Preparation and Characterization of Two Immunogens and Production of Polyclonal Antibody with High Affinity and Specificity for Darunavir
Abstract
1. Introduction
2. Results
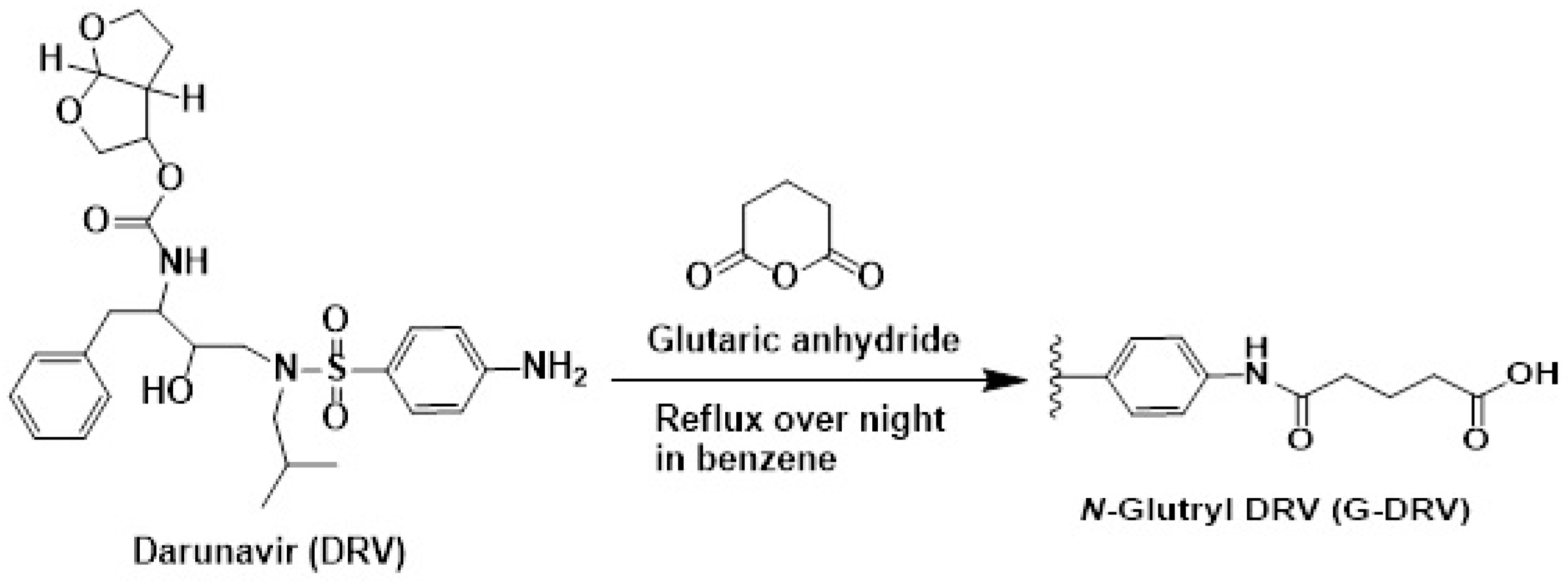
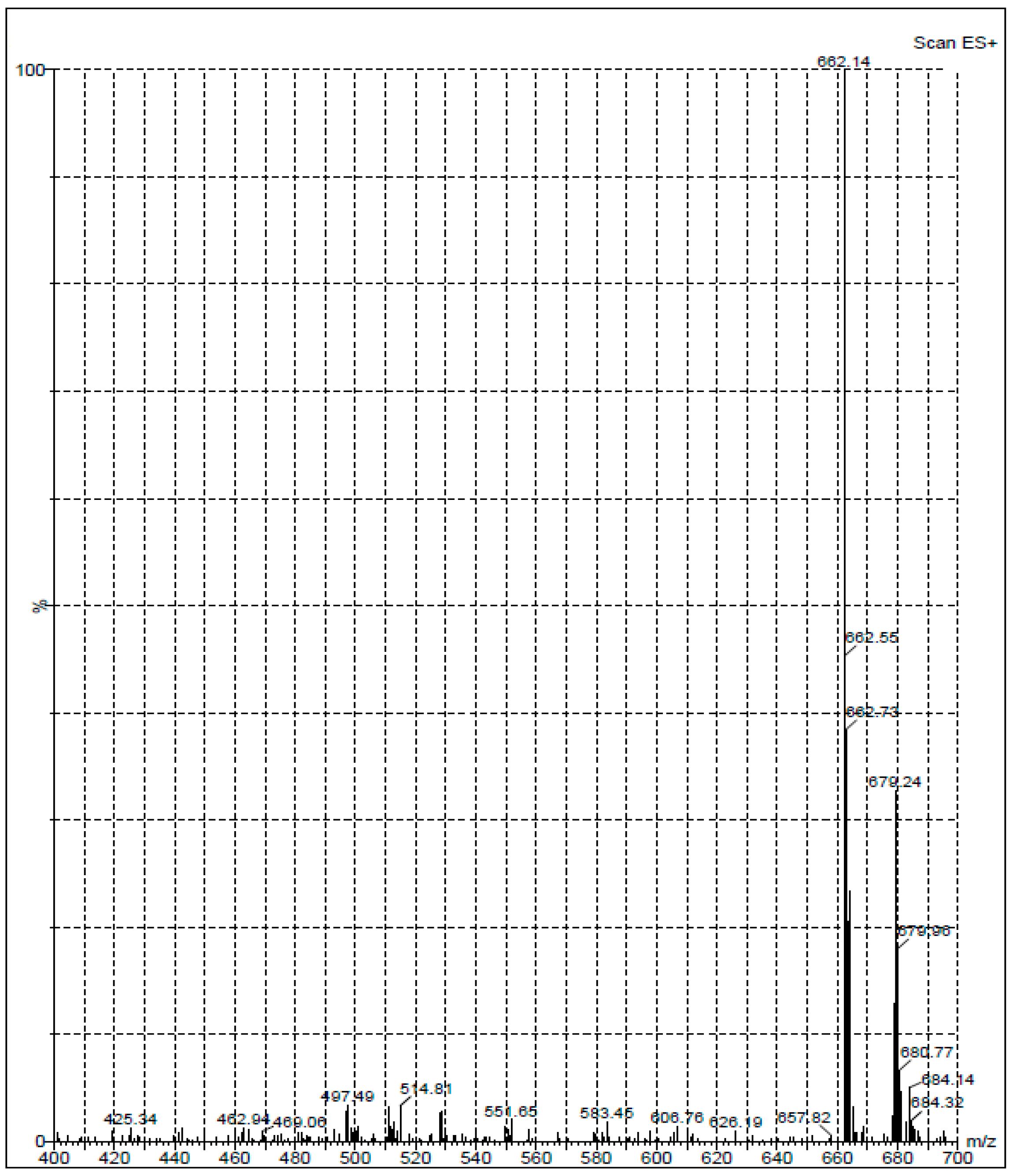
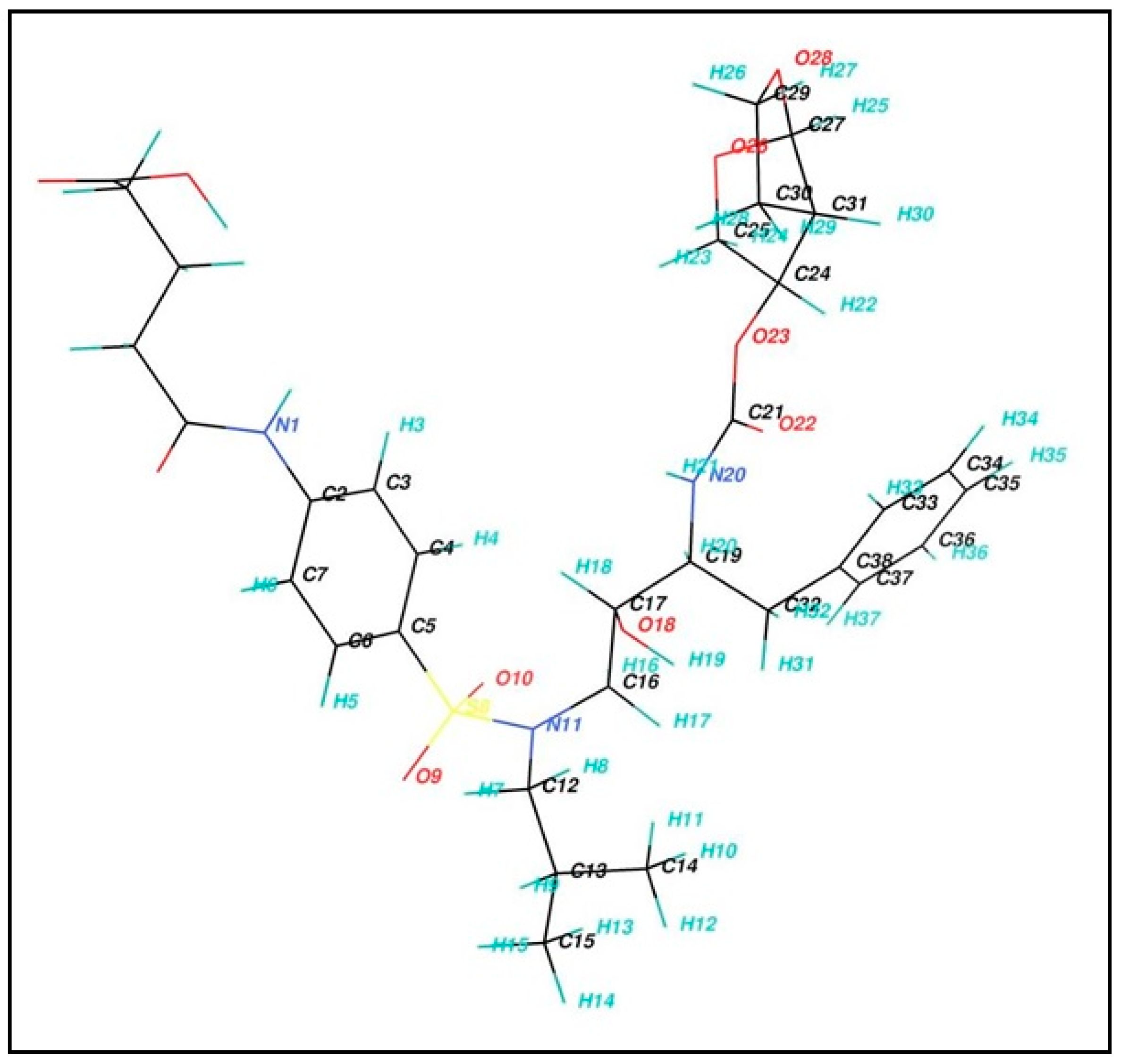
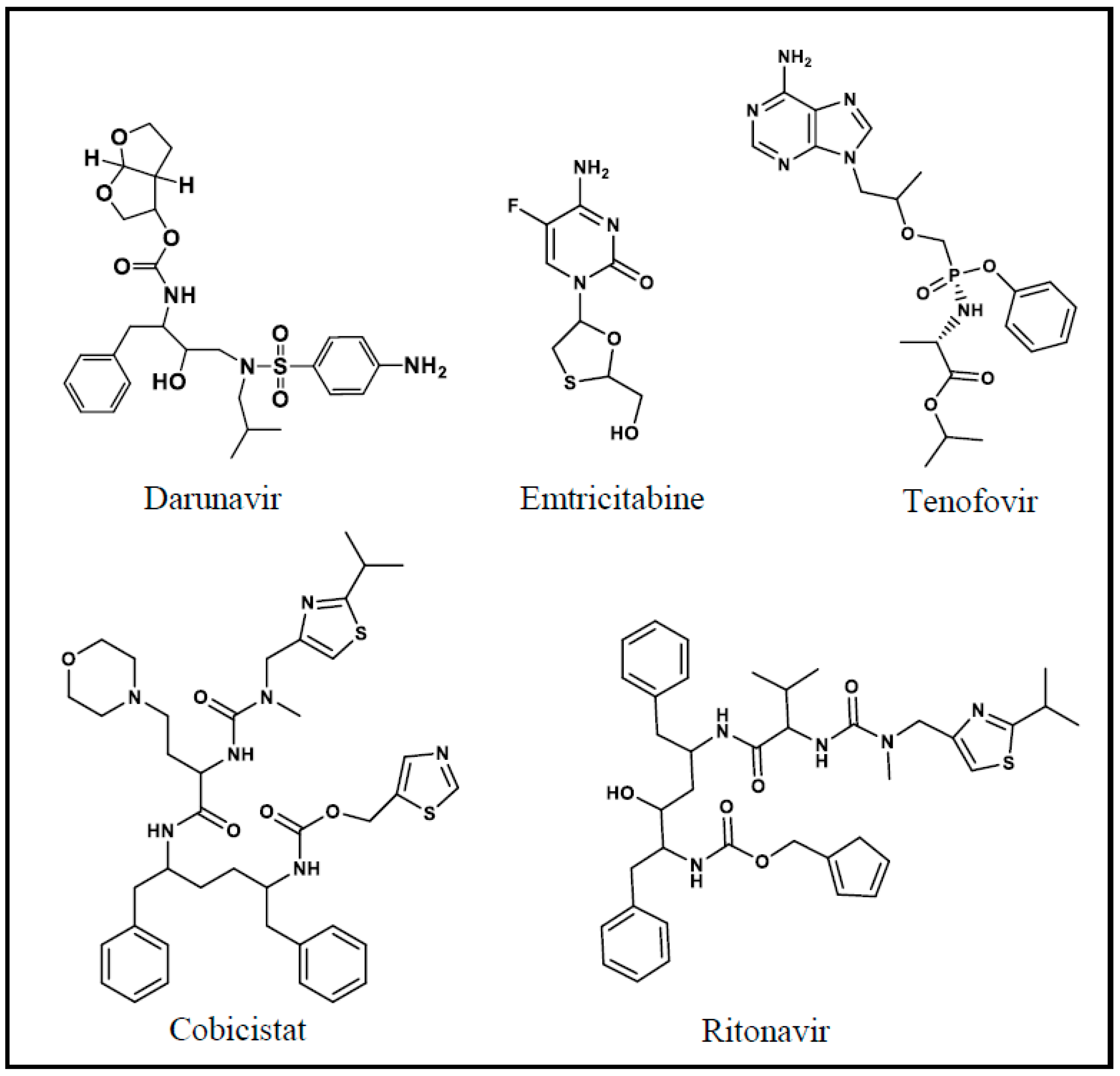
2.1. Synthesis and Structure Confirmation of DRV Hapten (N-Glutaryl-DRV)
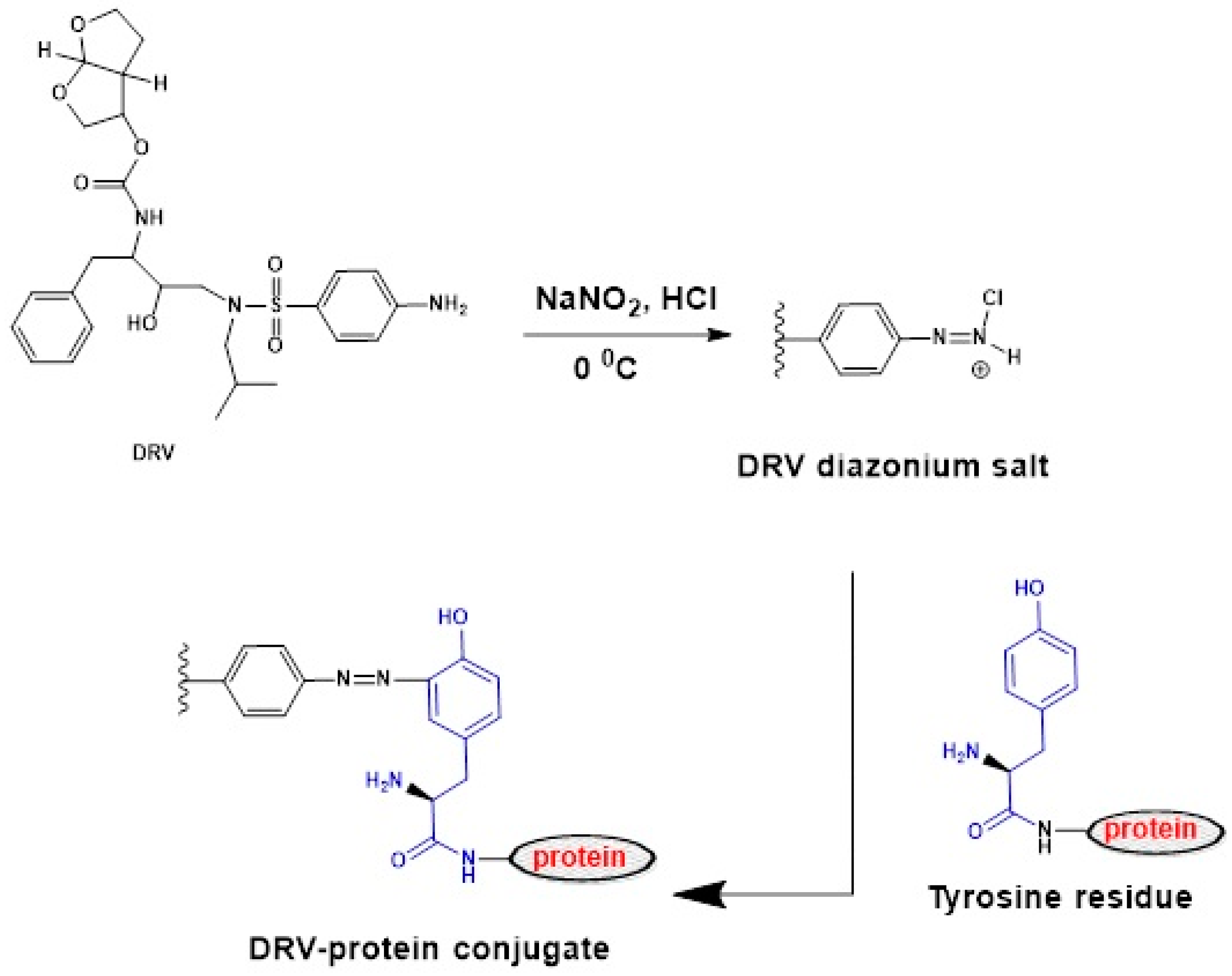
2.2. Preparation and Characterization of Protein Conjugates
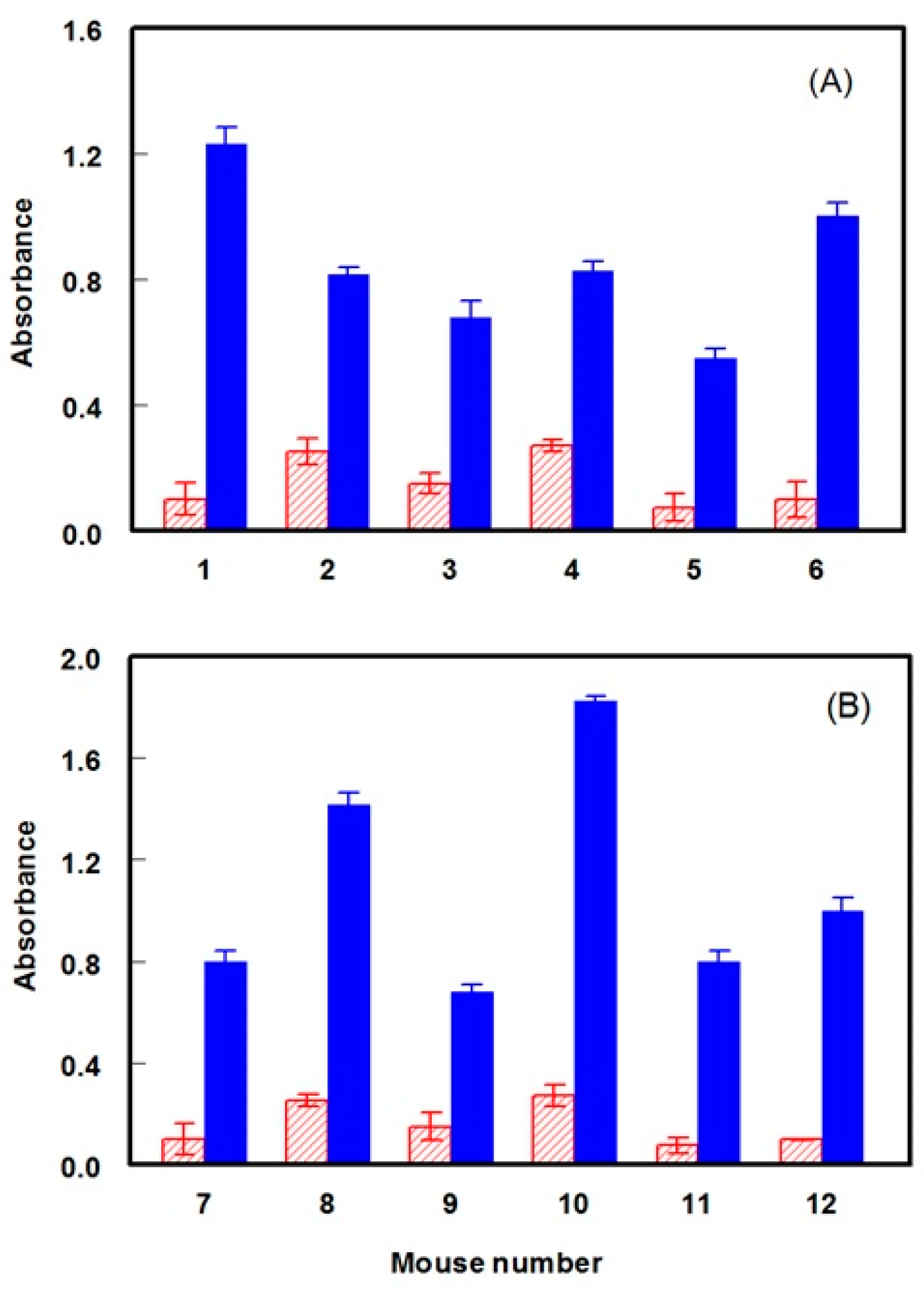
