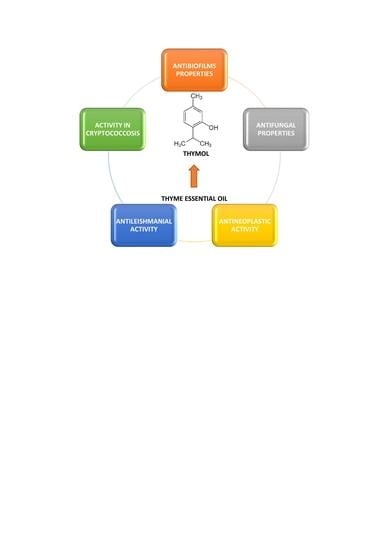
Thymol and Thyme Essential Oil—New Insights into Selected Therapeutic Applications
Abstract
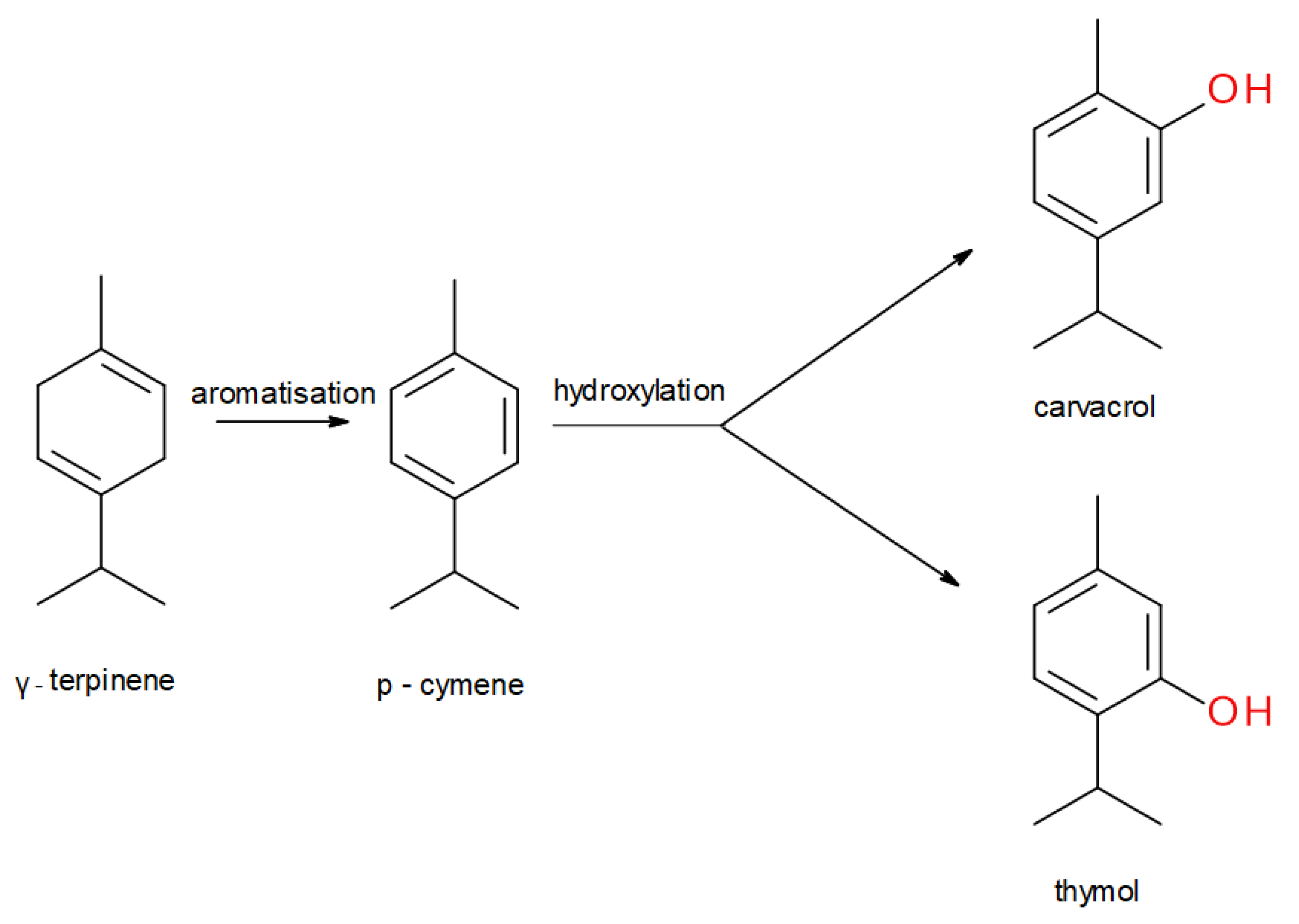
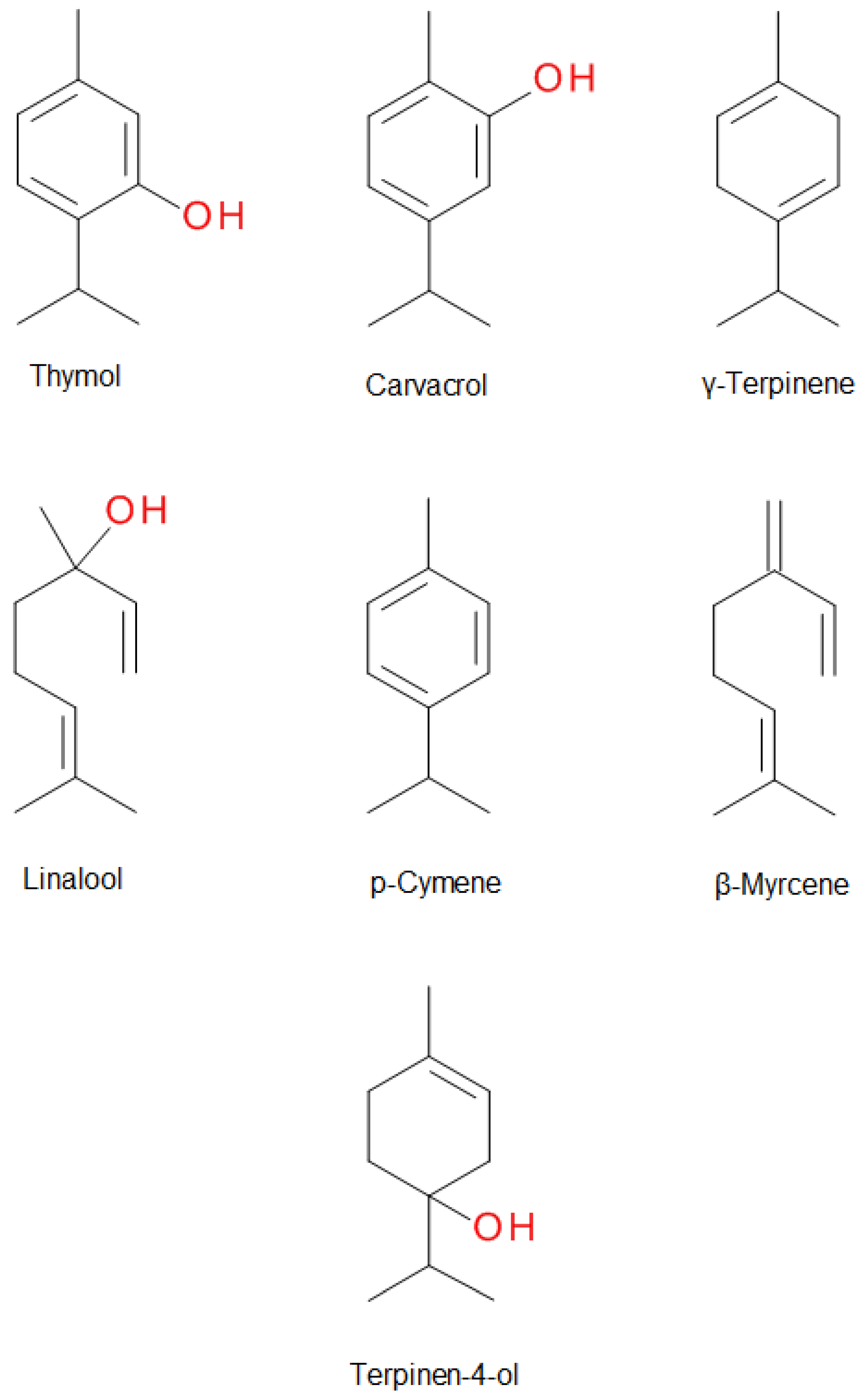
1. Introduction
2. Activity against Microbiological Biofilms
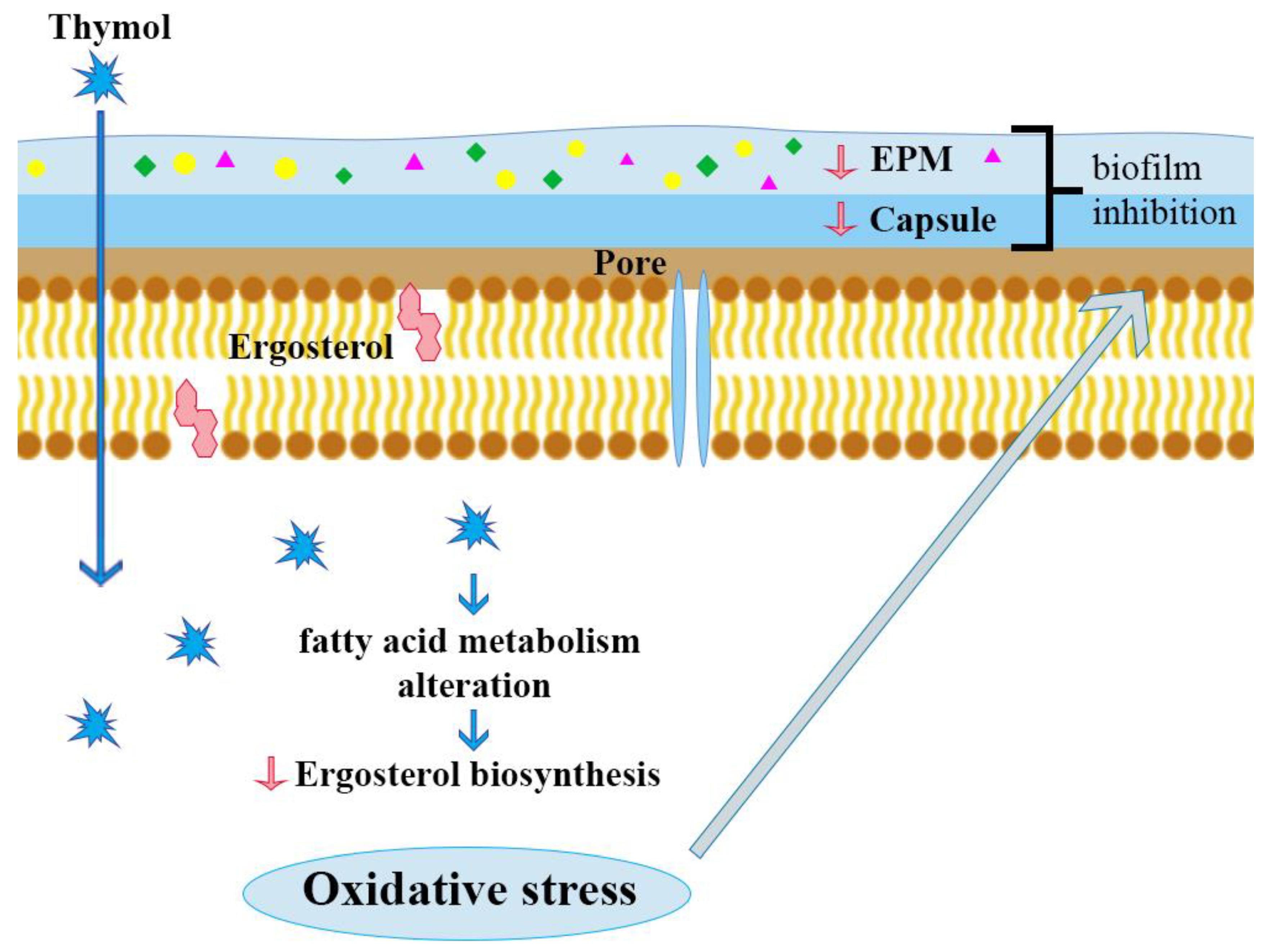
3. Antifungal Activity
3.1. Thymol Activity in Cryptococcosis
3.2. Other Antifungal Properties
4. Antileishmanial Properties
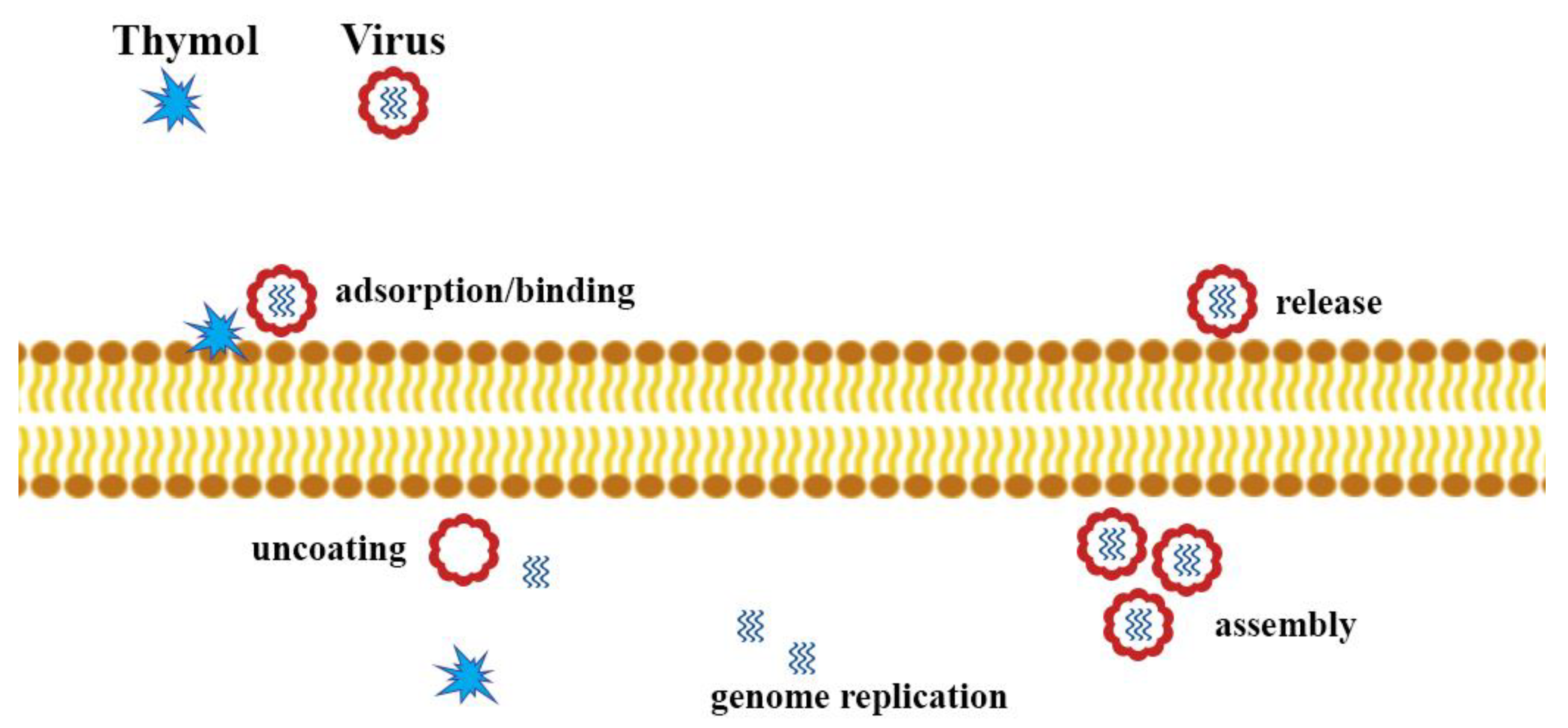
5. Antiviral Activity
5.1. Activity Against SARS-Cov-2
5.2. Other Antiviral Properties
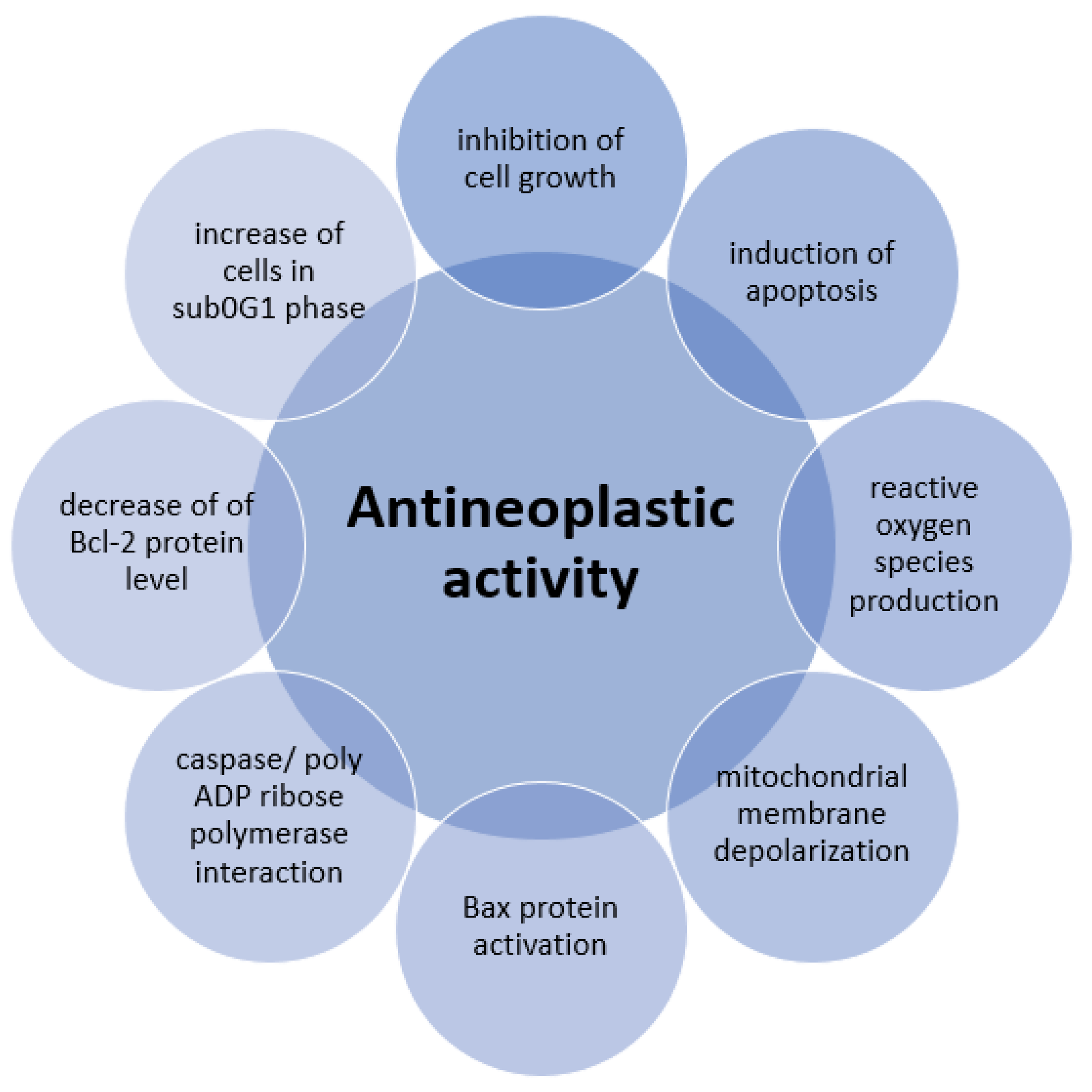
6. Antineoplastic Activity
7. New Forms of Therapeutics Using Thyme Essential Oil and Its Active Ingredients
7.1. Natural Polymers
7.2. Semi-Synthetic Polymers
7.3. Microfibres
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ghasemi, G.; Alirezalu, A.; Ghosta, Y.; Jarrahi, A.; Safavi, S.A.; Abbas-Mohammadi, M.; Barba, F.J.; Munekata, P.E.S.; Domínguez, R.; Lorenzo, J.M. Composition, Antifungal, Phytotoxic, and Insecticidal Activities of Thymus kotschyanus Essential Oil. Molecules 2020, 25, 1152. [Google Scholar] [CrossRef]
- Council of Europe. European Pharmacopoeia, 10th ed.; Council of Europe: Strasbourg, France, 2019; ISBN 928-718-505-0. [Google Scholar]
- European Medicines Agency. Assessment Report on Thymus vulgaris L., Thymus zygis Loefl. ex. L., aetheroleum. Available online: https://www.ema.europa.eu/en/documents/herbal-report/final-assessment-report-thymus-vulgaris-l-thymus-zygis-loefl-ex-l-aetheroleum_en.pdf (accessed on 15 March 2020).
