Cytotoxicity of Oleandrin Is Mediated by Calcium Influx and by Increased Manganese Uptake in Saccharomyces cerevisiae Cells
Abstract
:1. Introduction
2. Results
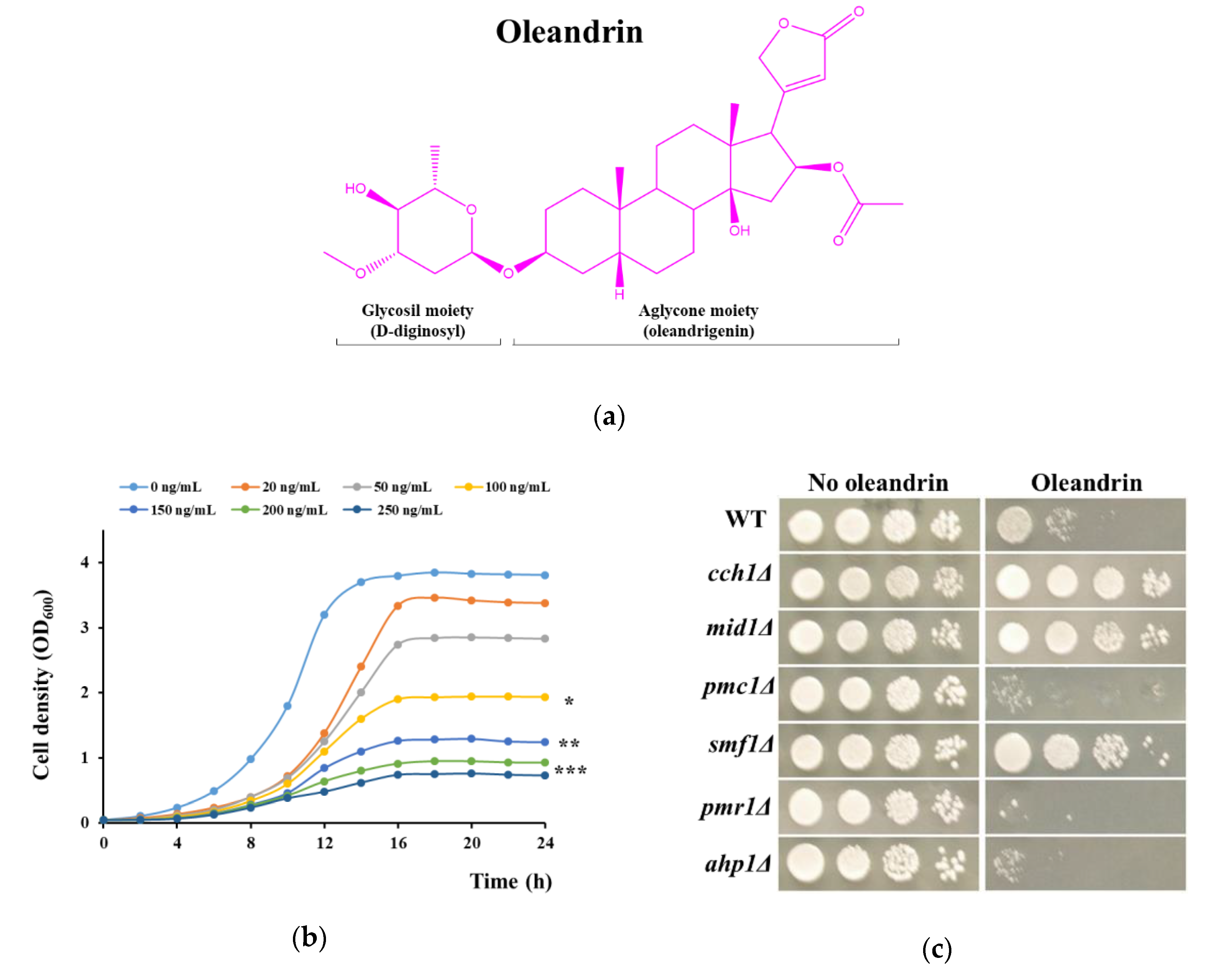
2.1. Toxicity of Oleandrin towards Saccharomyces cerevsiae Cells
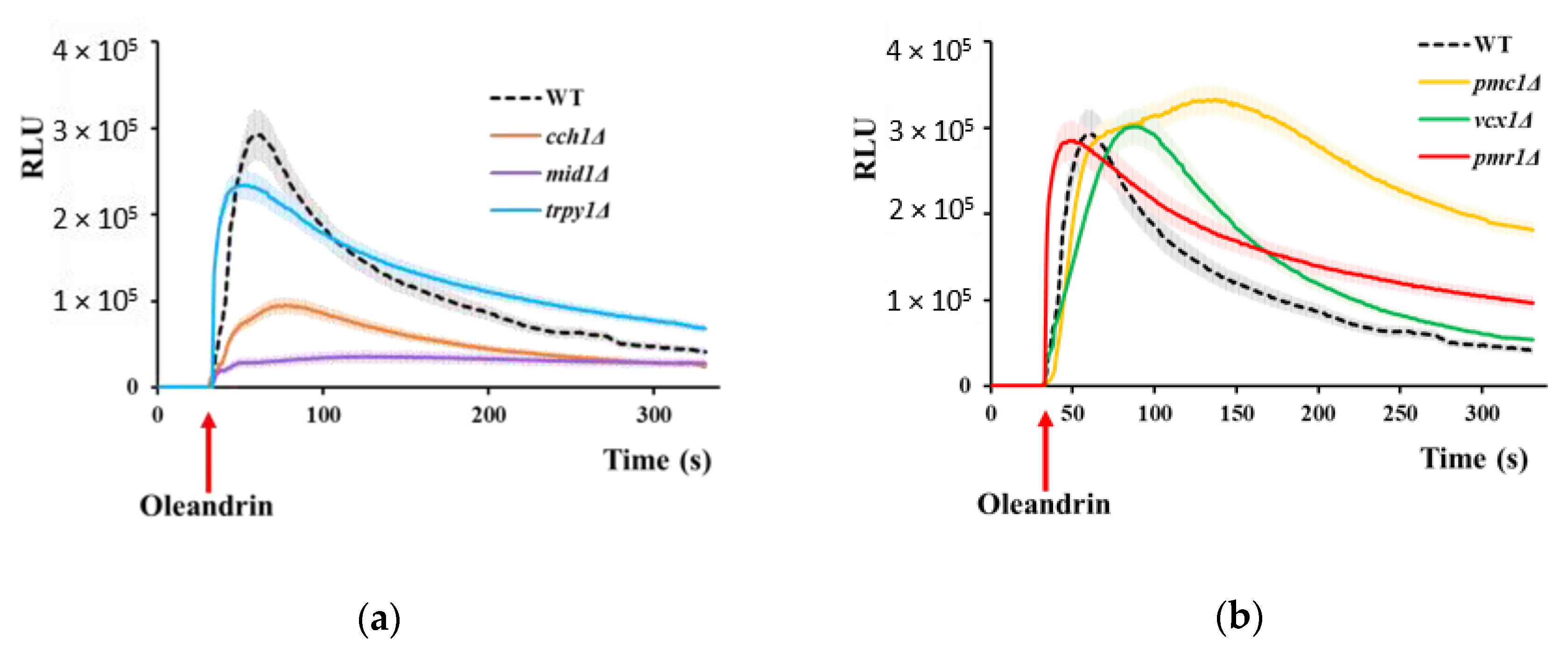
2.2. Oleandrin Induces Calcium Influx via Cch1/Mid1
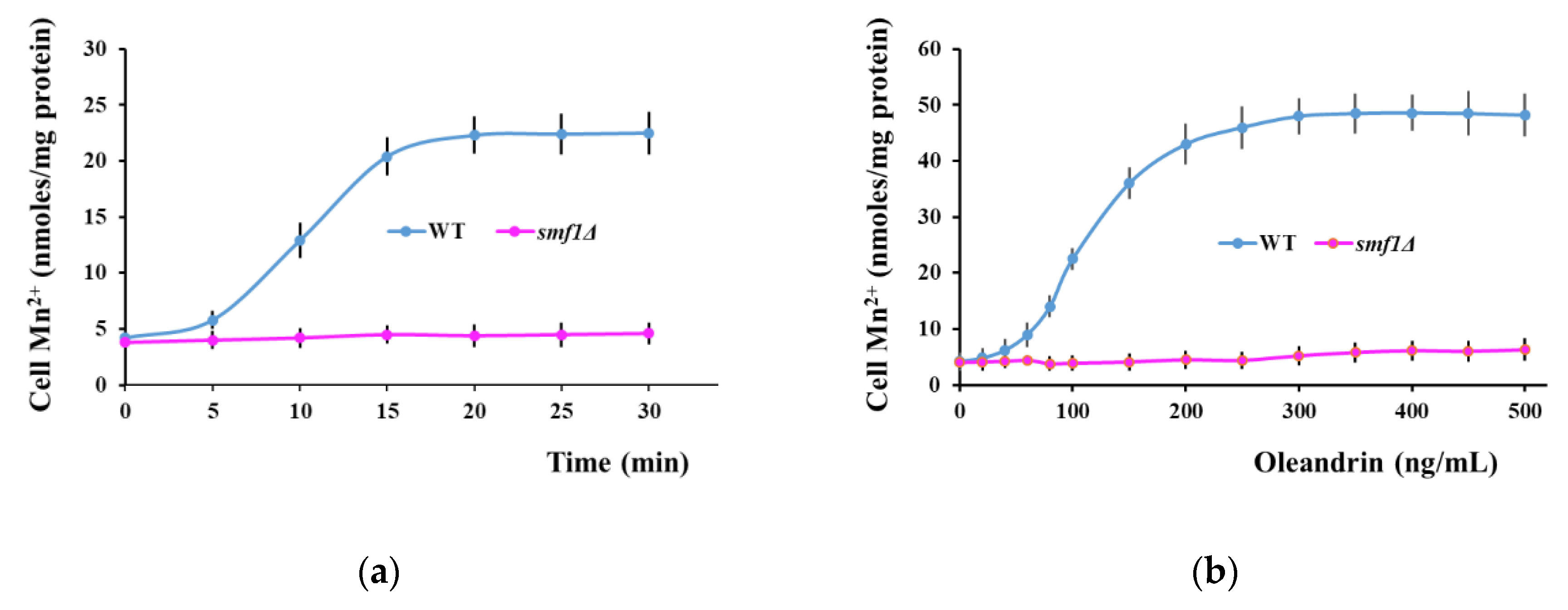
2.3. Oleandrin Exposure Induces Manganese Accumulation
2.4. Oleandrin Hypersensitivity of Mutants pmr1Δ and ahp1Δ Is Caused by Mn2+ Accumulation
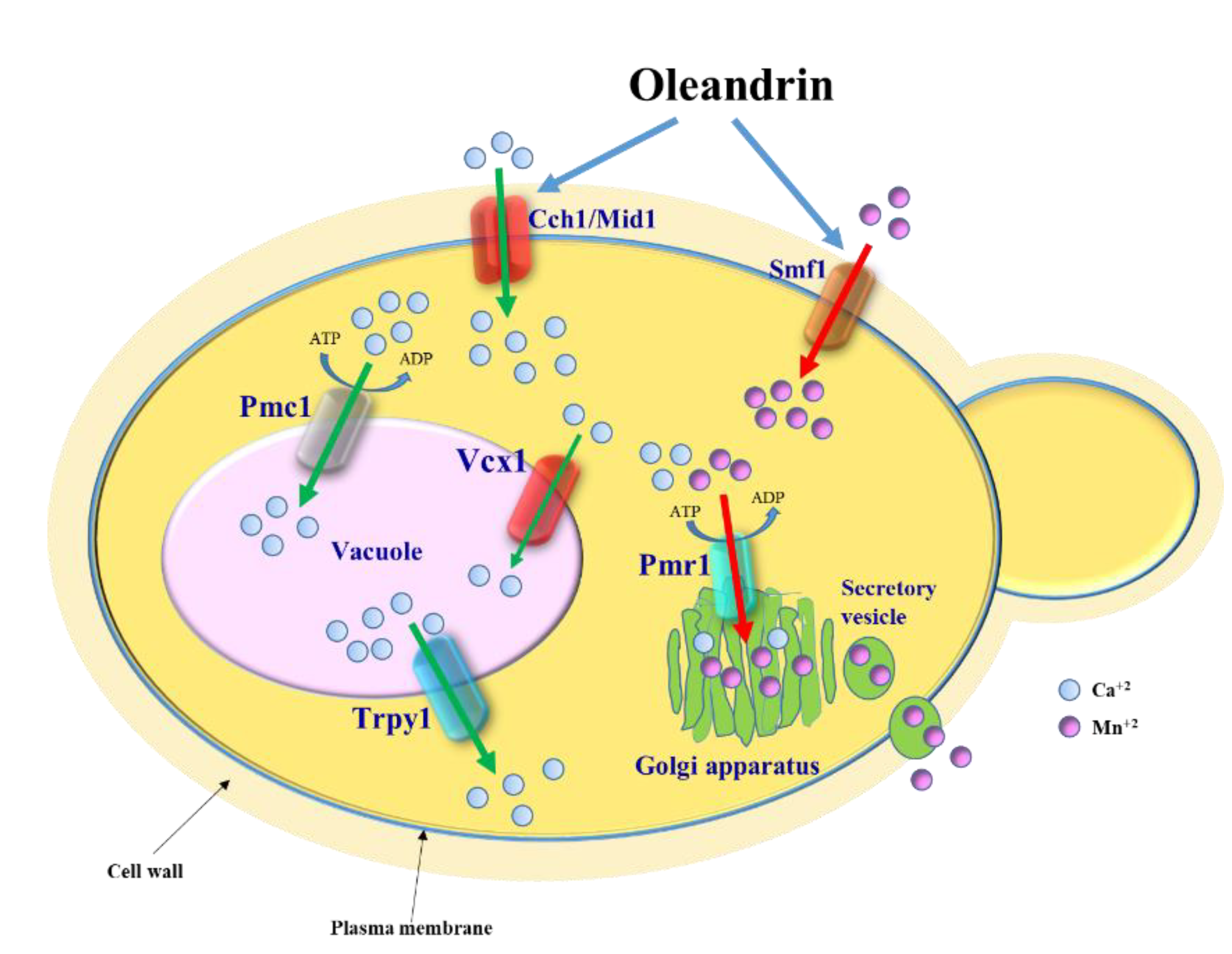
3. Discussion
4. Materials and Methods
4.1. Yeast Strains and Cultivation Media
4.2. Plasmid and Yeast Transformation
4.3. Detection of Oleandrin Effect on Yeast Cell Growth
4.4. Detection of [Ca2+]cyt by Recording Aequorin Luminescence
4.5. Multielemental Analysis of Yeast Cells
4.6. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dey, P. The pharmaco-toxicological conundrum of oleander: Potential role of gutmicrobiome. Biomed. Pharmacother. 2020, 129, 110422. [Google Scholar] [CrossRef] [PubMed]
- Langford, S.D.; Boor, P.J. Oleander toxicity: An examination of human and animal toxic exposures. Toxicology 1996, 109, 1–13. [Google Scholar] [CrossRef]
- Karthik, G.; Iyadurai, R.; Ralph, R.; Prakash, V.; Abhilash, K.P.P.; Sathyendra, S.; Abraham, O.C.; Truman, C.; Reginald, A. Acute oleander poisoning: A study of clinical profile from a tertiary care center in South India. J. Fam. Med. Prim. Care 2020, 9, 136–140. [Google Scholar] [CrossRef]
- Botelho, A.F.M.; Santos-Miranda, A.; Joca, H.C.; Mattoso, C.R.S.; de Oliveira, M.S.; Pierezan, F.; Cruz, J.S.; Soto-Blanco, B.; Melo, M.M. Hydroalcoholic extract from Nerium oleander L. (Apocynaceae) elicits arrhythmogenic activity. J. Ethnopharmacol. 2017, 206, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Rashan, L.J.; Franke, K.; Khine, M.M.; Kelter, G.; Fiebig, H.H.; Neumann, J.; Wessjohann, L.A. Characterization of the anticancer properties of monoglycosidic cardenolides isolated from Nerium oleander and Streptocaulon tomentosum. J. Ethnopharmacol. 2011, 134, 781–788. [Google Scholar] [CrossRef]
