.7, 23.9; HRMS (ESI+) found 350.1981 (calculated for C20H24N5O ([M + H]+): 350.1968).
N2-(2-methoxyethyl)-6-methyl-N4-(4-phenoxyphenyl)pyrimidine-2,4-diamine (Ba4). Yield: 70%; white solid; m.p. 145–146 °C; 1H-NMR (500 MHz, DMSO-d6) δ 9.06 (s, 1H), 7.70 (d, J = 8.6 Hz, 2H), 7.30 (t, J = 8.0 Hz, 2H), 7.03 (t, J = 7.5 Hz, 1H), 6.92 (d, J = 9.2 Hz, 4H), 6.54 (brs, 1H), 5.85 (s, 1H), 3.43-3.38 (m, 4H), 3.20 (s, 3H), 2.07 (s, 3H); 13C-NMR (125 MHz, DMSO-d6) δ 165.2, 162.2, 161.5, 158.2, 150.8, 137.3, 130.3, 123.2, 121.4, 120.0, 118.1, 95.3, 71.3, 58.4, 40.8, 24.1; HRMS (ESI+) found 351.1821 (calculated for C20H23N4O2 ([M + H]+): 351.1811).
N4-(4-(4-chlorophenoxy)phenyl)-N2-(2-methoxyethyl)-6-methylpyrimidine-2,4-diamine (Ba5). Yield: 74%; white solid; m.p. 143–144 °C; 1H-NMR (500 MHz, DMSO-d6) δ 9.05 (s, 1H) 7.70 (d, J = 8.6 Hz, 2H), 7.34 (dt, J = 9.2, 2.9 Hz, 2H), 6.96-6.83 (m, 4H), 6.53 (s, 1H), 5.83 (s, 1H), 3.43-3.34 (m, 4H), 3.20 (s, 3H), 2.07 (s, 3H); 13C-NMR (125 MHz, DMSO-d6) δ 165.4, 162.3, 161.5, 157.2, 150.2, 137.8, 130.2, 126.3, 121.3, 120.2, 119.6, 95.1, 71.3, 58.4, 40.8, 24.1; HRMS (ESI+) found 385.1431 (calculated for C20H21ClN4O2 ([M + H]+): 385.1422).
N2-(2-methoxyethyl)-6-methyl-N4-(4-(p-tolyloxy)phenyl)pyrimidine-2,4-diamine (Ba6). Yield: 77%; Gray solid; m.p. 140–142 °C; 1H-NMR (500 MHz, DMSO-d6) δ 8.96 (s, 1H), 7.64 (d, J = 8.6 Hz, 2H), 7.11 (d, J = 8.6 Hz, 2H), 6.86 (dt, J = 9.2, 2.9 Hz, 2H), 6.82 (dt, J = 8.6, 2.9 Hz, 2H), 6.48 (s, 1H), 5.80 (s, 1H), 3.42-3.29 (m, 4H), 3.20 (s, 3H), 2.23 (s, 3H), 2.05 (s, 3H); 13C-NMR (125 MHz, DMSO-d6) δ 162.7, 161.5, 155.8, 151.4, 137.0, 132.3, 130.7, 121.3, 119.4, 118.4, 71.3, 58.4, 40.8, 23.0, 20.7; HRMS (ESI+) found 365.1978 (calculated for C21H24N4O2 ([M + H]+): 365.1969).
2-((2-((4-isopropoxyphenyl)amino)-6-methylpyrimidin-4-yl)amino)ethan-1-ol (Ab1). White solid; 1H-NMR (500 MHz, DMSO-d6) δ 8.95 (s, 1H), 8.71 (s,1H), 7.61 (d, J = 9.5 Hz, 2H), 7.00 (brs, 1H), 6.74 (d, J = 8.6 Hz, 2H), 5.76 (s, 1H), 4.72 (s, 1H), 4.42 (heptet, J = 6.0 Hz, 1H), 3.52 (t, J = 5.5 Hz, 2H), 3.33 (brs, 2H), 2.07 (s, 3H), 1.18 (d, J = 5.8 Hz, 6H); 13C-NMR (125 MHz, CDCl3) δ 163.8, 159.8, 152.0, 135.1, 120.4, 116.3, 95.7, 69.9, 60.3, 43.5, 23.7, 22.4.
2-((6-methyl-2-((4-morpholinophenyl)amino)pyrimidin-4-yl)amino)ethan-1-ol (Ab2). Purple solid; m.p. 206–208 °C; 1H-NMR (500 MHz, DMSO-d6) δ 10.10 (s, 1H), 8.94 (s, 1H), 7.38 (d, J = 8.0 Hz, 2H), 6.91 (d, J = 8.1 Hz, 2H), 5.99 (s, 1H), 4.89 (brs, 1H), 3.69 (brs, 4H), 3.52 (brs, 2H), 3.39 (brs, 2H), 3.03 (brs, 4H), 2.18 (s, 3H); 13C-NMR (125 MHz, DMSO-d6) δ 163.3, 153.0, 152.0, 148.4, 129.4, 122.3, 115.9, 96.9, 66.6, 59.4, 49.1, 44.1, 18.9.
2-((6-methyl-2-((4-(phenylamino)phenyl)amino)pyrimidin-4-yl)amino)ethan-1-ol (Ab3). Purple solid; 1H-NMR (500 MHz, DMSO-d6) δ 10.25 (s, 1H), 9.14 (s, 1H), 8.26 (s, 1H), 7.37 (d, J = 8.6 Hz, 2H), 7.17 (t, J = 7.8 Hz, 2H), 7.06 (t, J = 8.6 Hz, 2H), 7.02 (d, J = 8.0 Hz, 2H), 6.75 (t, J = 7.2 Hz, 1H), 6.02 (s, 1H), 4.94 (brs, 1H), 3.53 (t, J = 5.5 Hz, 2H), 3.40 (q, J = 5.4 Hz, 2H), 2.19 (s, 3H); 13C-NMR (125 MHz, DMSO-d6) δ 163.3, 152.7, 151.4, 144.0, 140.6, 129.6, 122.5, 119.9, 117.7, 116.9, 96.9, 59.4, 44.2, 18.7.
2-((6-methyl-2-((4-phenoxyphenyl)amino)pyrimidin-4-yl)amino)ethan-1-ol (Ab4). White solid; m.p. 245–247 °C; 1H-NMR (500 MHz, DMSO-d6) δ 10.30 (s, 1H), 8.97 (s, 1H), 7.64 (d, J = 6.3 Hz, 2H), 7.34 (t, J = 7.2 Hz, 2H), 7.09 (t, J = 6.9 Hz, 1H), 7.00 (d, J = 8.1 Hz, 2H), 6.97 (d, J = 7.5 Hz, 2H), 6.04 (s, 1H), 4.87 (brs, 1H), 3.52 (brs, 2H), 3.40 (brs, 2H), 2.21 (s, 3H); 13C-NMR (125 MHz, DMSO-d6) δ 163.3, 157.5, 153.3, 153.1, 133.4, 130.5, 123.8, 122.8, 119.8, 118.8, 97.3, 59.4, 44.2, 19.0.