- European Medicines Agency. Assessment Report on Thymus vulgaris L., Thymus zygis L., herba. Available online: https://www.ema.europa.eu/en/documents/herbal-report/final-assessment-report-thymus-vulgaris-l-thymus-zygis -l-herba_en.pdf (accessed on 15 March 2020).
- Kosakowska, O.; Bączek, K.; Przybył, J.L.; Pawełczak, A.; Rolewska, K.; Węglarz, Z. Morphological and Chemical Traits as Quality Determinants of Common Thyme (Thymus vulgaris L.), on the Example of ‘Standard Winter’ Cultivar. Agronomy 2020, 10, 909. [Google Scholar] [CrossRef]
- Tohidi, B.; Rahimmalek, M.; Arzani, A.; Trindade, H. Sequencing and variation of terpene synthase gene (TPS2) as the major gene in biosynthesis of thymol in different Thymus species. Phytochemistry 2020, 169, 112126. [Google Scholar] [CrossRef] [PubMed]
- Bendif, H.; Peron, G.; Miara, M.D.; Sut, S.; Dall’Acqua, S.; Flamini, G.; Maggi, F. Total phytochemical analysis of Thymus munbyanus subsp. coloratus from Algeria by HS-SPME-GC-MS, NMR and HPLC-MSn studies. J. Pharmaceut. Biomed. 2020, 186, 113330. [Google Scholar] [CrossRef] [PubMed]
- PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Thymol (accessed on 15 April 2020).
- Sharifi-Rad, M.; Varoni, E.M.; Iriti, M.; Martorell, M.; Setzer, W.N.; Del Mar Contreras, M.; Salehi, B.; Soltani-Nejad, A.; Rajabi, S.; Tajbakhsh, M.; et al. Carvacrol and human health: A comprehensive review. Phytother. Res. 2018, 32, 1675–1687. [Google Scholar] [CrossRef] [PubMed]
