Wheat Bran Extract Regulates Mast Cell-Mediated Allergic Responses In Vitro and In Vivo
Abstract
1. Introduction
2. Results
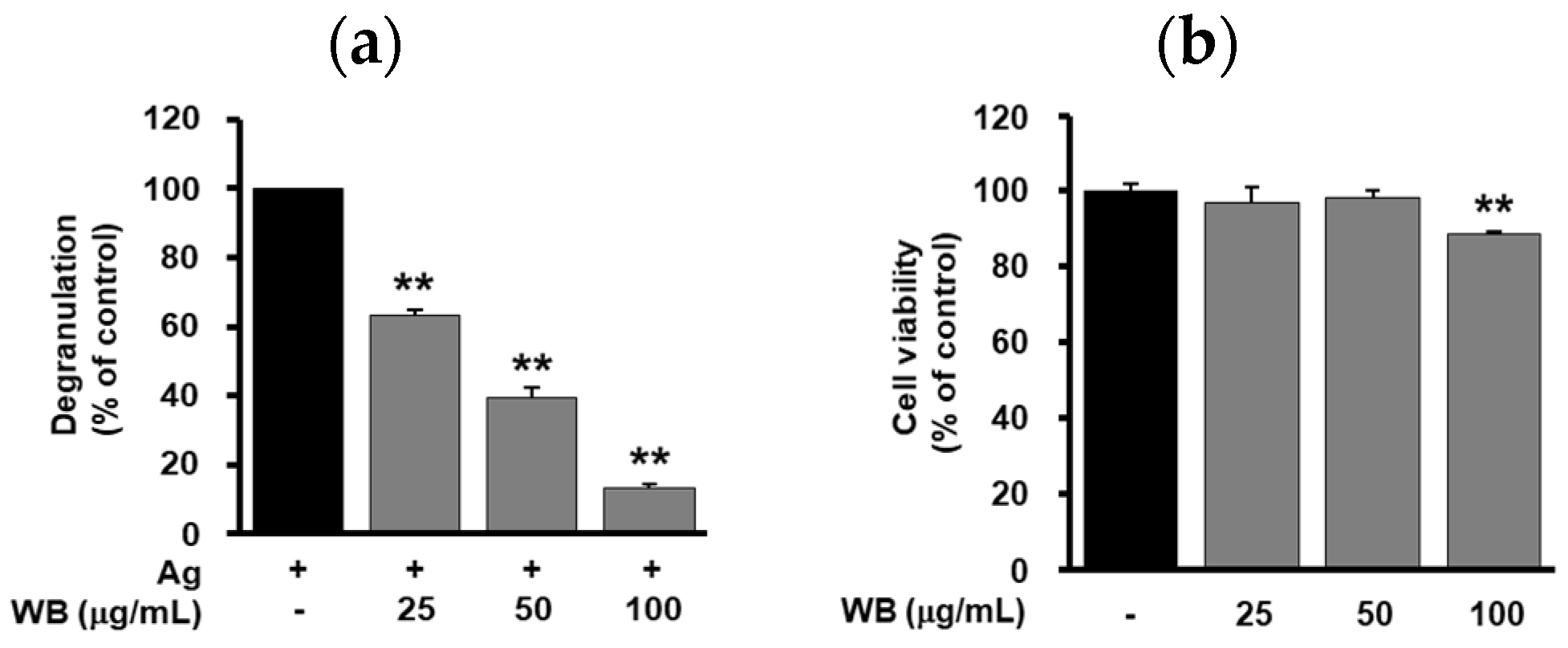
2.1. Effect of WB on Antigen-Induced Degranulation in RBL-2H3 Cells
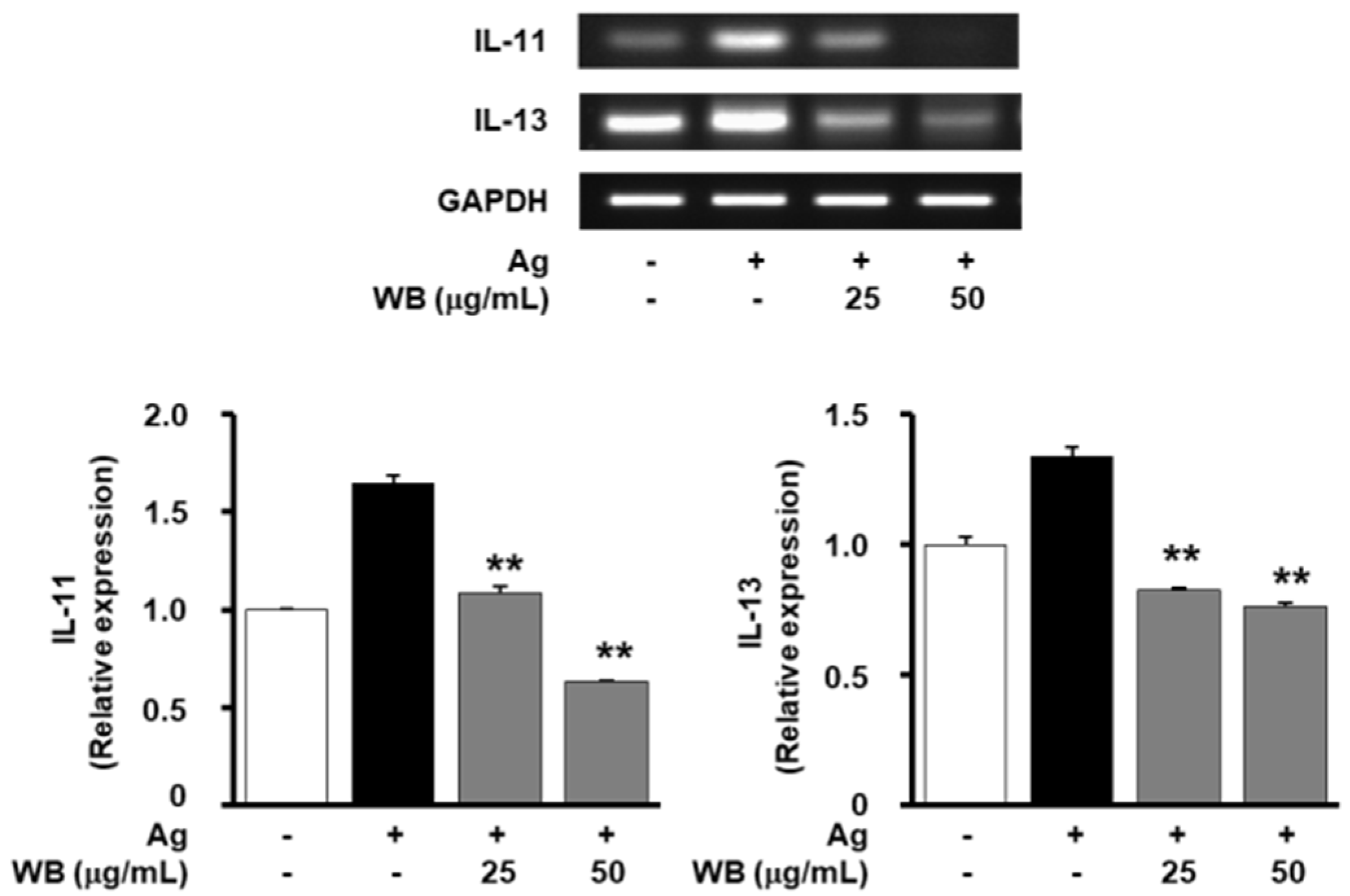
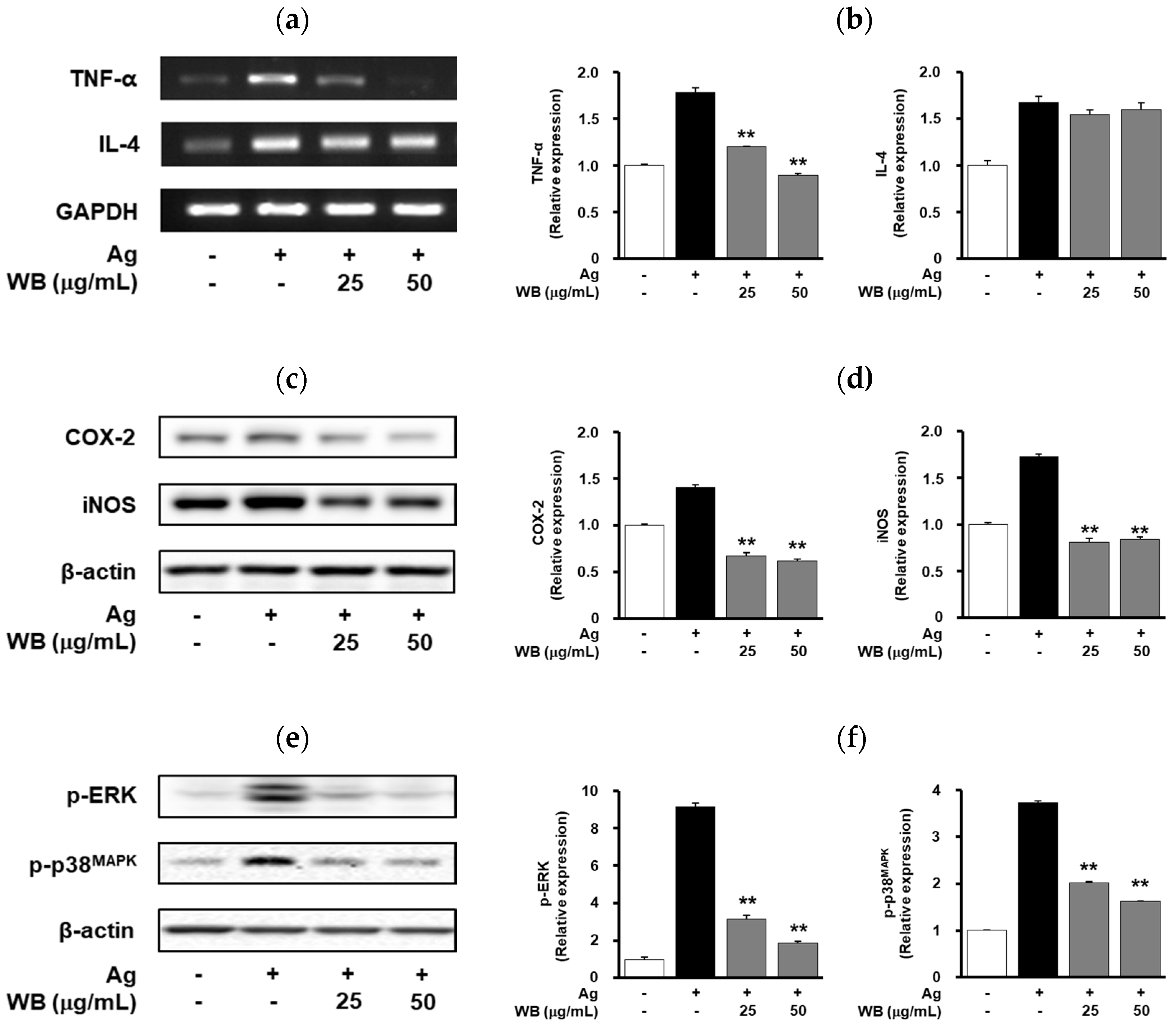
2.2. Effect of WB on Antigen-Induced Expression of Allergic and Inflammatory Mediators in RBL-2H3 Cells
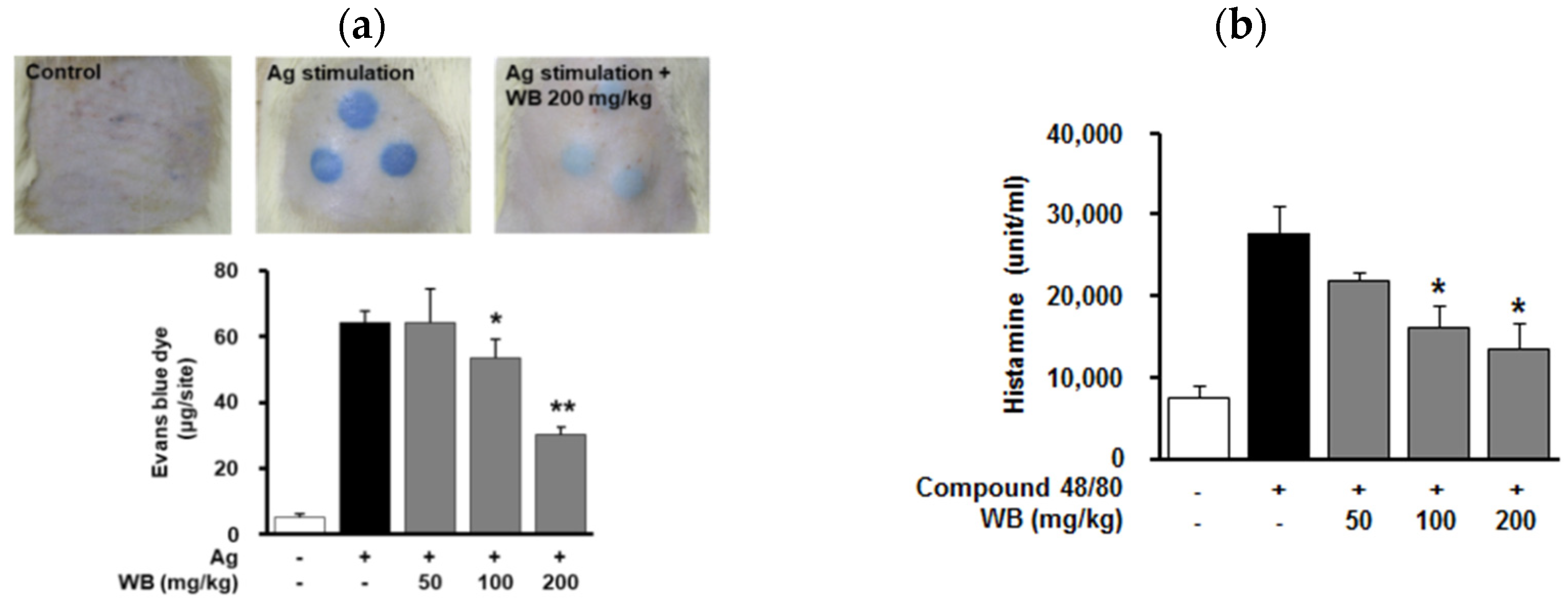
2.3. Effect of WB on IgE/Antigen-Induced Passive Cutaneous Anaphylaxis (PCA) in Rats
2.4. Effect of WB on Compound 48/80-Induced Systemic Anaphylaxis in Mice
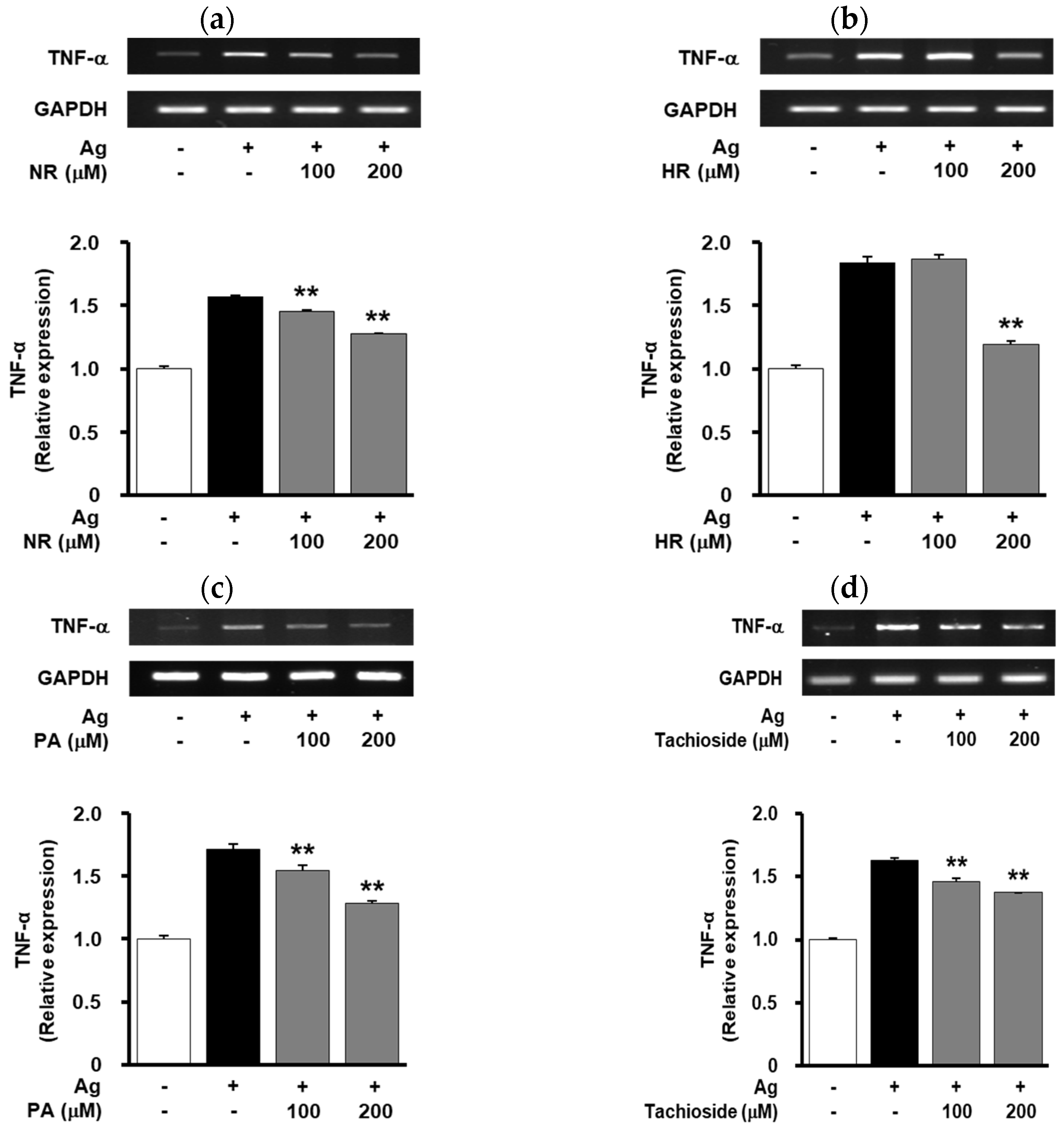
2.5. Effect of WB-Derived Compounds on the Release of β-Hexosaminidase and Expression of TNF-α in Antigen-Stimulated RBL-2H3 Cells
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Preparation of the Water Extract of Wheat Bran
4.3. Chromatographic Separation of the Water Extract of Wheat Bran
4.4. Cell Culture Conditions
4.5. Animals
4.6. Cytotoxicity Assay
4.7. Stimulation and Measurement of Degranulation in RBL-2H3 Cells
4.8. RNA Extraction and Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
4.9. Western Blot Analysis
4.10. IgE-Induced Passive Cutaneous Anaphylaxis (PCA)
