Wine’s Phenolic Compounds and Health: A Pythagorean View
Abstract
1. Introduction
2. Phenolic Compounds and Health
3. Classification and Amounts of Wine Phenolic Compounds
3.1. Flavonoids
3.2. Flavonols
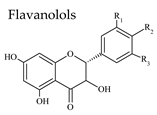
3.3. Flavan-3-Ols
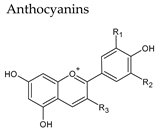
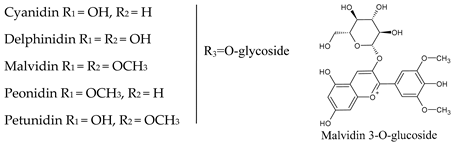
4. Anthocyanins
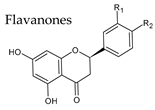
4.1. Flavanones
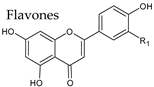
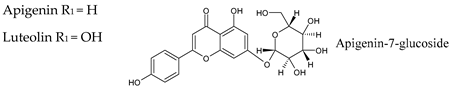
4.2. Flavones
5. Non-Flavonoids
6. Hydroxycinnamic Acids
7. Hydroxybenzoic Acids
8. Stilbenes
9. Effects on Human Health
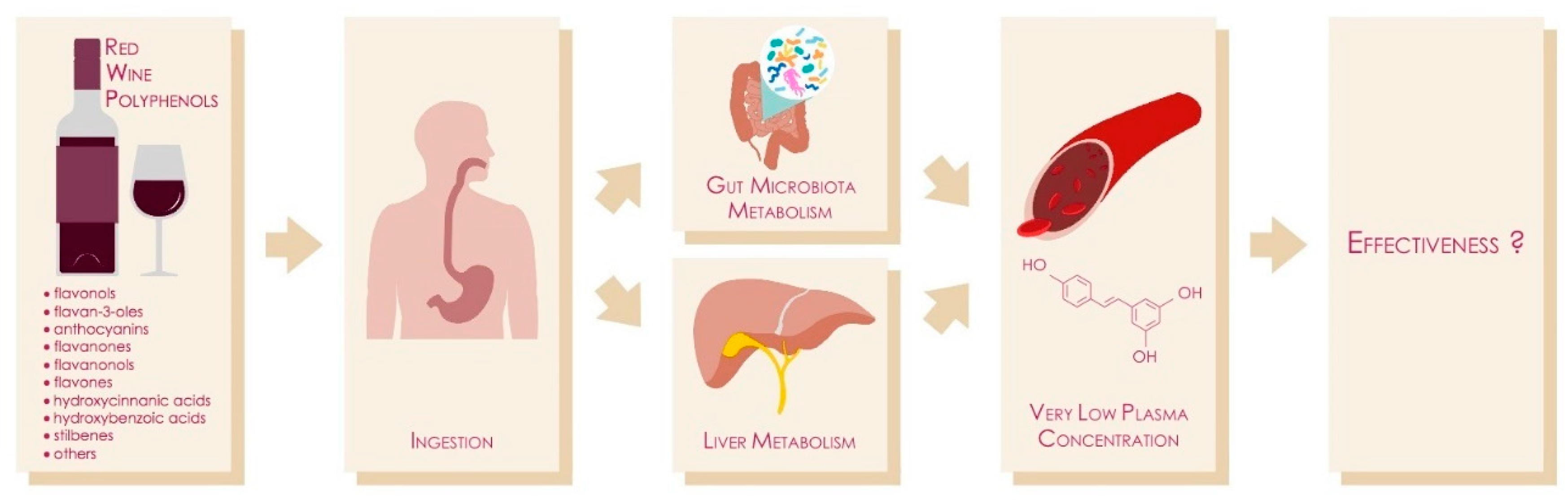
10. Human Studies of Resveratrol and Red Wine (Poly)Phenols
11. Wine vs. Other Alcoholic Beverages: Does Digestion Make the Difference?
12. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Poli, A.; Marangoni, F.; Avogaro, A.; Barba, G.; Bellentani, S.; Bucci, M.; Cambieri, R.; Catapano, A.L.; Costanzo, S.; Cricelli, C.; et al. Moderate alcohol use and health: A consensus document. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 487–504. [Google Scholar] [CrossRef] [PubMed]
- Holst, C.; Becker, U.; Jorgensen, M.E.; Gronbaek, M.; Tolstrup, J.S. Alcohol drinking patterns and risk of diabetes: A cohort study of 70,551 men and women from the general Danish population. Diabetologia 2017, 60, 1941–1950. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, S.; de Gaetano, G.; Di Castelnuovo, A.; Djousse, L.; Poli, A.; van Velden, D.P. Moderate alcohol consumption and lower total mortality risk: Justified doubts or established facts? Nutr. Metab. Cardiovasc. Dis. 2019, 29, 1003–1008. [Google Scholar] [CrossRef] [PubMed]
- Ditano-Vazquez, P.; Torres-Pena, J.D.; Galeano-Valle, F.; Perez-Caballero, A.I.; Demelo-Rodriguez, P.; Lopez-Miranda, J.; Katsiki, N.; Delgado-Lista, J.; Alvarez-Sala-Walther, L.A. The Fluid Aspect of the Mediterranean Diet in the Prevention and Management of Cardiovascular Disease and Diabetes: The Role of Polyphenol Content in Moderate Consumption of Wine and Olive Oil. Nutrients 2019, 11, 2833. [Google Scholar] [CrossRef]
- Weaver, S.R.; Rendeiro, C.; McGettrick, H.M.; Philp, A.; Lucas, S.J.E. Fine wine or sour grapes? A systematic review and meta-analysis of the impact of red wine polyphenols on vascular health. Eur. J. Nutr. 2020. [Google Scholar] [CrossRef]
- WHO. Diet, nutrition and the prevention of chronic diseases. World Health Organ. Tech. Rep. Ser. 2003, 916, 1–149. [Google Scholar]
- Yazdi, F.; Morreale, P.; Reisin, E. First Course DASH, Second Course Mediterranean: Comparing Renal Outcomes for Two “Heart-Healthy” Diets. Curr. Hypertens Rep. 2020, 22, 54. [Google Scholar] [CrossRef]
- Bhupathiraju, S.N.; Tucker, K.L. Coronary heart disease prevention: Nutrients, foods, and dietary patterns. Clin. Chim. Acta 2011, 412, 1493–1514. [Google Scholar] [CrossRef]
- Visioli, F.; Bogani, P.; Grande, S.; Detopoulou, V.; Manios, Y.; Galli, C. Local food and cardioprotection: The role of phytochemicals. Forum. Nutr. 2006, 59, 116–129. [Google Scholar] [CrossRef]
- Holst, B.; Williamson, G. Nutrients and phytochemicals: From bioavailability to bioefficacy beyond antioxidants. Curr. Opin. Biotechnol. 2008, 19, 73–82. [Google Scholar] [CrossRef]
- Barabási, A.; Menichetti, G.; Loscalzo, J. The unmapped chemical complexity of our diet. Nat. Food 2019, 1, 33–37. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Remesy, C.; Jimenez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed]
- Baiano, A.; Terracone, C.; Gambacorta, G.; La Notte, E. Phenolic content and antioxidant activity of Primitivo wine: Comparison among winemaking technologies. J. Food Sci. 2009, 74, C258–C267. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, X.; Li, Y.; Li, P.; Wang, H. Polyphenolic compounds and antioxidant properties of selected China wines. Food Chem. 2009, 112, 454–460. [Google Scholar] [CrossRef]
- Paixão, N.; Perestrelo, R.; Marques, J.C.; Câmara, J.S. Relationship between antioxidant capacity and total phenolic content of red, rosé and white wines. Food Chem. 2007, 105, 204–214. [Google Scholar] [CrossRef]
- Minussi, R.C.; Rossi, M.; Bologna, L.; Cordi, L.v.; Rotilio, D.; Pastore, G.M.; Durán, N. Phenolic compounds and total antioxidant potential of commercial wines. Food Chem. 2003, 82, 409–416. [Google Scholar] [CrossRef]
- Fernández-Pachón, M.S.; Villaño, D.; García-Parrilla, M.C.; Troncoso, A.M. Antioxidant activity of wines and relation with their polyphenolic composition. Anal. Chim. Acta 2004, 513, 113–118. [Google Scholar] [CrossRef]
- Katalinić, V.; Milos, M.; Modun, D.; Musić, I.; Boban, M. Antioxidant effectiveness of selected wines in comparison with (+)-catechin. Food Chem. 2004, 86, 593–600. [Google Scholar] [CrossRef]
- Kallithraka, S.; Tsoutsouras, E.; Tzourou, E.; Lanaridis, P. Principal phenolic compounds in Greek red wines. Food Chem. 2006, 99, 784–793. [Google Scholar] [CrossRef]
- Di Majo, D.; La Guardia, M.; Giammanco, S.; La Neve, L.; Giammanco, M. The antioxidant capacity of red wine in relationship with its polyphenolic constituents. Food Chem. 2008, 111, 45–49. [Google Scholar] [CrossRef]
