Production and Characterization of Molecular Dications: Experimental and Theoretical Efforts
Abstract
1. Introduction
2. Experimental Studies
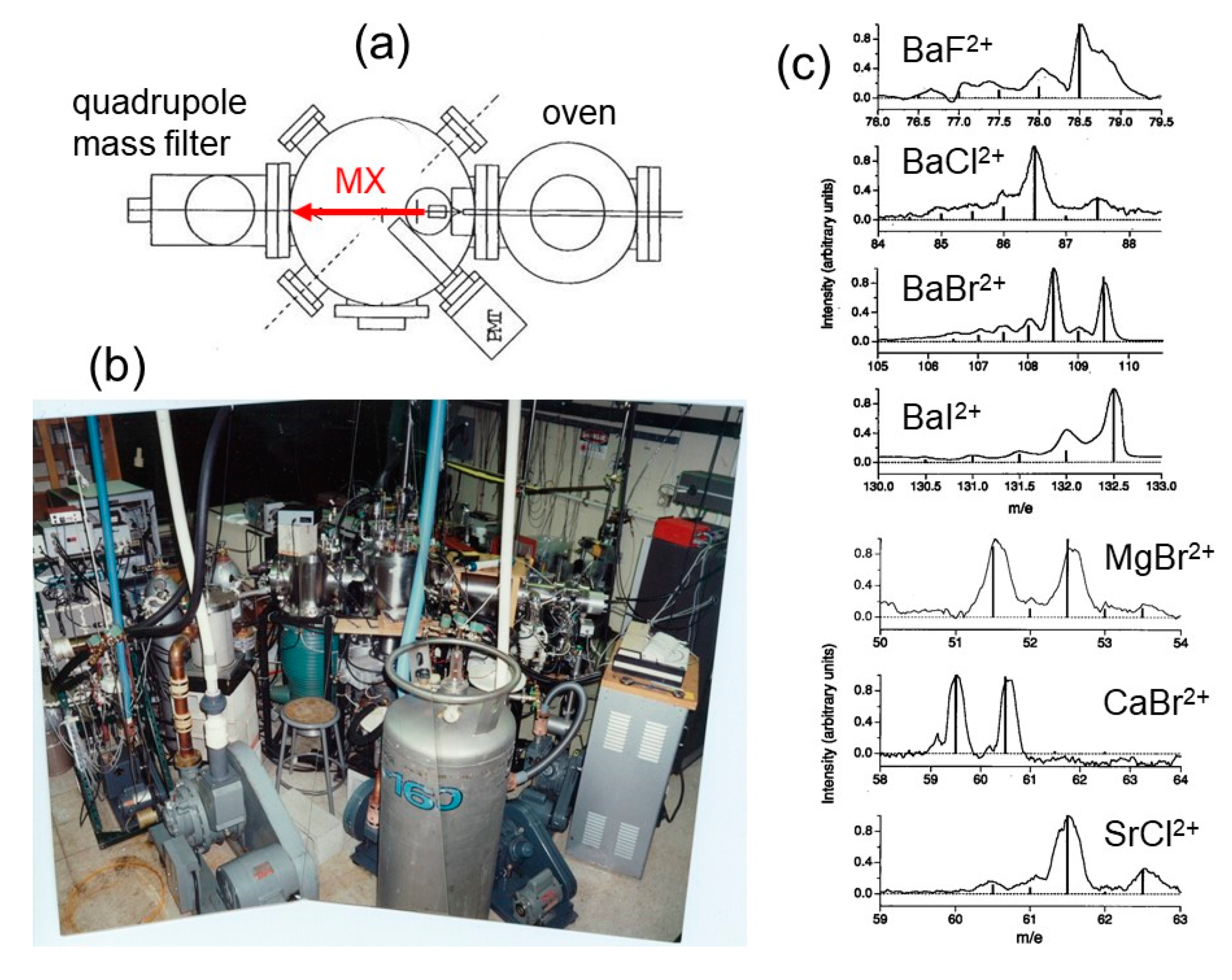
2.1. The Alkaline Earth Monohalide Dications Production by Electron Impact: The Stanford Experiment
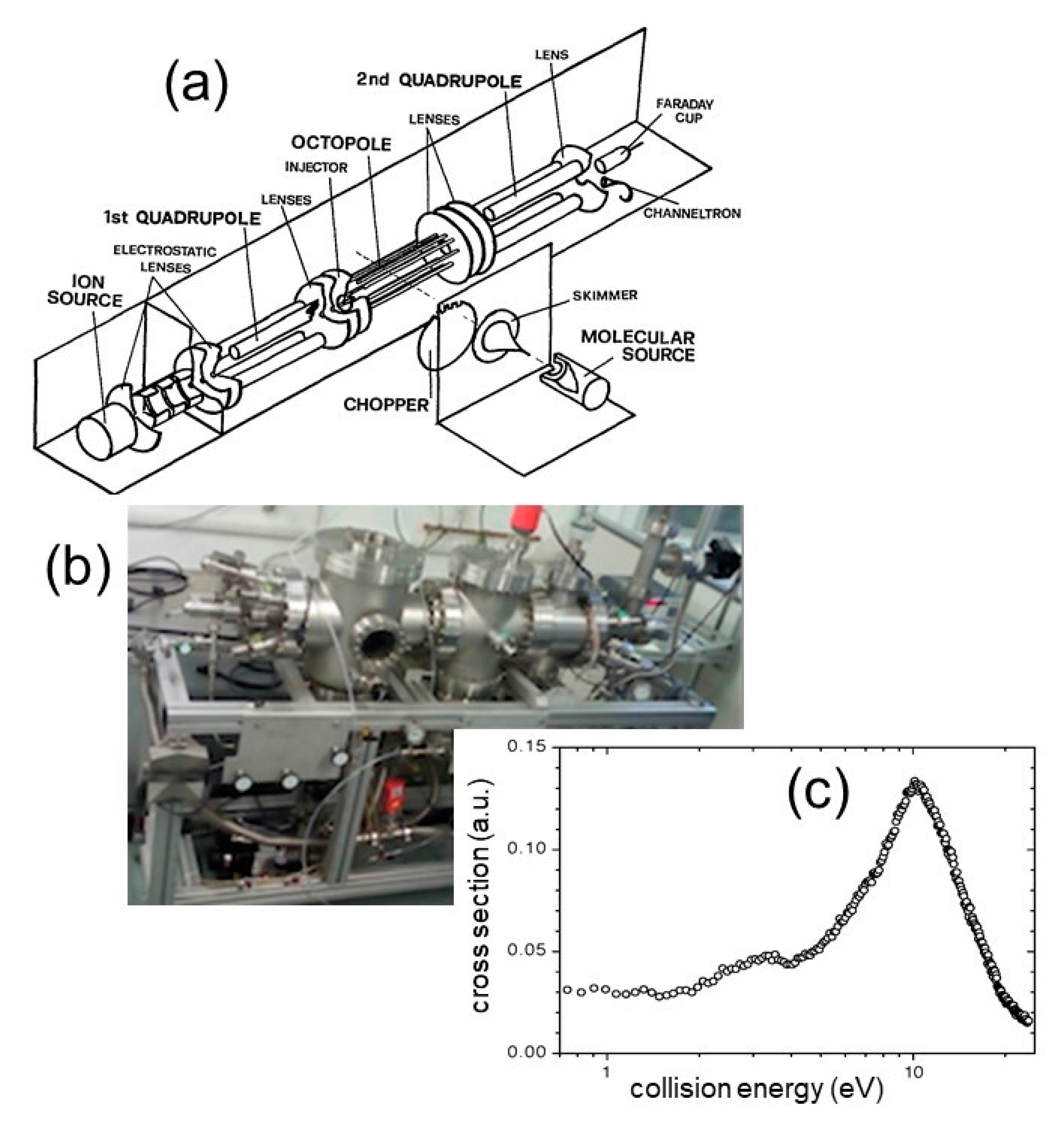
2.2. The Production of Metastable Dications by Ion-molecules Reactions: The Trento Experiment
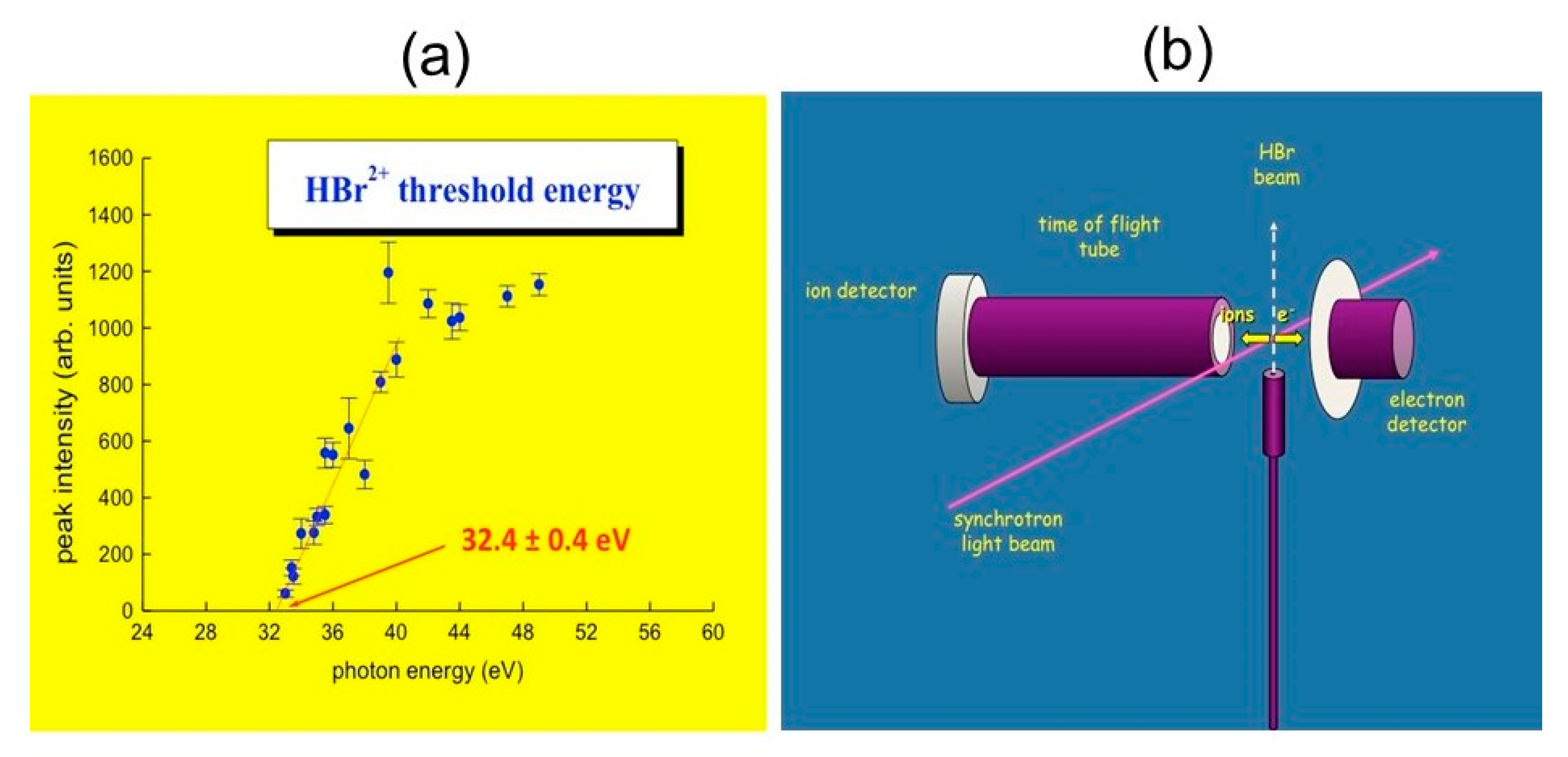
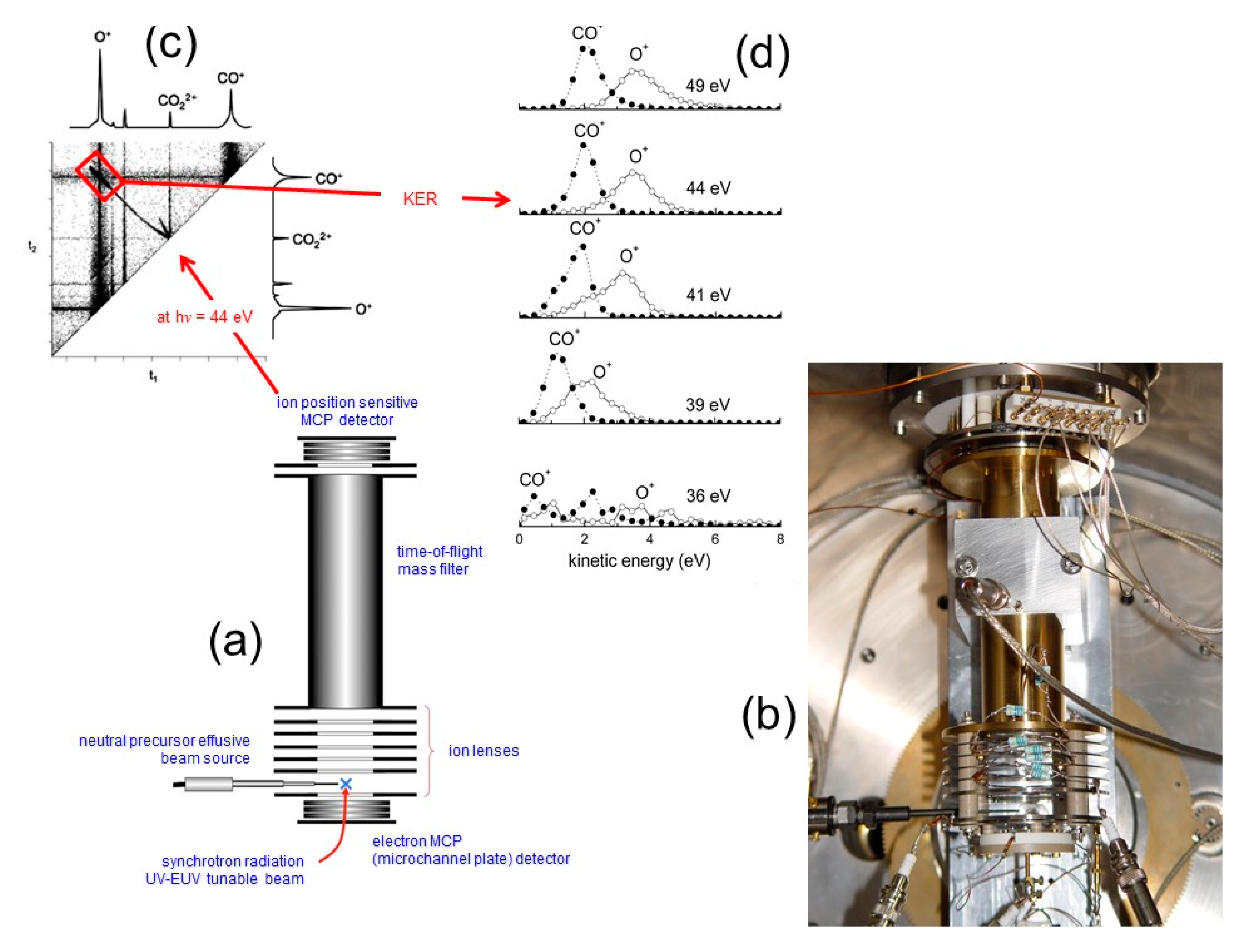
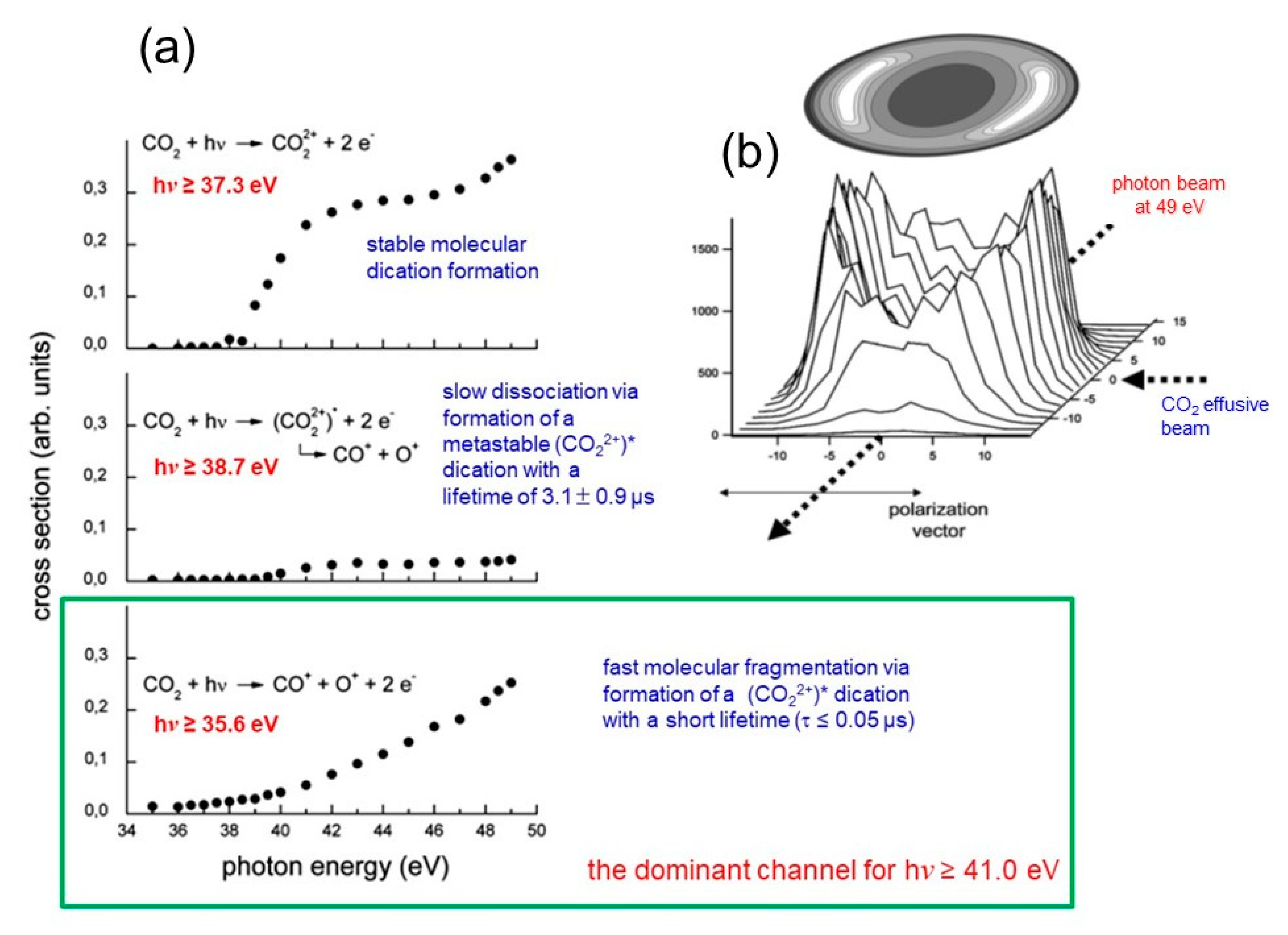
2.3. Molecular Dications by Double Photoionization Using Synchrotron Radiation: The Elettra Experiments
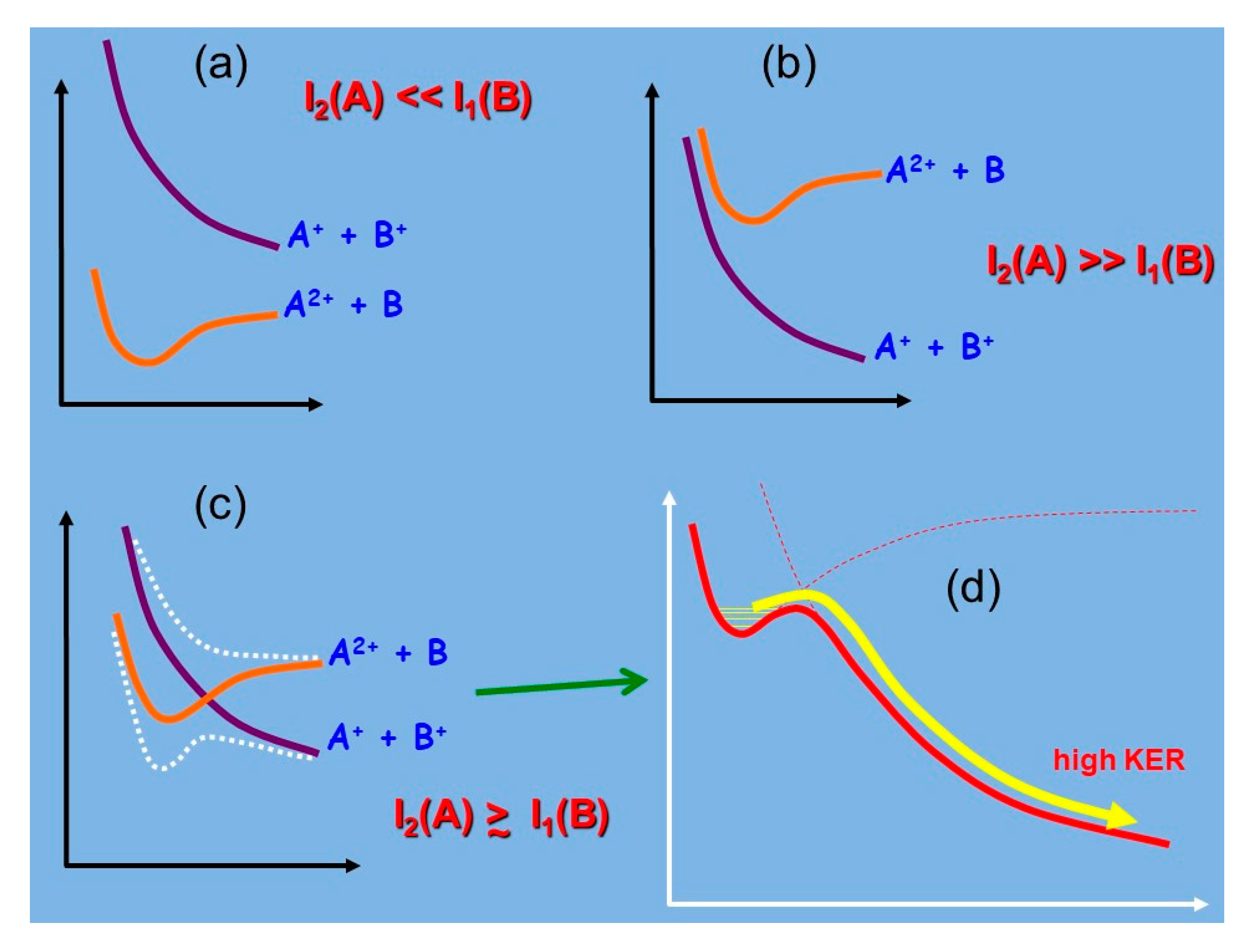
Looking at the Escape of Ions from Planetary Atmospheres
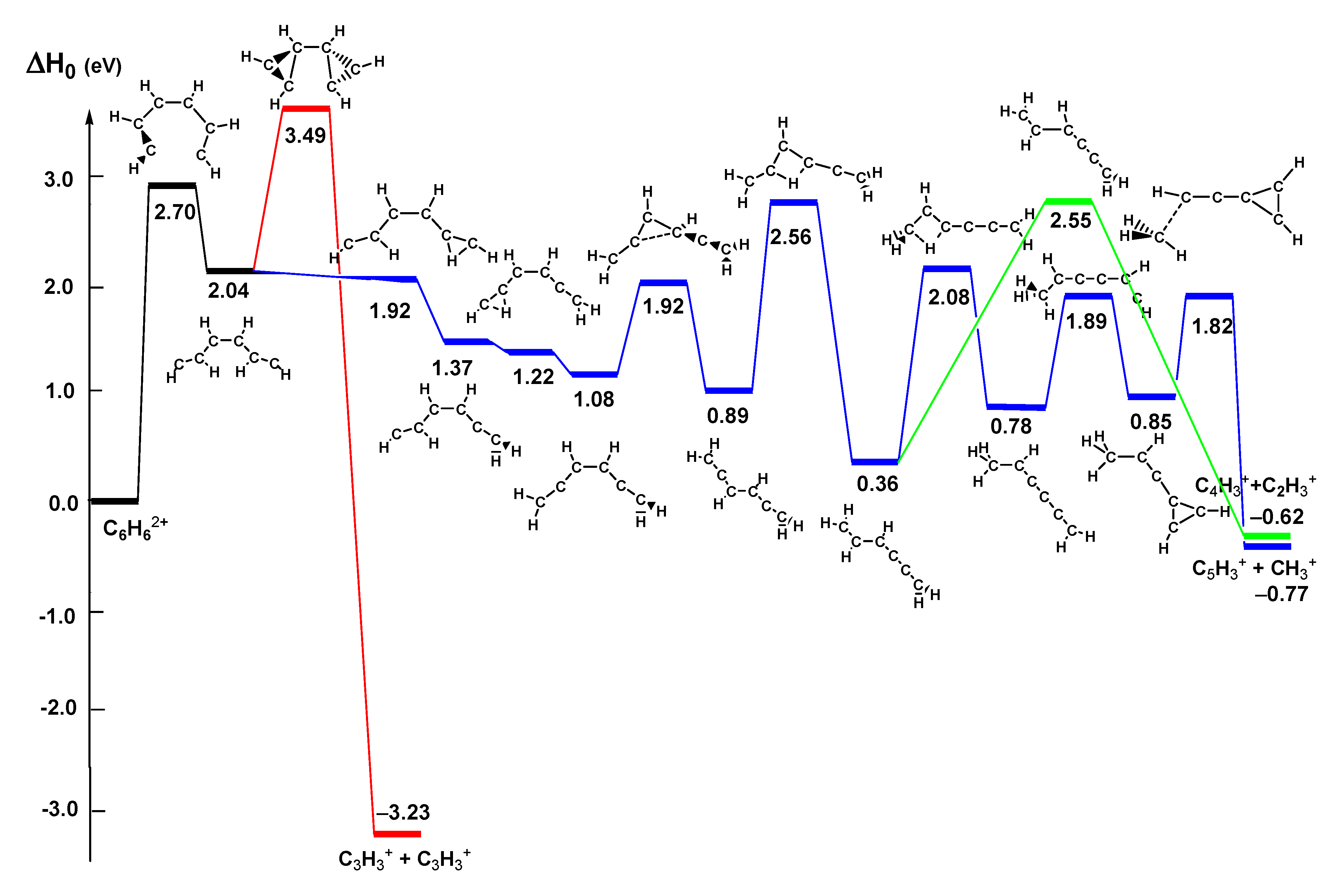
3. Theoretical and Computational Calculations
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Larsson, M.; Geppert, W.D.; Nyman, G. Ion chemistry in space. Rep. Prog. Phys. 2012, 75, 066901. [Google Scholar] [CrossRef]
- Alagia, M.; Balucani, N.; Candori, P.; Falcinelli, S.; Pirani, F.; Richter, R.; Rosi, M.; Stranges, S.; Vecchiocattivi, F. Production of ions at high energy and its role in extraterrestrial environments. Rend. Lincei Sci. Fis. Nat. 2013, 24, 53–65. [Google Scholar] [CrossRef]
- Falcinelli, S.; Pirani, F.; Vecchiocattivi, F. The possible role of penning ionization processes in planetary atmospheres. Atmosphere 2015, 6, 299–317. [Google Scholar] [CrossRef]
- Witasse, O.; Dutuit, O.; Lilensten, J.; Thissen, R.; Zabka, J.; Alcaraz, C.; Blelly, P.L.; Bougher, S.W.; Engel, S.; Andersen, L.H.; et al. Prediction of a CO2++ layer in the atmosphere of Mars. Geophys. Res. Lett. 2002, 29, 104. [Google Scholar] [CrossRef]
- Lilensten, J.; Witasse, O.; Simon, C.; Soldi-Lose, H.; Dutuit, O.; Thissen, R.; Alcaraz, C. Prediction of a N2++ layer in the atmosphere of Titan. Geophys. Res. Lett. 2005, 32, L03203. [Google Scholar] [CrossRef]
- Gronoff, G.; Lilensten, J.; Simon, C.; Witasse, O.; Thissen, R.; Dutuit, O.; Alcaraz, C. Modeling dications in the diurnal ionosphere of Venus. A&A 2007, 465, 641–645. [Google Scholar]
- Thissen, R.; Witasse, O.; Dutuit, O.; Wedlund, C.S.; Gronoff, G.; Lilensten, J. Doubly-charged ions in the planetary ionospheres: A review. Phys. Chem. Chem. Phys. 2011, 13, 18264–18287. [Google Scholar] [CrossRef]
- Lilensten, J.; Simon Wedlund, C.; Barthélémy, M.; Thissen, R.; Ehrenreich, D.; Gronoff, G.; Witasse, O. Dications and thermal ions in planetary atmospheric escape. Icarus 2013, 222, 169–187. [Google Scholar] [CrossRef]
- Falcinelli, S.; Pirani, F.; Alagia, M.; Schio, L.; Richter, R.; Stranges, S.; Balucani, N.; Vecchiocattivi, F. Molecular Dications in Planetary Atmospheric Escape. Atmosphere 2016, 7, 112. [Google Scholar] [CrossRef]
- Herman, Z. Dynamics of charge transfer and chemical reactions of doubly-charged ions at low collision energies. Int. Rev. Phys. Chem. 1996, 15, 299–324. [Google Scholar] [CrossRef]
- Sabzyan, H.; Keshavarz, E.; Noorisafa, Z. Diatomic dications and anions. J. Iran. Chem. Soc. 2014, 11, 871. [Google Scholar] [CrossRef]
- Price, S.D.; Fletcher, J.D.; Gossan, F.E.; Parkes, M.A. Bimolecular reactions of the dications and trications of atoms and small molecules in the gas-phase. Int. Rev. Phys. Chem. 2017, 36, 145–183. [Google Scholar] [CrossRef]
- Bates, D.R.; Carson, T.R. Doubly Charged Diatomic Molecular Ions. Proc. Phys. Soc. A 1955, 68, 1199–1202. [Google Scholar] [CrossRef]
- Thomson, J.J. Rays of Positive Electricity, 2nd ed.; Longmans, Green and Co.: London, UK, 1921; p. 84. [Google Scholar]