4.11. Compound 48/80 Induced Systemic Anaphylaxis
4.12. Histamine Release Assay
4.13. Statistical Analyses
Author Contributions
Funding
Conflicts of Interest
Appendix A
References
- Yu, W.; Freeland, D.M.H.; Nadeau, K.C. Food allergy: Immune mechanisms, diagnosis and immunotherapy. Nat. Rev. Immunol. 2016, 16, 751–765. [Google Scholar] [CrossRef] [PubMed]
- Demoly, P.; Adkinson, N.F.; Brockow, K.; Castells, M.; Chiriac, A.M.; Greenberger, P.A.; Khan, D.A.; Lang, D.M.; Park, H.S.; Pichler, W.; et al. International consensus on drug allergy. Allergy 2014, 69, 420–437. [Google Scholar] [CrossRef]
- Kim, B.J.; Lee, S.Y.; Kim, H.B.; Lee, E.; Hong, S.J. Environmental changes, microbiota, and allergic diseases. Allergy Asthma Immunol. Res. 2014, 6, 389–400. [Google Scholar] [CrossRef]
- Sicherer, S.H.; Sampson, H.A. Food allergy: A review and update on epidemiology, pathogenesis, diagnosis, prevention, and management. J. Allergy Clin. Immunol. 2018, 141, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Galli, S.J.; Tsai, M. IgE and mast cells in allergic disease. Nat. Med. 2012, 18, 693–704. [Google Scholar] [CrossRef]