- Staško, A.; Brezová, V.; Mazúr, M.; Čertík, M.; Kaliňák, M.; Gescheidt, G. A comparative study on the antioxidant properties of Slovakian and Austrian wines. LWT Food Sci. Technol. 2008, 41, 2126–2135. [Google Scholar] [CrossRef]
- Roussis, I.G.; Lambropoulos, I.; Tzimas, P.; Gkoulioti, A.; Marinos, V.; Tsoupeis, D.; Boutaris, I. Antioxidant activities of some Greek wines and wine phenolic extracts. J. Food Compos. Anal. 2008, 21, 614–621. [Google Scholar] [CrossRef]
- Lucena, A.P.S.; Nascimento, R.J.B.; Maciel, J.A.C.; Tavares, J.X.; Barbosa-Filho, J.M.; Oliveira, E.J. Antioxidant activity and phenolics content of selected Brazilian wines. J. Food Compos. Anal. 2010, 23, 30–36. [Google Scholar] [CrossRef]
- Jordão, A.M.; Gonçalves Fj Fau-Correia, A.C.; Correia Ac Fau-Cantão, J.; Cantão J Fau-Rivero-Pérez, M.D.; Rivero-Pérez Md Fau-González Sanjosé, M.L.; González Sanjosé, M.L. Proanthocyanidin content, antioxidant capacity and scavenger activity of Portuguese sparkling wines (Bairrada Appellation of Origin). J. Sci. Food Agric. 2010, 90, 2144–2152. [Google Scholar] [CrossRef]
- Šeruga, M.; Novak, I.; Jakobek, L. Determination of polyphenols content and antioxidant activity of some red wines by differential pulse voltammetry, HPLC and spectrophotometric methods. Food Chem. 2011, 124, 1208–1216. [Google Scholar] [CrossRef]
- Vrček, I.V.; Bojić, M.; Žuntar, I.; Mendaš, G.; Medić-Šarić, M. Phenol content, antioxidant activity and metal composition of Croatian wines deriving from organically and conventionally grown grapes. Food Chem. 2011, 124, 354–361. [Google Scholar] [CrossRef]
- Yoo, Y.J.; Prenzler Pd Fau-Saliba, A.J.; Saliba Aj Fau-Ryan, D.; Ryan, D. Assessment of some Australian red wines for price, phenolic content, antioxidant activity, and vintage in relation to functional food prospects. J. Food Sci. 2011, 76, C1355–C1364. [Google Scholar] [CrossRef]
- Ružić, I.; Škerget, M.; Knez, Ž.; Runje, M. Phenolic content and antioxidant potential of macerated white wines. Eur. Food Res. Technol. 2011, 233, 465. [Google Scholar] [CrossRef]
- Jiang, B.; Zhang, Z.W. Comparison on phenolic compounds and antioxidant properties of cabernet sauvignon and merlot wines from four wine grape-growing regions in China. Molecules 2012, 17, 8804–8821. [Google Scholar] [CrossRef]
- Radovanović, A.N.; Jovančićević Bs Fau-Radovanović, B.C.; Radovanović Bc Fau-Mihajilov-Krstev, T.; Mihajilov-Krstev, T.; Fau-Zvezdanović, J.B.; Zvezdanović, J.B. Antioxidant and antimicrobial potentials of Serbian red wines produced from international Vitis vinifera grape varieties. J. Sci. Food Agric. 2012, 92, 2154–2161. [Google Scholar] [CrossRef]
- Porgalı, E.; Büyüktuncel, E. Determination of phenolic composition and antioxidant capacity of native red wines by high performance liquid chromatography and spectrophotometric methods. Food Res. Int. 2012, 45, 145–154. [Google Scholar] [CrossRef]
- Agatonovic-Kustrin, S.; Hettiarachchi, C.G.; Morton, D.W.; Razic, S. Analysis of phenolics in wine by high performance thin-layer chromatography with gradient elution and high resolution plate imaging. J. Pharm. Biomed. Anal. 2015, 102, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Ferrazzano, G.F.; Amato, I.; Ingenito, A.; Zarrelli, A.; Pinto, G.; Pollio, A. Plant polyphenols and their anti-cariogenic properties: A review. Molecules 2011, 16, 1486–1507. [Google Scholar] [CrossRef] [PubMed]
- Stalikas, C.D. Extraction, separation, and detection methods for phenolic acids and flavonoids. J. Sep. Sci. 2007, 30, 3268–3295. [Google Scholar] [CrossRef]
- Yang, B.; Liu, H.; Yang, J.; Gupta, V.K.; Jiang, Y. New insights on bioactivities and biosynthesis of flavonoid glycosides. Trends Food Sci. Technol. 2018, 79, 116–124. [Google Scholar] [CrossRef]
- Perez-Vizcaino, F.; Duarte, J. Flavonols and cardiovascular disease. Mol. Aspects Med. 2010, 31, 478–494. [Google Scholar] [CrossRef]
- Ku, Y.S.; Ng, M.S.; Cheng, S.S.; Lo, A.W.; Xiao, Z.; Shin, T.S.; Chung, G.; Lam, H.M. Understanding the Composition, Biosynthesis, Accumulation and Transport of Flavonoids in Crops for the Promotion of Crops as Healthy Sources of Flavonoids for Human Consumption. Nutrients 2020, 12, 1717. [Google Scholar] [CrossRef]
- Jeffery, D.W.; Parker, M.; Smith, P.A. Flavonol composition of Australian red and white wines determined by high-performance liquid chromatography. Aust. J. Grape Wine Res. 2008, 14, 153–161. [Google Scholar] [CrossRef]
- Cueva, C.; Gil-Sanchez, I.; Ayuda-Duran, B.; Gonzalez-Manzano, S.; Gonzalez-Paramas, A.M.; Santos-Buelga, C.; Bartolome, B.; Moreno-Arribas, M.V. An Integrated View of the Effects of Wine Polyphenols and Their Relevant Metabolites on Gut and Host Health. Molecules 2017, 22, 99. [Google Scholar] [CrossRef]
- Li, S.Y.; Duan, C.Q. Astringency, bitterness and color changes in dry red wines before and during oak barrel aging: An updated phenolic perspective review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1840–1867. [Google Scholar] [CrossRef] [PubMed]