- Nagoor Meeran, M.F.; Javed, H.; Taee, H.A.; Azimullah, S.; Ojha, S.K. Pharmacological properties and molecular mechanisms of thymol: Prospects for its therapeutic potential and pharmaceutical development. Front. Pharmacol. 2017, 8, 380. [Google Scholar] [CrossRef]
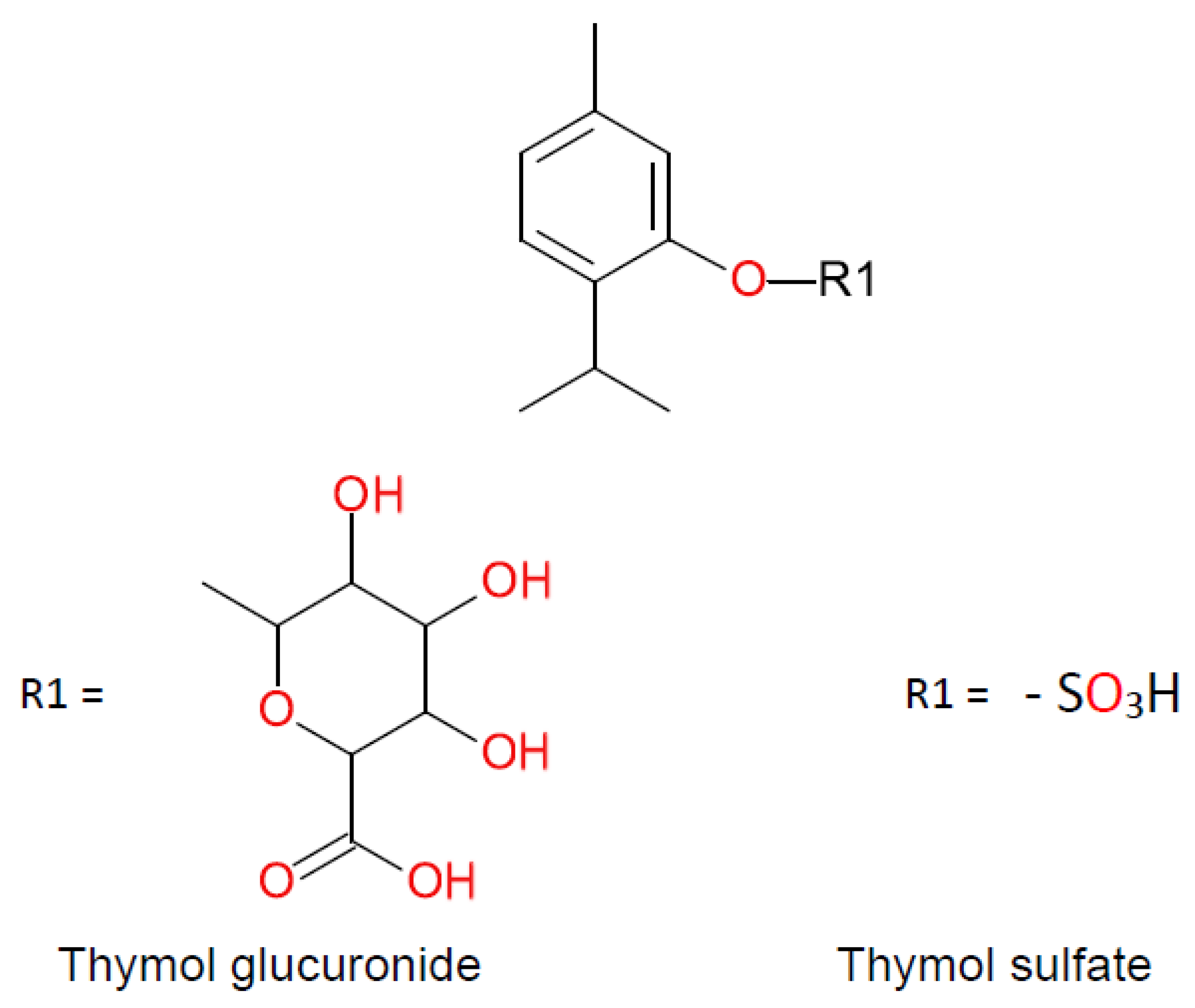
- Kohlert, C.; Schindler, G.; März, R.W.; Abel, G.; Brinkhaus, B.; Derendorf, H.; Gräfe, E.U.; Veit, M. Systemic Availability and Pharmacokinetics of Thymol in Humans. J. Clin. Pharmacol. 2002, 42, 731–737. [Google Scholar] [CrossRef]
- Nilima, T.; Silpi, B.; Rakesh, N.B.; Monali, R. Antimicrobial efficacy of five essential oils against oral pathogens: An in vitro study. Eur. J. Dent. 2013, 7, 71–77. [Google Scholar]
- Walther, C.; Schmidtke, M. Anti-rhinovirus and anti-influenza virus activities of mucoactive secretolytic agents and plant extracts—A comparative in vitro study. Res. Sq. 2020; in press. [Google Scholar] [CrossRef]
- Eva, L.; Christin, M.; Ahmed, M.; Julia, D.; Pumaree, K.; Sharmistha, D.; Ute, C.; Lars-Norbert, P.; Stephan, P. Authorised medicinal product Aspecton® Oral Drops containing thyme extract KMTv24497 shows antiviral activity against viruses which cause respiratory infections. J. Herb. Med. 2018, 13, 26–33. [Google Scholar]
- Salehi, B.; Mishra, A.P.; Shukla, I.; Sharifi-Rad, M.; del Mar Contreras, M.; Segura-Carretero, A.; Fathi, H.; Nasrabadi, N.N.; Kobarfard, F.; Sharifi-Rad, J. Thymol, thyme, and other plant sources: Health and potential uses. Phytother. Res. 2018, 32, 1688–1706. [Google Scholar] [CrossRef]