2-((2-((4-(4-chlorophenoxy)phenyl)amino)-6-methylpyrimidin-4-yl)amino)ethan-1-ol (Ab5). White solid; m.p. 244–246 °C; 1H-NMR (500 MHz, DMSO-d6) δ 8.96 (s, 1H), 7.80 (d, J = 9.2 Hz, 2H), 7.33 (dt, J = 9.2, 2.9 Hz, 2H), 7.01 (brs, 1H), 6.90 (d, J = 8.6 Hz, 4H), 5.79 (s, 1H), 4.68 (brs, 1H), 3.51 (t, J = 5.7 Hz, 2H), 4.33 (brs, 2H), 2.08 (s, 3H); 13C-NMR (125 MHz, CDCl3) δ 163.8, 159.9, 157.6, 149.2, 138.7, 130.1, 126.5, 120.2, 120.2, 119.3, 96.1, 60.3, 43.6, 23.8.
2-((6-methyl-2-((4-(p-tolyloxy)phenyl)amino)pyrimidin-4-yl)amino)ethan-1-ol (Ab6). Gray solid; 1H-NMR (500 MHz, DMSO-d6) δ 8.91 (s, 1H), 7.77 (d, J = 8.6 Hz, 2H), 7.08 (d, J = 8.1 Hz, 2H), 7.01 (brs, 1H), 6.85 (d, J = 9.2 Hz, 2H), 6.80 (d, J = 8.0 Hz, 2H), 5.80 (s, 1H), 4.71 (brs, 1H), 3.52 (t, J = 5.7 Hz, 2H), 3.34 (brs, 2H), 2.21 (s, 3H), 2.08 (s, 3H); 13C-NMR (125 MHz, CDCl3) δ 163.8, 159.9, 156.1, 150.3, 138.0, 132.0, 130.6, 120.2, 119.5, 118.0, 96.3, 60.3, 43.4, 23.8, 20.6.
2-((4-((4-isopropoxyphenyl)amino)-6-methylpyrimidin-2-yl)amino)ethan-1-ol (Bb1). Gray solid; m.p. 214–216 °C; 1H-NMR (500 MHz, DMSO-d6) δ 10.53 (s, 1H), 7.54 (m, 3H), 6.89 (d, J = 9.2 Hz, 2H), 6.05 (s, 1H), 4.89 (s, 1H), 4.45 (heptet, J = 6.0 Hz, 1H), 3.52 (t, J = 5.5 Hz, 2H), 3.39 (q, J = 5.7 Hz, 2H), 2.23 (s, 3H), 1.22 (d, J = 5.8 Hz, 6H); 13C-NMR (125 MHz, CDCl3) δ 160.9, 154.9, 154.7, 152.5, 131.3, 123.2, 116.2, 96.9, 69.9, 59.5, 44.0, 22.3, 18.8.
2-((4-methyl-6-((4-morpholinophenyl)amino)pyrimidin-2-yl)amino)ethan-1-ol (Bb2). White solid; 1H-NMR (500 MHz, DMSO-d6) δ 10.75 (s, 1H), 7.72 (s, 1H), 7.57 (d, J = 8.1 Hz, 2H), 6.91 (d, J = 8.6 Hz, 2H), 6.13 (s, 1H), 4.90 (brs, 1H), 3.69 (t, J = 4.6 Hz, 4H), 3.52 (t, J = 5.7 Hz, 2H), 3.40 (q, J = 5.2 Hz, 2H), 3.05 (m, 4H), 2.21 (s, 3H); 13C-NMR (125 MHz, DMSO-d6) δ 160.8, 154.9, 152.2, 148.3, 130.4, 122.5, 115.6, 97.1, 66.5, 59.5, 49.0, 43.8, 18.8.
N2-(2-methoxyethyl)-6-methyl-N4-(4-(phenylamino)phenyl)pyrimidine-2,4-diamine (Bb3). Blue solid; m.p. 228–230 °C; 1H-NMR (500 MHz, DMSO-d6) δ 10.63 (s, 1H), 8.22 (s, 1H), 7.66–7.53 (m, 2H), 7.19 (t, J = 7.2 Hz, 2H), 7.05 (t, J = 9.2 Hz, 2H), 7.03 (d, J = 8.0 Hz, 2H), 6.77 (t, J = 7.2 Hz, 1H), 6.08 (s, 1H), 4.89 (brs, 1H), 3.52 (t, J = 5.7 Hz, 2H), 3.40 (q, J = 5.3 Hz, 2H), 2.22 (s, 3H); 13C-NMR (125 MHz, DMSO-d6) δ 160.6, 154.9, 152.0, 143.9, 140.7, 130.8, 129.6, 122.8, 120.1, 117.3, 117.1, 96.9, 59.5, 43.8, 18.8.
2-((4-methyl-6-((4-phenoxyphenyl)amino)pyrimidin-2-yl)amino)ethan-1-ol (Bb4). Gray solid; 1H-NMR (500 MHz, DMSO-d6) δ 10.36 (s, 1H), 9.07 (s, 1H), 7.54 (d, J = 9.2 Hz, 2H), 7.35 (t, J = 8.0 Hz, 2H), 7.09 (t, J = 7.4 Hz, 1H), 7.01 (d, J = 9.2 Hz, 2H), 6.99 (d, J = 9.2 Hz, 2H), 6.05 (s, 1H), 4.80 (brs, 1H), 3.52 (t, J = 5.4 Hz, 2H), 3.40 (q, J = 5.4 Hz, 2H), 2.22 (s, 3H); 13C-NMR (125 MHz, CDCl3) δ 163.2, 157.4, 153.4, 152.7, 151.7, 133.2, 130.5, 123.9, 123.0, 119.8, 118.9, 97.4, 59.4, 44.2, 18.8.
2-((4-((4-(4-chlorophenoxy)phenyl)amino)-6-methylpyrimidin-2-yl)amino)ethan-1-ol (Bb5). Gray solid; 1H-NMR (500 MHz, DMSO-d6) δ 9.11 (s, 1H), 7.72 (d, J = 8.6 Hz, 2H), 7.34 (dt, J = 9.2, 2.9 Hz, 2H), 6.96–6.92 (m, 4H), 6.52 (s, 1H), 5.85 (s, 1H), 4.64 (brs, 1H), 3.49 (t, J = 6.0 Hz, 2H), 3.31 (q, J = 6.1 Hz, 2H), 2.07 (s, 3H); 13C-NMR (125 MHz, CDCl3) δ 164.9, 162.2, 161.5, 157.2, 150.3, 137.7, 130.2, 126.9, 121.4, 120.2, 119.6, 95.1, 60.7, 44.1, 24.0.