- Conrad, R. The appearance of doubly positively charged molecules in a beam of canal rays. Phys. Z. 1930, 31, 888–892. [Google Scholar]
- Aston, F.W. Mass-Spectra of Helium and Oxygen. Nature 1932, 130, 21–22. [Google Scholar] [CrossRef]
- Vaughan, A.L. Mass Spectrograph Analyses, and Critical Potentials for the Production of Ions by Electron Impact, in Nitrogen and Carbon Monoxide. Phys. Rev. 1931, 38, 1687–1695. [Google Scholar] [CrossRef]
- Pauling, L. The normal state of the helium molecule-ions He2+ and He2++. J. Chem. Phys. 1933, 1, 56–59. [Google Scholar] [CrossRef]
- Nicolaides, C.A. Energy generation from volcanic ground-states—Application to cold He22+. Chem. Phys. Lett. 1989, 161, 547–553. [Google Scholar] [CrossRef]
- Boldyrev, A.I.; Simons, J. Ab initio study of geometrically metastable multiprotonated species: MHnk+. J. Chem. Phys. 1992, 97, 4272–4281. [Google Scholar] [CrossRef]
- Hurley, A.C.; Maslen, V.W. Potential Curves for Doubly Positive Diatomic Ions. J. Chem. Phys. 1961, 34, 1919–1925. [Google Scholar] [CrossRef]
- Hurley, A.C. Potential energy curves for doubly positive diatomic ions: Part II. Predicted states and transitions of N22+, O22+, and NO22+. J. Molec. Spectrosc. 1962, 9, 18–29. [Google Scholar] [CrossRef]
- Wong, M.W.; Nobes, R.H.; Bouma, W.J.; Radom, L. Isoelectronic analogs of molecular nitrogen: Tightly bound multiply charged species. J. Chem. Phys. 1989, 91, 2971–2979. [Google Scholar] [CrossRef]
- Nobes, R.H.; Moncrieff, D.; Wong, M.W.; Radom, L.; Gill, P.M.W.; Pople, J.A. The structure and stability of the O2+2 dication: A dramatic failure of Møller—Plesset perturbation theory. Chem. Phys. Lett. 1991, 182, 216–224. [Google Scholar] [CrossRef]
- Bruna, P.J.; Wright, J.S. Strongly bound metastable states of B2+2. J. Chem. Phys. 1990, 93, 2617–2630. [Google Scholar] [CrossRef]
- Kolbuszewski, M.; Wright, J.S. Predicting thermodynamic stability of diatomic dications: A case study of BeF2+. Can. J. Chem. 1993, 71, 1562–1569. [Google Scholar] [CrossRef]
- Miller, P.J.; Rogers, S.A.; Senekowitsch, J.; O’Neil, S.V.; Leone, S.R.; Werner, H.-J.; Knowles, P.J. Multireference—Configuration interaction (MR-CI) calculations of HS2+ and experimental observation via electron impact ionization of H2S. Int. J. Mass Spectrom. Ion. Process. 1990, 100, 505–519. [Google Scholar] [CrossRef]
- Levasseur, N.; Millie, P.; Archirel, P.; Levy, B. Bond formation between positively charged species. Non-adiabatic analysis and valence-bond model in the CO2+ case. Chem. Phys. 1991, 153, 387–398. [Google Scholar] [CrossRef]
- Senekowitsch, J.; O’Neill, S. MetasTable 3Σ−g ground state of F2++ and the bonding in molecular dications. J. Chem. Phys. 1991, 95, 1847–1851. [Google Scholar] [CrossRef]
- Senekowitsch, J.; O’Neill, S.; Meyer, W. On the bonding in doubly charged diatomics. Theoret. Chim. Acta 1992, 84, 85–93. [Google Scholar] [CrossRef]
- Bauschlicher, C.W., Jr.; Langhoff, S.R. Theoretical study of the bonding in molecular transition-metal cations. Int. Rev. Phys. Chem. 1990, 9, 149–185. [Google Scholar] [CrossRef]
- Rosi, M.; Bauschlicher, C.W., Jr. On the bonding of La+ and La2+ to C2H2, C2H4, and C3H6. Chem. Phys. Lett. 1990, 166, 189–194. [Google Scholar] [CrossRef]
- Tarantelli, F.; Sgamellotti, A.; Cederbaum, L.S.; Shirmer, J. Theoretical investigation of many dicationic states and the Auger spectrum of benzene. J. Chem. Phys. 1987, 86, 2201–2206. [Google Scholar] [CrossRef]
- Ohrendorf, M.-L.; Tarantelli, F.; Cederbaum, L.S. Dicationic states of hydrocarbons and a statistical approach to their Auger spectra. J. Chem. Phys. 1990, 92, 2984–2999. [Google Scholar] [CrossRef]