- Tsao, R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Ilarduya, M.B.; Sanchez-Fernandez, C.; Garmon-Lobato, S.; Abad-Garcia, B.; Berrueta, L.A.; Gallo, B.; Vicente, F. Detection of non-coloured anthocyanin-flavanol derivatives in Rioja aged red wines by liquid chromatography-mass spectrometry. Talanta 2014, 121, 81–88. [Google Scholar] [CrossRef]
- Rentzsch, M.; Wilkens, A.; Winterhalter, P. Non-flavonoid Phenolic Compounds. In Wine Chemistry and Biochemistry; Moreno-Arribas, M.V., Polo, M.C., Eds.; Springer: New York, NY, USA, 2009; pp. 509–527. [Google Scholar] [CrossRef]
- Castaldo, L.; Narvaez, A.; Izzo, L.; Graziani, G.; Gaspari, A.; Minno, G.D.; Ritieni, A. Red Wine Consumption and Cardiovascular Health. Molecules 2019, 24, 3626. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Lima, N.; Vallverdu-Queralt, A.; Meudec, E.; Pinasseau, L.; Verbaere, A.; Bordignon-Luiz, M.T.; Le Guerneve, C.; Cheynier, V.; Sommerer, N. Quantification of hydroxycinnamic derivatives in wines by UHPLC-MRM-MS. Anal. Bioanal. Chem. 2018, 410, 3483–3490. [Google Scholar] [CrossRef] [PubMed]
- Garrido, J.; Borges, F. Wine and grape polyphenols—A chemical perspective. Food Res. Int. 2013, 54, 1844–1858. [Google Scholar] [CrossRef]
- Baderschneider, B.; Winterhalter, P. Isolation and characterization of novel benzoates, cinnamates, flavonoids, and lignans from Riesling wine and screening for antioxidant activity. J. Agric. Food Chem. 2001, 49, 2788–2798. [Google Scholar] [CrossRef] [PubMed]
- Neveu, V.; Perez-Jimenez, J.; Vos, F.; Crespy, V.; du Chaffaut, L.; Mennen, L.; Knox, C.; Eisner, R.; Cruz, J.; Wishart, D.; et al. Phenol-Explorer: An online comprehensive database on polyphenol contents in foods. Database (Oxford) 2010, 2010, bap024. [Google Scholar] [CrossRef] [PubMed]
- Kahn, C.H. Pythagoras and the Pythagoreans: A Brief History; Hackett Pub Co Inc.: Indianapolis, IN, USA, 2001. [Google Scholar]
- Ratcliffe, S. Oxford Essential Quotation, 4th ed.; Oxford University Press: Oxford, UK, 2016. [Google Scholar]
- Cochrane, A.L. Effectiveness and Efficiency: Random Reflections on Health Services; Nuffield Trust: New York, NY, USA, 1972. [Google Scholar]
- Koltover, V.K. Free Radical Timer of Aging: From Chemistry of Free Radicals to Systems Theory of Reliability. Curr. Aging Sci. 2017, 10, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Frankel, E.N.; Kanner, J.; German, J.B.; Parks, E.; Kinsella, J.E. Inhibition of oxidation of human low-density lipoprotein by phenolic substances in red wine. Lancet 1993, 341, 454–457. [Google Scholar] [CrossRef]
- Visioli, F.; Keaney, J.F.; Halliwell, B. Antioxidants and cardiovascular disease; panaceas or tonics for tired sheep? Cardiovasc. Res. 2000, 47, 409. [Google Scholar] [CrossRef]
- Forman, H.J.; Davies, K.J.; Ursini, F. How do nutritional antioxidants really work: Nucleophilic tone and para-hormesis versus free radical scavenging in vivo. Free Radic. Biol. Med. 2014, 66, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Lotito, S.B.; Frei, B. Consumption of flavonoid-rich foods and increased plasma antioxidant capacity in humans: Cause, consequence, or epiphenomenon? Free Radic. Biol. Med. 2006, 41, 1727–1746. [Google Scholar] [CrossRef]
- Sies, H. Polyphenols and health: Update and perspectives. Arch. Biochem. Biophys. 2010, 501, 2–5. [Google Scholar] [CrossRef] [PubMed]
- Visioli, F.; De La Lastra, C.A.; Andres-Lacueva, C.; Aviram, M.; Calhau, C.; Cassano, A.; D’Archivio, M.; Faria, A.; Fave, G.; Fogliano, V.; et al. Polyphenols and human health: A prospectus. Crit. Rev. Food Sci. Nutr. 2011, 51, 524–546. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Mateos, A.; Vauzour, D.; Krueger, C.G.; Shanmuganayagam, D.; Reed, J.; Calani, L.; Mena, P.; Del Rio, D.; Crozier, A. Bioavailability, bioactivity and impact on health of dietary flavonoids and related compounds: An update. Arch. Toxicol. 2014, 88, 1803–1853. [Google Scholar] [CrossRef]
- Tome-Carneiro, J.; Visioli, F. Polyphenol-based nutraceuticals for the prevention and treatment of cardiovascular disease: Review of human evidence. Phytomedicine 2016, 23, 1145–1174. [Google Scholar] [CrossRef]
- Castellano-Escuder, P.; Gonzalez-Dominguez, R.; Wishart, D.S.; Andres-Lacueva, C.; Sanchez-Pla, A. FOBI: An ontology to represent food intake data and associate it with metabolomic data. Database (Oxford) 2020, 2020. [Google Scholar] [CrossRef]
- Gonzalez-Dominguez, R.; Jauregui, O.; Mena, P.; Hanhineva, K.; Tinahones, F.J.; Angelino, D.; Andres-Lacueva, C. Quantifying the human diet in the crosstalk between nutrition and health by multi-targeted metabolomics of food and microbiota-derived metabolites. Int. J. Obes. (Lond.) 2020. [Google Scholar] [CrossRef]
- Scarmozzino, F.; Poli, A.; Visioli, F. Microbiota and cardiovascular disease risk: A scoping review. Pharmacol. Res. 2020, 159, 104952. [Google Scholar] [CrossRef]
- Soleas, G.J.; Diamandis, E.P.; Goldberg, D.M. Resveratrol: A molecule whose time has come? And gone? Clin. Biochem. 1997, 30, 91–113. [Google Scholar] [CrossRef]
- Visioli, F. The resveratrol fiasco. Pharmacol. Res. 2014, 90, 87. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.C.; Ng, Y.F.; Ho, S.; Gyda, M.; Chan, S.W. Resveratrol and cardiovascular health--promising therapeutic or hopeless illusion? Pharmacol. Res. 2014, 90, 88–115. [Google Scholar] [CrossRef] [PubMed]
