Features and Advantages of Supercritical CO2 Extraction of Sea Cucumber Cucumaria frondosa japonica Semper, 1868
Abstract
1. Introduction
2. Results
2.1. Sterol Compositions Identified in Supercritical CO2 Extract
2.2. Carotenoids Identified in Supercritical CO2 Extract
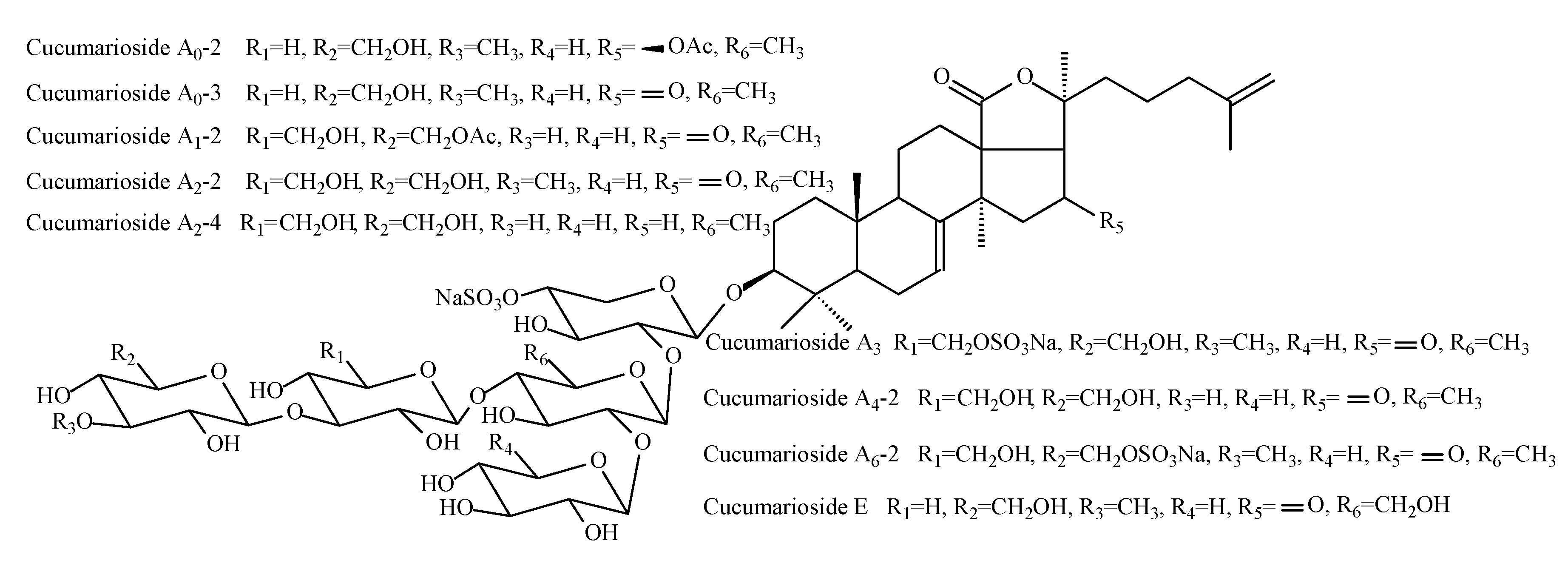
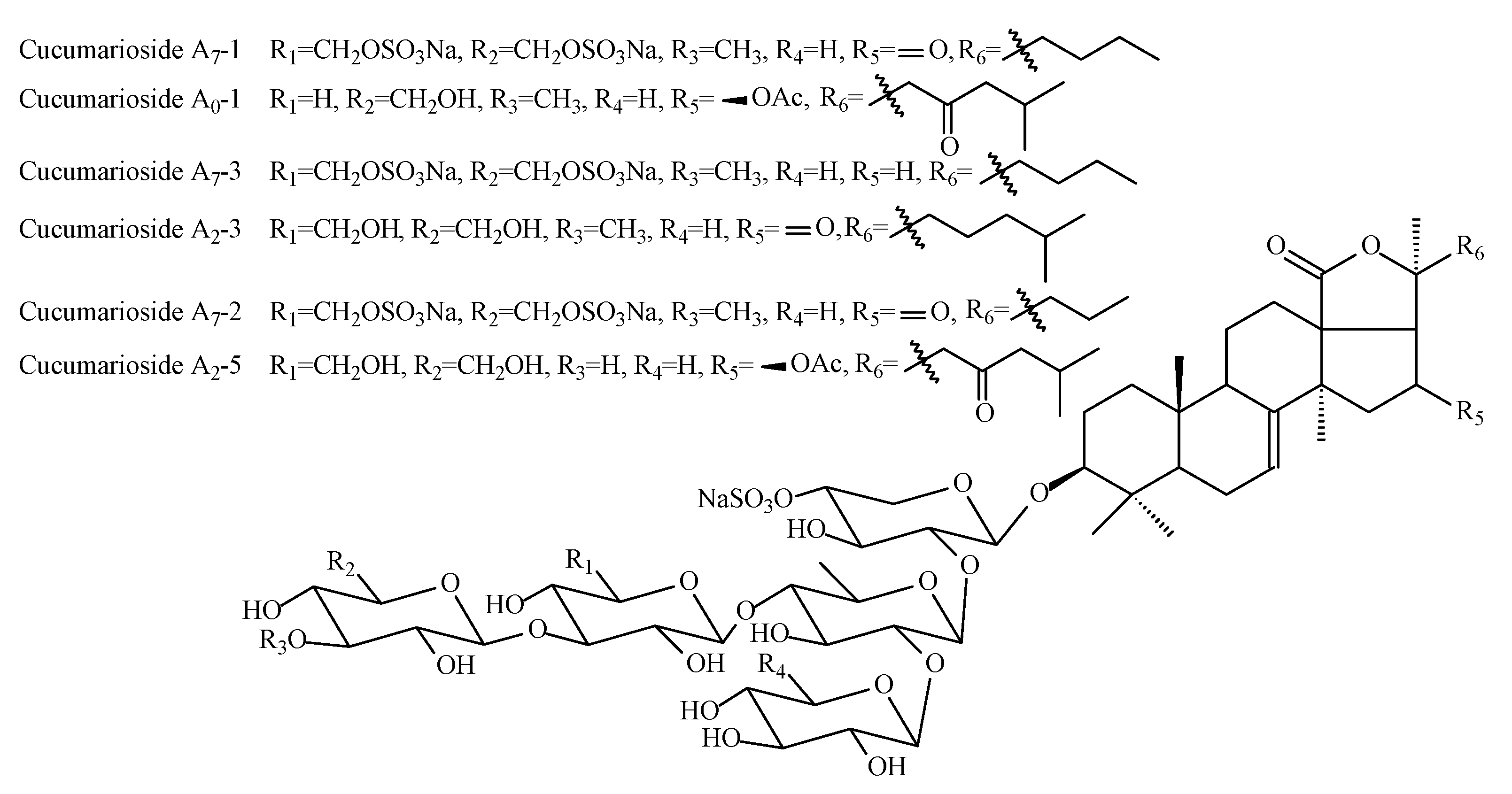
2.3. Triterpene Glycosides Identified in Supercritical CO2 Extract
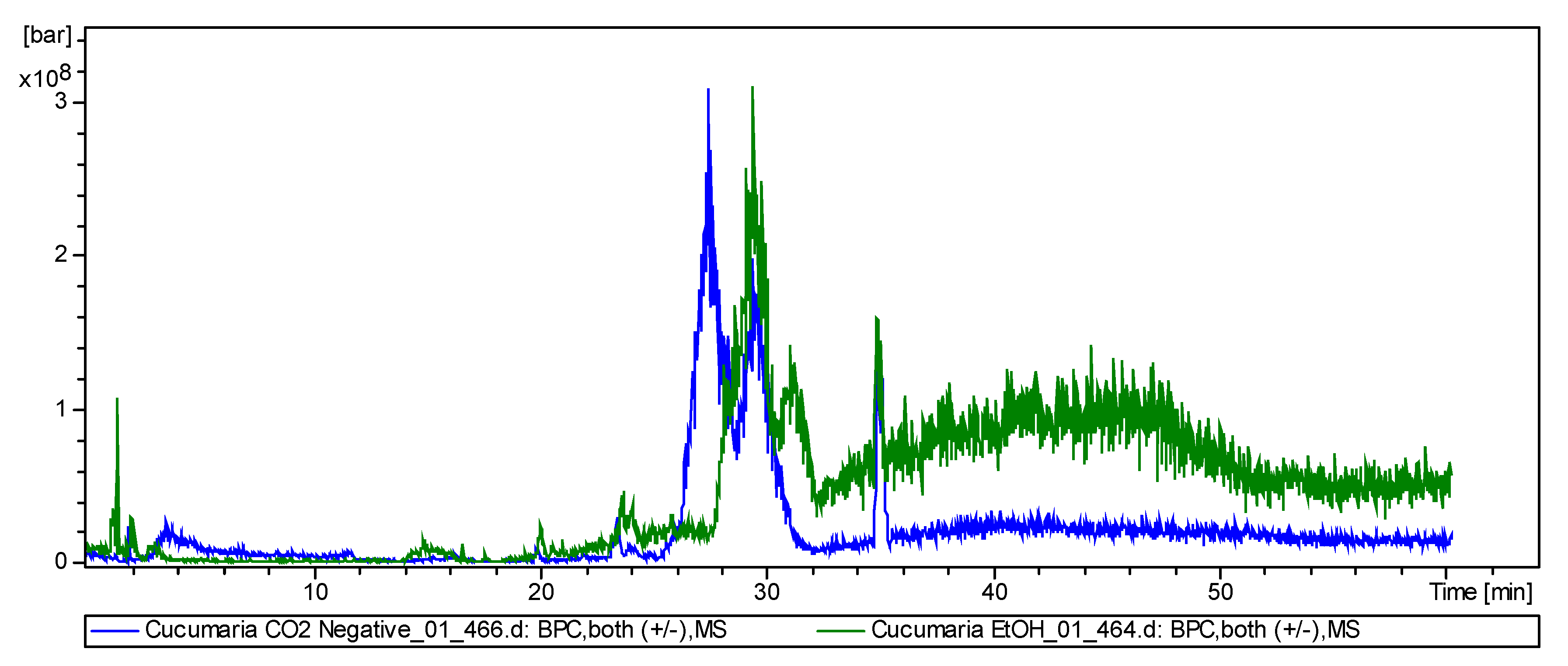
3. Discussion
4. Materials and Methods
4.1. Sea Cucumber
4.2. Chemicals
4.3. Supercritical Fluid Extraction
4.4. Ethanol Extraction
4.5. High-Performance Liquid Chromatography-Electrospray Ionization Tandem Mass Spectrometry
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Carcamo, P.F. Effects of food type and feeding frequency on the performance of early juveniles of the sea urchin Loxechinus albus (Echinodermata: Echinoidea): Implications for aquaculture and restocking. Aquaculture 2015, 436, 172–178. [Google Scholar] [CrossRef]
- Honey-Escandon, M.; Arreguin-Espinosa, R.; Solis-Marin, F.A.; Samyn, Y. Biological and taxonomic perspective of triterpenoid glycosides of sea cucumbers of the family Holothuriidae (Echinodermata, Holothuroidea). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2015, 180, 16–39. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, V.C.; Soyar, S.; Horam, S.; Raj, S.; Arockiaraj, J.; Pasupuleti, M.; Dikshit, D.K. Natural products from polar organisms: Structural diversity, bioactivities and potential pharmaceutical applications. Polar Sci. 2018, 18, 147–166. [Google Scholar] [CrossRef]
- Gomes, A.R.; Freitas, A.C.; Duarte, A.C.; Rocha-Santos, T.A.P. Echinoderms: A review of bioactive compounds with potential health effects. Stud. Nat. Prod. Chem. 2016, 49, 1–54. [Google Scholar] [CrossRef]
