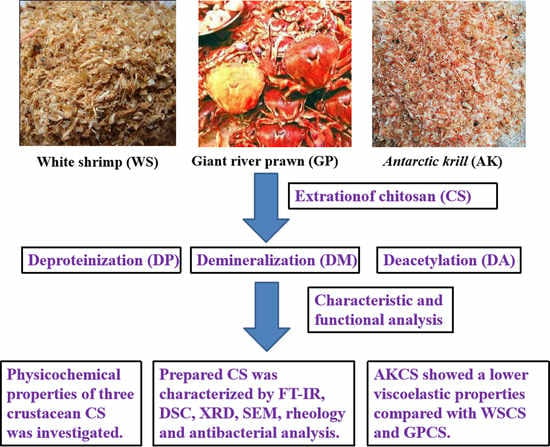
Insight into Physicochemical, Rheological, and Antibacterial Properties of Chitosan Extracted from Antarctic krill: A Comparative Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of WSCS, GPCS, and AKCS
2.3. Physicochemical Properties of Prepared CS
2.3.1. Yield and Solubility
2.3.2. Ash and Moisture Content
2.3.3. Viscosity Average Molecular Weight (MW) and Degree of Deacetylation (DDA)
2.4. Scanning Electron Microscopy (SEM) Analysis of WSCS, GPCS, and AKCS
2.5. Structural Properties of WSCS, GPCS, and AKCS
2.5.1. Fourier Transform Infrared (FT-IR) Analysis
2.5.2. Differential Scanning Calorimetry (DSC) Analysis
2.5.3. X-ray Diffraction (XRD) Analysis
2.6. Rheological Properties of WSCS, GPCS, and AKCS
2.7. Antimicrobial Activity of WSCS, GPCS, and AKCS
2.8. Data Analysis
3. Results and Discussion
3.1. Physicochemical Analysis of WSCS, GPCS, and AKCS
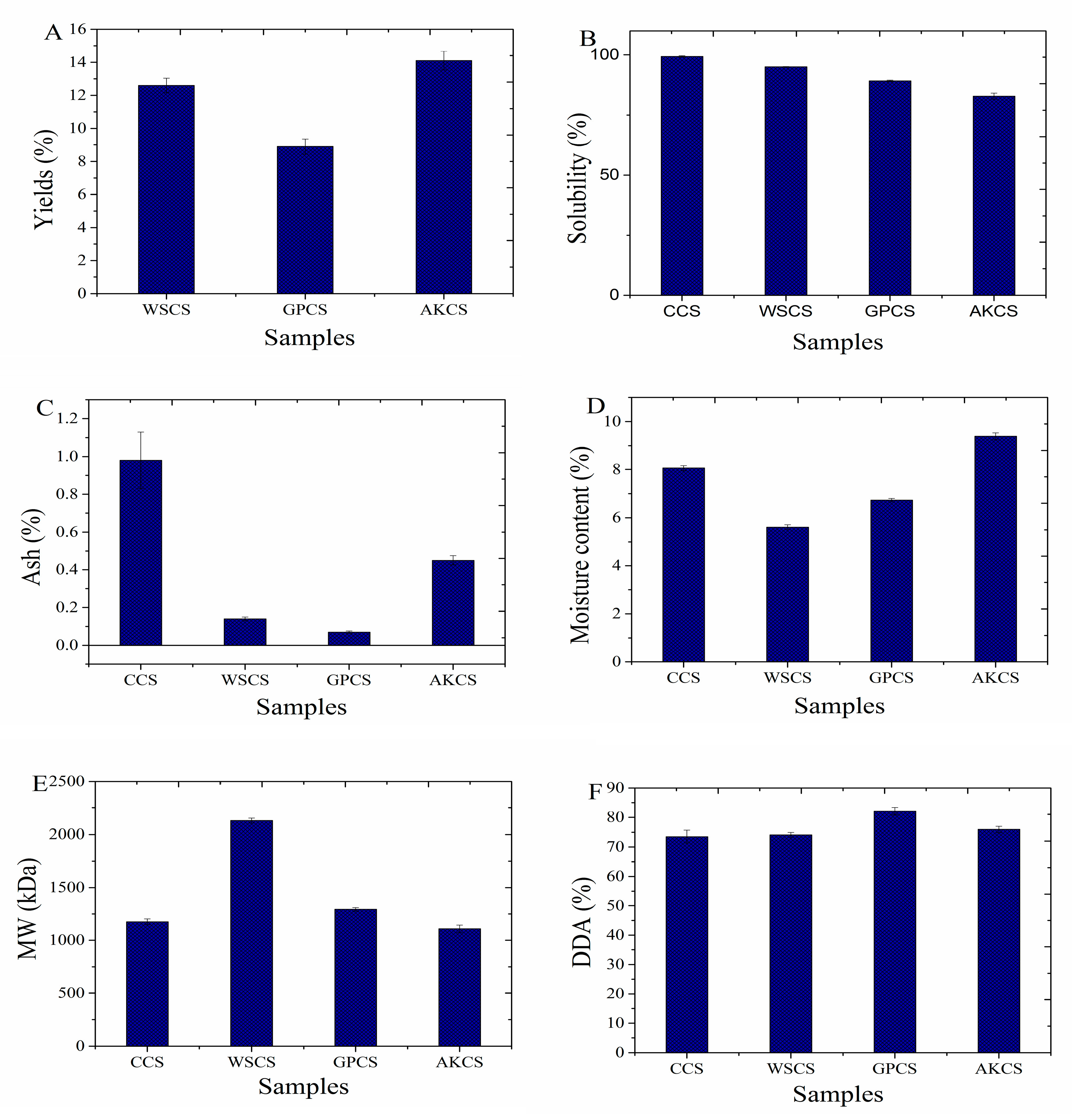
3.1.1. Yields and Solubility
3.1.2. Ash and Moisture Content
3.1.3. MW and DDA
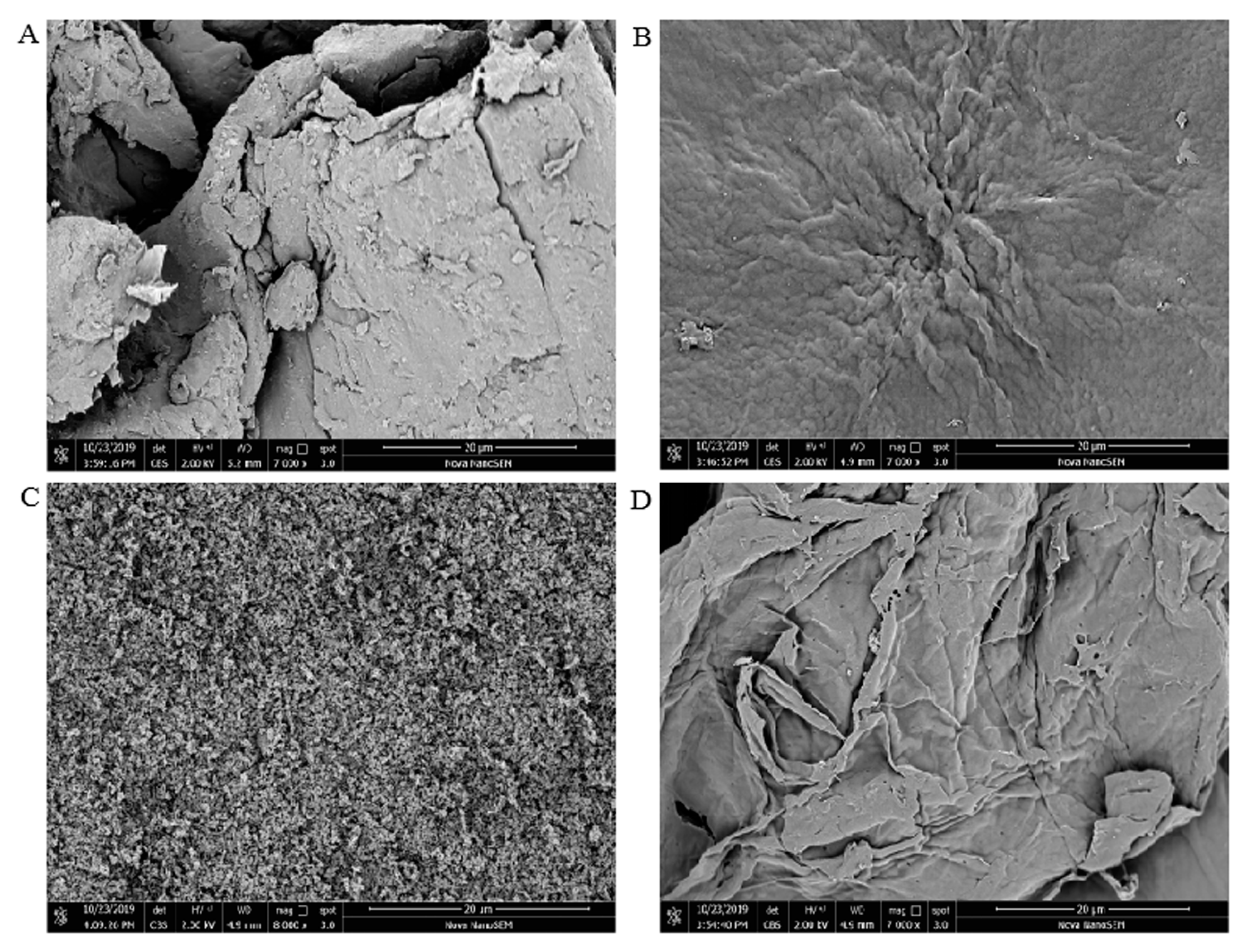
3.2. SEM Observation
3.3. Structural Analysis of CCS, WSCS, GPCS, and AKCS
3.3.1. FT-IR Analysis
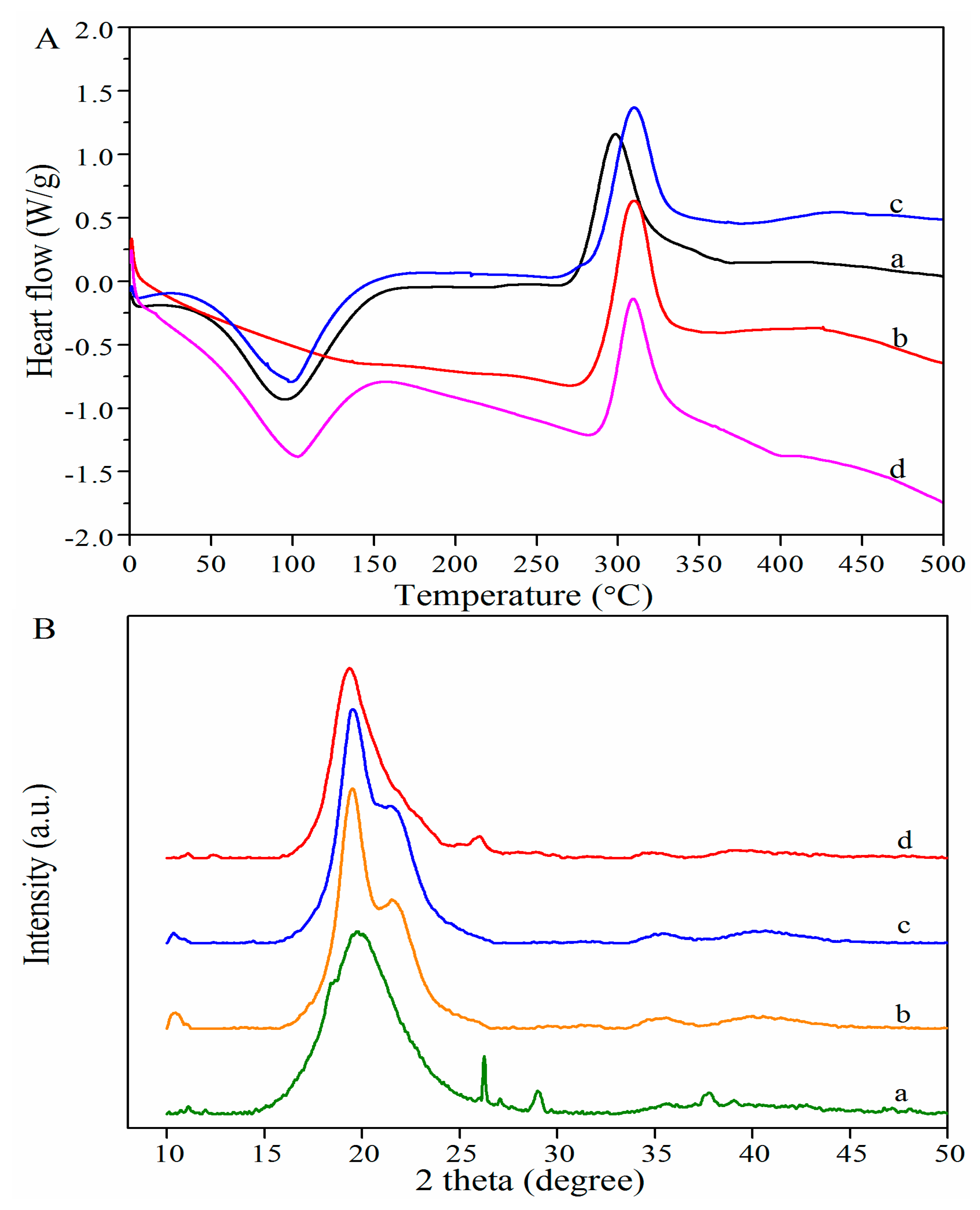
3.3.2. DSC Analysis
3.3.3. XRD Analysis
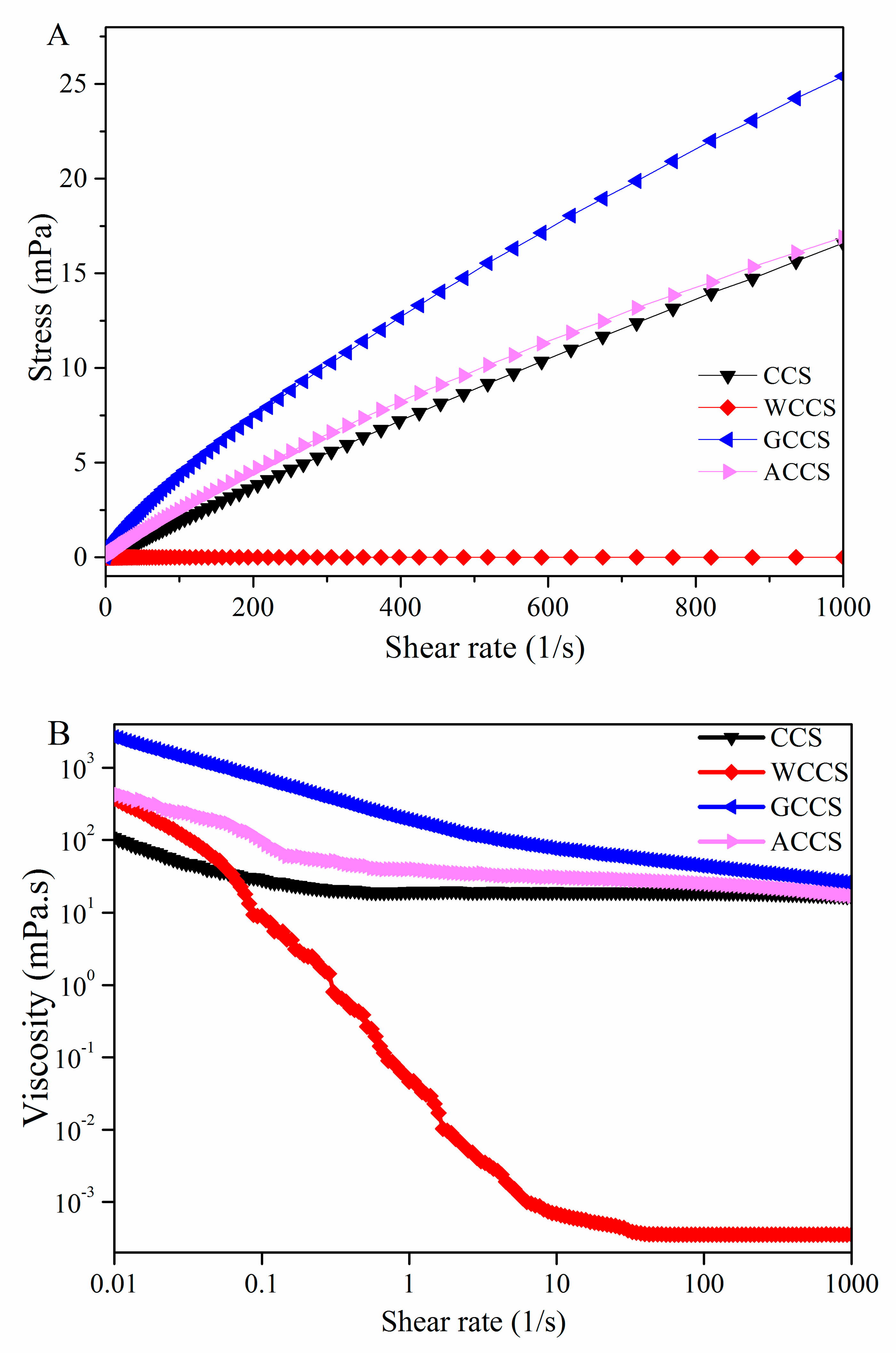
3.4. Rheological Measurements of CCS, WSCS, GPCS, and AKCS
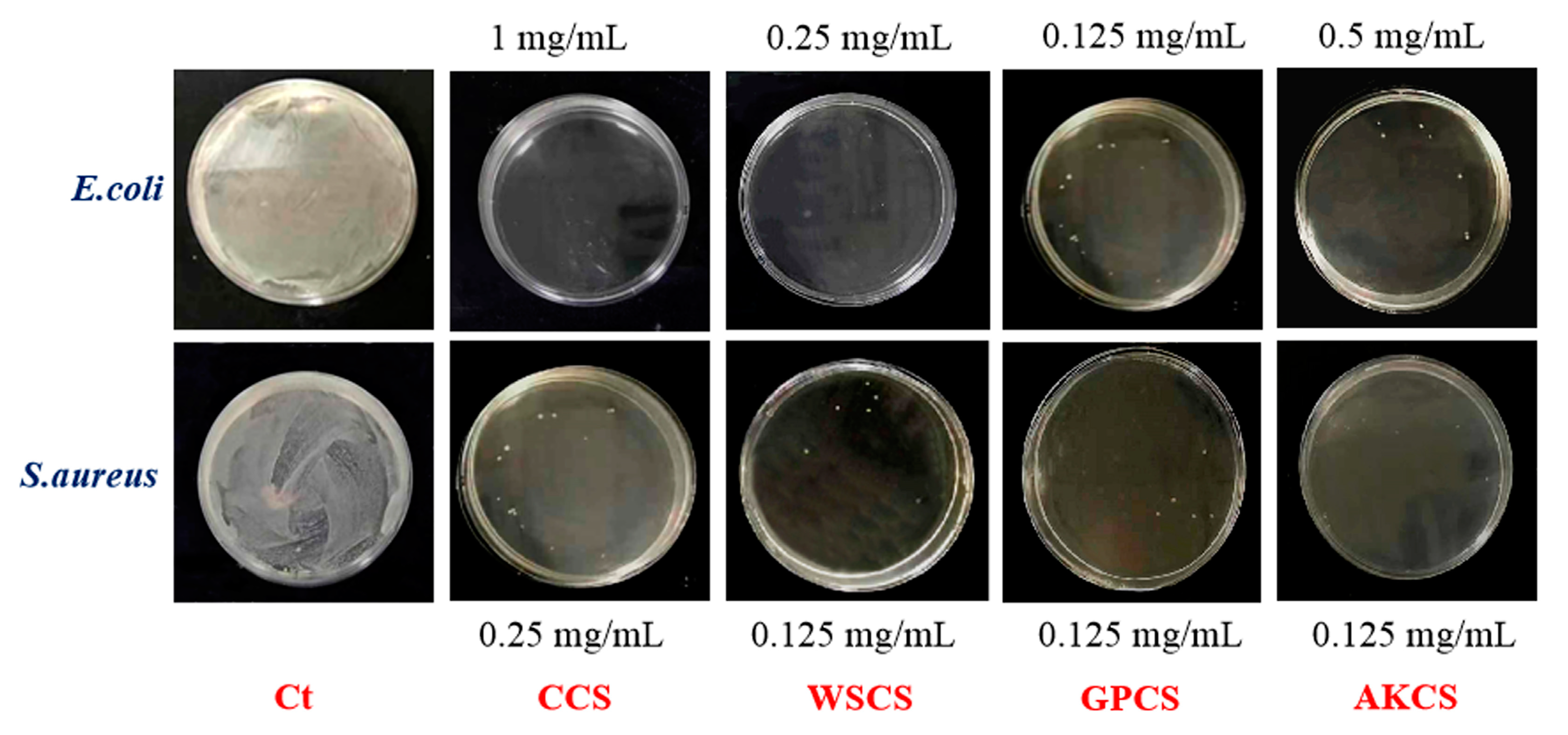
3.5. Antimicrobial Activity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Alfaro-Montoya, J.; Braga, A.L.; Umaña-Castro, R. Research frontiers in penaeid shrimp reproduction: Future trends to improve commercial production. Aquaculture 2019, 503, 70–87. [Google Scholar] [CrossRef]
- Kumari, S.; Rath, P.; Kumar, A.S.H.; Tiwari, T. Extraction and characterization of chitin and chitosan from fishery waste by chemical method. Environ. Technol. Innov. 2015, 3, 77–85. [Google Scholar] [CrossRef]