- Shaito, A.; Posadino, A.M.; Younes, N.; Hasan, H.; Halabi, S.; Alhababi, D.; Al-Mohannadi, A.; Abdel-Rahman, W.M.; Eid, A.H.; Nasrallah, G.K.; et al. Potential Adverse Effects of Resveratrol: A Literature Review. Int. J. Mol. Sci. 2020, 21, 2084. [Google Scholar] [CrossRef] [PubMed]
- Wilson, T.; Knight, T.J.; Beitz, D.C.; Lewis, D.S.; Engen, R.L. Resveratrol promotes atherosclerosis in hypercholesterolemic rabbits. Life Sci. 1996, 59, PL15–PL21. [Google Scholar] [CrossRef]
- Woerdeman, J.; Del Rio, D.; Calani, L.; Eringa, E.C.; Smulders, Y.M.; Serne, E.H. Red wine polyphenols do not improve obesity-associated insulin resistance: A randomized controlled trial. Diabetes Obes. Metab. 2018, 20, 206–210. [Google Scholar] [CrossRef]
- Most, J.; Tosti, V.; Redman, L.M.; Fontana, L. Calorie restriction in humans: An update. Ageing Res. Rev. 2017, 39, 36–45. [Google Scholar] [CrossRef]
- Bo, S.; Ponzo, V.; Ciccone, G.; Evangelista, A.; Saba, F.; Goitre, I.; Procopio, M.; Pagano, G.F.; Cassader, M.; Gambino, R. Six months of resveratrol supplementation has no measurable effect in type 2 diabetic patients. A randomized, double blind, placebo-controlled trial. Pharmacol. Res. 2016, 111, 896–905. [Google Scholar] [CrossRef]
- Milne, J.C.; Lambert, P.D.; Schenk, S.; Carney, D.P.; Smith, J.J.; Gagne, D.J.; Jin, L.; Boss, O.; Perni, R.B.; Vu, C.B.; et al. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature 2007, 450, 712–716. [Google Scholar] [CrossRef]
- Kaeberlein, M.; McDonagh, T.; Heltweg, B.; Hixon, J.; Westman, E.A.; Caldwell, S.D.; Napper, A.; Curtis, R.; DiStefano, P.S.; Fields, S.; et al. Substrate-specific activation of sirtuins by resveratrol. J. Biol. Chem. 2005, 280, 17038–17045. [Google Scholar] [CrossRef]
- Dang, W. The controversial world of sirtuins. Drug Discov. Today Technol. 2014, 12, e9–e17. [Google Scholar] [CrossRef]
- Pacholec, M.; Bleasdale, J.E.; Chrunyk, B.; Cunningham, D.; Flynn, D.; Garofalo, R.S.; Griffith, D.; Griffor, M.; Loulakis, P.; Pabst, B.; et al. SRT1720, SRT2183, SRT1460, and resveratrol are not direct activators of SIRT1. J. Biol. Chem. 2010, 285, 8340–8351. [Google Scholar] [CrossRef]
- Yuan, H.; Marmorstein, R. Biochemistry. Red wine, toast of the town (again). Science 2013, 339, 1156–1157. [Google Scholar] [CrossRef] [PubMed]
- Gliemann, L. Dodging physical activity and healthy diet: Can resveratrol take the edge off the consequences of your lifestyle? Am. J. Clin. Nutr. 2020. [Google Scholar] [CrossRef] [PubMed]
- Van de Burgwal, L.H.M.; van der Waal, M.B.; Claassen, E. Accelerating microbiota product development: The Societal Impact Value Cycle as a conceptual model to shape and improve public-private valorization processes. PharmaNutrition 2018, 6, 157–168. [Google Scholar] [CrossRef]
- Flach, J.; dos Ribeiro, C.S.; van der Waal, M.B.; van der Waal, R.X.; Claassen, E.; Van de Burgwal, L.H.M. The Nagoya Protocol on Access to Genetic Resources and Benefit Sharing: Best practices for users of Lactic Acid Bacteria. PharmaNutrition 2019, 9, 100158. [Google Scholar] [CrossRef]
- Clemente-Postigo, M.; Queipo-Ortuno, M.I.; Boto-Ordonez, M.; Coin-Araguez, L.; Roca-Rodriguez, M.M.; Delgado-Lista, J.; Cardona, F.; Andres-Lacueva, C.; Tinahones, F.J. Effect of acute and chronic red wine consumption on lipopolysaccharide concentrations. Am. J. Clin. Nutr. 2013, 97, 1053–1061. [Google Scholar] [CrossRef]
- Banini, A.E.; Boyd, L.C.; Allen, J.C.; Allen, H.G.; Sauls, D.L. Muscadine grape products intake, diet and blood constituents of non-diabetic and type 2 diabetic subjects. Nutrition 2006, 22, 1137–1145. [Google Scholar] [CrossRef]
- Chiva-Blanch, G.; Urpi-Sarda, M.; Llorach, R.; Rotches-Ribalta, M.; Guillen, M.; Casas, R.; Arranz, S.; Valderas-Martinez, P.; Portoles, O.; Corella, D.; et al. Differential effects of polyphenols and alcohol of red wine on the expression of adhesion molecules and inflammatory cytokines related to atherosclerosis: A randomized clinical trial. Am. J. Clin. Nutr. 2012, 95, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Queipo-Ortuno, M.I.; Boto-Ordonez, M.; Murri, M.; Gomez-Zumaquero, J.M.; Clemente-Postigo, M.; Estruch, R.; Cardona Diaz, F.; Andres-Lacueva, C.; Tinahones, F.J. Influence of red wine polyphenols and ethanol on the gut microbiota ecology and biochemical biomarkers. Am. J. Clin. Nutr. 2012, 95, 1323–1334. [Google Scholar] [CrossRef]
- Barden, A.; Shinde, S.; Phillips, M.; Beilin, L.; Mas, E.; Hodgson, J.M.; Puddey, I.; Mori, T.A. The effects of alcohol on plasma lipid mediators of inflammation resolution in patients with Type 2 diabetes mellitus. Prostaglandins Leukot Essent. Fat. Acids 2018, 133, 29–34. [Google Scholar] [CrossRef]
- Barden, A.E.; Chavez, V.; Phillips, M.; Mas, E.; Beilin, L.J.; Croft, K.D.; Mori, T.A.; Puddey, I.B. A Randomized Trial of Effects of Alcohol on Cytochrome P450 Eicosanoids, Mediators of Inflammation Resolution, and Blood Pressure in Men. Alcohol. Clin. Exp. Res. 2017, 41, 1666–1674. [Google Scholar] [CrossRef]