- Casas, L.; Mantell, C.; Rodríguez, M.; Torres, A.; Macias, F.A.; de la Ossa, E.M. Extraction of natural compounds with biological activity from sunflower leaves with supercritical carbon dioxide. Chem. Eng. J. 2009, 152, 301–306. [Google Scholar] [CrossRef]
- Herrero, M.; Mendiola, J.; Cifuentes, A.; Ibanez, E. Supercritical fluid extraction: Recent advances and applications. J. Chromatogr. A 2010, 1217, 2495–2511. [Google Scholar] [CrossRef] [PubMed]
- Plaza, M.; Herrero, M.; Cifuentes, A.; Ibanez, E. Innovative natural functional ingredients from microalgae. J. Agric. Food Chem. 2009, 57, 7159–7170. [Google Scholar] [CrossRef]
- Kumar, S.P.J.; Prasad, S.R.; Banerjee, R.; Agarwal, D.K.; Kulkarni, K.S.; Ramesh, K.V. Green solvents and technologies for oil extraction from oilseeds. Chem. Cent. J. 2017, 11, 7. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.-O.; Shastina, V.V.; Shin, S.-W.; Joo, Q.X.; Park, J.-I.; Rasskazov, V.A.; Avilov, S.A.; Fedorov, S.N.; Stonik, V.A.; Kwak, J.-Y. Differential effects of triterpene glycosides, frondoside A and cucumarioside A2-2 isolated from sea cucumbers on caspase activation and apoptosis of human leukemia cells. FEBS Lett. 2009, 583, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Hossain, A.; Dave, D.; Shahidi, F. Northern sea cucumber (Cucumaria frondosa): A potential candidate for functional food, nutraceutical, and pharmaceutical sector. Mar. Drugs 2020, 18, 274. [Google Scholar] [CrossRef] [PubMed]
- Aminin, D.L.; Agafonova, I.G.; Berdyshev, E.V.; Isachenko, E.G.; Avilov, S.A.; Stonik, V.A. Immunomodulatory properties of cucumariosides from the edible far-eastern holothurian Cucumaria japonica. J. Med. Food 2001, 4, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Silchenko, A.; Avilov, S.; Antonov, A.; Kalinovsky, A.; Dmitrenok, P.; Kalinin, V.; Woodward, C.; Collin, P. Glycosides from the sea cucumber Cucumaria frondosa. IV. structure of frondosides A2-4, A2-7, and A2-8, three new minor monosulfated triterpene glycosides. Can. J. Chem. 2005, 83, 2120–2126. [Google Scholar] [CrossRef]