- Sun, J.; Mao, X. An environmental friendly process for Antarctic krill (Euphausia superba) utilization using fermentation technology. J. Clean. Prod. 2016, 127, 618–623. [Google Scholar] [CrossRef]
- Floreto, E.; Brown, P.; Bayer, R. The effects of krill hydrolysate-supplemented soya-bean based diets on the growth, colouration, amino and fatty acid profiles of juvenile American lobster, Homarus americanus. Aquac. Nutr. 2001, 7, 33–43. [Google Scholar] [CrossRef]
- Mathiesen, A.M. The state of world fisheries and aquaculture, 2012. Choice Rev. Online 2013, 50, 50. [Google Scholar] [CrossRef] [Green Version]
- Gigliotti, J.C.; Davenport, M.P.; Beamer, S.K.; Tou, J.C.; Jaczynski, J. Extraction and characterisation of lipids from Antarctic krill (Euphausia superba). Food Chem. 2011, 125, 1028–1036. [Google Scholar] [CrossRef]
- Yuan, G.; Li, W.; Pan, Y.; Wang, C.; Chen, H. Shrimp shell wastes: Optimization of peptide hydrolysis and peptide inhibition of α-amylase. Food Biosci. 2018, 25, 52–60. [Google Scholar] [CrossRef]
- Davies, G.J.G.; Knight, D.P.; Vollrath, F. Chitin in the silk gland ducts of the spider nephila edulis and the silkworm bombyx mori. PLoS ONE 2013, 8, e73225. [Google Scholar] [CrossRef] [Green Version]
- Knidri, H.; Belaabed, R.; Addaou, A.; Laajeb, A.; Lahsini, A. Extraction, chemical modification and characterization of chitin and chitosan. Int. J. Boil. Macromol. 2018, 120, 1181–1189. [Google Scholar] [CrossRef]
- Kumari, S.; Annamareddy, S.H.K.; Abanti, S.; Rath, P.K. Physicochemical properties and characterization of chitosan synthesized from fish scales, crab and shrimp shells. Int. J. Boil. Macromol. 2017, 104, 1697–1705. [Google Scholar] [CrossRef]