- Mori, T.A.; Burke, V.; Zilkens, R.R.; Hodgson, J.M.; Beilin, L.J.; Puddey, I.B. The effects of alcohol on ambulatory blood pressure and other cardiovascular risk factors in type 2 diabetes: A randomized intervention. J. Hypertens 2016, 34, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Indias, I.; Sanchez-Alcoholado, L.; Perez-Martinez, P.; Andres-Lacueva, C.; Cardona, F.; Tinahones, F.; Queipo-Ortuno, M.I. Red wine polyphenols modulate fecal microbiota and reduce markers of the metabolic syndrome in obese patients. Food Funct. 2016, 7, 1775–1787. [Google Scholar] [CrossRef]
- Mori, T.A.; Burke, V.; Beilin, L.J.; Puddey, I.B. Randomized Controlled Intervention of the Effects of Alcohol on Blood Pressure in Premenopausal Women. Hypertension 2015, 66, 517–523. [Google Scholar] [CrossRef]
- Boto-Ordonez, M.; Urpi-Sarda, M.; Queipo-Ortuno, M.I.; Tulipani, S.; Tinahones, F.J.; Andres-Lacueva, C. High levels of Bifidobacteria are associated with increased levels of anthocyanin microbial metabolites: A randomized clinical trial. Food Funct. 2014, 5, 1932–1938. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Giron, A.; Queipo-Ortuno, M.I.; Boto-Ordonez, M.; Munoz-Gonzalez, I.; Sanchez-Patan, F.; Monagas, M.; Martin-Alvarez, P.J.; Murri, M.; Tinahones, F.J.; Andres-Lacueva, C.; et al. Comparative study of microbial-derived phenolic metabolites in human feces after intake of gin, red wine, and dealcoholized red wine. J. Agric. Food Chem. 2013, 61, 3909–3915. [Google Scholar] [CrossRef] [PubMed]
- Chiva-Blanch, G.; Urpi-Sarda, M.; Ros, E.; Valderas-Martinez, P.; Casas, R.; Arranz, S.; Guillen, M.; Lamuela-Raventos, R.M.; Llorach, R.; Andres-Lacueva, C.; et al. Effects of red wine polyphenols and alcohol on glucose metabolism and the lipid profile: A randomized clinical trial. Clin. Nutr. 2013, 32, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Chiva-Blanch, G.; Urpi-Sarda, M.; Ros, E.; Arranz, S.; Valderas-Martinez, P.; Casas, R.; Sacanella, E.; Llorach, R.; Lamuela-Raventos, R.M.; Andres-Lacueva, C.; et al. Dealcoholized red wine decreases systolic and diastolic blood pressure and increases plasma nitric oxide: Short communication. Circ. Res. 2012, 111, 1065–1068. [Google Scholar] [CrossRef]
- Vazquez-Fresno, R.; Llorach, R.; Alcaro, F.; Rodriguez, M.A.; Vinaixa, M.; Chiva-Blanch, G.; Estruch, R.; Correig, X.; Andres-Lacueva, C. (1)H-NMR-based metabolomic analysis of the effect of moderate wine consumption on subjects with cardiovascular risk factors. Electrophoresis 2012, 33, 2345–2354. [Google Scholar] [CrossRef]
- Schrieks, I.C.; van den Berg, R.; Sierksma, A.; Beulens, J.W.; Vaes, W.H.; Hendriks, H.F. Effect of red wine consumption on biomarkers of oxidative stress. Alcohol. Alcohol. 2013, 48, 153–159. [Google Scholar] [CrossRef]
- Noguer, M.A.; Cerezo, A.B.; Donoso Navarro, E.; Garcia-Parrilla, M.C. Intake of alcohol-free red wine modulates antioxidant enzyme activities in a human intervention study. Pharmacol. Res. 2012, 65, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Imhof, A.; Plamper, I.; Maier, S.; Trischler, G.; Koenig, W. Effect of drinking on adiponectin in healthy men and women: A randomized intervention study of water, ethanol, red wine, and beer with or without alcohol. Diabetes Care 2009, 32, 1101–1103. [Google Scholar] [CrossRef] [PubMed]
- Ellinger, S.; Arendt, B.M.; Fimmers, R.; Stehle, P.; Spengler, U.; Goerlich, R. Bolus ingestion but not regular consumption of native or dealcoholized red wine modulates selected immunological functions of leukocytes in healthy volunteers. Ann. Nutr. Metab. 2008, 52, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Beulens, J.W.; van Beers, R.M.; Stolk, R.P.; Schaafsma, G.; Hendriks, H.F. The effect of moderate alcohol consumption on fat distribution and adipocytokines. Obes. (Silver Spring) 2006, 14, 60–66. [Google Scholar] [CrossRef]
- Arendt, B.M.; Ellinger, S.; Kekic, K.; Geus, L.; Fimmers, R.; Spengler, U.; Muller, W.U.; Goerlich, R. Single and repeated moderate consumption of native or dealcoholized red wine show different effects on antioxidant parameters in blood and DNA strand breaks in peripheral leukocytes in healthy volunteers: A randomized controlled trial (ISRCTN68505294). Nutr. J. 2005, 4, 33. [Google Scholar] [CrossRef][Green Version]
- Zilkens, R.R.; Burke, V.; Hodgson, J.M.; Barden, A.; Beilin, L.J.; Puddey, I.B. Red wine and beer elevate blood pressure in normotensive men. Hypertension 2005, 45, 874–879. [Google Scholar] [CrossRef]
- Watzl, B.; Bub, A.; Pretzer, G.; Roser, S.; Barth, S.W.; Rechkemmer, G. Daily moderate amounts of red wine or alcohol have no effect on the immune system of healthy men. Eur. J. Clin. Nutr. 2004, 58, 40–45. [Google Scholar] [CrossRef]
- Abu-Amsha Caccetta, R.; Burke, V.; Mori, T.A.; Beilin, L.J.; Puddey, I.B.; Croft, K.D. Red wine polyphenols, in the absence of alcohol, reduce lipid peroxidative stress in smoking subjects. Free Radic. Biol. Med. 2001, 30, 636–642. [Google Scholar] [CrossRef]
- McDonald, J.T.; Margen, S. Wine versus ethanol in human nutrition. IV. Zinc balance. Am. J. Clin. Nutr. 1980, 33, 1096–1102. [Google Scholar] [CrossRef]