- Cao, Y.L.; Zhang, M.H.; Lu, Y.F.; Li, C.Y.; Tang, J.S.; Jiang, M.M. Cardenolides from the leaves of Nerium oleander. Fitoterapia 2018, 127, 293–300. [Google Scholar] [CrossRef]
- Botelho, A.F.M.; Pierezan, F.; Soto-Blanco, B.; Melo, M.M. A review of cardiac glycosides: Structure, toxicokinetics, clinical signs, diagnosis and antineoplastic potential. Toxicon 2019, 158, 63–68. [Google Scholar] [CrossRef]
- Hutchison, T.; Yapindi, L.; Malu, A.; Newman, R.A.; Sastry, K.J.; Harrod, R. The botanical glycoside oleandrin inhibits human T-cell leukemia virus type-1 infectivity and Env-dependent virological synapse formation. J. Antivir. Antiretrovir. 2019, 11, 184. [Google Scholar] [CrossRef] [Green Version]
- Available online: https://pubchem.ncbi.nlm.nih.gov/compound/11541511 (accessed on 10 August 2020).
- Lin, Y.; Ho, D.H.; Newman, R.A. Human tumor cell sensitivity to oleandrin is dependent on relative expression of Na+,K+-ATPase subunits. J. Exp. Ther. Oncol. 2010, 8, 271–286. [Google Scholar]
- Botelho, A.F.M.; Miranda, A.L.S.; Freitas, T.G.; Milani, P.F.; Barreto, T.; Cruz, J.S.; Melo, M.M. Comparative cardiotoxicity of low doses of digoxin, ouabain, and oleandrin. Cardiovasc. Toxicol. 2020. [Google Scholar] [CrossRef]
- Bao, Z.; Tian, B.; Wang, X.; Feng, H.; Liang, Y.; Chen, Z.; Li, W.; Shen, H.; Ying, S. Oleandrin induces DNA damage responses in cancer cells by suppressing the expression of Rad51. Oncotarget 2016, 7, 59572–59579. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Zhang, Y.; Zhao, W.; Zhou, X.; Wang, C.; Deng, F. The cardiac glycoside oleandrin induces apoptosis in human colon cancer cells via the mitochondrial pathway. Cancer Chemother. Pharmacol. 2017, 80, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.S.; Rugira, T.; Jin, H.; Park, S.W.; Kim, H.J. Oleandrin and its derivative odoroside a, both cardiac glycosides, exhibit anticancer effects by inhibiting invasion via suppressing the STAT-3 signaling pathway. Int. J. Mol. Sci. 2018, 19, 3350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yong, L.; Ma, Y.; Liang, C.; He, G.; Zhao, Z.; Yang, C.; Hai, B.; Pan, X.; Liu, Z.; Liu, X. Oleandrin sensitizes human osteosarcoma cells to cisplatin by preventing degradation of the copper transporter 1. Phytother. Res. 2019, 33, 1837–1850. [Google Scholar] [CrossRef]