N2-(2-methoxyethyl)-6-methyl-N4-(4-(p-tolyloxy)phenyl)pyrimidine-2,4-diamine (Bb6). Gray solid; m.p. 108–110 °C; 1H-NMR (500 MHz, DMSO-d6) δ 10.77 (s, 1H), 7.69 (brs, 3H), 7.16 (d, J = 8.0 Hz, 2H), 6.95 (d, J = 8.6 Hz, 2H), 6.89 (dt, J = 8.6 Hz, 2H), 6.13 (s, 1H), 4.88 (brs, 1H), 3.51 (t, J = 5.5 Hz, 2H), 3.40 (q, J = 5.2 Hz, 2H), 2.49 (s, 6H); 13C-NMR (125 MHz, CDCl3) δ 161.3, 154.9, 154.7, 154.2, 152.9, 133.8, 133.2, 130.9, 123.2, 119.3, 118.9, 97.1, 59.5, 43.8, 20.7, 18.9.
3-((2-((4-isopropoxyphenyl)amino)-6-methylpyrimidin-4-yl)amino)propan-1-ol (Ac1). Gray solid; m.p. 156–158 °C; 1H-NMR (500 MHz, DMSO-d6) δ 10.19 (s, 1H), 9.1 (s, 1H), 7.42 (d, J = 9.6 Hz, 2H), 6.88 (d, J = 9.2 Hz, 2H), 5.98 (s, 1H), 4.54 (s, 1H), 4.52 (heptet, J = 6.0 Hz, 1H), 3.41 (t, J = 6.0 Hz, 2H), 3.36 (q, J = 6.3 Hz, 2H), 2.19 (s, 2H), 1.65 (quint., J = 6.6 Hz, 2H), 1.21 (d, J = 6.3 Hz, 6H); 13C-NMR (125 MHz, DMSO-d6) δ 161.2, 155.6, 154.6, 131.6, 123.2, 116.2, 96.2, 69.9, 58.8, 38.6, 32.3, 22.3, 19.4.
2-((6-methyl-2-((4-morpholinophenyl)amino)pyrimidin-4-yl)amino)propan-1-ol (Ac2). Black solid; m.p. 135–137 °C; 1H-NMR (500 MHz, DMSO-d6) δ 9.04 (s, 1H), 7.54 (d, J = 7.5 Hz, 2H), 6.82 (d, J = 8.0 Hz, 2H), 5.77 (s, 1H), 4.47 (s, 1H), 3.68 (brs, 4H), 3.43 (s, 2H), 3.33 (s, 2H), 2.97 (s, 3H), 2.08 (s, 3H), 1.64 (quint., J = 6.6 Hz, 2H); 13C-NMR (125 MHz, DMSO-d6) δ 163.6, 146.5, 133.2, 120.7, 116.1, 95.6, 66.7, 58.9, 49.8, 38.0, 32.6, 22.3.
2-((6-methyl-2-((4-(phenylamino)phenyl)amino)pyrimidin-4-yl)amino)propan-1-ol (Ac3). Blue solid; m.p. 113–115 °C; 1H-NMR (500 MHz, DMSO-d6) δ 9.59 (s, 1H), 8.25 (s, 1H), 8.04 (s, 1H), 7.50 (d, J = 8.6 Hz, 2H), 7.14 (t, J = 8.0 Hz, 2H), 7.02 (d, J = 8.0 Hz, 2H), 6.71 (t, J = 7.2 Hz, 2H), 5.89 (s, 1H), 4.53 (s, 1H), 3.44 (d, J = 6.3 Hz, 2H), 3.36-3.33 (m, 2H), 2.14 (s, 3H), 1.66 (quint., J = 6.6 Hz, 2H); 13C-NMR (125 MHz, DMSO-d6) δ 163.3, 155.8, 144.7, 138.9, 131.9, 129.6, 121.7, 119.4, 118.4, 116.2, 96.5, 58.8, 38.3, 32.4, 20.6.
2-((6-methyl-2-((4-phenoxyphenyl)amino)pyrimidin-4-yl)amino)propan-1-ol (Ac4). White solid; 1H-NMR (500 MHz, DMSO-d6) δ 8.94 (s, 1H), 7.81 (dt, J = 9.2, 2.6 Hz, 2H), 7.28 (m, 2H), 7.00 (t, J = 7.5 Hz, 1H), 6.89 (d, J = 9.2 Hz, 4H), 5.76 (s, 1H), 4.47 (brs, 1H), 3.45 (t, J = 6.3 Hz, 2H), 3.32 (brs, 2H), 2.08 (s, 3H), 1.67 (quint., J = 6.7 Hz, 2H); 13C-NMR (125 MHz, DMSO-d6) δ 163.7, 159.9, 158.6, 149.6, 138.4, 130.3, 122.8, 120.2, 120.1, 117.7, 95.8, 59.1, 37.9, 32.8, 23.8.
2-((2-((4-(4-chlorophenoxy)phenyl)amino)-6-methylpyrimidin-4-yl)amino)propan-1-ol (Ac5). White solid; 1H-NMR (500 MHz, DMSO-d6) δ 8.96 (s, 1H), 7.83 (dt, J = 9.2, 2.6 Hz, 2H), 7.32 (dt, J = 8.6, 2.9 Hz, 2H), 6.88 (dt, J = 9.2, 2.6 Hz, 2H), 6.98 (brs, 1H), 6.92-6.88 (m, 4H), 5.76 (s, 1H), 4.47 (s, 1H), 3.46 (t, J = 6.0 Hz, 2H), 3.32 (brs, 2H), 2.08 (s, 3H), 1.67 (quint., J = 6.6 Hz, 2H); 13C-NMR (125 MHz, DMSO-d6) δ 163.7, 159.9, 157.6, 149.1, 138.8, 130.1, 126.5, 120.2, 120.2, 119.2, 96.6, 59.1, 37.8, 32.8, 23.8.
2-((6-methyl-2-((4-(p-tolyloxy)phenyl)amino)pyrimidin-4-yl)amino)propan-1-ol (Ac6). White solid; 1H-NMR (500 MHz, DMSO-d6) δ 10.37 (s, 1H), 9.05 (s, 1H), 7.56 (d, J = 8.6 Hz, 2H), 7.14 (d, J = 8.0 Hz, 2H), 6.95 (d, J = 9.2 Hz, 2H), 6.86 (t, J = 8.0 Hz, 2H), 6.01 (s, 1H), 4.54 (s, 1H), 3.41 (t, J = 6.0 Hz, 2H), 3.38 (q, J = 6.3 Hz, 2H), 2.23 (s, 3H), 2.20 (s, 1H), 1.66 (quint., J = 6.6 Hz, 2H); 13C-NMR (125 MHz, DMSO-d6) δ 163.0, 155.0, 153.7, 152.9, 151.9, 133.1, 132.9, 130.9, 122.7, 119.3, 118.9, 97.2, 58.6, 38.5, 32.0, 20.7, 18.9.