- McDonald, J.T.; Margen, S. Wine versus ethanol in human nutrition. III. Calcium, phosphorous, and magnesium balance. Am. J. Clin. Nutr. 1979, 32, 823–833. [Google Scholar] [CrossRef]
- McDonald, J.T.; Margen, S. Wine versus ethanol in human nutrition. II. Fluid, sodium, and potassium balance. Am. J. Clin. Nutr. 1979, 32, 817–822. [Google Scholar] [CrossRef]
- McDonald, J.T.; Margen, S. Wine versus ethanol in human nutrition. I. Nitrogen and calorie balance. Am. J. Clin. Nutr. 1976, 29, 1093–1103. [Google Scholar] [CrossRef] [PubMed]
- Perez-Mana, C.; Farre, M.; Rodriguez-Morato, J.; Papaseit, E.; Pujadas, M.; Fito, M.; Robledo, P.; Covas, M.I.; Cheynier, V.; Meudec, E.; et al. Moderate consumption of wine, through both its phenolic compounds and alcohol content, promotes hydroxytyrosol endogenous generation in humans. A randomized controlled trial. Mol. Nutr. Food Res. 2015, 59, 1213–1216. [Google Scholar] [CrossRef] [PubMed]
- Barden, A.E.; Croft, K.D.; Beilin, L.J.; Phillips, M.; Ledowski, T.; Puddey, I.B. Acute effects of red wine on cytochrome P450 eicosanoids and blood pressure in men. J. Hypertens 2013, 31, 2195–2202. [Google Scholar] [CrossRef] [PubMed]
- Kiviniemi, T.O.; Saraste, A.; Lehtimaki, T.; Toikka, J.O.; Saraste, M.; Raitakari, O.T.; Hartiala, J.J.; Viikari, J.; Koskenvuo, J.W. Decreased endothelin-1 levels after acute consumption of red wine and de-alcoholized red wine. Atherosclerosis 2010, 211, 283–286. [Google Scholar] [CrossRef]
- Kiviniemi, T.O.; Saraste, A.; Lehtimaki, T.; Toikka, J.O.; Saraste, M.; Raitakari, O.T.; Parkka, J.P.; Hartiala, J.J.; Viikari, J.; Koskenvuo, J.W. High dose of red wine elicits enhanced inhibition of fibrinolysis. Eur J Cardiovasc. Prev. Rehabil. 2009, 16, 161–163. [Google Scholar] [CrossRef]
- Kiviniemi, T.O.; Saraste, A.; Toikka, J.O.; Saraste, M.; Raitakari, O.T.; Parkka, J.P.; Lehtimaki, T.; Hartiala, J.J.; Viikari, J.; Koskenvuo, J.W. A moderate dose of red wine, but not de-alcoholized red wine increases coronary flow reserve. Atherosclerosis 2007, 195, e176–e181. [Google Scholar] [CrossRef]
- Modun, D.; Music, I.; Vukovic, J.; Brizic, I.; Katalinic, V.; Obad, A.; Palada, I.; Dujic, Z.; Boban, M. The increase in human plasma antioxidant capacity after red wine consumption is due to both plasma urate and wine polyphenols. Atherosclerosis 2008, 197, 250–256. [Google Scholar] [CrossRef]
- Karatzi, K.; Papamichael, C.; Karatzis, E.; Papaioannou, T.G.; Voidonikola, P.T.; Lekakis, J.; Zampelas, A. Acute smoking induces endothelial dysfunction in healthy smokers. Is this reversible by red wine’s antioxidant constituents? J. Am. Coll. Nutr. 2007, 26, 10–15. [Google Scholar] [CrossRef]
- Boban, M.; Modun, D.; Music, I.; Vukovic, J.; Brizic, I.; Salamunic, I.; Obad, A.; Palada, I.; Dujic, Z. Red wine induced modulation of vascular function: Separating the role of polyphenols, ethanol, and urates. J. Cardiovasc. Pharmacol. 2006, 47, 695–701. [Google Scholar] [CrossRef]
- Karatzi, K.N.; Papamichael, C.M.; Karatzis, E.N.; Papaioannou, T.G.; Aznaouridis, K.A.; Katsichti, P.P.; Stamatelopoulos, K.S.; Zampelas, A.; Lekakis, J.P.; Mavrikakis, M.E. Red wine acutely induces favorable effects on wave reflections and central pressures in coronary artery disease patients. Am. J. Hypertens 2005, 18, 1161–1167. [Google Scholar] [CrossRef]
- Pal, S.; Naissides, M.; Mamo, J. Polyphenolics and fat absorption. Int. J. Obes. Relat. Metab. Disord. 2004, 28, 324–326. [Google Scholar] [CrossRef] [PubMed]
- Papamichael, C.; Karatzis, E.; Karatzi, K.; Aznaouridis, K.; Papaioannou, T.; Protogerou, A.; Stamatelopoulos, K.; Zampelas, A.; Lekakis, J.; Mavrikakis, M. Red wine’s antioxidants counteract acute endothelial dysfunction caused by cigarette smoking in healthy nonsmokers. Am. Heart J. 2004, 147, E5. [Google Scholar] [CrossRef]
- Watzl, B.; Bub, A.; Briviba, K.; Rechkemmer, G. Acute intake of moderate amounts of red wine or alcohol has no effect on the immune system of healthy men. Eur. J. Nutr. 2002, 41, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Bub, A.; Watzl, B.; Heeb, D.; Rechkemmer, G.; Briviba, K. Malvidin-3-glucoside bioavailability in humans after ingestion of red wine, dealcoholized red wine and red grape juice. Eur. J. Nutr. 2001, 40, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Bell, J.R.; Donovan, J.L.; Wong, R.; Waterhouse, A.L.; German, J.B.; Walzem, R.L.; Kasim-Karakas, S.E. (+)-Catechin in human plasma after ingestion of a single serving of reconstituted red wine. Am. J. Clin. Nutr. 2000, 71, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Caccetta, R.A.; Croft, K.D.; Beilin, L.J.; Puddey, I.B. Ingestion of red wine significantly increases plasma phenolic acid concentrations but does not acutely affect ex vivo lipoprotein oxidizability. Am. J. Clin. Nutr. 2000, 71, 67–74. [Google Scholar] [CrossRef]
- Agewall, S.; Wright, S.; Doughty, R.N.; Whalley, G.A.; Duxbury, M.; Sharpe, N. Does a glass of red wine improve endothelial function? Eur. Heart J. 2000, 21, 74–78. [Google Scholar] [CrossRef]