2.3. Production of Anti-DRV Polyclonal Antibody
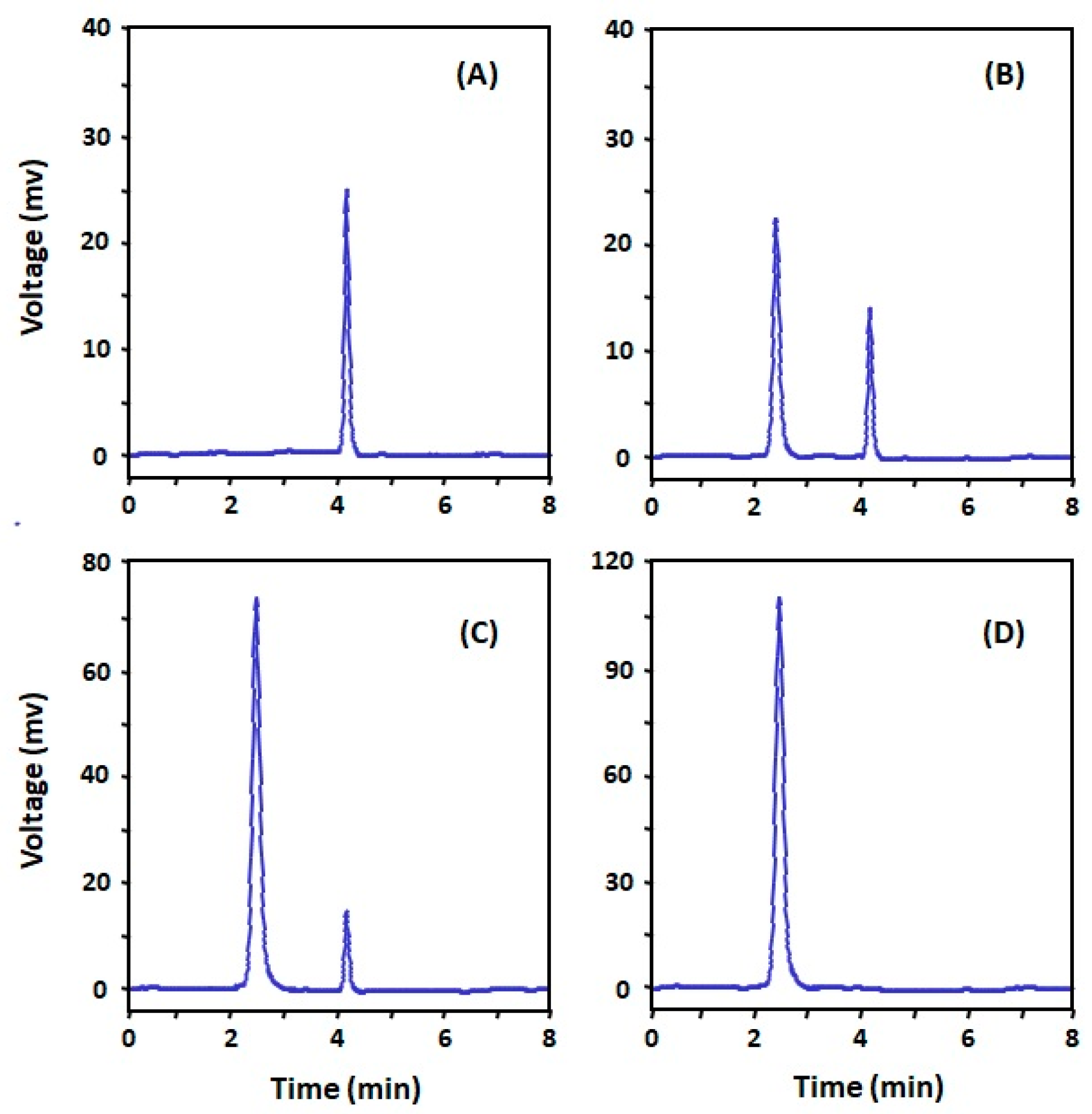
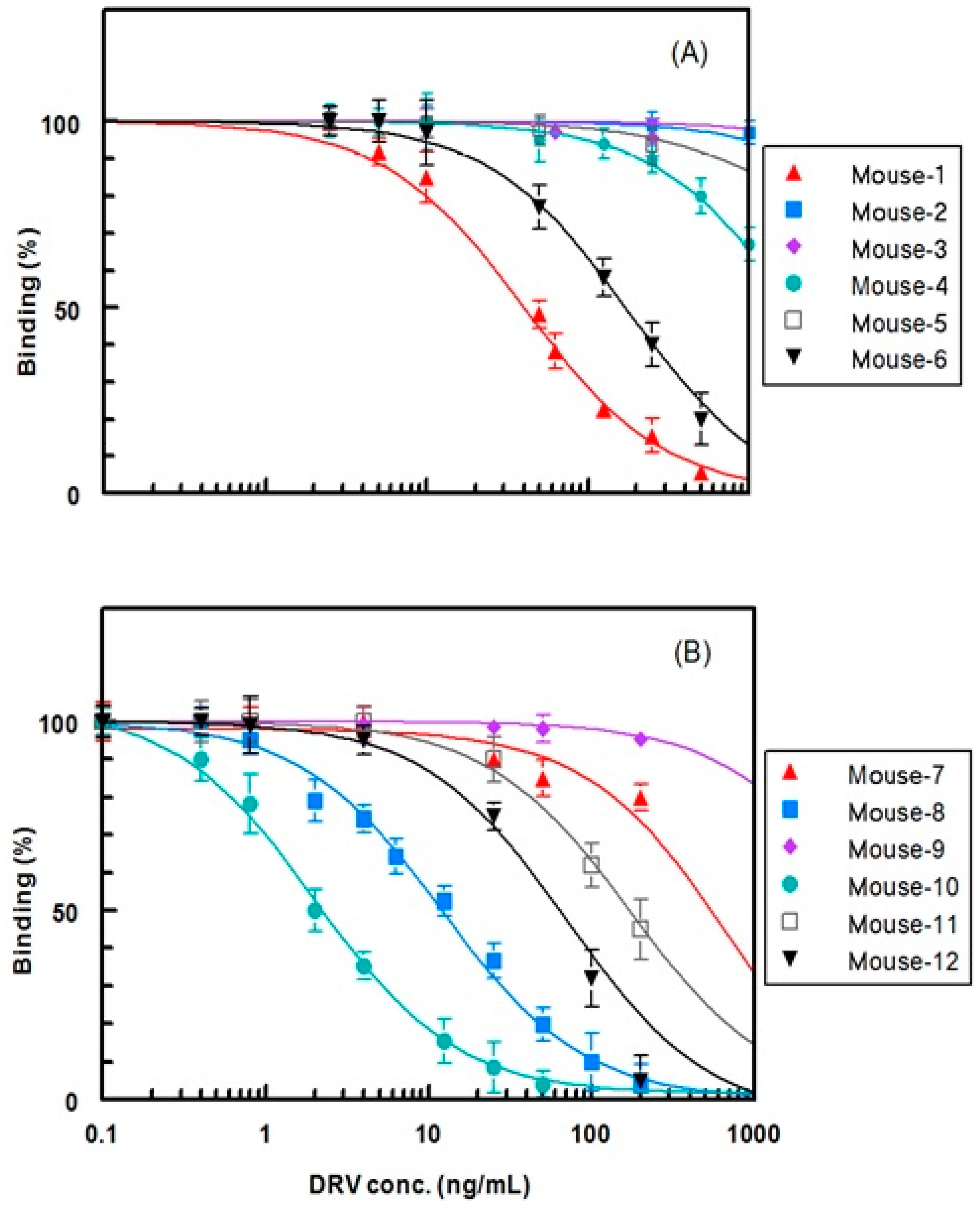
2.4. Selection of the Antibody with the Highest Affinity to DRV
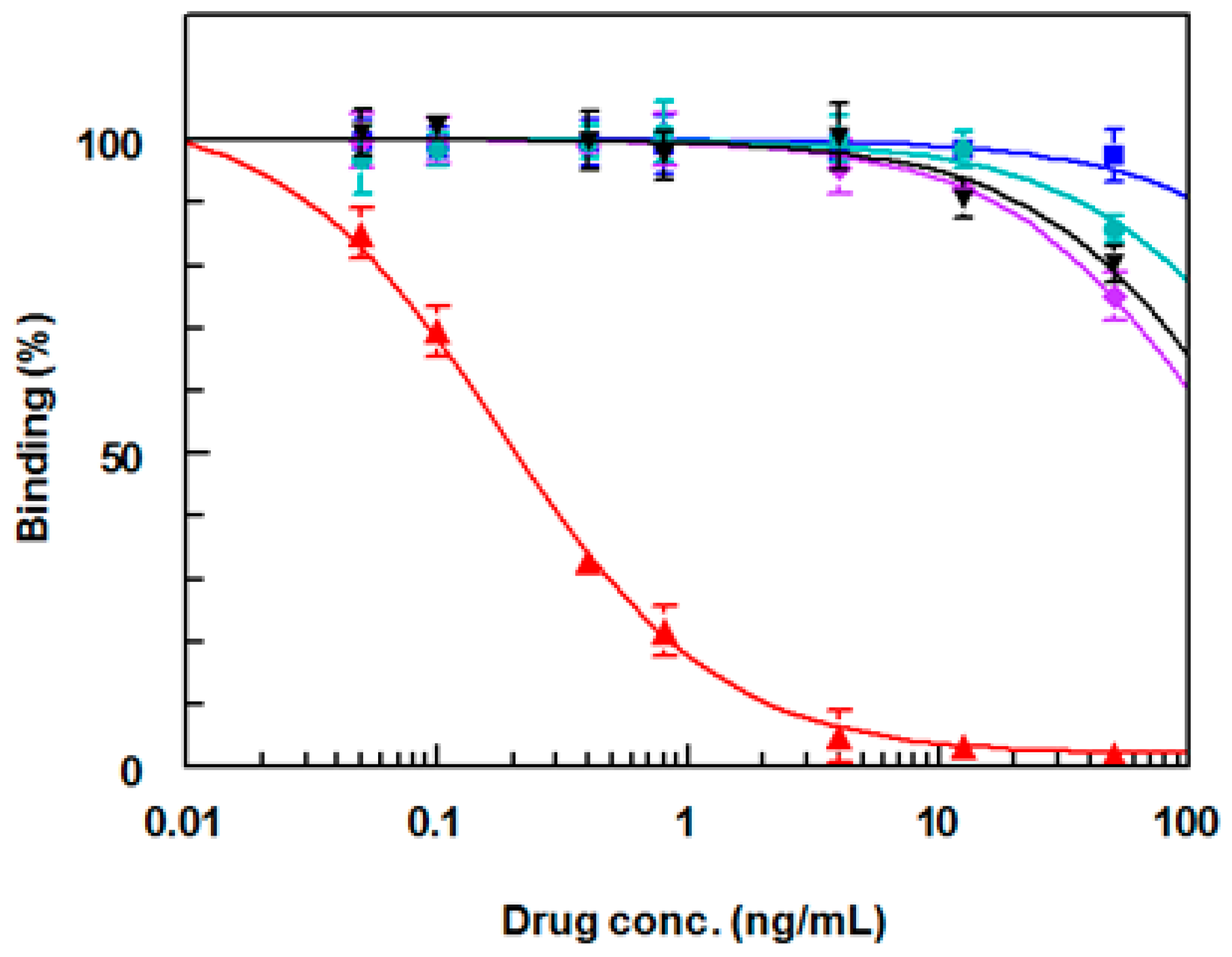
2.5. Effect of Type of Plate-Coating Conjugate on the Affinity and Specificity of Anti-DRV Antibody
2.6. Applications of the Anti-DRV Polyclonal Antibody
3. Experimental
3.1. Instruments
3.2. Materials
3.3. Procedures
3.3.1. Synthesis of Hapten (N-Glutaryl Darunavir; G-DRV)
3.3.2. Preparation of DRV–Protein Conjugates by Diazotization/Coupling Reaction