- Li, X.X.; Wang, D.Q.; Sui, C.G.; Meng, F.D.; Sun, S.L.; Zheng, J.; Jiang, Y.H. Oleandrin induces apoptosis via activating endoplasmic reticulum stress in breast cancer cells. Biomed. Pharmacother. 2020, 124, 109852. [Google Scholar] [CrossRef]
- Singh, S.; Shenoy, S.; Nehete, P.N.; Yang, P.; Nehete, B.; Fontenot, D.; Yang, G.; Newman, R.A.; Sastry, K.J. Nerium oleander derived cardiac glycoside oleandrin is a novel inhibitor of HIV infectivity. Fitoterapia 2013, 84, 32–39. [Google Scholar] [CrossRef]
- Yang, C.W.; Chang, H.Y.; Hsu, H.Y.; Lee, Y.Z.; Chang, H.S.; Chen, I.S.; Lee, S.J. Identification of anti-viral activity of the cardenolides, Na(+)/K(+)-ATPase inhibitors, against porcine transmissible gastroenteritis virus. Toxicol. Appl. Pharmacol. 2017, 332, 129–137. [Google Scholar] [CrossRef]
- Plante, K.S.; Plante, J.A.; Fernandez, D.; Mirchandani, D.; Bopp, N.; Aguilar, P.V.; Sastry, K.J.; Newman, R.A.; Weaver, S.C. Prophylactic and therapeutic inhibition of in vitro SARS-CoV-2 replication by Oleandrin. bioRxiv 2020. [Google Scholar] [CrossRef]
- Kanwal, N.; Rasul, A.; Hussain, G.; Anwar, H.; Shah, M.A.; Sarfraz, I.; Riaz, A.; Batool, R.; Shahba, M.; Hussain, A.; et al. Oleandrin: A bioactive phytochemical and potential cancer killer via multiple cellular signaling pathway. Food Chem. Toxicol. 2020, 143, 111570. [Google Scholar] [CrossRef]
- Newman, R.A.; Yang, P.; Hittelman, W.N.; Lu, T.; Ho, D.H.; Ni, D.; Chan, D.; Vijjeswarapu, M.; Cartwright, C.; Dixon, S.; et al. Oleandrin-mediated oxidative stress in human melanoma cells. J. Exp. Ther. Oncol. 2006, 5, 167–181. [Google Scholar]
- Castrillo, J.I.; Oliver, S. Yeast as a touchstone in post-genomic research: Strategies for integrative analysis in functional genomics. J. Biochem. Mol. Biol. 2004, 37, 93–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matuo, R.; Sousa, F.G.; Soares, D.G.; Bonatto, D.; Saffi, J.; Escargueil, A.E.; Larsen, A.K.; Henriques, J.A. Saccharomyces cerevisiae as a model system to study the response to anticancer agents. Cancer Chemother. Pharmacol. 2012, 70, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, S.C.; Sá-Correia, I. Yeast toxicogenomics: Lessons from a eukaryotic cell model and cell factory. Curr. Opin. Biotechnol. 2015, 33, 183–191. [Google Scholar] [CrossRef]