2-((4-((4-isopropoxyphenyl)amino)-6-methylpyrimidin-2-yl)amino)propan-1-ol (Bc1). Gray solid; m.p. 176–178 °C; 1H-NMR (500 MHz, DMSO-d6) δ 10.25 (s, 1H), 7.52 (s, 1H), 6.86 (d, J = 9.2 Hz, 2H), 5.97 (s, 1H), 4.57 (s, 1H), 4.53 (heptet, J = 6.0 Hz, 2H), 3.45 (t, J = 6.0 Hz, 2H), 3.36 (q, J = 5.8 Hz, 2H), 2.18 (s, 2H), 1.66 (quint., J = 6.5 Hz, 2H), 1.22 (d, J = 6.5 Hz, 6H); 13C-NMR (125 MHz, DMSO-d6) δ 161.2, 155.6, 154.6, 131.6, 123.2, 116.2, 96.2, 69.9, 58.8, 38.6, 32.3, 22.3, 19.4.
2-((4-methyl-6-((4-morpholinophenyl)amino)pyrimidin-2-yl)amino)propan-1-ol (Bc2). Gray solid; m.p. 162–166 °C; 1H-NMR (500 MHz, DMSO-d6) δ 9.19 (s, 1H), 7.48 (d, J = 8.0 Hz, 2H), 6.85 (d, J = 9.2 Hz, 2H), 5.77 (s, 1H), 4.54 (s, 1H), 3.69 (t, J = 4.6 Hz, 4H), 3.42 (t, J = 6.0 Hz, 2H), 3.27 (q, J = 6.3 Hz, 2H), 2.99 (t, J = 4.6 Hz, 2H), 2.06 (s, 1H), 1.63 (quint., J = 6.3 Hz, 6H); 13C-NMR (125 MHz, DMSO-d6) δ 161.2, 155.6, 154.6, 131.6, 123.2, 116.2, 96.2, 69.9, 58.8, 38.6, 32.3, 22.3, 19.4.
2-((4-methyl-6-((4-(phenylamino)phenyl)amino)pyrimidin-2-yl)amino)propan-1-ol (Bc3). Blue solid; m.p. 104–108 °C; 1H-NMR (500 MHz, DMSO-d6) δ 9.77 (s, 1H), 8.05 (s, 1H), 7.54 (d, J = 8.6 Hz, 2H), 7.15 (t, J = 7.7 Hz, 2H), 7.01 (t, J = 8.6 Hz, 2H), 6.98 (t, J = 8.0 Hz, 2H), 6.72 (t, J = 7.2 Hz, 2H), 5.90 (s, 1H), 4.59 (s, 1H), 3.44 (t, J = 6.0 Hz, 4H), 3.32 (q, J = 5.7 Hz, 2H), 2.01 (s, 3H), 1.65 (quint., J = 6.3 Hz, 2H); 13C-NMR (125 MHz, DMSO-d6) δ 172.7, 161.4, 158.7, 144.5, 139.0, 132.3, 129.6, 122.2, 119.5, 118.3, 116.3, 95.3, 59.0, 38.6, 32.7, 21.6.
2-((4-methyl-6-((4-phenoxyphenyl)amino)pyrimidin-2-yl)amino)propan-1-ol (Bc4). White solid; 1H-NMR (500 MHz, DMSO-d6) δ 9.10 (s, 1H), 7.71 (d, J = 8.6 Hz, 2H), 7.31 (td, J = 9.2, 2.3 Hz, 2H), 7.03 (t, J = 7.4 Hz, 1H), 6.93-6.91 (m, 4H), 6.63 (brs, 1H), 5.83 (s, 1H), 4.44 (s, 1H), 3.43 (t, J = 6.3 Hz, 2H), 3.28 (q, J = 6.9 Hz, 2H), 2.06 (s, 3H), 1.64 (quint., J = 6.4 Hz, 2H); 13C-NMR (125 MHz, DMSO-d6) δ 164.7, 162.2, 161.5, 158.2, 150.7, 137.3, 130.4, 123.2, 121.4, 120.0, 118.1, 95.0, 59.2, 38.5, 33.1, 23.9.
2-((4-((4-(4-chlorophenoxy)phenyl)amino)-6-methylpyrimidin-2-yl)amino)propan-1-ol (Bc5). White solid; m.p. 142–144 °C; 1H-NMR (500 MHz, DMSO-d6) δ 9.10 (s, 1H), 7.73 (d, J = 9.2 Hz, 2H), 7.34 (dt, J = 9.2, 2.9 Hz, 2H), 6.94 (d, J = 8.6 Hz, 2H), 6.93 (dt, J = 9.2, 2.3 Hz, 2H), 6.62 (brs, 1H), 5.82 (s, 1H), 4.41 (brs, 1H), 3.43 (t, J = 6.3 Hz, 2H), 3.29 (q, J = 6.5 Hz, 2H), 2.06 (s, 3H), 1.64 (quint., J = 6.3 Hz, 2H), 13C-NMR (125 MHz, DMSO-d6) δ 164.9, 162.2, 161.5, 157.3, 150.2, 137.7, 130.2, 126.8, 121.3, 120.2, 119.6, 94.7, 59.2, 49.1, 38.4, 33.1, 23.8.
3-((4-methyl-6-((4-(p-tolyloxy)phenyl)amino)pyrimidin-2-yl)amino)propan-1-ol (Bc6). White solid; m.p. 136–138 °C; 1H-NMR (500 MHz, DMSO-d6) δ 9.03 (s, 1H), 7.68 (d, J = 8.6 Hz, 2H), 7.10 (d, J = 8.6 Hz, 2H), 6.88 (dt, J = 9.2, 2.6 Hz, 2H), 6.82 (dt, J = 8.6, 2.3 Hz, 2H), 6.59 (brs, 1H), 5.81 (s, 1H), 4.43 (s, 1H), 3.43 (t, J = 6.3 Hz, 2H), 3.28 (q, J = 6.5 Hz, 2H), 2.22 (s, 3H), 2.06 (s, 3H), 1.64 (quint., J = 6.4 Hz, 2H); 13C-NMR (125 MHz, DMSO-d6) δ 164.9, 162.3, 161.5, 155.8, 151.4, 137.0, 132.3, 130.7, 121.4, 119.5, 118.3, 94.6, 59.2, 38.5, 33.1, 24.0, 20.7.