- Zhang, H.C.; Yun, S.Y.; Song, L.L.; Zhang, Y.W.; Zhao, Y.Y. The preparation and characterization of chitin and chitosan under large-scale submerged fermentation level using shrimp by-products as substrate. Int. J. Boil. Macromol. 2017, 96, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Mohanasrinivasan, V.; Mishra, M.; Paliwal, J.S.; Singh, S.K.; Selvarajan, E.; Suganthi, V.; Devi, C.S. Studies on heavy metal removal efficiency and antibacterial activity of chitosan prepared from shrimp shell waste. 3 Biotech 2013, 4, 167–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.C.; Yu, H.N.; Chen, S.S. Production of chitin and chitosan using successive three-step microbial fermentation. J. Polym. Mater. 2017, 34, 123–127. [Google Scholar]
- Zhang, H.C.; Yang, S.F.; Fang, J.Y.; Deng, Y.; Zhao, Y.Y. Optimization of the fermentation conditions of Rhizopus raponicas M193 for the production of chitin deacetylase and chitosan. Carbohydr. Polym. 2014, 101, 57–67. [Google Scholar] [CrossRef]
- Tammineni, N.; Rasco, B.; Powers, J.; Nindo, C.; Unlu, G. Bovine and fish gelatin coatings incorporating tannins: Effect on physical properties and oxidative stability of salmon fillets. J. Food Chem. Nutr. 2014, 2, 93–102. [Google Scholar]
- Weska, R.; De Moura, J.M.; Batista, L.; Rizzi, J.; Pinto, L.A. Optimization of deacetylation in the production of chitosan from shrimp wastes: Use of response surface methodology. J. Food Eng. 2007, 80, 749–753. [Google Scholar] [CrossRef]
- Cai, J.; Yang, J.; Du, Y.; Fan, L.; Qiu, Y.; Li, J.; Kennedy, J.F. Purification and characterization of chitin deacetylase from Scopulariopsis brevicaulis. Carbohydr. Polym. 2006, 65, 211–217. [Google Scholar] [CrossRef]
- Kaya, M.; Baran, T.; Erdogan, S.; Menteş, A.; Özüsağlam, M.A.; Cakmak, Y.S. Physicochemical comparison of chitin and chitosan obtained from larvae and adult Colorado potato beetle (Leptinotarsa decemlineata). Mater. Sci. Eng. C 2014, 45, 72–81. [Google Scholar] [CrossRef]
- Gałkowska, D.; Długosz, M.; Juszczak, L. Effect of high methoxy pectin and sucrose on pasting, rheological, and textural properties of modified starch systems. Starch-Stärke 2013, 65, 499–508. [Google Scholar] [CrossRef]