- Manna, S.K.; Sah, N.K.; Newman, A.; Cisneros, A.; Aggarwal, B.B. Oleandrin suppresses activation of nuclear transcription factor-kB, activator protein-1, and c-Jun N-terminal kinase. Cancer Res. 2000, 60, 3838–3847. [Google Scholar] [PubMed]
- Ariño, J.; Ramos, J.; Sychrova, H. Monovalent cation transporters at the plasma membrane in yeasts. Yeast 2019, 36, 177–193. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.K.; Ellsmore, A.J.; Cessna, S.G.; Low, P.S.; Pardo, J.M.; Bressan, R.A.; Hasegawa, P.M. An osmotically induced cytosolic Ca2+ transient activates calcineurin signaling to mediate ion homeostasis and salt tolerance of Saccharomyces cerevisiae. J. Biol. Chem. 2002, 277, 33075–33080. [Google Scholar] [CrossRef] [Green Version]
- Viladevall, L.; Serrano, R.; Ruiz, A.; Domenech, G.; Giraldo, J.; Barceló, A.; Ariño, J. Characterization of the calcium-mediated response to alkaline stress in Saccharomyces cerevisiae. J. Biol. Chem. 2004, 279, 43614–43624. [Google Scholar] [CrossRef] [Green Version]
- Bootman, M.D.; Berridge, M.J.; Putney, J.W.; Roderick, H.L. Calcium Signaling; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2011; 449p, ISBN 978-0-87969-903-1. [Google Scholar]
- Batiza, A.F.; Schulz, T.; Masson, P.H. Yeast respond to hypotonic shock with a calcium pulse. J. Biol. Chem. 1996, 271, 23357–23362. [Google Scholar] [CrossRef] [Green Version]
- Denis, V.; Cyert, M.S. Internal Ca(2+) release in yeast is triggered by hypertonic shock and mediated by a TRP channel homologue. J. Cell Biol. 2002, 156, 29–34. [Google Scholar] [CrossRef]
- Palmer, C.P.; Zhou, X.; Lin, J.; Loukin, S.H.; Kung, C.; Saimi, Y. A TRP homolog in Saccharomyces cerevisiae forms an intracellular Ca(2+)-permeable channel in the yeast vacuolar membrane. Proc. Natl. Acad. Sci. USA 2001, 98, 7801–7805. [Google Scholar] [CrossRef] [Green Version]