- Li, Y.; Wena, J.; Du, C.; Hu, S.; Chen, J.; Zhang, S.; Zhang, N.; Gao, F.; Li, S.; Mao, X.; et al. Thymol inhibits bladder cancer cell proliferation via inducing cell cycle arrest and apoptosis. Biochem. Biophys. Res. Commun. 2017, 491, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Codruta, H.S.; Lorena, F.; Oliviu, V.; Cristina, M.; Doina, M.; Adela, I.C.; Mirela, M. Essential Oil-Bearing Plants from Balkan Peninsula: Promising Sources for New Drug Candidates for the Prevention and Treatment of Diabetes Mellitus and Dyslipidemia. Front. Pharmacol. 2020, 11, 989. [Google Scholar]
- Tariq, S.; Wani, S.; Rasool, W.; Shafi, K.; Bhat, M.A.; Prabhakar, A.; Shalla, A.H.; Rather, M.A. A Comprehensive Review of the Antibacterial, Antifungal and Antiviral Potential of Essential Oils and Their Chemical Constituents Against Drug-Resistant Microbial Pathogens. Microb. Pathog. 2019, 134, 103580. [Google Scholar] [CrossRef] [PubMed]
- Schnitzler, P. Essential Oils for the Treatment of Herpes Simplex Virus Infections. Chemotherapy 2019, 64, 1–7. [Google Scholar] [CrossRef]
- Memar, M.Y.; Raei, P.; Alizadeh, N.; Aghdam, M.A.; Kafil, H.S. Carvacrol and Thymol: Strong Antimicrobial Agents Against Resistant Isolates. Rev. Med. Microbiol. 2017, 28, 63–68. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, G.; Hou, S.; Zhang, T.; Li, Z.; Liang, F. Distribution of ARGs and MGEs among glacial soil, permafrost, and sediment using metagenomic analysis. Environ. Pollut. 2018, 234, 339–346. [Google Scholar] [CrossRef]