- Hochlaf, M.; Bennett, F.R.; Chambaud, G.; Rosmus, P. Theoretical study of the electronic states of CO22+. J. Phys. B At. Mol. Opt. Phys. 1998, 31, 2163–2175. [Google Scholar] [CrossRef]
- Sharma, V.; Bapat, B.; Mondal, J.; Hochlaf, M.; Giri, K.; Sathyamurthy, N. Dissociative double ionization of CO2: Dynamics, energy levels, and lifetime. J. Phys. Chem. A 2007, 111, 10205–10211. [Google Scholar] [CrossRef]
- De Melo, G.F.; Ornellas, F.R. The thermodynamic stability of strontium monohalides dications: A theoretical exploration of the electronic states and spectroscopic parameters of SrF2+ and SrCl2+. Chem. Phys. Lett. 2018, 712, 118–122. [Google Scholar] [CrossRef]
- De Melo, G.F.; Ornellas, F.R. A high-level theoretical characterization of the electronic states and spectroscopic parameters of SrBr2+ and SrI2+, and thermodynamic stability in the family of strontium monohalides dications. Chem. Phys. Lett. 2019, 722, 12–17. [Google Scholar] [CrossRef]
- De Melo, G.F.; Ornellas, F.R. Thermodynamic stability and spectroscopic properties of alkaline earth monobromides: The cases of MgBr2+ and BaBr2+. Comput. Theor. Chem. 2020, 1178, 112792. [Google Scholar] [CrossRef]
- Dorman, F.H.; Morrison, J.D. Double and Triple Ionization in Molecules Induced by Electron Impact. J. Chem. Phys. 1961, 35, 575–581. [Google Scholar] [CrossRef]
- Johnsen, R.; Biondi, M.A. Thermal-energy charge transfer, quenching, and association reactions of doubly charged ions in the rare gases. Phys. Rev. A 1979, 20, 87–97. [Google Scholar] [CrossRef]
- Helm, H.; Stephan, K.; Märk, T.D.; Huestis, D.L. Double ionization of van der Waals dimers: NeXe and ArXe. J. Chem. Phys. 1981, 74, 3844–3851. [Google Scholar] [CrossRef]
- Rogers, S.A.; Miller, P.J.; Leone, S.R.; Brehm, B. Observation of the NF2+ dication in the electron impact ionization mass spectrum of NF3. Chem. Phys. Lett. 1990, 166, 137–140. [Google Scholar]
- Imamura, T.; Brion, C.E.; Koyano, I. Dissociative single, double, and triple photoionization of silicon tetrafluoride in the valence shell and silicon 2p regions (hν = 33–133 eV). J. Chem. Phys. 1991, 94, 4936–4948. [Google Scholar] [CrossRef]
- Guo, X.; Cooper, G.; Chan, W.-F.; Burton, G.R.; Brion, C.E. Absolute oscillator strengths for the photoabsorption, photoionization and ionic photofragmentation of silicon tetrafluoride. I. The valence shell. Chem. Phys. 1992, 161, 453–470. [Google Scholar] [CrossRef]
- Kolbuszewski, M.; Wright, J.S. Thermodynamically stable dications: AlF2+ and SiF2+. J. Phys. Chem. 1995, 99, 3455–3460. [Google Scholar] [CrossRef]
- Cossart, D.; Launay, F.; Robbe, J.M.; Gandara, G. The optical spectrum of the doubly charged molecular nitrogen ion: Rotational analysis of the (0-0) and (1-1) bands of the D1Σu+-X1Σg+ transition of N22+: Comparison of observations with Ab-initio calculation results. J. Molec. Spectrosc. 1985, 113, 142–158. [Google Scholar] [CrossRef]
- Cossart, D.; Launay, F. The vacuum UV emission spectrum of the 15N22+ molecular ion. J. Molec. Spectrosc. 1985, 113, 159–166. [Google Scholar] [CrossRef]
- Cossart, D.; Bonneau, M. Optical emission spectrum of the NO2+ dication. J. Molec. Spectrosc. 1987, 125, 413–427. [Google Scholar] [CrossRef]
- Chatterjee, B.K.; Johnsen, R. Thermal-energy reactions of O22+ ions with O2, N2, CO2, NO, and Ne. J. Chem. Phys. 1989, 91, 1378–1379. [Google Scholar] [CrossRef]
- Van der Kamp, A.B.; Athmer, P.B.; Hiemstra, R.S.; Peterson, J.R.; van der Zande, W.J. Evidence for quasi bound states in highly excited CO+ and NO+. Chem. Phys. 1995, 193, 181–191. [Google Scholar] [CrossRef]