3.3.3. Preparation of G-DRV–Protein Conjugates by Carbodiimide Method
3.3.4. Immunization of Animals and Production of Anti-DRV Polyclonal Antibody
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sepkowitz, K.A. AIDS—The first 20 years. N. Engl. J. Med. 2001, 344, 1764–1772. [Google Scholar] [CrossRef] [PubMed]
- FDA. U.S. Food and Drug Administration. (HIV/AIDS historical time line 1981–1990). 2018. Available online: https://www.fda.gov/patients/hiv-timeline-and-history-approvals/hivaids-historical-time-line-1981-1990 (accessed on 12 August 2020).
- Sharp, P.M.; Hahn, B.H. Origins of HIV and the AIDS pandemic. Cold. Spring Harb. Perspect. Med. 2011, 1, a006841. [Google Scholar]
- Fischer, J.; Ganellin, C.R.; Ganesan, A.; Proudfoot, J. Analogue-Based Drug Discovery; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2010. [Google Scholar]
- Wang, S.; Milne, G.W.; Yan, X.; Posey, I.J.; Nicklaus, M.C.; Graham, L.; Rice, W.G. Discovery of novel, non-peptide HIV-1 protease inhibitors by pharmacophore searching. J. Med. Chem. 1996, 39, 2047–2054. [Google Scholar] [CrossRef] [PubMed]