- Cunningham, K.W. Acidic calcium stores of Saccharomyces cerevisiae. Cell Calcium 2011, 50, 129–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paidhungat, M.; Garrett, S. A homolog of mammalian, voltage-gated calcium channels mediates yeast pheromone-stimulated Ca2+ uptake and exacerbates the cdc1(Ts) growth defect. Mol. Cell. Biol. 1997, 17, 6339–6347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iida, K.; Teng, J.; Cho, T.; Yoshikawa-Kimura, S.; Iida, H. Post-translational processing and membrane translocation of the yeast regulatory Mid1 subunit of the Cch1/VGCC/NALCN cation channel family. J. Biol. Chem. 2017, 292, 20570–20582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cunningham, K.W.; Fink, G.R. Calcineurin-dependent growth control in Saccharomyces cerevisiae mutants lacking PMC1, a homolog of plasma membrane Ca2+ ATPases. J. Cell Biol. 1994, 124, 351–363. [Google Scholar] [CrossRef]
- Cunningham, K.W.; Fink, G.R. Calcineurin inhibits VCX1-dependent H+/Ca2+ exchange and induces Ca2+ ATPases in Saccharomyces cerevisiae. Mol. Cell. Biol. 1996, 16, 2226–2237. [Google Scholar] [CrossRef] [Green Version]
- Miseta, A.; Kellermayer, R.; Aiello, D.P.; Fu, L.; Bedwell, D.M. The vacuolar Ca2+/H+ exchanger Vcx1p/Hum1p tightly controls cytosolic Ca2+ levels in S. cerevisiae. FEBS Lett. 1999, 451, 132–136. [Google Scholar] [CrossRef] [Green Version]
- Sorin, A.; Rosas, G.; Rao, R. PMR1, a Ca2+-ATPase in yeast Golgi, has properties distinct from sarco/endoplasmic reticulum and plasma membrane calcium pumps. J. Biol. Chem. 1997, 272, 9895–9901. [Google Scholar] [CrossRef] [Green Version]
- Dürr, G.; Strayle, J.; Plemper, R.; Elbs, S.; Klee, S.K.; Catty, P.; Wolf, D.H.; Rudolph, H.K. The medial-Golgi ion pump Pmr1 supplies the yeast secretory pathway with Ca2+ and Mn2+ required for glycosylation, sorting, and endoplasmic reticulum-associated protein degradation. Mol. Biol. Cell 1998, 9, 1149–1162. [Google Scholar] [CrossRef] [Green Version]
- Culotta, V.C.; Yang, M.; Hall, M.D. Manganese transport and trafficking: Lessons learned from Saccharomyces cerevisiae. Eukaryot. Cell 2005, 4, 1159–1165. [Google Scholar] [CrossRef] [Green Version]
- Kellermayer, R. Hailey-Hailey disease as an orthodisease of PMR1 deficiency in Saccharomyces cerevisiae. FEBS Lett. 2005, 579, 2021–2025. [Google Scholar] [CrossRef] [Green Version]