- Reber, L.L.; Hernandez, J.D.; Galli, S.J. The pathophysiology of anaphylaxis. J. Allergy Clin. Immunol. 2017, 140, 335–348. [Google Scholar] [CrossRef]
- Bulfone-Paus, S.; Nilsson, G.; Draber, P.; Blank, U.; Levi-Schaffer, F. Positive and negative signals in mast cell activation. Trends Immunol. 2017, 38, 657–667. [Google Scholar] [CrossRef]
- Sibilano, R.; Frossi, B.; Pucillo, C.E. Mast cell activation: A complex interplay of positive and negative signaling pathways. Eur. J. Immunol. 2014, 44, 2558–2566. [Google Scholar] [CrossRef]
- Gomez, G. Current strategies to inhibit high affinity FcεRI-mediated signaling for the treatment of allergic disease. Front. Immunol. 2019, 10, 175. [Google Scholar] [CrossRef]
- Laddomada, B.; Caretto, S.; Mita, G. Wheat bran phenolic acids: Bioavailability and stability in whole wheat-based foods. Molecules 2015, 20, 15666–15685. [Google Scholar] [CrossRef]
- Stevenson, L.; Phillips, F.; O’Sullivan, K.; Walton, J. Wheat bran: Its composition and benefits to health, a European perspective. Int. J. Food Sci. Nutr. 2012, 63, 1001–1013. [Google Scholar] [CrossRef] [PubMed]
- Fardet, A. New hypotheses for the health-protective mechanisms of whole-grain cereals: What is beyond fibre? Nutr. Res. Rev. 2010, 23, 65–134. [Google Scholar] [CrossRef] [PubMed]