- Kalinin, V.I.; Silchenko, A.S.; Avilov, S.A.; Stonik, V.A.; Smirnov, A.V. Sea cucumbers triterpene glycosides, the recent progress in structural elucidation and chemotaxonomy. Phytochem. Rev. 2005, 4, 221–236. [Google Scholar] [CrossRef]
- Popov, R.S.; Ivanchina, N.V.; Silchenko, A.S.; Avilov, S.A.; Kalinin, V.I.; Dolmatov, I.Y.; Stonik, V.A.; Dmitrenok, P.S. Metabolite profiling of triterpene glycosides of the far eastern sea cucumber eupentacta fraudatrix and their distribution in various body components using LC-ESI QTOF-MS. Mar. Drugs 2017, 15, 302. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, Y.; Zhang, W.; MM Franco, C. Distribution of saponins in the sea cucumber holothuria lessoni; the body wall versus the viscera, and their biological activities. Mar. Drugs 2018, 16, 423. [Google Scholar] [CrossRef] [PubMed]
- Silchenko, A.S.; Kalinovsky, A.I.; Dmitrenok, P.S.; Kalinin, V.I.; Mazeika, A.N.; Vorobieva, N.S.; Sanina, N.M.; Kostetsky, E.Y. Cucumarioside E from the far eastern sea cucumber cucumaria japonica (cucumariidae, dendrochirotida), new minor monosulfated holostane triterpene pentaoside with glucose as the second monosaccharide residue. Nat. Prod. Commun. 2015, 10, 877–880. [Google Scholar] [CrossRef] [PubMed]
- Avilov, S.A.; Stonik, V.A.; Kalinovskii, A.I. Structures of four new triterpene glycosides from the holothurian Cucumaria japonica. Chem. Nat. Compd. 1990, 26, 670–675. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available. |
| № | Sterol | Molecular Formula | [M+H]+ (m/z) | MS2 (m/z) | Yield, % |
|---|---|---|---|---|---|
| 1 | 24-nor-5α-cholesta-7,22-dien-3β-ol (Asterosterol) | C26H42O | 371.32 | 303.26; 275.23; 235.20 | 1.34 |