4-((2-((4-isopropoxyphenyl)amino)-6-methylpyrimidin-4-yl)amino)butan-1-ol (Ad1). Gray solid; m.p. 170–172 °C; 1H-NMR (500 MHz, DMSO-d6) δ 10.19 (s, 1H), 9.04 (s, 1H), 7.41 (d, J = 8.6 Hz, 2H), 6.87 (d, J = 8.6 Hz, 2H), 5.99 (s, 1H), 4.51 (heptet, J = 5.9 Hz, 1H), 4.46 (s, 1H), 3.36 (t, J = 6.0 Hz, 2H), 3.29 (m, 2H), 2.18 (s, 3H), 1.53 (quint., J = 7.0 Hz, 2H), 1.41 (quint., J = 6.7 Hz, 2H), 1.20 (quint., J = 5.8 Hz, 6H); 13C-NMR (125 MHz, DMSO-d6) δ 163.0, 154.5, 153.1, 151.9, 130.5, 122.7, 116.4, 96.9, 69.9, 60.8, 41.1, 30.4, 25.6, 22.3, 18.9.
4-((6-methyl-2-((4-morpholinophenyl)amino)pyrimidin-4-yl)amino)butan-1-ol (Ad2). Black solid; m.p. 160–162 °C; 1H-NMR (500 MHz, DMSO-d6) δ 9.95 (s, 1H), 8.70 (s, 1H), 7.41 (d, J = 8.6 Hz, 2H), 6.89 (d, J = 8.1 Hz, 2H), 5.92 (s, 1H), 4.44 (s, 1H), 3.69 (brs, 4H), 3.37 (t, J = 6.3 Hz, 2H), 3.30 (s, 2H), 3.02 (brs, 4H), 2.17 (s, 3H), 1.53 (quint., J = 7.2 Hz, 2H), 1.42 (quint., J = 7.2 Hz, 2H); 13C-NMR (125 MHz, DMSO-d6) δ 163.1, 153.8, 148.1, 130.1, 122.1, 115.9, 96.7, 66.6, 49.2, 41.0, 30.4, 25.7, 19.4.
4-((6-methyl-2-((4-(phenylamino)phenyl)amino)pyrimidin-4-yl)amino)butan-1-ol (Ad3). Dark blue solid; m.p. 222–224 °C; 1H-NMR (500 MHz, DMSO-d6) δ 10.08 (s, 1H), 8.99 (s, 1H), 8.23 (s, 1H), 7.41 (d, J = 6.3 Hz, 2H), 7.15 (t, J = 7.2 Hz, 2H), 7.06 (d, J = 8.0 Hz, 2H), 7.01 (d, J = 7.5 Hz, 2H), 6.73 (t, J = 6.9 Hz, 1H), 5.99 (s, 1H), 4.50 (s, 1H), 3.38 (t, J = 5.8 Hz, 2H), 3.30 (brs, 2H), 2.16 (s, 3H), 1.53 (quint., J = 6.3 Hz, 2H), 1.43 (quint., J = 6.9 Hz, 2H); 13C-NMR (125 MHz, DMSO-d6) δ 163.0, 153.3, 152.4, 144.2, 140.2, 130.2, 129.6, 122.3, 119.7, 117.9, 116.6, 96.8, 60.8, 41.1, 30.4, 25.7, 19.2.
4-((6-methyl-2-((4-phenoxyphenyl)amino)pyrimidin-4-yl)amino)butan-1-ol (Ad4). White solid; m.p. 156–158 °C; 1H-NMR (500 MHz, DMSO-d6) δ 10.41 (s, 1H), 9.10 (s, 1H), 7.56 (d, J = 8.6 Hz, 2H), 7.34 (t, J = 8.0 Hz, 2H), 7.07 (d, J = 7.2 Hz, 2H), 7.01 (d, J = 8.6 Hz, 2H), 6.95 (t, J = 7.5 Hz, 1H), 6.02 (s, 1H), 4.43 (s, 1H), 3.35 (t, J = 6.6 Hz, 2H), 3.30 (q, J = 6.6 Hz, 2H), 2.21 (s, 3H), 1.53 (quint., J = 7.3 Hz, 2H), 1.41 (quint., J = 6.9 Hz, 2H); 13C-NMR (125 MHz, DMSO-d6) δ 163.0, 157.5, 153.1, 153.0, 151.9, 133.4, 130.5, 123.7, 122.8, 119.9, 118.6, 97.2, 60.8, 41.2, 30.4, 25.6, 18.9.
4-((2-((4-(4-chlorophenoxy)phenyl)amino)-6-methylpyrimidin-4-yl)amino)butan-1-ol (Ad5). Gray solid; m.p. 142–144 °C; 1H-NMR (500 MHz, DMSO-d6) δ 10.42 (s, 1H), 9.08 (s, 1H), 7.57 (d, J = 9.2 Hz, 2H), 7.37 (d, J = 8.6 Hz, 2H), 7.04 (d, J = 8.6 Hz, 2H), 6.98 (d, J = 8.6 Hz, 2H), 6.02 (s, 1H), 4.43 (s, 1H), 3.34 (t, J = 6.3 Hz, 2H), 3.32 (q, J = 6.3 Hz, 2H), 2.21 (s, 3H), 1.53 (quint., J = 7.3 Hz, 2H), 1.41 (quint., J = 6.9 Hz, 2H); 13C-NMR (125 MHz, DMSO-d6) δ 163.0, 156.6, 152.9, 152.7, 151.9, 133.8, 130.3, 127.4, 122.9, 120.2, 120.2, 97.3, 60.8, 41.2, 30.4, 25.6, 18.9.
4-((6-methyl-2-((4-(p-tolyloxy)phenyl)amino)pyrimidin-4-yl)amino)butan-1-ol (Ad6). White solid; m.p. 122–124 °C; 1H-NMR (500 MHz, DMSO-d6) δ 10.32 (s, 1H), 9.03 (s, 1H), 7.52 (d, J = 8.1 Hz, 2H), 7.14 (t, J = 7.5 Hz, 2H), 6.96 (d, J = 8.0 Hz, 2H), 6.86 (d, J = 7.5 Hz, 2H), 6.00 (s, 1H), 4.42 (s, 1H), 3.34-3.32 (m, 4H), 2.24 (s, 3H), 2.21 (s, 3H), 1.53 (quint., J = 6.9 Hz, 2H), 1.41 (m, 2H); 13C-NMR (125 MHz, DMSO-d6) δ 163.0, 155.0, 153.9, 152.9, 151.9, 133.0, 130.9, 123.0, 119.3, 118.9, 97.2, 60.8, 41.2, 30.3, 25.6, 20.7, 18.8.