- Qi, L.; Xu, Z.; Jiang, X.; Hu, C.; Zou, X. Preparation and antibacterial activity of chitosan nanoparticles. Carbohydr. Res. 2004, 339, 2693–2700. [Google Scholar] [CrossRef]
- Bajaj, M.; Winter, J.; Gallert, C. Effect of deproteination and deacetylation conditions on viscosity of chitin and chitosan extracted from Crangon crangon shrimp waste. Biochem. Eng. J. 2011, 56, 51–62. [Google Scholar] [CrossRef]
- Samar, M.M.; El-Kalyoubi, M.; Khalaf, M.; El-Razik, M.A. Physicochemical, functional, antioxidant and antibacterial properties of chitosan extracted from shrimp wastes by microwave technique. Ann. Agric. Sci. 2013, 58, 33–41. [Google Scholar] [CrossRef] [Green Version]
- Luo, Q.; Wang, Y.; Han, Q.; Ji, L.; Zhang, H.; Fei, Z.; Wang, Y. Comparison of the physicochemical, rheological, and morphologic properties of chitosan from four insects. Carbohydr. Polym. 2019, 209, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Aguirre-Loredo, R.Y.; Hernández, A.I.R.; Morales-Sánchez, E.; Gómez-Aldapa, C.A.; Velazquez, G. Effect of equilibrium moisture content on barrier, mechanical and thermal properties of chitosan films. Food Chem. 2016, 196, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Pan, I.; Maté, J.; Gardrat, C.; Coma, V. Effect of chitosan molecular weight on the antimicrobial activity and release rate of carvacrol-enriched films. Food Hydrocoll. 2015, 51, 60–68. [Google Scholar] [CrossRef]
- Tolaimate, A.; Desbrières, J.; Rhazi, M.; Alagui, A.; Vincendon, M.; Vottero, P. On the influence of deacetylation process on the physicochemical characteristics of chitosan from squid chitin. Polymer 2000, 41, 2463–2469. [Google Scholar] [CrossRef]
- Younes, I.; Hajji, S.; Frachet, V.; Rinaudo, M.; Jellouli, K.; Nasri, M. Chitin extraction from shrimp shell using enzymatic treatment. Antitumor, antioxidant and antimicrobial activities of chitosan. Int. J. Boil. Macromol. 2014, 69, 489–498. [Google Scholar] [CrossRef]