| 2 | 24-nor-5α-cholesta-22-en-3β-ol (24-nordehydrocholestanol) | C26H44O | 373.34 | 305.28; 277.25; 237.21 | 1.48 |
| 3 | 5α-cholesta-7,22-en-3β-ol | C27H44O | 385.34 | 329.28; 303.26; 235.20; 167.14; 143.14 | 9.26 |
| 4 | 5α-cholest-22-en-3β-ol (trans-22-dehydrocholestanol) | C27H46O | 387.35 | 331.29; 305.28; 237.21; 169.15; 143.14 | 6.06 |
| 5 | 5α-cholest-7-en-3β-ol (lathosterol) | C27H46O | 387.35 | 331.29; 303.26; 235.20; 167.14; 143.14; | 3.24 |
| 6 | 5α-cholestan-3β-ol (cholestanol) | C27H48O | 389.37 | 333.31; 305.28; 237.21; 169.15; 143.14 | 13.20 |
| 7 | 24-methyl-5α-cholesta-trien-3β-ol | C28H44O | 397.34 | 327.26; 299.23; 231.17; 165.12; 141.12 | 1.94 |
| 8 | 24-methyl-5α-cholesta-7,22-dien-3β-ol (diatomsterol) | C28H46O | 399.35 | 329.27; 303.26; 235.20; 167.14; 141.12 | 20.22 |
| 9 | 24-methylen-5α-cholest-7-en-3β-ol | C28H46O | 399.35 | 331.29; 235.20; 167.14; 141.12 | 2.40 |
| 10 | 24-methyl-5α-cholest-7-en-3β-ol | C28H48O | 401.37 | 331.29; 235.20; 167.14; 141.12 | 1.88 |
| 11 | 24-methyl-5α-cholest-22-en-3β-ol (diatomstanol) | C28H48O | 401.37 | 331.29; 237.21; 169.15; 143.14 | 2.78 |
| 12 | 24-methylen-5α-cholestan-3β-ol | C28H48O | 401.37 | 333.31; 237.21; 169.15; 143.14 | 9.28 |
| 13 | 24-methyl-5α-cholestan-3β-ol (campestanol) | C28H50O | 403.39 | 333.31; 237.21; 169.15; 143.14 | 3.64 |
| 14 | 24-ethyl-5α-cholesta-trien-3β-ol (Provitamin D6) | C29H46O | 411.36 | 327.26; 233.18; 165.12; 140.12 | 0.64 |
| 15 | 24-ethyl-5α-cholest-7,22-dien-3β-ol (Spinasterol) | C29H48O | 413.38 | 329.28; 235.20; 167.14; 141.12 | 4.44 |
| 16 | 24-ethyl-5α-cholest-7-en-3β-ol (Scottenol) | C29H50O | 415.39 | 331.29; 235.20; 167.14; 141.12 | 9.06 |
| 17 | 24-ethyl-5α-cholest-22-en-3β-ol (stigmast-22E-en-3β-ol) | C29H50O | 415.39 | 331.29; 237.21; 169.15; 143.14 | 2.66 |
| 18 | 24-ethyl-5α-cholestan-3β-ol (sitostanol) | C29H52O | 417.40 | 333.31; 237.21; 169.15; 143.14 | 7.82 |