- Costabile, A.; Klinder, A.; Fava, F.; Napolitano, A.; Fogliano, V.; Leonard, C.; Gibson, G.R.; Tuohy, K.M. Whole-grain wheat breakfast cereal has a prebiotic effect on the human gut microbiota: A double-blind, placebo-controlled, crossover study. Br. J. Nutr. 2008, 99, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Qu, H.; Madl, R.L.; Takemoto, D.J.; Baybutt, R.C.; Wang, W. Lignans are involved in the antitumor activity of wheat bran in colon cancer SW480 cells. J. Nutr. 2005, 135, 598–602. [Google Scholar] [CrossRef] [PubMed]
- Bijkerk, C.J.; de Wit, N.J.; Muris, J.W.; Whorwell, P.J.; Knottnerus, J.A.; Hoes, A.W. Soluble or insoluble fibre in irritable bowel syndrome in primary care? Randomised placebo controlled trial. Br. Med. J. 2009, 339, b3154. [Google Scholar] [CrossRef]
- Jensen, M.K.; Koh-Banerjee, P.; Hu, F.B.; Franz, M.; Sampson, L.; Grønbaek, M.; Rimm, E.B. Intakes of whole grains, bran, and germ and the risk of coronary heart disease in men. Am. J. Clin. Nutr. 2004, 80, 1492–1499. [Google Scholar] [CrossRef]
- Belobrajdic, D.P.; Bird, A.R. The potential role of phytochemicals in wholegrain cereals for the prevention of type-2 diabetes. Nutr. J. 2013, 12, 62. [Google Scholar] [CrossRef]