- Van Ho, A.; Ward, D.M.; Kaplan, J. Transition metal transport in yeast. Annu. Rev. Microbiol. 2002, 56, 237–261. [Google Scholar] [CrossRef] [PubMed]
- Dancis, A.; Haile, D.; Yuan, D.S.; Klausner, R.D. The Saccharomyces cerevisiae copper transport protein (Ctr1p). Biochemical characterization, regulation by copper, and physiologic role in copper uptake. J. Biol. Chem. 1994, 269, 25660–25667. [Google Scholar] [PubMed]
- Eide, D.J. The molecular biology of metal ion transport in Saccharomyces cerevisiae. Annu. Rev. Nutr. 1998, 18, 441–469. [Google Scholar] [CrossRef] [PubMed]
- Hassett, R.; Dix, D.R.; Eide, D.J.; Kosman, D.J. The Fe(II) permease Fet4p functions as a low affinity copper transporter and supports normal copper trafficking in Saccharomyces cerevisiae. Biochem. J. 2000, 351 Pt 2, 477–484. [Google Scholar] [CrossRef]
- Supek, F.; Supekova, L.; Nelson, H.; Nelson, N. A yeast manganese transporter related to the macrophage protein involved in conferring resistance to mycobacteria. Proc. Natl. Acad. Sci. USA 1996, 93, 5105–5110. [Google Scholar] [CrossRef] [Green Version]
- Jensen, L.T.; Ajua-Alemanji, M.; Culotta, V.C. The Saccharomyces cerevisiae high affinity phosphate transporter encoded by PHO84 also functions in manganese homeostasis. J. Biol. Chem. 2003, 278, 42036–42040. [Google Scholar] [CrossRef] [Green Version]
- Ofiteru, A.M.; Ruta, L.L.; Rotaru, C.; Dumitru, I.; Ene, C.D.; Neagoe, A.; Farcasanu, I.C. Overexpression of the PHO84 gene causes heavy metal accumulation and induces Ire1p-dependent unfolded protein response in Saccharomyces cerevisiae cells. Appl. Microbiol. Biotechnol. 2012, 94, 425–435. [Google Scholar] [CrossRef]
- Zhao, H.; Eide, D. The yeast ZRT1 gene encodes the zinc transporter protein of a high-affinity uptake system induced by zinc limitation. Proc. Natl. Acad. Sci. USA 1996, 93, 2454–2458. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.; Eide, D. The ZRT2 gene encodes the low affinity zinc transporter in Saccharomyces cerevisiae. J. Biol. Chem. 1996, 271, 23203–23210. [Google Scholar] [CrossRef] [Green Version]
- Available online: https://www.yeastgenome.org/ (accessed on 10 August 2020).