- Høiby, N.; Bjarnsholt, T.; Givskov, M.; Molin, S.; Ciofu, O. Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents 2010, 35, 322–332. [Google Scholar]
- Al-Shuneigat, J.; Al-Sarayreh, S.; Al-Saraireh, Y.; Al-Qudah, M.; Al-Tarawneh, I. Effects of wild Thymus vulgaris essential oil on clinical isolates biofilm-forming bacteria. J. Dent. Med. Sci. 2014, 13, 62–66. [Google Scholar] [CrossRef]
- Bazargania, M.M.; Rohloff, J. Antibiofilm activity od essential oils and plant extracts against Staphylococcus aureus and Escherichia coli biofilms. Food Control 2016, 61, 156–164. [Google Scholar] [CrossRef]
- Hayat, S.; Muzammil, S.; Aslam, B.; Siddique, M.H.; Saqalein, M.; Nisar, M.A. Quorum quenching: Role of nanoparticles as signal jammers in Gram-negative bacteria. Future Microbiol. 2019, 14, 61–72. [Google Scholar] [CrossRef]
- Singh, S.; Singh, S.K.; Chowdhury, I.; Singh, R. Understanding the Mechanism of Bacterial Biofilms Resistance to Antimicrobial Agents. Open Microbiol. J. 2017, 11, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Van Acker, H.; Van Dijck, P.; Coenye, T. Molecular mechanisms of antimicrobial tolerance and resistance in bacterial and fungal biofilms. Trends Microbiol. 2014, 22, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. WHO Pathogens Priority List Working Group. Discovery, research and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- Antibiotic Resistance Threats in the United States. 2019. Available online: https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf. (accessed on 15 March 2020).