- Hussain, R. Determination of degree of deacetylation of chitosan and their effect on the release behavior of essential oil from chitosan and chitosan-gelatin complex microcapsules. Int. J. Adv. Eng. Appl. 2013, 2, 4–12. [Google Scholar]
- Knidri, H.; Dahmani, J.; Addaou, A.; Laajeb, A.; Lahsini, A. Rapid and efficient extraction of chitin and chitosan for scale-up production: Effect of process parameters on deacetylation degree and molecular weight. Int. J. Boil. Macromol. 2019, 139, 1092–1102. [Google Scholar] [CrossRef]
- Hargono, H.; Djaeni, M. Utilization of chitosan prepared from shrimp shell as fat diluent. Indones. J. Chem. 2003, 5, 130–134. [Google Scholar]
- Patria, A. Production and characterization of chitosan from shrimp shells waste. Aquac. Aquar. Conserv. Legis. 2013, 6, 339–344. [Google Scholar]
- Cahyaningrum, S.E.; Amaria, A. Utilization of penaus monodon shrimp shell waste as adsorbent of cadmium(II) in water medium. Indones. J. Chem. 2010, 5, 130–134. [Google Scholar] [CrossRef]
- Qin, Y.; Xing, R.; Liu, S.; Li, K.; Meng, X.; Li, R.; Cui, J.; Li, B.; Li, P. Novel thiosemicarbazone chitosan derivatives: Preparation, characterization, and antifungal activity. Carbohydr. Polym. 2012, 87, 2664–2670. [Google Scholar] [CrossRef]
- Kumar, R.; Xavier, K.A.M.; Lekshmi, M.; Balange, A.; Gudipati, V. Fortification of extruded snacks with chitosan: Effects on techno functional and sensory quality. Carbohydr. Polym. 2018, 194, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Akpan, E.I.; Gbenebor, O.; Adeosun, S. Synthesis and characterisation of chitin from periwinkle (Tympanotonus fusatus (L.)) and snail (Lissachatina fulica (Bowdich)) shells. Int. J. Boil. Macromol. 2018, 106, 1080–1088. [Google Scholar] [CrossRef]