- Nakajima-Shimada, J.; Iida, H.; Tsuji, F.I.; Anraku, Y. Monitoring of intracellular calcium in Saccharomyces cerevisiae with an apoaequorine cDNA expression system. Proc. Natl. Acad. Sci. USA 1991, 88, 6878–6882. [Google Scholar] [CrossRef] [Green Version]
- Tisi, R.; Baldassa, S.; Belotti, F.; Martegani, E. Phospholipase C is required for glucose-induced calcium influx in budding yeast. FEBS Lett. 2002, 520, 133–138. [Google Scholar] [CrossRef]
- Mandal, D.; Woolf, T.B.; Rao, R. Manganese selectivity of Pmr1, the yeast secretory pathway ion pump, is defined by residue gln783 in transmembrane segment 6. Residue Asp778 is essential for cation transport. J. Biol. Chem. 2000, 275, 23933–23938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.; Spector, D.; Godon, C.; Labarre, J.; Toledano, M.B. A new antioxidant with alkyl hydroperoxide defense properties in yeast. J. Biol. Chem. 1999, 274, 4537–4544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farcasanu, I.C.; Hirata, D.; Tsuchiya, E.; Mizuta, K.; Miyakawa, T. Involvement of thioredoxin peroxidase type II (Ahp1p) of Saccharomyces cerevisiae in Mn2+ homeostasis. Biosci. Biotechnol. Biochem. 1999, 63, 1871–1881. [Google Scholar] [CrossRef] [Green Version]
- Kanzaki, M.; Nagasawa, M.; Kojima, I.; Sato, C.; Naruse, K.; Sokabe, M.; Iida, H. Molecular identification of a eukaryotic, stretch-activated nonselective cation channel. Science 1999, 5429, 882–886. [Google Scholar] [CrossRef]
- Erikson, K.M.; Aschner, M. Manganese: Its role in disease and health. In Essential Metals in Medicine: Therapeutic Use and Toxicity of Metal Ions in the Clinic; Sigel, A., Freisinger, E., Sigel, R.K.O., Carver, P.L., Eds.; De Gruyter GmbH: Berlin, Germany, 2019; pp. 253–266. [Google Scholar] [CrossRef]
- Farcasanu, I.C.; Mizunuma, M.; Nishiyama, F.; Miyakawa, T. Role of L-histidine in conferring tolerance to Ni2+ in Sacchromyces cerevisiae cells. Biosci. Biotechnol. Biochem. 2005, 69, 2343–2348. [Google Scholar] [CrossRef]
- Oprea, E.; Ruta, L.L.; Nicolau, I.; Popa, C.V.; Neagoe, A.D.; Farcasanu, I.C. Vaccinium corymbosum L. (blueberry) extracts exhibit protective action against cadmium toxicity in Saccharomyces cerevisiae cells. Food Chem. 2014, 152, 516–521. [Google Scholar] [CrossRef]
- Lapinskas, P.J.; Cunningham, K.W.; Liu, X.F.; Fink, G.R.; Culotta, V.C. Mutations in PMR1 suppress oxidative damage in yeast cells lacking superoxide dismutase. Mol. Cell. Biol. 1995, 15, 1382–1388. [Google Scholar] [CrossRef] [Green Version]
- Au, C.; Benedetto, A.; Aschner, M. Manganese transport in eukaryotes: The role of DMT1. Neurotoxicology 2008, 29, 569–576. [Google Scholar] [CrossRef] [Green Version]
- Courville, P.; Chaloupka, R.; Cellier, M.F. Recent progress in structure-function analyses of Nramp proton-dependent metal-ion transporters. Biochem. Cell Biol. 2006, 84, 960–978. [Google Scholar] [CrossRef]
- Calhoun, E.S.; McGovern, R.M.; Janney, C.A.; Cerhan, J.R.; Iturria, S.J.; Smith, D.I.; Gostout, B.S.; Persing, D.H. Host genetic polymorphism analysis in cervical cancer. Clin. Chem. 2002, 48, 1218–1224. [Google Scholar] [CrossRef] [PubMed]
- Lenormand, C.; Couteau, J.; Nouhaud, F.X.; Maillet, G.; Bou, J.; Gobet, F.; Pfister, C. Predictive value of NRAMP1 and HGPX1 gene polymorphism for maintenance BCG response in non-muscle-invasive bladder cancer. Anticancer Res. 2016, 36, 1737–1743. [Google Scholar] [PubMed]