4-((4-((4-isopropoxyphenyl)amino)-6-methylpyrimidin-2-yl)amino)butan-1-ol (Bd1). Purple solid; m.p. 153–155 °C; 1H-NMR (500 MHz, DMSO-d6) δ 10.74 (s, 1H), 7.92 (s, 1H), 7.58 (brs, 2H), 6.88 (d, J = 8.6 Hz, 2H), 6.10 (s, 1H), 4.54 (heptet, J = 6.1 Hz, 2H), 4.45 (s, 1H), 3.38 (t, J = 6.3 Hz, 2H), 3.32 (q, J = 6.3 Hz, 2H), 2.21 (s, 3H), 1.55 (quint., J = 7.3 Hz, 2H), 1.43 (quint., J = 7.0 Hz, 2H), 1.21 (d, J = 5.7 Hz, 6H); 13C-NMR (125 MHz, DMSO-d6) δ 161.0, 154.8, 152.6, 131.3, 127.1, 123.3, 116.2, 96.6, 69.9, 60.8, 41.2, 30.2, 25.9, 22.3, 18.9.
4-((4-methyl-6-((4-morpholinophenyl)amino)pyrimidin-2-yl)amino)butan-1-ol (Bd2). Pink solid; m.p. 208–210 °C; 1H-NMR (500 MHz, DMSO-d6) δ 10.77 (s, 1H), 7.94 (s, 1H), 7.57 (brs, 2H), 6.90 (d, J = 9.2 Hz, 2H), 6.11 (s, 1H), 4.47 (s, 1H), 3.68 (t, J = 4.6 Hz, 4H), 3.37 (t, J = 6.3 Hz, 2H), 3.32 (q, J = 6.1 Hz, 2H), 3.03 (t, J = 4.3 Hz, 2H), 2.20 (s, 3H), 1.53 (quint., J = 7.2 Hz, 2H), 1.43 (quint., J = 6.9 Hz, 2H); 13C-NMR (125 MHz, DMSO-d6) δ 160.7, 154.9, 152.2, 148.5, 130.5, 122.5, 115.6, 96.7, 66.5, 60.5, 49.0, 41.1, 30.2, 25.9, 18.9.
4-((4-methyl-6-((4-(phenylamino)phenyl)amino)pyrimidin-2-yl)amino)butan-1-ol (Bd3). Dark blue solid; m.p. 192–194 °C; 1H-NMR (500 MHz, DMSO-d6) δ 10.77 (s, 1H), 8.25 (s, 1H), 7.93 (brs, 2H), 7.17 (t, J = 7.4 Hz, 2H), 7.05 (d, J = 8.6 Hz, 2H), 7.02 (d, J = 8.0 Hz, 2H), 6.76 (t, J = 7.2 Hz, 1H), 6.13 (s, 1H), 4.47 (s, 1H), 3.38 (t, J = 6.0 Hz, 2H), 3.32 (q, J = 5.8 Hz, 2H), 2.21 (s, 3H), 1.55 (quint., J = 7.0 Hz, 2H), 1.44 (quint., J = 6.7 Hz, 2H); 13C-NMR (125 MHz, DMSO-d6) δ 160.7, 154.8, 152.2, 143.9, 140.6, 130.8, 129.6, 122.8, 120.0, 117.4, 116.9, 96.8, 60.8, 41.2, 30.3, 25.9, 18.8.
4-((4-methyl-6-((4-phenoxyphenyl)amino)pyrimidin-2-yl)amino)butan-1-ol (Bd4). White solid; m.p. 140–142 °C; 1H-NMR (500 MHz, DMSO-d6) δ 9.12 (s, 1H), 7.70 (d, J = 9.2 Hz, 2H), 7.31 (t, J = 8.0 Hz, 2H), 7.04 (t, J = 7.4 Hz, 1H), 6.93 (d, J = 8.6 Hz, 2H), 6.92 (d, J = 8.0 Hz, 2H), 6.71 (brs, 1H), 5.82 (s, 1H), 4.36 (brs, 1H), 3.37 (t, J = 6.6 Hz, 2H), 3.21 (q, J = 6.7 Hz, 2H), 2.06 (s, 1H), 1.51 (quint., J = 7.2 Hz, 2H), 1.42 (quint., J = 7.0 Hz, 2H); 13C-NMR (125 MHz, DMSO-d6) δ 164.5, 161.9, 161.5, 158.2 150.7, 137.3, 130.4, 123.2, 121.4, 120.0, 118.0, 94.7, 61.1, 41.2, 30.6, 26.5, 23.8.
4-((4-((4-(4-chlorophenoxy)phenyl)amino)-6-methylpyrimidin-2-yl)amino)butan-1-ol (Bd5). White solid; m.p. 164–166 °C; 1H-NMR (500 MHz, DMSO-d6) δ 10.41 (s, 1H), 9.10 (s, 1H), 7.56 (d, J = 8.6 Hz, 2H), 7.34 (t, J = 8.0 Hz, 2H), 7.07 (d, J = 7.2 Hz, 2H), 7.01 (d, J = 8.6 Hz, 2H), 6.95 (t, J = 7.5 Hz, 1H), 6.02 (s, 1H), 4.43 (s, 1H), 3.35 (t, J = 6.6 Hz, 2H), 3.30 (q, J = 6.6 Hz, 2H), 2.21 (s, 3H), 1.53 (quint., J = 7.3 Hz, 2H), 1.41 (quint., J = 6.9 Hz, 2H); 13C-NMR (125 MHz, DMSO-d6) δ 163.0, 157.5, 153.1, 153.0, 151.9, 133.4, 130.5, 123.7, 122.8, 119.9, 118.6, 97.2, 60.8, 41.2, 30.4, 25.6, 18.9.
4-((4-methyl-6-((4-(p-tolyloxy)phenyl)amino)pyrimidin-2-yl)amino)butan-1-ol (Bd6). White solid; 1H-NMR (500 MHz, DMSO-d6) δ 9.22 (s, 1H), 7.69 (d, J = 9.2 Hz, 2H), 7.10 (d, J = 8.0 Hz, 2H), 6.89 (d, J = 9.2 Hz, 2H), 6.82 (d, J = 8.6 Hz, 2H), 6.74 (s, 1H), 5.84 (s, 1H), 4.34 (brs, 1H), 3.38 (t, J = 6.3 Hz, 2H), 3.23 (q, J = 6.7 Hz, 2H), 2.21 (s, 3H), 2.07 (s, 3H), 1.52 (quint., J = 7.2 Hz, 2H), 1.43 (quint., J = 7.0 Hz, 2H); 13C-NMR (125 MHz, DMSO-d6) δ 163.8, 161.5, 155.7, 151.5, 136.8, 132.3, 130.7, 121.5, 119.5, 118.3, 94.7, 61.2, 41.2, 30.6, 26.5, 23.2, 20.7.