- Fišer, J.; Franzreb, K.; Lörinčík, J.; Williams, P. Oxygen-containing diatomic dications in the gas phase. Eur. J. Mass Spectrom. 2009, 15, 315–324. [Google Scholar] [CrossRef]
- Brites, V.; K Franzreb, K.; Hochlaf, M. Metastable ClO2+ and ClO3+ ions in the gas phase: A combined theoretical and mass spectrometric investigation. Phys. Chem. Chem. Phys. 2011, 13, 18315–18321. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves dos Santos, L.; Franzreb, K.; Ornellas, F. Thermodynamically stable diatomic dications: The cases of SrO2+ and SrH2+. J. Chem. Phys. 2018, 148, 124306. [Google Scholar] [CrossRef]
- Price, S.D.; Eland, J.H.D.; Fournier, P.G.; Fournier, J.; Millié, P. Electronic states and decay mechanisms of the N2O2+ dication. J. Chem. Phys. 1988, 88, 1511–1515. [Google Scholar] [CrossRef]
- Thissen, R.; Delwiche, J.; Robbe, J.M.; Duflot, D.; Flament, J.P.; Eland, J.H.D. Dissociations of the ethyne dication C2H22+. J. Chem. Phys. 1993, 99, 6590–6599. [Google Scholar] [CrossRef]
- Slattery, A.E.; Field, T.A.; Ahmad, M.; Hall, R.I.; Lambourne, J.; Penent, F.; Lablanquie, P.; Eland, J.H. Spectroscopy and metastability of CO22+ molecular ions. J. Chem. Phys. 2005, 122, 084317. [Google Scholar] [CrossRef]
- Eland, J.H.D. Dynamics of Double Photoionization in Molecules and Atoms. In Advances in Chemical Physics; Rice, S.A., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; Volume 141, pp. 103–150. [Google Scholar]
- Hankin, S.M.; Villeneuve, D.M.; Corkum, P.B.; Rayner, D.M. Intense-field laser ionization rates in atoms and molecules. Phys. Rev. A 2001, 64, 013405. [Google Scholar] [CrossRef]
- Agostini, P.; DiMauro, L.F. Chapter 3—Atomic and Molecular Ionization Dynamics in Strong Laser Fields: From Optical to X-rays. Adv. At. Mol. Opt. Phys. 2012, 61, 117–158. [Google Scholar]
- Yatsuhashi, T.; Nakashima, N. Multiple ionization and Coulomb explosion of molecules, molecular complexes, clusters and solid surfaces. J. Photochem. Photobiol. C Photochem. Rev. 2018, 34, 52–84. [Google Scholar] [CrossRef]
- Kolbuszewski, M.; Wright, J.S. Thermodynamically stable diatomic dications: MgN2+, MgO2+, MgF2+ and MgNe2+. Chem. Phys. Lett. 1994, 218, 338–342. [Google Scholar] [CrossRef]
- Falcinelli, S.; Fernandez-Alonso, F.; Kalogerakis, K.; Zare, R.N. Mass spectrometric detection of alkaline earth monohalide dications. Mol. Phys. 1996, 88, 663–672. [Google Scholar] [CrossRef]
- Tosi, P.; Correale, R.; Lu, W.; Falcinelli, S.; Bassi, D. Production of the molecular dication ArN2+ in the reaction Ar2+ + N2. Phys. Rev. Lett. 1999, 82, 450–452. [Google Scholar] [CrossRef]
- Tosi, P.; Lu, W.; Correale, R.; Bassi, D. Production of the molecular dication ArC2+ by ion-molecule reactions. Chem. Phys. Lett. 1999, 310, 180–182. [Google Scholar] [CrossRef]
- Alagia, M.; Boustimi, M.; Brunetti, B.G.; Candori, P.; Falcinelli, S.; Richter, R.; Stranges, S.; Vecchiocattivi, F. Mass Spectrometric Study of Double Photoionization of HBr Molecules. J. Chem. Phys. 2002, 117, 1098–1102. [Google Scholar] [CrossRef]
- Teixidor, M.M.; Pirani, F.; Candori, P.; Falcinelli, S.; Vecchiocattivi, F. Predicted Structure and Energetics of HCl2+. Chem. Phys. Lett. 2003, 379, 139–146. [Google Scholar] [CrossRef]
- Alagia, M.; Biondini, F.; Brunetti, B.G.; Candori, P.; Falcinelli, S.; Teixidor, M.M.; Pirani, F.; Richter, R.; Stranges, S.; Vecchiocattivi, F. The double photoionization of HCl: An ion-electron coincidence study. J. Chem. Phys. 2004, 121, 10508–10512. [Google Scholar] [CrossRef]