| № | Identity | Molecular Formula | MS (m/z) | MS2 (m/z) | Yield, (mg/100 g) |
|---|---|---|---|---|---|
| 1 | β-Carotene | C40H56 | 537.5 | 445.4; 379.4; 346.3; 308.3; 268.3; 224.2; 203.2; 178.2; 133.2; 119.1; 107.1; 95.1 | 4.2 |
| 2 | β-Echinenone | C40H54O | 551.5 | 347.3; 265.3; 209.2; 203.2;157.2; 133.1; 119.1; 95.1; 81.1; 69.1 | 0.9 |
| 3 | Canthaxanthin | C40H52O2 | 565.5 | 548.5; 413.3; 404.0; 363.3; 307.3; 231.2; 215.2; 203.2; 145.2; 133.2; 95.2; 69.1 | 25.4 |
| 4 | (3R)-, (3S)-phoenicoxanthin | C40H52O3 | 581.5 | 565.5; 562.5; 488.4; 157.0; 119.0; 105.0; 91.0; 55.0 | 1.6 |
| 5 | Lutein | C40H56O2 | 568.5 | 550.5; 476.4; 430.4; 367.0; 336.3; 323.3; 175.2; 145.2; 133.2; 107.1; 95.2 | 0.5 |
| 6 | Diatoxanthin | C40H54O2 | 567.5 | 551.5; 533.5; 459.4; 413.4; 329.3; 263.3; 217.2; 199.2; 175.2; 133.2; 109.2 | 1.8 |
| 7 | Alloxanthin | C40H52O2 | 565.5 | 547.5; 491.4; 465.4; 411.3; 393.0; 249.2; 209.2; 199.2; 173.2; 157.2; 119.1; 109.2; 81.1 | 2.3 |
| 8 | Pectenolone | C40H52O3 | 581.5 | 563.5; 315.3; 27.2; 217.2; 199.2; 173.2; 147.2; 119.1; 107.1 | 2.2 |
| 9 | (3S,3′S)-7,8-Didehydroastaxanthin | C40H50O4 | 595.5 | 577.5; 565.5; 441.6; 425.4; 375.4; 359.4; 165.2 | 1.7 |
| 10 | Fucoxanthin | C42H58O6 | 659.5 | 641.5; 623.5; 599.5; 581.5; 567.4; 549.4; 489.4; 433.3; 355.3; 239.2; 149.2; 109.2 | 1.4 |
| 11 | Fucoxanthinol | C40H56O5 | 617.5 | 598.3; 447.4; 285.2; 233.2; 143.0; 109.2; 91.0; 43.0 | 1.6 |
| 12 | Cucumariaxanthin A | C40H56O2 | 568.5 | 550.2; 476.4; 462.3 | 56.1 |
| 13 | Cucumariaxanthin B | C40H58O2 | 571.1 | 552.4; 478.1; 464.3 | 13.7 |
| 14 | Cucumariaxanthin C | C40H60O2 | 573.6 | 556.2; 536.3; 480.5; 466.2 | 5.1 |
| № | Identity and Retention Time * | Molecular Formula | Adducts | MS (m/z) | MS2 (m/z) | MS3 (m/z) |
|---|---|---|---|---|---|---|