- Pereira, M.A.V.; Fonseca, G.D.; Silva-Júnior, A.A.; Fernandes-Pedrosa, M.F.; Moura, M.D.F.V.D.; Barbosa, E.G.; Gomes, A.P.B.; Dos Santos, K.S.C.R.; Fernandes-Pedrosa, M.D.F. Compatibility study between chitosan and pharmaceutical excipients used in solid dosage forms. J. Therm. Anal. Calorim. 2014, 116, 1091–1100. [Google Scholar] [CrossRef]
- Rao, V.; Johns, J. Thermal behavior of chitosan/natural rubber latex blends TG and DSC analysis. J. Therm. Anal. Calorim. 2008, 92, 801–806. [Google Scholar] [CrossRef]
- Guinesi, L.S.; Cavalheiro, E. The use of DSC curves to determine the acetylation degree of chitin/chitosan samples. Thermochim. Acta 2006, 444, 128–133. [Google Scholar] [CrossRef]
- Yen, M.T.; Mau, J.L. Selected physical properties of chitin prepared from shiitake stipes. LWT-Food Sci. Technol. 2007, 40, 558–563. [Google Scholar] [CrossRef]
- Jung, W.J.; Kuk, J.H.; Kim, K.Y.; Park, R.D. Demineralization of red crab shellwaste by lactic acid fermentation. Appl. Microbiol. Biotechnol. 2005, 67, 851–854. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Julkapli, N.M.; Akil, H.M. X-Ray Powder Diffraction (XRD) studies on kenaf dust filled chitosan bio-composites. Proc. AIP Conf. Proc. 2008, 989, 111–114. [Google Scholar]
- Schmitt, D.R.; Schmitt, D.R. Ultrasonic shear wave reflectometry applied to the determination of the shear moduli and viscosity of a viscoelastic bitumen. Fuel 2018, 232, 506–518. [Google Scholar] [CrossRef]
- Flores, A.; Ania, F.; Baltá-Calleja, F.J. From the glassy state to ordered polymer structures: A microhardness study. Polymer 2009, 50, 729–746. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Ge, X.; Lu, Y.; Dong, S.; Zhao, Y.; Zeng, M. Effects of chitosan molecular weight and degree of deacetylation on the properties of gelatine-based films. Food Hydrocoll. 2012, 26, 311–317. [Google Scholar] [CrossRef]
- Hamdi, M.; Nasri, R.; Hajji, S.; Nigen, M.; Li, S.; Nasri, M. Acetylation degree, a key parameter modulating chitosan rheological, thermal and film-forming properties. Food Hydrocoll. 2019, 87, 48–60. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, X.; Zhao, R.; Guo, D.; Zhang, J. Preparation and properties of chitosan/guar gum/nanocrystalline cellulose nanocomposite films. Carbohydr. Polym. 2018, 197, 128–136. [Google Scholar] [CrossRef]