- Alagia, M.; Brunetti, B.G.; Candori, P.; Falcinelli, S.; Teixidor, M.M.; Pirani, F.; Richter, R.; Stranges, S.; Vecchiocattivi, F. The double photoionization of hydrogen iodide molecules. J. Chem. Phys. 2006, 124, 204318. [Google Scholar] [CrossRef]
- Alagia, M.; Brunetti, B.G.; Candori, P.; Falcinelli, S.; Teixidor, M.M.; Pirani, F.; Richter, R.; Stranges, S.; Vecchiocattivi, F. Threshold-photoelectron-spectroscopy-coincidence study of the double photoionization of HBr. J. Chem. Phys. 2004, 120, 6980–6984. [Google Scholar] [CrossRef]
- Candori, P.; Falcinelli, S.; Pirani, F.; Tarantelli, F.; Vecchiocattivi, F. Interaction components in the hydrogen halide dications. Chem. Phys. Lett. 2007, 436, 322–326. [Google Scholar] [CrossRef]
- Lavollée, M. A new detector for measuring three-dimensional momenta of charged particles in coincidence. Rev. Sci. Instrum. 1990, 70, 2968–2974. [Google Scholar] [CrossRef]
- Lundqvist, M.; Baltzer, P.; Edvardsson, D.; Karlsson, L.; Wannberg, B. Novel time of flight instrument for doppler free kinetic energy release spectroscopy. Phys. Rev. Lett. 1995, 75, 1058–1061. [Google Scholar] [CrossRef] [PubMed]
- Field, T.A.; Eland, J.H.D. Lifetimes of metastable molecular doubly charged ions. Chem. Phys. Lett. 1993, 211, 436–442. [Google Scholar] [CrossRef]
- Zare, R.N.; Herschbach, D.R. Angular Distribution of Products in Molecular Photodissociation. Bull. Am. Phys. Soc. 1962, 7, 458. [Google Scholar]
- Zare, R.N. Photoejection Dynamics. Mol. Photochem. 1972, 4, 1–37. [Google Scholar]
- Alagia, M.; Candori, P.; Falcinelli, S.; Lavollée, M.; Pirani, F.; Richter, R.; Stranges, S.; Vecchiocattivi, F. Double photoionization of CO2 molecules in the 34–50 eV Energy range. J. Phys. Chem. A 2009, 113, 14755–14759. [Google Scholar] [CrossRef]
- Alagia, M.; Candori, P.; Falcinelli, S.; Lavollèe, M.; Pirani, F.; Richter, R.; Stranges, S.; Vecchiocattivi, F. Dissociative double photoionization of CO2 molecules in the 36–49 eV energy range: Angular and energy distribution of ion products. Phys. Chem. Chem. Phys. 2010, 12, 5389–5395. [Google Scholar] [CrossRef]
- Alagia, M.; Candori, P.; Falcinelli, S.; Lavollée, M.; Pirani, F.; Richter, R.; Stranges, S.; Vecchiocattivi, F. Double photoionization of N2O molecules in the 28–40 eV energy range. Chem. Phys. Lett. 2006, 432, 398–402. [Google Scholar] [CrossRef]
- Alagia, M.; Candori, P.; Falcinelli, S.; Lavollée, M.; Pirani, F.; Richter, R.; Stranges, S.; Vecchiocattivi, F. Anisotropy of the angular distribution of fragment ions in dissociative double photoionization of N2O molecules in the 30–50 eV energy range. J. Chem. Phys. 2007, 126, 201101. [Google Scholar] [CrossRef]
- Alagia, M.; Candori, P.; Falcinelli, S.; Pirani, F.; Mundim, M.S.P.; Richter, R.; Rosi, M.; Stranges, S.; Vecchiocattivi, F. Dissociative double photoionization of benzene molecules in the 26–33 eV energy range. Phys. Chem. Chem. Phys. 2011, 13, 8245–8250. [Google Scholar] [CrossRef]
- Alagia, M.; Candori, P.; Falcinelli, S.; Mundim, M.S.P.; Pirani, F.; Richter, R.; Rosi, M.; Stranges, S.; Vecchiocattivi, F. Dissociative double photoionization of singly deuterated benzene molecules in the 26–33 eV energy range. J. Chem. Phys. 2011, 135, 144304. [Google Scholar] [CrossRef]
- Alagia, M.; Callegari, C.; Candori, P.; Falcinelli, S.; Pirani, F.; Richter, R.; Stranges, S.; Vecchiocattivi, F. Angular and energy distribution of fragment ions in dissociative double photoionization of acetylene molecules at 39 eV. J. Chem. Phys. 2012, 136, 204302. [Google Scholar] [CrossRef]