| 1 | cucumarioside A0-1 27.6 min | C60H93O30SNa | [MNa–Na]− | 1325.55 | 1193.50; 797.20 | 1017.44; 885.39; 739.34; 665.16; 489.09; 357.05; 211.00 |
| 2 | cucumarioside A0-2 30.3 min | C60H93O29SNa | [MNa–Na]− | 1309.55 | 1177.51; 797.20 | 1001.44; 869.40; 723.34; 665.16; 489.09; 375.05; 211.00 |
| 3 | cucumarioside A0-3 30.8 min | C58H89O28SNa | [MNa–Na]− | 1265.53 | 1133.48; 797.20 | 957.42; 825.37; 679.32; 665.16; 489.09; 375.05; 211.00 |
| 4 | cucumarioside A1-2 28.4 min | C60H91O30SNa | [MNa–Na]− | 1323.53 | 1191.49; 855.21 | 987.43; 825.37; 723.17; 519.10; 357.05; 211.00 |
| 5 | cucumarioside A2-2 28.6 min | C59H91O29SNa | [MNa–Na]− | 1296.41 | 1163.49; | 987.43; 827.21; 825.37; 695.17; 679.32; 519.10; 357.05; 211.00 |
| 6 | cucumarioside A2-3 28.5 min | C59H93O29SNa | [MNa–Na]− | 1297.55 | 1165.51; 827.21 | 989.44; 827.39; 695.17; 681.33; 519.10; 357.05; 211.00 |
| 7 | cucumarioside A2-4 29.0 min | C58H91O28SNa | [MNa–Na]− | 1267.54 | 1135.50; | 974.45; 813.20; 811.39; 681.15; 665.33; 519.10; 357.05; 211.00 |
| 8 | cucumarioside A2-5 28.5 min | C60H93O31SNa | [MNa–Na]− | 1341.54 | 1209.50; 813.20 | 1047.45; 885.39; 739.34; 681.15; 519.10; 357.05; 211.00 |
| 9 | cucumarioside A3 29.4 min | C59H90O32S2Na2 | [MNa2–Na]− | 1397.48 | 1265.43; 929.15 | 1089.36; 825.37; 797.11; 679.32; 621.04; 357.05; 211.00 |
| 10 | cucumarioside A4-2 29.6 min | C58H89O29SNa | [MNa–Na]− | 1281.52 | 1149.48; 813.20 | 987.43; 825.37; 681.15; 519.10; 357.05; 211.00 |
| 11 | cucumarioside A6-2 29.2 min | C59H90O32S2Na2 | [MNa2–Na]− | 1397.48 | 1265.43; 929.15 | 987.43; 825.37; 797.11; 679.32; 519.10; 357.05; 211.00 |
| 12 | cucumarioside A7-1 27.3 min | C57H87O35S3Na3 | [MNa3–Na]− | 1473.40 | 1341.35; 1031.09 | 1063.35; 899.05; 799.35; 659.19; 653.30; 621.04; 357.05; 211.00 |
| 13 | cucumarioside A7-2 26.9 min | C56H85O35S3Na3 | [MNa3–Na]− | 1459.38 | 1327.34; 1031.09 | 1049.33; 899.05; 785.34; 639.28; 621.04; 357.05; 211.00 |