- Hernroth, B.; Holm, I.; Gondikas, A.; Tassidis, H. Manganese inhibits viability of prostate cancer cells. Anticancer Res. 2018, 38, 137–145. [Google Scholar] [CrossRef] [Green Version]
- Doble, P.A.; Miklos, G.L.G. Distributions of manganese in diverse human cancers provide insights into tumour radioresistance. Metallomics 2018, 10, 1191–1210. [Google Scholar] [CrossRef] [Green Version]
- Brachmann, C.B.; Davies, A.; Cost, G.J.; Caputo, E.; Li, J.; Hieter, P.; Boeke, J.D. Designer deletion strains derived from Saccharomyces cerevisiae S288C: A useful set of strains and plasmids for PCR-mediated gene disruption and other applications. Yeast 1998, 14, 115–132. [Google Scholar] [CrossRef]
- Sherman, F. Getting started with yeast. Methods Enzymol. 2002, 350, 3–41. [Google Scholar] [CrossRef]
- Cohen, R.; Engelberg, D. Commonly used Saccharomyces cerevisiae strains (e.g., BY4741, W303) are growth sensitive on synthetic complete medium due to poor leucine uptake. FEMS Microbiol. Lett. 2007, 273, 239–243. [Google Scholar] [CrossRef] [Green Version]
- Dohmen, R.J.; Strasser, A.W.M.; Honer, C.B.; Hollenberg, C.P. An efficient transformation procedure enabling long-term storage of competent cells of various yeast genera. Yeast 1991, 7, 691–692. [Google Scholar] [CrossRef]
- Tisi, R.; Martegani, E.; Brandão, R.L. Monitoring yeast intracellular Ca2+ levels using an in vivo bioluminescence assay. Cold Spring Harb. Protoc. 2015, 2015, 210–213. [Google Scholar] [CrossRef] [PubMed]
- Ruta, L.L.; Kissen, R.; Nicolau, I.; Neagoe, A.D.; Petrescu, A.J.; Bones, A.M.; Farcasanu, I.C. Heavy metal accumulation by Saccharomyces cerevisiae cells armed with metal binding hexapeptides targeted to the inner face of the plasma membrane. Appl. Microbiol. Biotechnol. 2017, 101, 5749–5763. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
Sample Availability: Samples of the oleandrin stock solution used are available from the authors. |

| Metal Detected | Cellular Metal Content (nmoles/mg Total Cell Protein) | |
|---|---|---|
| No Oleandrin | Oleandrin | |
| Co2+ | 1.32 ± 0.24 | 1.44 ± 0.31 |
| Cu2+ | 5.84 ± 0.42 | 5.52 ± 0.82 |
| Fe3+ | 52.82 ± 3.24 | 54.33 ± 3.84 |
| Mn2+ | 4.25 ± 0.62 | 9.92 ± 1.82 * |
| Ni2+ | 0.24 ± 0.12 | 0.22 ± 0.21 |
| Zn2+ | 12.42 ± 1.14 | 11.88 ± 1.43 |
| Li+ | 1.14 ± 0.32 | 1.21 ± 0.22 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruta, L.L.; Popa, C.V.; Farcasanu, I.C. Cytotoxicity of Oleandrin Is Mediated by Calcium Influx and by Increased Manganese Uptake in Saccharomyces cerevisiae Cells. Molecules 2020, 25, 4259. https://doi.org/10.3390/molecules25184259
Ruta LL, Popa CV, Farcasanu IC. Cytotoxicity of Oleandrin Is Mediated by Calcium Influx and by Increased Manganese Uptake in Saccharomyces cerevisiae Cells. Molecules. 2020; 25(18):4259. https://doi.org/10.3390/molecules25184259
Chicago/Turabian StyleRuta, Lavinia L., Claudia V. Popa, and Ileana C. Farcasanu. 2020. "Cytotoxicity of Oleandrin Is Mediated by Calcium Influx and by Increased Manganese Uptake in Saccharomyces cerevisiae Cells" Molecules 25, no. 18: 4259. https://doi.org/10.3390/molecules25184259