- Falcinelli, S.; Alagia, M.; Farrar, J.M.; Kalogerakis, K.S.; Pirani, F.; Richter, R.; Schio, L.; Stranges, S.; Rosi, M.; Vecchiocattivi, F. Angular and energy distributions of fragment ions in dissociative double photoionization of acetylene molecules in the 31.9–50.0 eV photon energy range. J. Chem. Phys. 2016, 145, 114308. [Google Scholar] [CrossRef]
- Falcinelli, S.; Vecchiocattivi, F.; Alagia, M.; Schio, L.; Richter, R.; Stranges, S.; Catone, D.; Arruda, M.S.; Mendes, L.A.V.; Palazzetti, F.; et al. Double photoionization of propylene oxide: A coincidence study of the ejection of a pair of valence-shell electrons. J. Chem. Phys. 2018, 148, 114302. [Google Scholar] [CrossRef]
- Falcinelli, S.; Rosi, M.; Pirani, F.; Bassi, D.; Alagia, M.; Schio, L.; Richter, R.; Stranges, S.; Balucani, N.; Lorent, V.; et al. Angular Distribution of Ion Products in the Double Photoionization of Propylene Oxide. Front. Chem. 2019, 7, 621. [Google Scholar] [CrossRef]
- Falcinelli, S.; Vecchiocattivi, F.; Pirani, F.; Alagia, M.; Schio, L.; Richter, R.; Stranges, S.; Zhaunerchyk, V.; Balucani, N.; Rosi, M. The Fragmentation Dynamics of Simple Organic Molecules of Astrochemical Interest Interacting with VUV Photons. ACS Earth Space Chem. 2019, 3, 1862–1872. [Google Scholar] [CrossRef]
- Dobrijevic, M.; Hébrard, E.; Loison, J.C.; Hickson, K.M. Coupling of oxygen, nitrogen, and hydrocarbon species in the photochemistry of Titan’s atmosphere. Icarus 2014, 228, 324–346. [Google Scholar] [CrossRef]
- Falcinelli, S.; Rosi, M.; Candori, P.; Vecchiocattivi, F.; Farrar, J.M.; Pirani, F.; Balucani, N.; Alagia, M.; Richter, R.; Stranges, S. Kinetic energy release in molecular dications fragmentation after VUV and EUV ionization and escape from planetary atmospheres. Plan. Space Sci. 2014, 99, 149–157. [Google Scholar] [CrossRef]
- Falcinelli, S.; Pirani, F.; Alagia, M.; Schio, L.; Richter, R.; Stranges, S.; Vecchiocattivi, F. The escape of O+ ions from the atmosphere: An explanation of the observed ion density profiles on Mars. Chem. Phys. Lett. 2016, 666, 1–6. [Google Scholar] [CrossRef]
- McGuire, B.A.; Carroll, P.B.; Loomis, R.A.; Finneran, I.A.; Jewell, P.R.; Remijan, A.J. Discovery of the interstellar chiral molecule propylene oxide (CH3CHCH2O). Science 2016, 352, 1449–1452. [Google Scholar] [CrossRef]
- Brunetti, B.G.; Candori, P.; Cappelletti, D.; Falcinelli, S.; Pirani, F.; Stranges, D.; Vecchiocattivi, F. Penning Ionization Electron Spectroscopy of water molecules by metastable neon atoms. Chem. Phys. Lett. 2012, 539−540, 19–23. [Google Scholar] [CrossRef]
- Cavallotti, C.; Leonori, F.; Balucani, N.; Nevrly, V.; Bergeat, A.; Falcinelli, S.; Vanuzzo, G.; Casavecchia, P. Relevance of the Channel Leading to Formaldehyde + Triplet Ethylidene in the O(3P) + Propene Reaction under Combustion Conditions. J. Phys. Chem. Lett. 2014, 5, 4213–4218. [Google Scholar] [CrossRef]
- Rosi, M.; Bauschlicher, C.W., Jr.; Bakes, E.L.O. The Stability of C6H6+2: The Implication for Polycyclic Aromatic Hydrocarbons Dications. Astrophys. J. 2004, 609, 1192–1196. [Google Scholar] [CrossRef][Green Version]
- Allamandola, L.J.; Tielens, A.G.G.M.; Barker, J.R. Interstellar Polycyclic Aromatic Hydrocarbons: The Infrared Emission Bands, the Excitation/Emission Mechanism, and the Astrophysical Implications. Astrophys. J. Suppl. 1989, 71, 733–775. [Google Scholar] [CrossRef]
- Szczepanski, J.; Vala, M. Laboratory Evidence for Ionized Polycyclic Aromatic Hydrocarbons in the Interstellar Medium. Nature 1993, 363, 699–701. [Google Scholar] [CrossRef]
- Puget, J.L.; Léger, A. A New Component of the Interstellar Matter: Small Grains and Large Aromatic Molecules. Annu. Rev. Astron. Astrophys. 1989, 27, 161–198. [Google Scholar] [CrossRef]