- Donovan, J.L.; Bell, J.R.; Kasim-Karakas, S.; German, J.B.; Walzem, R.L.; Hansen, R.J.; Waterhouse, A.L. Catechin is present as metabolites in human plasma after consumption of red wine. J. Nutr. 1999, 129, 1662–1668. [Google Scholar] [CrossRef]
- Yang, C.S.; Ho, C.T.; Zhang, J.; Wan, X.; Zhang, K.; Lim, J. Antioxidants: Differing Meanings in Food Science and Health Science. J. Agric. Food Chem. 2018, 66, 3063–3068. [Google Scholar] [CrossRef]
- Gorelik, S.; Ligumsky, M.; Kohen, R.; Kanner, J. The stomach as a “bioreactor”: When red meat meets red wine. J. Agric. Food Chem. 2008, 56, 5002–5007. [Google Scholar] [CrossRef] [PubMed]
- Timmers, P.; Wilson, J.F.; Joshi, P.K.; Deelen, J. Multivariate genomic scan implicates novel loci and haem metabolism in human ageing. Nat. Commun. 2020, 11, 3570. [Google Scholar] [CrossRef] [PubMed]
- Tirosh, O.; Shpaizer, A.; Kanner, J. Lipid Peroxidation in a Stomach Medium Is Affected by Dietary Oils (Olive/Fish) and Antioxidants: The Mediterranean versus Western Diet. J. Agric. Food Chem. 2015, 63, 7016–7023. [Google Scholar] [CrossRef] [PubMed]
- Natella, F.; Ghiselli, A.; Guidi, A.; Ursini, F.; Scaccini, C. Red wine mitigates the postprandial increase of LDL susceptibility to oxidation. Free Radic. Biol. Med. 2001, 30, 1036–1044. [Google Scholar] [CrossRef]
- Kanner, J.; Gorelik, S.; Roman, S.; Kohen, R. Protection by polyphenols of postprandial human plasma and low-density lipoprotein modification: The stomach as a bioreactor. J. Agric. Food Chem. 2012, 60, 8790–8796. [Google Scholar] [CrossRef]
- Kanner, J.; Selhub, J.; Shpaizer, A.; Rabkin, B.; Shacham, I.; Tirosh, O. Redox homeostasis in stomach medium by foods: The Postprandial Oxidative Stress Index (POSI) for balancing nutrition and human health. Redox. Biol. 2017, 12, 929–936. [Google Scholar] [CrossRef]
- Ellinger, S.; Muller, N.; Stehle, P.; Ulrich-Merzenich, G. Consumption of green tea or green tea products: Is there an evidence for antioxidant effects from controlled interventional studies? Phytomedicine 2011, 18, 903–915. [Google Scholar] [CrossRef]
- Higdon, J.V.; Frei, B. Coffee and health: A review of recent human research. Crit. Rev. Food Sci. Nutr. 2006, 46, 101–123. [Google Scholar] [CrossRef]
- van Dam, R.M.; Hu, F.B.; Willett, W.C. Coffee, Caffeine, and Health. N. Engl. J. Med. 2020, 383, 369–378. [Google Scholar] [CrossRef]
- Comporti, M.; Signorini, C.; Leoncini, S.; Gardi, C.; Ciccoli, L.; Giardini, A.; Vecchio, D.; Arezzini, B. Ethanol-induced oxidative stress: Basic knowledge. Genes Nutr. 2010, 5, 101–109. [Google Scholar] [CrossRef]
- Wynder, E.L.; Higgins, I.T.; Harris, R.E. The wish bias. J. Clin. Epidemiol. 1990, 43, 619–621. [Google Scholar] [CrossRef]
- Ioannidis, J.P. We need more randomized trials in nutrition-preferably large, long-term, and with negative results. Am. J. Clin. Nutr. 2016, 103, 1385–1386. [Google Scholar] [CrossRef] [PubMed]
- Trepanowski, J.F.; Ioannidis, J.P.A. Perspective: Limiting Dependence on Nonrandomized Studies and Improving Randomized Trials in Human Nutrition Research: Why and How. Adv. Nutr. 2018, 9, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Laville, M.; Segrestin, B.; Alligier, M.; Ruano-Rodriguez, C.; Serra-Majem, L.; Hiesmayr, M.; Schols, A.; La Vecchia, C.; Boirie, Y.; Rath, A.; et al. Evidence-based practice within nutrition: What are the barriers for improving the evidence and how can they be dealt with? Trials 2017, 18, 425. [Google Scholar] [CrossRef] [PubMed]
- Visioli, F. Can experimental pharmacology be always applied to human nutrition? Int. J. Food Sci. Nutr. 2012, 63 (Suppl. 1), 10–13. [Google Scholar] [CrossRef]
| Group | Subgroup | Main Parent Compounds and Representative Derivatives |
|---|---|---|
Flavonoids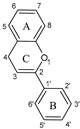 | 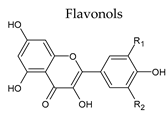 | 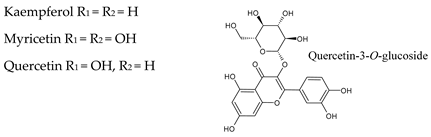 |
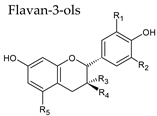 | 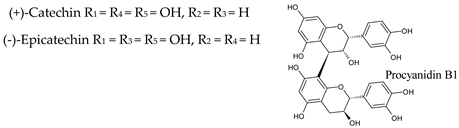 | |
 |  | |
 |  | |
 |  | |
 |  | |
| Non-flavonoids | 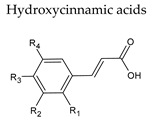 | 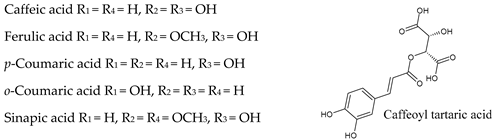 |
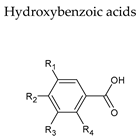 | 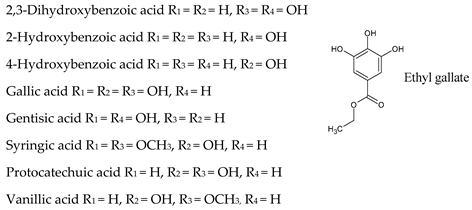 | |
 | 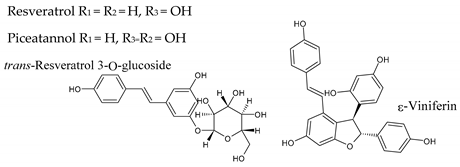 |
| Red Wine | White Wine | ||||||
|---|---|---|---|---|---|---|---|
| Total Phenolic Content a | Total Phenolic Content a | ||||||
| Range | Range | ||||||
| Min. | Max. | n | Min. | Max. | n | Reference | |
| 1615 | 4177 | 7 | 216 | 854 | 7 | [16] | |
| 1313 | 2389 | 16 | 89 | 407 | 17 | [17] | |
| 2193 | 3183 | 6 | 292 | 402 | 4 | [18] | |
| 622 | 3200 | 20 | - | - | - | [19] | |
| 1724 | 1936 | 5 | 282 | 434 | 5 | [15] | |
| 2340 | 3730 | 23 | - | - | - | [20] | |
| 1460 | 3380 | 39 | 210 | 390 | 47 | [21] | |
| 2082 | 3184 | 3 | 213 | 277 | 5 | [22] | |
| 1402 | 3180 | 24 | 189 | 425 | 11 | [14] | |
| 3200 | 5900 | 8 | - | - | - | [23] | |
| 1788 | 3070 | 4 | 55 | 370 | 20 | [24] | |
| 1012 | 3264 | 11 | - | - | - | [25] | |
| 554 | 2669 | 2 | 167 | 347 | 3 | [26] | |
| 1181 | 3589 | 23 | - | - | - | [27] | |
| - | - | - | 291 | 2103 | 14 | [28] | |
| 860 | 2710 | 8 | - | - | - | [29] | |
| 1602 | 1968 | 7 | - | - | - | [30] | |
| 1837 | 3467 | 6 | - | - | - | [31] | |