- Dierynck, I.; De Wit, M.; Gustin, E.; Keuleers, I.; Vandersmissen, J.; Hallenberger, S.; Hertogs, K. Binding kinetics of darunavir to human immunodeficiency virus type 1 protease explain the potent antiviral activity and high genetic barrier. J. Virol. 2007, 81, 13845–13851. [Google Scholar] [CrossRef]
- Mocroft, A.; Ledergerber, B.; Katlama, C.; Kirk, O.; Reiss, P.D.; Monforte, A.D.A.; Knysz, B.; Dietrich, M.; Phillips, A.N.; Lundgren, J.D.; et al. Decline in the AIDS and death rates in the EuroSIDA study: An observational study. Lancet 2003, 362, 22–29. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Kincaid, J.F.; Cho, W.; Walters, D.E.; Krishnan, K.; Hussain, K.A.; Koo, Y.; Cho, H.; Rudall, C.; Holland, L.; et al. Potent HIV protease inhibitors incorporating high-affinity P2-ligands and (R)-(hydroxyethylamino)sulfonamide isostere. Bioorg. Med. Chem. Lett. 1998, 8, 687–690. [Google Scholar] [CrossRef]
- CenterWatch. Prezista (Darunavir). Available online: https://www.centerwatch.com/drug-information/fda-approved-drugs/drug/902/prezista-darunavir (accessed on 12 August 2020).