- Qaralleh, H. Thymol Rich Thymbra capitata Essential Oil Inhibits Quorum Sensing, Virulence and Biofilm Formation of Beta Lactamase Producing Pseudomonas aeruginosa. Nat. Prod. Sci. 2019, 25, 172–180. [Google Scholar] [CrossRef]
- Kryvtsova, M.V.; Salamon, I.; Koscova, J.; Bucko, D.; Spivak, M. Antimicrobial, antibiofilm and biochemichal properties of Thymus vulgaris essential oil against clinical isolates of opportunistic infections. Biosyst. Divers. 2019, 27, 270–275. [Google Scholar] [CrossRef]
- Perez, A.P.; Perez, N.; Lozano, C.M.S.; Altube, M.J.; de Farias, M.A.; Portugal, R.V.; Buzzola, F.; Morilla, M.J.; Romero, E.L. The anti MRSA biofilm activity of Thymus vulgaris essential oil in nanovesicle. Phytomedicine 2019, 57, 339–351. [Google Scholar] [CrossRef]
- Nazar1, F.N.; Videla, E.A.; Marin, R.H. Thymol supplementation effects on adrenocortical, immune and biochemical variables recovery in Japanese quail after exposure to chronic heat stress. Animal 2019, 13, 318–325. [Google Scholar] [CrossRef]
- De Lira Mota, K.S.; de Oliveira Pereira, F.; de Oliveira, W.A.; Lima, I.O.; de Oliveira Lima, E. Antifungal Activity of Thymus vulgaris L. Essential Oil and its Constituent Phytochemicals against Rhizopus oryzae: Interaction with Ergosterol. Molecules 2012, 17, 14418–14433. [Google Scholar] [CrossRef]
- Ahmad, A.; Khan, A.; Akhtar, F.; Yousuf, S.; Xess, I.; Khan, L.A.; Manzoor, N. Fungicidal activity of thymol and carvacrol by disrupting ergosterol biosynthesis and membrane integrity against Candida. Eur. J. Clin. Microbiol. Infect. Dis. 2011, 30, 41–50. [Google Scholar] [CrossRef]
- Lioliosa, C.C.; Gortzib, O.; Lalasb, S.; Tsaknis, J.; Chinoua, I. Liposomal incorporation of carvacrol and thymol isolated from the essential oil of Origanum dictamnus L. and in vitro antimicrobial activity. Food Chem. 2009, 112, 77–83. [Google Scholar] [CrossRef]
- Tohidpour, A.; Sattari, M.; Omidbaigi, R.; Yadegar, A.; Nazemi, J. Antibacterial effect of essential oils from two medicinal plants against Methicillin-resistant Staphylococcus aureus. Phytomedicine 2010, 17, 142–145. [Google Scholar] [CrossRef] [PubMed]
- Zhongwei, Y.; Yuyun, D.; Ping, O.; Tayyab, R.; Sajjad, H.; Tianyi, Z.; Zhongqiong, Y.; Hualin, F.; Juchun, L.; Changliang, H.; et al. Thymol Inhibits Biofilm Formation, Eliminates Pre-Existing Biofilms and Enhances Clearance of Methicillin-Resistant Staphylococcus aureus (MRSA) in a Mouse Peritoneal Implant Infection Model. Microorganisms 2020, 8, 99. [Google Scholar]
- Kostoglou, D.; Protopappas, I.; Giaouris, E. Common Plant-Derived Terpenoids Present Increased Anti-Biofilm Potential against Staphylococcus Bacteria Compared to a Quaternary Ammonium Biocide. Foods 2020, 9, 697. [Google Scholar] [CrossRef] [PubMed]
- Okshevsky, M.; Regina, V.R.; Meyer, R.L. Extracellular DNA as a target for biofilm control. Curr. Opin. Biotechnol. 2015, 33, 73–80. [Google Scholar] [CrossRef]
- Kerekes, E.B.; Deák, É.; Takó, M.; Tserennadmid, R.; Petkovits, T.; Vágvölgyi, C.; Krisch, J. Anti-biofilm forming and anti-quorum sensing activity of selected essential oils and their main components on food-related micro-organisms. J. Appl. Microbiol. 2013, 115, 933–942. [Google Scholar] [CrossRef]
- Kerekes, E.B.; Vidács, A.; Takó, M.; Petkovits, T.; Vágvölgyi, C.; Horváth, G.; Balázs, V.L.; Krisch, J. Anti-Biofilm Effect of Selected Essential Oils and Main Components on Mono- and Polymicrobic Bacterial Cultures. Microorganisms 2019, 7, 345. [Google Scholar] [CrossRef]
- Navon-Venezia, S.; Kondratyeva, K.; Carattoli, A. Klebsiella pneumoniae: A major worldwide source and shuttle for antibiotic resistance. FEMS Microbiol. Rev. 2017, 41, 252–275. [Google Scholar] [CrossRef]
- Mohamed, S.H.; Mohamed, M.S.M.; Khalil, M.S.; Azmy, M.; Mabrouk, M.I. Combination of essential oil and ciprofloxacin to inhibit/eradicate biofilms in multidrug-resistant Klebsiella pneumonia. J. Appl. Microbiol. 2018, 125, 84–95. [Google Scholar] [CrossRef]
- Virella, G. Mikrobiologia i Choroby Zakaźne, 1st ed.; Edra Urban & Partner: Wroclaw, Poland, 1999; pp. 388–389. [Google Scholar]
- Zavala, S.; Baddley, J.W. Cryptococcosis. Sem. Resp. Crit. Care M. 2020, 41, 69–79. [Google Scholar] [CrossRef]
- Rohatgi, S.; Pirofski, L.A. Host immunity to Cryptococcus neoformans. Future Microbiol. 2015, 10, 565–581. [Google Scholar] [CrossRef]
- Quintero, O.; Trachuk, P.; Lerner, M.Z.; Sarungbam, J.; Pirofski, L.P.; Park, S.O. Risk factors of laryngeal cryptococcosis: A case report. Med. Mycol. Case Rep. 2019, 24, 82–85. [Google Scholar] [CrossRef] [PubMed]