5-((2-((4-isopropoxyphenyl)amino)-6-methylpyrimidin-4-yl)amino)pentan-1-oll (Ae1). Gray solid; 1H-NMR (500 MHz, DMSO-d6) δ 8.71 (s, 1H), 7.61 (dt, J = 8.6, 3.5 Hz, 2H), 7.03 (s, 1H), 6.74 (dt, J = 9.2, 3.5 Hz, 2H), 5.70 (s, 1H), 4.44 (heptet, J = 6.0 Hz, 1H), 4.34 (s, 1H), 3.35–3.32 (m, 4H), 2.05 (s, 3H), 1.49 (quint., J = 7.3 Hz, 2H), 1.41 (quint., J = 7.0 Hz, 2H), 1.31 (quint., J = 7.2 Hz, 2H), 1.19 (d, J = 6.3 Hz, 2H); 13C-NMR (125 MHz, DMSO-d6) δ 163.6, 159.7, 152.0, 135.1, 120.4, 116.2, 69.9, 61.2, 40.5, 32.8, 29.5, 23.7, 22.4.
5-((6-methyl-2-((4-morpholinophenyl)amino)pyrimidin-4-yl)amino)pentan-1-ol (Ae2). Gray solid; m.p. 148–150 °C; 1H-NMR (500 MHz, DMSO-d6) δ 8.71 (s, 1H), 7.58 (d, J = 8.6 Hz, 2H), 7.09 (s, 1H), 6.80 (d, J = 9.2 Hz, 2H), 5.70 (s, 1H), 4.34 (brs, 1H), 3.68 (t, J = 4.6 Hz, 4H), 3.35 (brs, 2H), 3.23 (brs, 2H), 2.96 (t, J = 4.6 Hz, 4H), 2.06 (s, 3H), 1.49 (quint., J = 7.2 Hz, 2H), 1.41 (quint., J = 7.0 Hz, 2H), 1.30 (quint., J = 7.3 Hz, 2H); 13C-NMR (125 MHz, DMSO-d6) δ 163.6, 159.5, 146.0, 134.3, 120.2, 116.1, 95.8, 66.7, 61.2, 49.9, 40.3, 32.8, 29.5, 23.7, 23.4.
5-((6-methyl-2-((4-(phenylamino)phenyl)amino)pyrimidin-4-yl)amino)pentan-1-ol (Ae3). Dark gray solid; m.p. 238–240 °C; 1H-NMR (500 MHz, DMSO-d6) δ 9.32 (s, 1H), 8.02 (s, 2H), 7.53 (s, 2H), 7.13 (s, 2H), 6.97-6.95 (m, 4H), 6.68 (s, 1H), 5.83 (s, 1H), 4.39 (brs, 1H), 3.34–3.25 (m 4H), 2.11 (s, 3H), 1.50 (s, 2H), 1.30 (2H), 1.19 (s, 2H); 13C-NMR (125 MHz, DMSO-d6) δ 163.4, 157.4, 145.0, 137.6, 133.5, 129.5, 124.7, 120.7, 119.0, 118.8, 115.8, 96.0, 61.1, 40.9, 32.7, 29.2, 23.6, 21.6.
5-((6-methyl-2-((4-phenoxyphenyl)amino)pyrimidin-4-yl)amino)pentan-1-ol (Ae4). White solid; 1H-NMR (500 MHz, DMSO-d6) δ 8.94 (s, 1H), 7.79 (dt, J = 9.2, 2.6 Hz, 2H), 7.29 (t, J = 8.0 Hz, 2H), 7.04 (brs, 1H), 7.00 (t, J = 7.2 Hz, 1H), 6.90-6.88 (m, 4H), 5.75 (s, 1H), 4.34 (brs, 1H), 3.35 (t, J = 6.3 Hz, 2H), 3.24 (brs, 2H), 2.08 (s, 3H), 1.50 (quint., J = 7.5 Hz, 2H), 1.40 (quint., J = 7.0 Hz, 2H), 1.31 (quint., J = 7.3 Hz, 2H); 13C-NMR (125 MHz, DMSO-d6) δ 163.7, 159.9, 158.6, 149.6, 138.3, 130.3, 122.9, 120.2, 120.0, 117.7, 96.3, 61.2, 49.0, 40.7, 32.8, 29.4, 23.7, 23.7.
5-((2-((4-(4-chlorophenoxy)phenyl)amino)-6-methylpyrimidin-4-yl)amino)pentan-1-ol (Ae5). Gray solid; m.p. 146–148 °C; 1H-NMR (500 MHz, DMSO-d6) δ 8.94 (s, 1H), 7.81 (d, J = 9.2 Hz, 2H), 7.32 (dt, J = 8.6, 2.6 Hz, 2H), 6.98 (s, 1H), 6.90 (d, J = 9.2 Hz, 2H), 6.90 (d, J = 8.6 Hz, 2H), 5.74 (s, 1H), 4.34 (brs, 1H), 4.35 (t, J = 6.3 Hz, 2H), 3.24 (brs, 2H), 2.07 (s, 3H), 1.50 (quint., J = 7.3 Hz, 2H), 1.40 (quint., J = 6.9 Hz, 2H), 1.31 (quint., J = 7.3 Hz, 2H); 13C-NMR (125 MHz, DMSO-d6) δ 163.7, 160.0, 157.6, 149.1, 138.8, 130.1, 126.5, 120.2, 120.1, 119.2, 96.0, 61.2, 49.0, 40.7, 32.8, 29.5, 23.8, 23.7.
5-((6-methyl-2-((4-(p-tolyloxy)phenyl)amino)pyrimidin-4-yl)amino)pentan-1-ol (Ae6). White solid; 1H-NMR (500 MHz, DMSO-d6) δ 8.90 (s, 1H), 7.78 (dt, J = 9.2, 2.6 Hz, 2H), 7.08 (d, J = 8.0 Hz, 2H), 7.00 (s, 1H), 6.85 (dt, J = 9.2, 2.6 Hz, 2H), 6.79 (dt, J = 8.6, 2.6 Hz, 2H), 5.74 (s, 1H), 4.36 (brs, 1H), 3.36 (t, J = 6.3 Hz, 2H), 3.27 (brs, 2H), 2.20 (s, 3H), 2.07 (s, 3H), 1.50 (quint., J = 7.3 Hz, 2H), 1.41 (quint., J = 6.9 Hz, 2H), 1.31 (quint., J = 7.3 Hz, 2H); 13C-NMR (125 MHz, DMSO-d6) δ 163.7, 159.9, 156.2, 150.3, 138.0, 131.9, 130.6, 120.2, 119.5, 117.9, 96.3, 61.2, 49.1, 40.8, 32.8, 29.5, 23.7, 23.7, 20.6.