| 896 | 6319 | 38 | 77 | 83 | 2 | [32] | |
| Mean ± SD | 1538 ± 664 | 3406 ± 1139 | 189 ± 85 | 554 ± 545 | |||
| Median (Q25–Q75) | 1531 (983–1898) | 3192 (2700–3624) | 210 (89–282) | 402 (347–434) | |||
| Red Wine | White Wine | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phenol Explorer a | USDA b | Phenol Explorer a | USDA b | ||||||||||
| mean | min | max | mean | min | max | mean | min | max | mean | min | max | ||
| Flavonoids | Main representatives | ||||||||||||
| Anthocyanins | Cyanidin c | 2.9 | 0.6 | 11.9 | 1.9 | 0.0 | 45.0 | - | - | - | - | - | - |
| Delphinidin c | 16.6 | 2.4 | 40.1 | 20.1 | 0.2 | 57.1 | - | - | - | - | - | - | |
| Malvidin c | 156 | 12.4 | 541 | 138 | 0.0 | 536 | 0.4 | 0.0 | 3.5 | 0.6 | 0.0 | 2.4 | |
| Peodinin c | 18.1 | 2.5 | 80.9 | 12.5 | 0.2 | 50.3 | - | - | - | - | - | - | |
| Petunidin c | 23.6 | 3.4 | 61.8 | 19.8 | 0.2 | 56.6 | - | - | - | - | - | - | |
| Total | 217 | 21.3 | 736 | 193 | 0.6 | 745 | 0.4 | 0.0 | 3.5 | 0.6 | 0.0 | 2.4 | |
| Dihydroflavonols | Dihydromyricetin 3-O-rhamnoside | 44.7 | 44.7 | 44.7 | - | - | - | 3.0 | 3.0 | 3.0 | - | - | - |
| Total | 54.4 | 45.8 | 59.8 | - | - | - | 5.7 | 3.7 | 15.9 | - | - | - | |
| Flavanols | (+)-Catechin | 68.1 | 13.8 | 390 | 71.4 | 0.0 | 390 | 10.8 | 0.0 | 46.0 | 7.7 | 0.0 | 58.0 |
| (-)-Epicatechin | 37.8 | 0.0 | 165 | 37.9 | 0.0 | 165 | 9.5 | 0.0 | 60.0 | 5.5 | 0.5 | 60.0 | |
| Proanthocyanidins | 355 | 99.7 | 560 | 296 | 63.1 | 1354 | 0.2 | 0.0 | 1.5 | 3.9 | 0.6 | 7.3 | |
| Total | 470 | 114 | 1131 | 407 | 63.1 | 1917 | 20.8 | 0.0 | 109 | 17.1 | 1.1 | 125 | |
| Flavanones | Naringenin c | 8.0 | 7.3 | 8.8 | 17.7 | 10.3 | 25.1 | 2.3 | 1.7 | 2.9 | 3.8 | 0.0 | 7.7 |
| Total | 8.5 | 7.8 | 9.4 | 24.0 | 13.0 | 35.0 | 2.3 | 1.7 | 2.9 | 7.8 | 3.2 | 12.5 | |
| Flavones | Apigenin | - | - | - | 1.3 | 0.0 | 4.7 | - | - | - | - | - | - |
| Total | - | - | - | 1.7 | 0.0 | 8.7 | - | - | - | - | - | - | |
| Flavonols | Isorhamnetin c | 5.9 | 1.7 | 11.6 | 0.2 | 0.0 | 1.6 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.2 |
| Kaempferol c | 10.2 | 5.7 | 14.4 | 0.9 | 0.0 | 13.7 | 0.2 | 0.0 | 2.6 | 0.1 | 0.0 | 2.7 | |
| Myricetin c | 8.3 | 0.0 | 17.9 | 4.2 | 0.0 | 17.9 | 0.0 | 0.0 | 0.0 | 0.1 | 0.0 | 1.0 | |
| Quercetin c | 44.2 | 12.3 | 110 | 10.4 | 0.0 | 33.6 | 4.6 | 1.3 | 20.8 | 0.4 | 0.0 | 8.4 | |
| Total | 68.6 | 19.7 | 154 | 15.7 | 0.0 | 66.8 | 4.8 | 1.3 | 23.4 | 0.5 | 0.0 | 9.4 | |
| Non-flavonoids | |||||||||||||
| Hydroxybenzoic acids | Gallic | 35.9 | 0.0 | 126 | NA | NA | NA | 2.2 | 0.0 | 11.0 | NA | NA | NA |
| Gentisic | 4.6 | 0.0 | 8.0 | NA | NA | NA | 18.2 | 0.0 | 20.0 | NA | NA | NA | |
| Protocatechuic | 1.7 | 0.0 | 9.6 | NA | NA | NA | 3.3 | 0.1 | 13.0 | NA | NA | NA | |
| Syringic | 2.7 | 0.0 | 23.3 | NA | NA | NA | 0.5 | 0.0 | 0.2 | NA | NA | NA | |
| Vanillic | 3.2 | 0.0 | 7.5 | NA | NA | NA | 0.4 | 0.1 | 1.2 | NA | NA | NA | |
| Total | 70.1 | 13.7 | 221 | NA | NA | NA | 24.8 | 0.4 | 46.8 | NA | NA | NA | |
| Hydroxycinnamic acids | Caffeic | 18.8 | 0.0 | 77.0 | NA | NA | NA | 2.4 | 0.0 | 7.0 | NA | NA | NA |
| Caftaric | 33.5 | 1.4 | 179 | NA | NA | NA | 21.5 | 21.4 | 22.0 | NA | NA | NA | |
| Ferulic | 0.8 | 0.0 | 10.4 | NA | NA | NA | 0.9 | 0.3 | 2.1 | NA | NA | NA | |
| (o- and p-) Coumaric | 5.8 | 0.2 | 40.4 | NA | NA | NA | 1.8 | 0.0 | 5.6 | NA | NA | NA | |
| Sinapic | 0.7 | 0.0 | 5.4 | NA | NA | NA | 0.6 | 0.0 | 2.8 | NA | NA | NA | |
| Total | 100 | 14.9 | 378 | NA | NA | NA | 28.2 | 21.7 | 42.4 | NA | NA | NA | |
| Stilbenes | Resveratrol d | 5.8 | 0.0 | 61.5 | NA | NA | NA | 0.9 | 0.0 | 3.2 | NA | NA | NA |
| Resveratrol 3-O-glucoside d | 12.5 | 0.0 | 88.0 | NA | NA | NA | 5.0 | 0.4 | 13.1 | NA | NA | NA | |
| Piceatannol c | 15.3 | 6.3 | 38.8 | NA | NA | NA | 4.6 | 1.4 | 8.0 | NA | NA | NA | |
| Viniferins (δ-. ε-) | 7.9 | 0.1 | 26.7 | NA | NA | NA | 0.6 | 0.0 | 0.1 | NA | NA | NA | |
| Pallidol | 2.0 | 0.0 | 2.5 | NA | NA | NA | 0.7 | 0.0 | 0.3 | NA | NA | NA | |
| Total | 43.5 | 6.4 | 218 | NA | NA | NA | 10.6 | 1.4 | 24.7 | NA | NA | NA | |
| Other polyphenols | Hydroxybenzaldehydes | 7.1 | 0.0 | 45.6 | NA | NA | NA | 4.1 | 2.4 | 5.8 | NA | NA | NA |
| Tyrosols | 36.5 | 6.4 | 54.3 | NA | NA | NA | 4.2 | 2.7 | 5.7 | NA | NA | NA | |
| Total | 43.6 | 6.4 | 99.9 | NA | NA | NA | 8.3 | 5.1 | 11.5 | NA | NA | NA | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Visioli, F.; Panaite, S.-A.; Tomé-Carneiro, J. Wine’s Phenolic Compounds and Health: A Pythagorean View. Molecules 2020, 25, 4105. https://doi.org/10.3390/molecules25184105
Visioli F, Panaite S-A, Tomé-Carneiro J. Wine’s Phenolic Compounds and Health: A Pythagorean View. Molecules. 2020; 25(18):4105. https://doi.org/10.3390/molecules25184105
Chicago/Turabian StyleVisioli, Francesco, Stefan-Alexandru Panaite, and Joao Tomé-Carneiro. 2020. "Wine’s Phenolic Compounds and Health: A Pythagorean View" Molecules 25, no. 18: 4105. https://doi.org/10.3390/molecules25184105
APA StyleVisioli, F., Panaite, S.-A., & Tomé-Carneiro, J. (2020). Wine’s Phenolic Compounds and Health: A Pythagorean View. Molecules, 25(18), 4105. https://doi.org/10.3390/molecules25184105