- Janssen Therapeutics. U.S. FDA approves Prezista® (darunavir) for Use in Pregnant Women with HIV. PRNewswire. Available online: http://www.prnewswire.com/news-releases/us-fda-approves-prezista-darunavir-for-use-in-pregnant-women-with-hiv-300299618.html (accessed on 12 August 2020).
- Busse, K.H.; Penzak, S.R. Darunavir: A second-generation protease inhibitor. Am. J. Health Syst. Pharm. 2007, 64, 1593–1602. [Google Scholar] [CrossRef]
- Justesen, U.S. Therapeutic drug monitoring and human immunodeficiency virus (HIV) antiretroviral therapy. Basic Clin. Pharmacol. Toxicol. 2006, 98, 20–31. [Google Scholar] [CrossRef]
- Correa, J.C.; D’Arcy, D.M.; Serra, C.H.; Salgado, H.R. A critical review of properties of darunavir and analytical methods for its determination. Crit. Rev. Anal. Chem. 2014, 44, 16–22. [Google Scholar] [CrossRef]
- García, S.P.; Tunica, D.G.; Serra, M.B. Development and assessment of a method for the determination of darunavir in plasma by LC-MS/MS. Rev. Lab. Clin. 2011, 4, 127–133. [Google Scholar]
- Muller, D.M.; Rentsch, K.M. Therapeutic drug monitoring by LC-MS-MS with special focus on anti-infective drugs. Anal. Bioanal. Chem. 2010, 398, 2573–2594. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Darwish, I.A.; Al-Obaid, A.R.; Al-Malaq, H.A. A highly sensitive and specific polyclonal antibody-based enzyme immunoassay for therapeutic monitoring and pharmacokinetic studies of atorvastatin. Microchim. Acta. 2010, 170, 67–74. [Google Scholar] [CrossRef]
- Darwish, I.A.; Alzoman, N.Z.; Abuhejail, R.M.; El-Samani, T.E. Synthesis of hapten and preparation of specific polyclonal antibody with high affinity for lenalidomide, the potent drug for treatment of multiple myeloma. Chem. Cent. J. 2012, 6, 125. [Google Scholar] [CrossRef] [PubMed]
- Darwish, I.A.; Al-Obaid, A.R.; Al-Malaq, H.A. New highly sensitive enzyme immunoassay for the determination of pravastatin in human plasma. Talanta 2009, 79, 1478–1483. [Google Scholar] [CrossRef] [PubMed]
- Alzoman, N.Z.; Darwish, I.A.; Abuhejail, R.M. A highly sensitive polyclonal antibody-based ELISA for therapeutic monitoring and pharmacokinetic studies of lenalidomide. J. Immunoass. Immunochem. 2014, 35, 130–138. [Google Scholar] [CrossRef]
- Darwish, I.A. Immunoassay methods and their applications in pharmaceutical analysis: Basic methodology and recent advances. Int. J. Biomed. Sci. 2006, 2, 217–235. [Google Scholar] [PubMed]
- Hermanson, G.T. Bioconjugate Techniques; Academic Press San Diego: Cambridge, MA, USA, 2013. [Google Scholar]
- Weeks, I. Chemiluminescence Immunoassay; Sevehla, G., Ed.; Elsevier: Amsterdam, The Netherlands, 1992; Volume XXIX, pp. 18–118. [Google Scholar]
- Erlanger, B.F. Principles and methods for the preparation of drug protein conjugates for immunological studies. Pharmacol. Rev. 1973, 25, 271–280. [Google Scholar]
- Shan, G.; Lipton, C.; Gee, S.; Hammock, B. In Handbook of Residue Analytical Methods for Agrochemicals, Ed. Philip W. Lee; John Wiley & Sons: Chichester, UK, 2002; p. 640. [Google Scholar]
- Xue, F.; Gu, Z.; Feng, J.A. Linker: A web server to generate peptide sequences with extended conformation. Nucleic Acids Res. 2004, 32 (Suppl. 2), W562–W565. [Google Scholar] [CrossRef]
- Buer, B.C.; Marsh, E.N. Design, synthesis, and study of fluorinated proteins. Methods Mol. Biol. 2014, 1216, 89–116. [Google Scholar]
- Ruela Correa, J.C.; D’Arcy, D.M.; dos Reis Serra, C.H.; Nunes Salgado, H.R. Darunavir: A critical review of its properties, use and drug interactions. Pharmacology 2012, 90, 102–109. [Google Scholar] [CrossRef]
- AIDSinfo. Darunavir/Cobicistat/Emtricitabine/Tenofovir Alafenamide. 2019. Available online: https://aidsinfo.nih.gov/drugs/597/symtuza/209/professional (accessed on 12 August 2020).