- Draine, B.T. Photoelectric Heating of Interstellar Gas. Astrophys. J. Suppl. 1978, 36, 595–619. [Google Scholar] [CrossRef]
- Bakes, E.L.O.; Tielens, A.G.G.M. The Photoelectric Heating Mechanism for Very Small Graphitic Grains and Polycyclic Aromatic Hydrocarbons. Astrophys. J. 1994, 427, 822–838. [Google Scholar] [CrossRef]
- Vuitton, V.; Dutuit, O.; Smith, M.; Balucani, N. Chemistry of Titan’s Atmosphere. In Titan: Interior, Surface, Atmosphere, and Space Environment; Müller-Wodarg, I., Griffith, C., Lellouch, E., Cravens, T., Eds.; Cambridge Planetary Science, Cambridge University Press: Cambridge, UK, 2014; Chapter 7 ; pp. 224–284. [Google Scholar] [CrossRef]
- Balucani, N. Elementary reactions of N atoms with hydrocarbons: First steps towards the formation of prebiotic N-containing molecules in planetary atmospheres. Chem. Soc. Rev. 2012, 41, 5473–5483. [Google Scholar] [CrossRef]
- Brown, R.; Lebreton, J.P.; Waite, J. (Eds.) Titan from Cassini-Huygens; Springer: Dordrecht, The Netherlands, 2010. [Google Scholar] [CrossRef]
- Vuitton, V.; Yelle, R.V.; Cui, J. Formation and Distribution of Benzene on Titan. J. Geophys. Res. 2008, 113, E05007. [Google Scholar] [CrossRef]
- Clark, R.N.; Chrurcin, J.M.; Barnes, J.W.; Jaumann, R.; Soderblom, L.; Cruikshank, D.P.; Brown, R.H.; Rodriguez, S.; Lunine, J.; Stephan, K.; et al. Detection and mapping of hydrocarbon deposits on Titan. J. Geophys. Res. 2010, 115, E10005. [Google Scholar] [CrossRef]
- Becke, A.D. Density functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Stephens, P.J.; Devlin, F.J.; Chablowski, C.F.; Frisch, M.J. Ab Initio Calculation of Vibrational Absorption and Circular Dichroism Spectra Using Density Functional Force Fields. J. Phys. Chem. 1994, 98, 11623–11627. [Google Scholar] [CrossRef]
- Krishnan, R.; Binkley, J.S.; Seeger, R.; Pople, J.A. Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J. Chem. Phys. 1980, 72, 650–654. [Google Scholar] [CrossRef]
- Frisch, M.J.; Pople, J.A.; Binkley, J.S. Self-consistent molecular orbital methods 25. Supplementary functions for Gaussian basis sets. J. Chem. Phys. 1984, 80, 3265–3269. [Google Scholar] [CrossRef]
- Gonzalez, C.; Schlegel, H.B. An improved algorithm for reaction path following. J. Chem. Phys. 1989, 90, 2154–2161. [Google Scholar] [CrossRef]
- Gonzalez, C.; Schlegel, H.B. Reaction path following in mass-weighted internal coordinates. J. Phys. Chem. 1990, 94, 5523–5527. [Google Scholar] [CrossRef]
- Bartlett, R.J. Many-Body Perturbation Theory and Coupled Cluster Theory for Electron Correlation in Molecules. Annu. Rev. Phys. Chem. 1981, 32, 359–401. [Google Scholar] [CrossRef]
- Raghavachari, K.; Trucks, G.W.; Pople, J.A.; Head-Gordon, M. Quadratic configuration interaction. A general technique for determining electron correlation energies. Chem. Phys. Lett. 1989, 157, 479–483. [Google Scholar] [CrossRef]
- Olsen, J.; Jorgensen, P.; Koch, H.; Balkova, A.; Bartlett, R.J. Full configuration–interaction and state of the art correlation calculations on water in a valence double-zeta basis with polarization functions. J. Chem. Phys. 1996, 104, 8007–8015. [Google Scholar] [CrossRef]
- Leonori, F.; Petrucci, R.; Balucani, N.; Casavecchia, P.; Rosi, M.; Berteloite, C.; Le Picard, S.; Canosa, A.; Sims, I.R. Observation of organosulfur products (thiovinoxy, thioketene and thioformyl) in crossed-beam experiments and low temperature rate coefficients for the reaction S(1D) + C2H4. Phys. Chem. Chem. Phys. 2009, 11, 4701–4706. [Google Scholar] [CrossRef]