- Martinez, L.R.; Casadevall, A. Biofilm formation by Cryptococcus neoformans. Microbiol. Spectr. 2015, 3, 1–11. [Google Scholar]
- Ajesh, K.; Sreejith, K. Cryptococcus laurentii biofilms: Structure, development and antifungal drug resistance. Mycopathologia 2012, 174, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Kumari, P.; Mishra, R.; Arora, N.; Chatrath, A.; Gangwar, R.; Roy, P.; Prasad, R. Antifungial and anti-biofilm activity of essential oil active components against Cryptococcus neoformans and Cryprococcus laurentii. Front. Microbiol. 2017, 8, 2161. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.V.N.; Capello, T.M.; Siqueira, L.J.A.; Lago, J.H.G.; Caseli, J. Mechanism of Action of Thymol on Cell Membranes Investigated through Lipid Monolayers at the Air–Water Interface and Molecular Simulation. Langmuir 2016, 32, 3234–3241. [Google Scholar] [CrossRef] [PubMed]
- Soković, M.D.; Vukojević, J.; Marin, P.D.; Brkić, D.D.; Vajs, V.; van Griensven, L.J.L.D. Chemical Composition of Essential Oils of Thymus and Mentha Species and Their Antifungal Activities. Molecules 2009, 14, 238–249. [Google Scholar] [CrossRef] [PubMed]
- Poonam, K.; Neha, A.; Apurva, C.; Rashmi, G.; Vikas, P.; Poluri, K.M.; Prasad, R. Delineating the Biofilm Inhibition Mechanisms of Phenolic and Aldehydic Terpenes against Cryptococcus neoformans. ACS Omega 2019, 4, 17634–17648. [Google Scholar]
- Al-Shahrani, M.H.; Mahfoud, M.; Anvarbatcha, R.; Athar, M.T.; Al Asmari, A. Evaluation of antifungal activity and cytotoxicity of Thymus vulgaris essential oil. Pharmacogn. Commun. 2017, 7, 34–40. [Google Scholar] [CrossRef]
- Pohl, C.H.; Kock, J.L.F.; Thibane, V.S. Antifungal free fatty acids: A Review In Science Against Microbial Pathogens: Communicating Current Research and Technological Advances; Méndez-Vilas, A., Ed.; Formatex Research Center: Badajoz, Spain, 2011; pp. 61–71. [Google Scholar]
- Avis, J.T.; Bélanger, R.R. Specificity and Mode of Action of the Antifungal Fatty Acid cis-9-Heptadecenoic Acid Produced by Pseudozyma flocculosa. Appl. Environ. Microb. 2001, 67, 956–996. [Google Scholar] [CrossRef]
- Bae, Y.S.; Rhee, M.S. Short-Term Antifungal Treatments of Caprylic Acid with Carvacrol or Thymol Induce Synergistic 6-Log Reduction of Pathogenic Candida albicans by Cell Membrane Disruption and Efflux Pump Inhibition. Cell Physiol. Biochem. 2019, 53, 285–300. [Google Scholar]
- Ibrahim, H.M.S.; Baraka, M.A. Prevalence of intestinal protozoan parasitic infections among people attending Sebha Central Laboratory in Sebha, Libya: A retrospective study. Int. J. Appl. Sci. 2019, 1, 374–385. [Google Scholar]
- World Health Organization. Leishmaniasis. Available online: https://www.who.int/news-room/fact-sheets/detail/leishmaniasis (accessed on 21 April 2020).
- Youssefi, M.R.; Moghaddas, E.; Tabari, M.A.; Moghadamnia, A.A.; Hosseini, S.M.; Farash, B.R.H.; Ebrahimi, M.A.; Mousavi, N.N.; Fata, A.; Maggi, F.; et al. In Vitro and In Vivo Effectiveness of Carvacrol, Thymol and Linalool against Leishmania infantum. Molecules 2019, 24, 2072. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.L.; Chuang, H.S.; Lee, M.H.; Wei, C.L.; Lin, C.F.; Tsai, Y.C. Inhibition of herpes simplex virus type 1 by thymol-related monoterpenoids. Planta Med. 2012, 78, 1636–1638. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Yao, L. Antiviral Effects of Plant-Derived Essential Oils and Their Components: An Updated Review. Molecules 2020, 25, 2627. [Google Scholar] [CrossRef] [PubMed]
- Gordon, D.E.; Jang, G.M.; Bouhaddou, M.; Xu, J.; Obernier, K.; White, K.M.; O’Meara, M.J.; Rezelj, V.V.; Guo, J.Z.; Swaney, D.L.; et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature 2020, 583, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Kutateladze, T.G. Molecular structure analyses suggest strategies to therapeutically target SARS-CoV-2. Nat. Commun. 2020, 11, 2920. [Google Scholar] [CrossRef] [PubMed]
- Walls, A.C.; Park, Y.-J.; Tortorici, M.A.; Wall, A.; McGuire, A.T.; Veesler, D. Structure function and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell 2020, 181, 281–292. [Google Scholar] [CrossRef]
- Kulkarni, S.A.; Nagarajan, S.K.; Ramesh, V.; Palaniyandi, V.; Selvam, S.P.; Madhavan, T. Computational evaluation of major components from plant essential oils as potent inhibitors of SARS-CoV-2 spike protein. J. Mol. Struct. 2020, 1221, 128823. [Google Scholar] [CrossRef]
- List N: Disinfectants for Use Against SARS-CoV-2 (COVID-19). Available online: https://www.epa.gov/pesticide-registration/list-n-disinfectants-use-against-sars-cov-2-covid-19 (accessed on 24 August 2020).