- Mukai, K.; Tsai, M.; Saito, H.; Galli, S.J. Mast cells as sources of cytokines, chemokines, and growth factors. Immunol. Rev. 2018, 282, 121–150. [Google Scholar] [CrossRef]
- Nam, S.T.; Kim, H.W.; Kim, H.S.; Park, Y.H.; Lee, D.; Lee, M.B.; Min, K.Y.; Kim, Y.M.; Choi, W.S. Furaltadone suppresses IgE-mediated allergic response through the inhibition of Lyn/Syk pathway in mast cells. Eur. J. Pharmacol. 2018, 828, 119–125. [Google Scholar] [CrossRef]
- Duan, W.; Wong, W.S. Targeting mitogen-activated protein kinases for asthma. Curr. Drug Targets 2006, 7, 691–698. [Google Scholar] [CrossRef]
- Hong, S.S.; Oh, J.S. Inhibitors of antigen-induced degranulation of RBL-2H3 cells isolated from wheat bran. J. Korean Soc. Appl. Biol. Chem. 2012, 55, 69–74. [Google Scholar] [CrossRef]
- Anh, N.H.; Ripperger, H.; Schmidt, J.; Porzel, A.; Sung, T.V.; Adam, G. Resorcinol derivatives from two Ardisia species. Planta Med. 1996, 62, 479–480. [Google Scholar] [CrossRef]
- Feresin, G.E.; Tapia, A.; Sortino, M.; Zacchino, S.; de Arias, A.R.; Inchausti, A.; Yaluff, G.; Rodriguez, J.; Theoduloz, C.; Schmeda-Hirschmann, G. Bioactive alkyl phenols and embelin from Oxalis erythrorhiza. J. Ethnopharmacol. 2003, 88, 241–247. [Google Scholar] [CrossRef]