- Leonori, F.; Petrucci, R.; Balucani, N.; Hickson, K.M.; Hamberg, M.; Geppert, W.D.; Casavecchia, P.; Rosi, M. Crossed-beam and theoretical studies of the S(1D) + C2H2 reaction. J. Phys. Chem. A 2009, 113, 4330–4339. [Google Scholar] [CrossRef]
- De Petris, G.; Cartoni, A.; Rosi, M.; Barone, V.; Puzzarini, C.; Troiani, A. The proton affinity and gas-phase basicity of sulfur dioxide. ChemPhysChem 2011, 12, 112–115. [Google Scholar] [CrossRef]
- Berteloite, C.; Le Picard, S.D.; Sims, I.R.; Rosi, M.; Leonori, F.; Petrucci, R.; Balucani, N.; Wang, X.; Casavecchia, P. Low temperature kinetics, crossed beam dynamics and theoretical studies of the reaction S(1D) + CH4 and low temperature kinetics of S(1D) + C2H2. Phys. Chem. Chem. Phys. 2011, 13, 8485–8501. [Google Scholar] [CrossRef]
- Rosi, M.; Falcinelli, S.; Balucani, N.; Casavecchia, P.; Leonori, F.; Skouteris, D. Theoretical study of reactions relevant for atmospheric models of Titan: Interaction of excited nirogen atoms with small hydrocarbons. LNCS 2012, 7333, 331–344. [Google Scholar]
- Skouteris, D.; Balucani, N.; Faginas-Lago, N.; Falcinelli, S.; Rosi, M. Dimerization of methanimine and its charged species in the atmosphere of Titan and interstellar/cometary ice analogs. Astronomy Astrophysics 2015, 584, A76. [Google Scholar] [CrossRef]
- Falcinelli, S.; Rosi, M.; Cavalli, S.; Pirani, F.; Vecchiocattivi, F. Stereoselectivity in autoionization reactions of hydrogenated molecules by metastable gas atoms: The role of electronic couplings. Chemistry Eur. J. 2016, 22, 12518–12526. [Google Scholar] [CrossRef]
- Troiani, A.; Rosi, M.; Garzoli, S.; Salvitti, C.; de Petris, G. Vanadium Hydroxide Cluster Ions in the Gas Phase: Bond-Forming Reactions of Doubly-Charged Negative Ions by SO2-Promoted V-O Activation. Chem. Eur. J. 2017, 23, 11752–11756. [Google Scholar] [CrossRef]
- Bauschlicher, C.W., Jr.; Rosi, M. On the Bonding in Be22+. Chem. Phys. Letters 1989, 159, 485–488. [Google Scholar] [CrossRef]
- Bauschlicher, C.W., Jr.; Rosi, M. Addendum to “On the Bonding in Be22+”. Chem. Phys. Letters 1990, 165, 501–502. [Google Scholar] [CrossRef]



| Ions | Measured KER Range (eV) | Escape Energy (eV) from the Atmosphere of Some Planets | |||
|---|---|---|---|---|---|
| Earth | Venus | Mars | Titan | ||
| H+ | 2.8–6.0 1; 2.8–6.0 2 | 0.62 | 0.53 | 0.13 | 0.02 |
| C+ | 1.7–3.3 1. | 7.4 | 6.4 | 1.5 | 0.28 |
| CH+ | 1.3–3.2 1. | 8.0 | 6.9 | 1.6 | 0.30 |
| CH2+ | 1.6–2.9 1. | 8.6 | 7.5 | 1.8 | 0.32 |
| N+ | 2.3–5.2 3. | 8.6 | 7.5 | 1.8 | 0.32 |
| CH3+ | 2.7–4.8 2. | 9.2 | 8.1 | 1.9 | 0.34 |
| O+ | 1.0–5.2 3; 2.2–3.8 4. | 9.8 | 8.6 | 2.0 | 0.37 |
| C2H+ | 0.1–0.4 1. | 15.4 | 13.3 | 3.1 | 0.58 |
| CO+ | 0.4–2.6 4. | 17.3 | 14.9 | 3.5 | 0.65 |
| N2+ | 0.4–2.9 3. | 17.3 | 14.9 | 3.5 | 0.65 |
| NO+ | 1.0–2.9 3. | 18.5 | 16.0 | 3.75 | 0.70 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Falcinelli, S.; Rosi, M. Production and Characterization of Molecular Dications: Experimental and Theoretical Efforts. Molecules 2020, 25, 4157. https://doi.org/10.3390/molecules25184157
Falcinelli S, Rosi M. Production and Characterization of Molecular Dications: Experimental and Theoretical Efforts. Molecules. 2020; 25(18):4157. https://doi.org/10.3390/molecules25184157
Chicago/Turabian StyleFalcinelli, Stefano, and Marzio Rosi. 2020. "Production and Characterization of Molecular Dications: Experimental and Theoretical Efforts" Molecules 25, no. 18: 4157. https://doi.org/10.3390/molecules25184157
APA StyleFalcinelli, S., & Rosi, M. (2020). Production and Characterization of Molecular Dications: Experimental and Theoretical Efforts. Molecules, 25(18), 4157. https://doi.org/10.3390/molecules25184157