| 14 | cucumarioside A7-3 27.1 min | C57H89O34S3Na3 | [MNa3–Na]− | 1459.42 | 1327.38; 1031.09 | 1049.37; 899.05; 785.38; 639.32; 621.04; 357.05, 211.00 |
| 15 | cucumarioside E 34.5 min | C58H89O29SNa | [MNa–Na]− | 1281.52 | 1149.51; 813.22; | 1105.51; 973.42; 841.41; 679.32; 637.19; 505.2 |
| [MNa+Na]+ | 1327.48 | 1207.51 | 1075.42; 899.29 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zakharenko, A.; Romanchenko, D.; Thinh, P.D.; Pikula, K.; Hang, C.T.T.; Yuan, W.; Xia, X.; Chaika, V.; Chernyshev, V.; Zakharenko, S.; et al. Features and Advantages of Supercritical CO2 Extraction of Sea Cucumber Cucumaria frondosa japonica Semper, 1868. Molecules 2020, 25, 4088. https://doi.org/10.3390/molecules25184088
Zakharenko A, Romanchenko D, Thinh PD, Pikula K, Hang CTT, Yuan W, Xia X, Chaika V, Chernyshev V, Zakharenko S, et al. Features and Advantages of Supercritical CO2 Extraction of Sea Cucumber Cucumaria frondosa japonica Semper, 1868. Molecules. 2020; 25(18):4088. https://doi.org/10.3390/molecules25184088
Chicago/Turabian StyleZakharenko, Alexander, Denis Romanchenko, Pham Duc Thinh, Konstantin Pikula, Cao Thi Thuy Hang, Wenpeng Yuan, Xuekui Xia, Vladimir Chaika, Valery Chernyshev, Svetlana Zakharenko, and et al. 2020. "Features and Advantages of Supercritical CO2 Extraction of Sea Cucumber Cucumaria frondosa japonica Semper, 1868" Molecules 25, no. 18: 4088. https://doi.org/10.3390/molecules25184088
APA StyleZakharenko, A., Romanchenko, D., Thinh, P. D., Pikula, K., Hang, C. T. T., Yuan, W., Xia, X., Chaika, V., Chernyshev, V., Zakharenko, S., Razgonova, M., Chung, G., & Golokhvast, K. (2020). Features and Advantages of Supercritical CO2 Extraction of Sea Cucumber Cucumaria frondosa japonica Semper, 1868. Molecules, 25(18), 4088. https://doi.org/10.3390/molecules25184088