- Kim, J.S.; Yean, M.H.; Seo, H.K.; Lee, J.H.; Kang, S.S. Phytochemical studies on Lonicera caulis (2)–aliphatic and phenolic compounds. Korean J. Pharm. 2009, 40, 326–333. [Google Scholar]
- Sayama, K.; Diehn, M.; Matsuda, K.; Lunderius, C.; Tsai, M.; Tam, S.Y.; Botstein, D.; Brown, P.O.; Galli, S.J. Transcriptional response of human mast cells stimulated via the FcεRI and identification of mast cells as a source of IL-11. BMC Immunol. 2002, 3, 5. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhang, T.; Han, S.; Cao, J.; Chen, Q.; Wang, S. The inhibitory effect of piperine from Fructus piperis extract on the degranulation of RBL-2H3 cells. Fitoterapia 2014, 99, 218–226. [Google Scholar] [CrossRef]
- Hong, S.S.; Jeong, W.; Kim, J.K.; Kwon, J.G.; Lee, J.Y.; Ahn, E.K.; Oh, J.; Seo, D.W.; Oh, J.S. Neolignan inhibitors of antigen-induced degranulation in RBL-2H3 cells from the needles of Pinus Thunbergii. Fitoterapia 2014, 99, 347–351. [Google Scholar] [CrossRef]
- Park, J.H.; Hwang, M.H.; Cho, Y.R.; Hong, S.S.; Kang, J.S.; Kim, W.H.; Yang, S.H.; Seo, D.W.; Oh, J.S.; Ahn, E.K. Combretum quadrangulare extract attenuates atopic dermatitis-like skin lesions through modulation of MAPK signaling in BALB/c mice. Molecules 2020, 25, 2003. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |

| Treatment | Compound 48/80 (8 mg/kg) | Mortality (%) |
|---|---|---|
| Untreated | + | 100 |
| WB (50 mg/kg) | + | 100 |
| WB (100 mg/kg) | + | 60 |
| WB (200 mg/kg) | + | 0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.Y.; Ahn, E.-K.; Park, J.-H.; Oh, J.S. Wheat Bran Extract Regulates Mast Cell-Mediated Allergic Responses In Vitro and In Vivo. Molecules 2020, 25, 3997. https://doi.org/10.3390/molecules25173997
Lee JY, Ahn E-K, Park J-H, Oh JS. Wheat Bran Extract Regulates Mast Cell-Mediated Allergic Responses In Vitro and In Vivo. Molecules. 2020; 25(17):3997. https://doi.org/10.3390/molecules25173997
Chicago/Turabian StyleLee, Jae Yeon, Eun-Kyung Ahn, Ju-Hyoung Park, and Joa Sub Oh. 2020. "Wheat Bran Extract Regulates Mast Cell-Mediated Allergic Responses In Vitro and In Vivo" Molecules 25, no. 17: 3997. https://doi.org/10.3390/molecules25173997
APA StyleLee, J. Y., Ahn, E.-K., Park, J.-H., & Oh, J. S. (2020). Wheat Bran Extract Regulates Mast Cell-Mediated Allergic Responses In Vitro and In Vivo. Molecules, 25(17), 3997. https://doi.org/10.3390/molecules25173997