5-((4-((4-isopropoxyphenyl)amino)-6-methylpyrimidin-2-yl)amino)pentan-1-ol (Be1). Black solid; m.p. 138–140 °C; 1H-NMR (500 MHz, DMSO-d6) δ 8.67 (s, 1H), 7.62 (dt, J = 8.6, 2.6 Hz, 2H), 6.96 (s, 1H), 6.73 (dt, J = 9.2, 2.6 Hz, 2H), 5.70 (s, 1H), 4.43 (heptet, J = 5.0 Hz, 1H), 4.35 (t, J = 6.6 Hz, 2H), 3.23 (brs, 2H), 2.05 (s, 1H), 1.49 (quint., J = 7.3 Hz, 2H), 1.42 (quint., J = 7.0 Hz, 2H), 1.31 (quint., J = 7.6 Hz, 2H), 1.18 (d, J = 5.8 Hz, 2H); 13C-NMR (125 MHz, DMSO-d6) δ 163.7, 160.0, 151.9, 135.2, 120.3, 116.2, 95.7, 69.9, 61.2, 49.1, 32.8, 29.5, 23.7, 22.4.
5-((4-methyl-6-((4-morpholinophenyl)amino)pyrimidin-2-yl)amino)pentan-1-ol (Be2). White solid; 1H-NMR (500 MHz, DMSO-d6) δ 9.07 (s, 1H), 7.49 (d, J = 8.6 Hz, 2H), 6.84 (d, J = 9.2 Hz, 2H), 6.74 (s, 1H), 5.77 (s, 1H), 4.35 (brs, 1H), 3.68 (t, J = 4.6 Hz, 4H), 3.35 (t, J = 6.3 Hz, 2H), 3.20 (q, J = 6.9 Hz, 2H), 2.99 (t, J = 4.6 Hz, 2H), 2.05 (s, 3H), 1.48 (quint., J = 7.3 Hz, 2H), 1.41 (quint., J = 7.0 Hz, 2H), 1.29 (quint., J = 7.5 Hz, 2H); 13C-NMR (125 MHz, DMSO-d6) δ 161.6, 146.9, 142.9, 142.7, 121.5, 118.0, 116.0, 115.3, 66.8, 66.6, 61.2, 51.1, 49.6, 49.1, 41.3, 32.9, 29.7, 23.6.
2-((4-methyl-6-((4-(phenylamino)phenyl)amino)pyrimidin-2-yl)amino)propan-1-ol (Be3). White solid; 1H-NMR (500 MHz, DMSO-d6) δ 8.73 (s, 1H), 7.80 (s, 1H), 7.62 (d, J = 9.2 Hz, 2H), 7.11 (t, J = 7.7 Hz, 2H), 6.99 (brs, 1H), 6.93 (dt, J = 8.6, 2.6 Hz, 2H), 6.90 (dd, J = 8.6, 1.2 Hz, 2H), 6.66 (t, J = 7.5 Hz, 1H), 5.70 (s, 1H), 4.33 (t, J = 5.2 Hz, 1H), 3.35 (q, J = 5.9 Hz, 2H), 3.23 (brs, 2H), 2.06 (s, 3H), 1.50 (quint., J = 7.5 Hz, 2H), 1.41 (quint., J = 6.9 Hz, 2H), 1.30 (quint., J = 7.6 Hz, 2H); 13C-NMR (125 MHz, DMSO-d6) δ 163.6, 145.5, 136.5, 135.6, 129.5, 120.0, 119.4, 118.6, 115.3, 104.0, 61.2, 32.8, 29.5, 23.7.
5-((4-methyl-6-((4-phenoxyphenyl)amino)pyrimidin-2-yl)amino)pentan-1-ol (Be4). White solid; 1H-NMR (500 MHz, DMSO-d6) δ 9.16 (s, 1H), 7.71 (d, J = 9.2 Hz, 2H), 7.30 (t, J = 8.0 Hz, 2H), 7.04 (t, J = 7.2 Hz, 2H), 6.92–6.91 (m, 4H), 6.75 (brs, 1H), 5.82 (s, 1H), 4.32 (s, 1H), 3.33 (t, J = 6.3 Hz, 2H), 3.20 (q, J = 6.9 Hz, 2H), 2.07 (s, 3H), 1.48 (quint., J = 7.3 Hz, 2H), 1.39 (quint., J = 6.9 Hz, 2H), 1.28 (quint., J = 7.5 Hz, 2H); 13C-NMR (125 MHz, DMSO-d6) δ 164.3, 161.9, 161.5, 158.2, 150.8, 137.2, 130.4, 123.2, 121.4, 120.0, 118.1, 94.7, 61.2, 49.1, 41.3, 32.8, 29.6, 23.6.
5-((4-((4-(4-chlorophenoxy)phenyl)amino)-6-methylpyrimidin-2-yl)amino)pentan-1-ol (Be5). White solid; m.p. 136–138 °C; 1H-NMR (500 MHz, DMSO-d6) δ 9.16 (s, 1H), 7.73 (d, J = 9.2 Hz, 2H), 7.33 (dt, J = 9.2, 2.9 Hz, 2H), 6.95–6.90 (m, 4H), 6.75 (s, 1H), 5.83 (s, 1H), 4.28 (s, 1H), 3.34 (t, J = 6.3 Hz, 2H), 3.21 (q, J = 6.7 Hz, 2H), 2.07 (s, 3H), 1.49 (quint., J = 7.3 Hz, 2H), 1.39 (quint., J = 6.9 Hz, 2H), 1.29 (quint., J = 7.3 Hz, 2H); 13C-NMR (125 MHz, DMSO-d6) δ 164.6, 162.0, 161.5, 157.2, 150.3, 137.7, 130.2, 126.9, 121.3, 120.2, 119.6, 94.9, 61.2, 41.3, 32.9, 29.6, 23.6.
5-((4-methyl-6-((4-(p-tolyloxy)phenyl)amino)pyrimidin-2-yl)amino)pentan-1-ol (Be6). White solid; 1H-NMR (500 MHz, DMSO-d6) δ 9.44 (s, 1H), 7.68 (d, J = 9.2 Hz, 2H), 7.11 (d, J = 8.6 Hz, 2H), 6.92 (s, 1H), 6.88 (d, J = 8.6 Hz, 2H), 6.83 (d, J = 8.6 Hz, 2H), 5.86 (s, 1H), 4.32 (brs, 1H), 3.33 (t, J = 6.3 Hz, 2H), 3.21 (q, J = 6.7 Hz, 2H), 2.22 (s, 3H), 2.08 (s, 3H), 1.48 (quint., J = 7.5 Hz, 2H), 1.39 (quint., J = 7.1 Hz, 2H), 1.28 (quint., J = 7.8 Hz, 2H); 13C-NMR (125 MHz, DMSO-d6) δ 161.5, 155.6, 151.9, 136.7, 132.4, 130.7, 121.7, 119.3, 118.5, 95.1, 61.2, 41.3, 32.8, 29.5, 23.6, 22.5, 20.7.