- Garcia, L.G.S.; Guedes, G.M.D.M.; Fonseca, X.M.Q.C.; Pereira-Neto, W.A.; Castelo-Branco, D.S.C.M.; Sidrim, J.J.D.C.; Cordeiro, R.D.A.; Rocha, M.F.G.; Vieira, R.S.; Brilhante, R.S.N. Antifungal activity of different molecular weight chitosans against planktonic cells and biofilm of Sporothrix brasiliensis. Int. J. Boil. Macromol. 2020, 143, 341–348. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Chung, Y. Comparison of Acid-Soluble and Water-Soluble Chitosan as Coagulants in Removing Bentonite Suspensions. Water Air Soil Pollut. 2010, 217, 603–610. [Google Scholar] [CrossRef]
- Sun, Z.; Shi, C.; Wang, X.; Fang, Q.; Huang, J. Synthesis, characterization, and antimicrobial activities of sulfonated chitosan. Carbohydr. Polym. 2017, 155, 321–328. [Google Scholar] [CrossRef] [Green Version]
- Gadkari, R.R.; Suwalka, S.; Yogi, M.R.; Ali, W.; Das, A.; Alagirusamy, R. Green synthesis of chitosan-cinnamaldehyde cross-linked nanoparticles: Characterization and antibacterial activity. Carbohydr. Polym. 2019, 226, 115298. [Google Scholar] [CrossRef] [PubMed]
- Kong, M.; Chen, X.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.C.; Jung, J.; Zhao, Y.Y. Preparation, characterization and evaluation of antibacterial activity of different particle sizes of β-chitosan nanoparticles encapsulated catechins and catechins-Zn complex. Carbohydr. Polym. 2016, 137, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, M.; Shigemune, N.; Tsugukuni, T.; Jun, H.; Matsushita, T.; Mekada, Y.; Kurahachi, M.; Miyamoto, T. Mechanism of the combined antibacterial effect of green teaextract and NaCl against Staphylococcus aureus and Escherichia coli O157:H7. Food Control. 2012, 25, 225–232. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |


| Samples | DSC | XRD | |||
|---|---|---|---|---|---|
| To (°C) ++ | Tp (°C) | Tc (°C) | ∆H (J/g) | CrI (%) | |
| CCS + | 276.75 ± 6.57 a+++ | 298.39 ± 4.68 a | 371.54 ± 10.24 a,b | 235.07 ± 5.38 c | 89.86 ± 1.31 b |
| WSCS | 288.20 ± 6.45 a | 309.60 ± 5.32 a | 359.62 ± 8.35 a | 193.00 ± 6.24 b | 83.25 ± 1.54 a |
| GPCS | 293.06 ± 8.24 a | 309.96 ± 7.38 a | 355.24 ± 9.15 a | 154.09 ± 7.05 a,b | 84.27 ± 1.86 a |
| AKCS | 292.51 ± 7.39 a | 309.14 ± 9.05 a | 353.27 ± 7.68 a | 145.08 ± 5.39 a | 90.39 ± 1.44 b |
| Samples | MIC (mg/mL) | MBC (mg/mL) | ||
|---|---|---|---|---|
| E. coli | S. aureus | coli | S. aureus | |
| CCS | 0.5 | 0.25 | 1 | 0.25 |
| WSCS | 0.125 | 0.125 | 0.25 | 0.125 |
| GPCS | 0.0625 | 0.0625 | 0.125 | 0.125 |
| AKCS | 0.25 | 0.125 | 0.5 | 0.125 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, Y.; Chen, L.; Shi, W.; Wang, Z.; Zhang, H. Insight into Physicochemical, Rheological, and Antibacterial Properties of Chitosan Extracted from Antarctic krill: A Comparative Study. Molecules 2020, 25, 4074. https://doi.org/10.3390/molecules25184074
Yuan Y, Chen L, Shi W, Wang Z, Zhang H. Insight into Physicochemical, Rheological, and Antibacterial Properties of Chitosan Extracted from Antarctic krill: A Comparative Study. Molecules. 2020; 25(18):4074. https://doi.org/10.3390/molecules25184074
Chicago/Turabian StyleYuan, Yuan, Luzhu Chen, Wenzheng Shi, Zhihe Wang, and Hongcai Zhang. 2020. "Insight into Physicochemical, Rheological, and Antibacterial Properties of Chitosan Extracted from Antarctic krill: A Comparative Study" Molecules 25, no. 18: 4074. https://doi.org/10.3390/molecules25184074
APA StyleYuan, Y., Chen, L., Shi, W., Wang, Z., & Zhang, H. (2020). Insight into Physicochemical, Rheological, and Antibacterial Properties of Chitosan Extracted from Antarctic krill: A Comparative Study. Molecules, 25(18), 4074. https://doi.org/10.3390/molecules25184074