- Almehizia, A.A.; Herqash, R.N.; Darwish, I.A. Development of a highly sensitive ELISA for determination of darunavir in plasma samples using a polyclonal antibody with high affinity and specificity. Bioanalysis 2020, 12, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Habeeb, A.F. Determination of free amino groups in proteins by trinitrobenzenesulfonic acid. Anal. Biochem. 1966, 14, 328–336. [Google Scholar] [CrossRef]
- Al-Shehri, M.M.; El-Gendy, M.A.; El-Azab, A.S.; Hamidaddin, M.A.; Darwish, I.A. Development and validation of an ELISA with high sensitivity for therapeutic monitoring of afatinib. Bioanalysis 2018, 10, 1511–1523. [Google Scholar] [CrossRef] [PubMed]
- Darwish, I.; Akizawa, T.; Hirose, K.; Omura, K.; Nawal, E.-R.; Yoshioka, M. Preparation of a specific monoclonal antibody against 2′-deoxycytidine. Anal. Chim. Acta. 1998, 365, 121–128. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
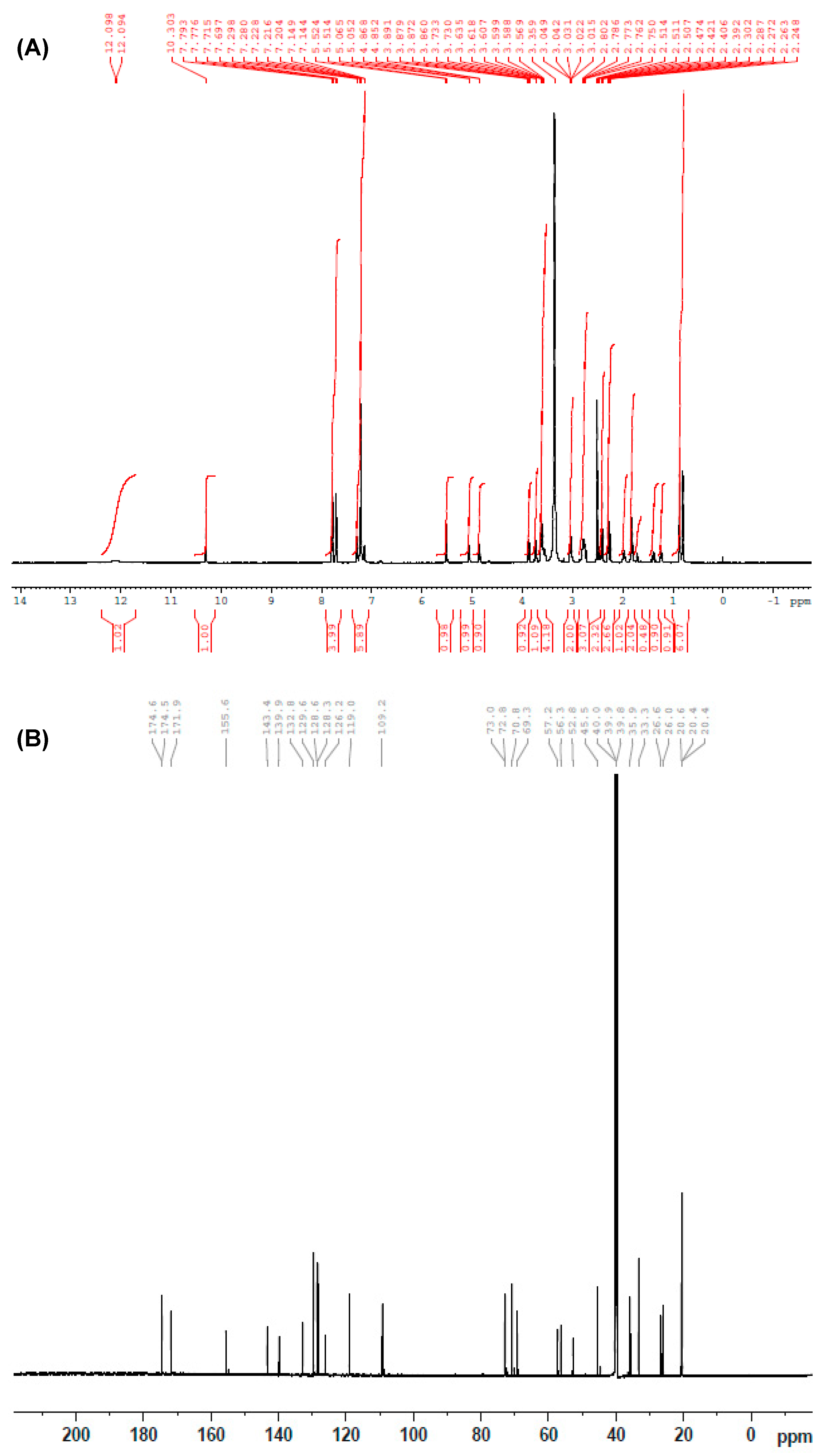


| Signal | Location (δ) | Shape | Integration | Corresponding Carbon Number |
|---|---|---|---|---|
| 1 | 0.78 | d, J = 6.5 Hz | 3H | C14 |
| 2 | 0.86 | d, J = 6.5 Hz | 3H | C15 |
| 3 | 1.42 | m | 1H | C13 |
| 4 | 1.82 | m | 3H | C30,C31 |
| 5 | 1.95 | m | 2H | COCH2CH2CH2COOH |
| 6 | 2.23–2.30 | m | 2H | C32 |
| 7 | 2.39–2.47 | m | 4H | COCH2CH2CH2COOH |
| 8 | 2.75–2.80 | m | 2H | C12 |
| 9 | 3.03 | d | 2H | C16 |
| 10 | 3.56–3.89 | m | 5H | C17, C19, C25, C29 |
| 11 | 4.86 | m | 1H | C24 |
| 12 | 7.05 | m | 1H | C27 |
| 13 | 5.51 | s | 1H | OCONH-C< |
| 14 | 7.14–7.29 | m | 5H | C6H5-CH2- |
| 15 | 7.74 | dd | 4H | C6H4-SO2- |
| 16 | 10.30 | s | 1H | -NH-CO-glutaryl |
| 17 | 12.9 | s | 1H | COOH |
| Group 1: Immunized with DRV-KLH | Group 2: Immunized with G-DRV-KLH | ||
|---|---|---|---|
| Mouse No. | IC50 (ng mL−1) | Mouse No. | IC50 (ng mL−1) |
| 1 | 40 | 7 | >1000 |
| 2 | >1000 | 8 | 12 |
| 3 | >1000 | 9 | 500 |
| 4 | >1000 | 10 | 2 |
| 5 | >1000 | 11 | 180 |
| 6 | 160 | 12 | 60 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Darwish, I.A.; Almehizia, A.A.; Radwan, A.A.; Herqash, R.N. Preparation and Characterization of Two Immunogens and Production of Polyclonal Antibody with High Affinity and Specificity for Darunavir. Molecules 2020, 25, 4075. https://doi.org/10.3390/molecules25184075
Darwish IA, Almehizia AA, Radwan AA, Herqash RN. Preparation and Characterization of Two Immunogens and Production of Polyclonal Antibody with High Affinity and Specificity for Darunavir. Molecules. 2020; 25(18):4075. https://doi.org/10.3390/molecules25184075
Chicago/Turabian StyleDarwish, Ibrahim A., Abdulrahman A. Almehizia, Awwad A. Radwan, and Rashed N. Herqash. 2020. "Preparation and Characterization of Two Immunogens and Production of Polyclonal Antibody with High Affinity and Specificity for Darunavir" Molecules 25, no. 18: 4075. https://doi.org/10.3390/molecules25184075
APA StyleDarwish, I. A., Almehizia, A. A., Radwan, A. A., & Herqash, R. N. (2020). Preparation and Characterization of Two Immunogens and Production of Polyclonal Antibody with High Affinity and Specificity for Darunavir. Molecules, 25(18), 4075. https://doi.org/10.3390/molecules25184075