- Hard-Surface Disinfectants and Hand Sanitizers (COVID-19): List of Disinfectants with Evidence for Use against COVID-19. Available online: https://www.canada.ca/en/health-canada/services/drugs-health-products/disinfectants/covid-19/list.html (accessed on 24 August 2020).
- Feriotto, G.; Marchetti, N.; Costa, V.; Beninati, S.; Tagliati, F.; Mischiati, C. Chemical Composition of Essential Oils from Thymus vulgaris, Cymbopogon citratus, and Rosmarinus officinalis, and Their Effects on the HIV-1 Tat Protein Function. Chem. Biodivers. 2018, 15, 1700436. [Google Scholar] [CrossRef]
- Toujani, M.M.; Rittà, M.; Civra, A.; Genovese, S.; Epifano, F.; Ghram, A.; Lembo, D.; Donalisio, M. Inhibition of HSV-2 infection by pure compounds from Thymus capitatus. Phytother. Res. 2018, 32, 1555–1563. [Google Scholar] [CrossRef]
- Word Health Organization. Cancer. Available online: http://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 20 April 2020).
- Baptista-Silva, S.; Borges, S.; Ramos, O.L.; Pintado, M.; Sarmento, B. The progress of essential oils as potential therapeutic agents: A review. J. Essent. Oil Res. 2020, 32, 279–295. [Google Scholar] [CrossRef]
- Islam, M.T.; Khalipha, A.B.R.; Bagchi, R.; Mondal, M.; Smrity, S.Z.; Uddin, S.J.; Shilpi, J.A.; Rouf, R. Anticancer Activity of Thymol: A Literature Based Review and Docking Study with Emphasis on its Anticancer Mechanisms. Iubmb Life 2019, 71, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Elbe, H.; Yigitturk, G.; Cavusoglu, T.; Uyanikgil, Y.; Ozturk, F. Apoptotic effects of thymol, a novel monoterpene phenol, on different types of cancer. Bratisl. Med. J. 2020, 121, 122–128. [Google Scholar] [CrossRef]
- Kang, S.-H.; Kim, Y.-S.; Kim, E.-K.; Hwang, J.-W.; Jeong, J.-H.; Dong, X.; Lee, J.-W.; Moon, S.-H.; Jeon, B.-T.; Park, P.-J. Anticancer Effect of Thymol on AGS Human Gastric Carcinoma Cells. J. Microbiol. Biotechnol. 2016, 26, 28–37. [Google Scholar] [CrossRef]
- Günes-Bayir, A.; Kocyigit, A.; Güler, E.M.; Kiziltan, H.S. Effects of Thymol, a Natural Phenolic Compound, on Human Gastric Adenocarcinoma Cells In Vitro. Altern. Ther. Health Med. 2019, 25, 12–21. [Google Scholar] [PubMed]
- Pinna, R.; Filigheddu, E.; Juliano, C.; Palmieri, A.; Manconi, M.; D’hallewin, G.; Petretto, G.; Maioli, M.; Caddeo, C.; Manca, M.L.; et al. Antimicrobial Effect of Thymus capitatus and Citrus limon var. pompia as Raw Extracts and Nanovesicles. Pharmaceutics 2019, 11, 234. [Google Scholar] [CrossRef]
- Piombino, C.; Lange, H.; Sabuzi, F.; Galloni, P.; Conte, V.; Crestini, C. Lignosulfonate Microcapsules for Delivery and Controlled Release of Thymol and Derivatives. Molecules 2020, 25, 866. [Google Scholar] [CrossRef]
- Chen, Y.; Qiu, Y.; Chen, W.; Wei, Q. Electrospun thymol-loaded porous cellulose acetate fibers with potential biomedical applications. Mat. Sci. Eng. C 2020, 109, 110536. [Google Scholar] [CrossRef]
- Rassu, G.; Nieddu, M.; Bosi, P.; Trevisi, P.; Colombo, M.; Priori, D.; Manconi, P.; Giunchedi, P.; Gavini, E.; Boatto, G. Encapsulation and modified-release of thymol from oral microparticles as adjuvant or substitute to current medications. Phytomedicine 2014, 21, 1627–1632. [Google Scholar] [CrossRef]
- Zamani, Z.; Alipour, D.; Moghimi, H.R.; Mortazavi, S.A.R.; Saffary, M. Development and Evaluation of Thymol Microparticles Using Cellulose Derivatives as Controlled Release Dosage form. Iran J. Pharm. Res. 2015, 14, 1032–1040. [Google Scholar]
- Gámez, E.; Elizondo-Castillo, H.; Tascon, J.; García-Salinas, S.; Navascues, N.; Mendoza, G.; Arruebo, M.; Irusta, S. Antibacterial Effect of Thymol Loaded SBA-15 Nanorods Incorporated in PCL Electrospun Fibers. Nanomaterials 2020, 10, 616. [Google Scholar] [CrossRef] [PubMed]
- Nayak, B.S.; Ghosh, S.K.; Patro, K.T.B. Preparation and characterization of famotidine microcapsule employing mucoadhesive polymers in combination to enhance gastro retention for oral delivery. Int. J. Pharm. 2009, 1, 112–120. [Google Scholar]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowalczyk, A.; Przychodna, M.; Sopata, S.; Bodalska, A.; Fecka, I. Thymol and Thyme Essential Oil—New Insights into Selected Therapeutic Applications. Molecules 2020, 25, 4125. https://doi.org/10.3390/molecules25184125
Kowalczyk A, Przychodna M, Sopata S, Bodalska A, Fecka I. Thymol and Thyme Essential Oil—New Insights into Selected Therapeutic Applications. Molecules. 2020; 25(18):4125. https://doi.org/10.3390/molecules25184125
Chicago/Turabian StyleKowalczyk, Adam, Martyna Przychodna, Sylwia Sopata, Agnieszka Bodalska, and Izabela Fecka. 2020. "Thymol and Thyme Essential Oil—New Insights into Selected Therapeutic Applications" Molecules 25, no. 18: 4125. https://doi.org/10.3390/molecules25184125
APA StyleKowalczyk, A., Przychodna, M., Sopata, S., Bodalska, A., & Fecka, I. (2020). Thymol and Thyme Essential Oil—New Insights into Selected Therapeutic Applications. Molecules, 25(18), 4125. https://doi.org/10.3390/molecules25184125