Kaempferol as a Dietary Anti-Inflammatory Agent: Current Therapeutic Standing
Abstract
1. Introduction
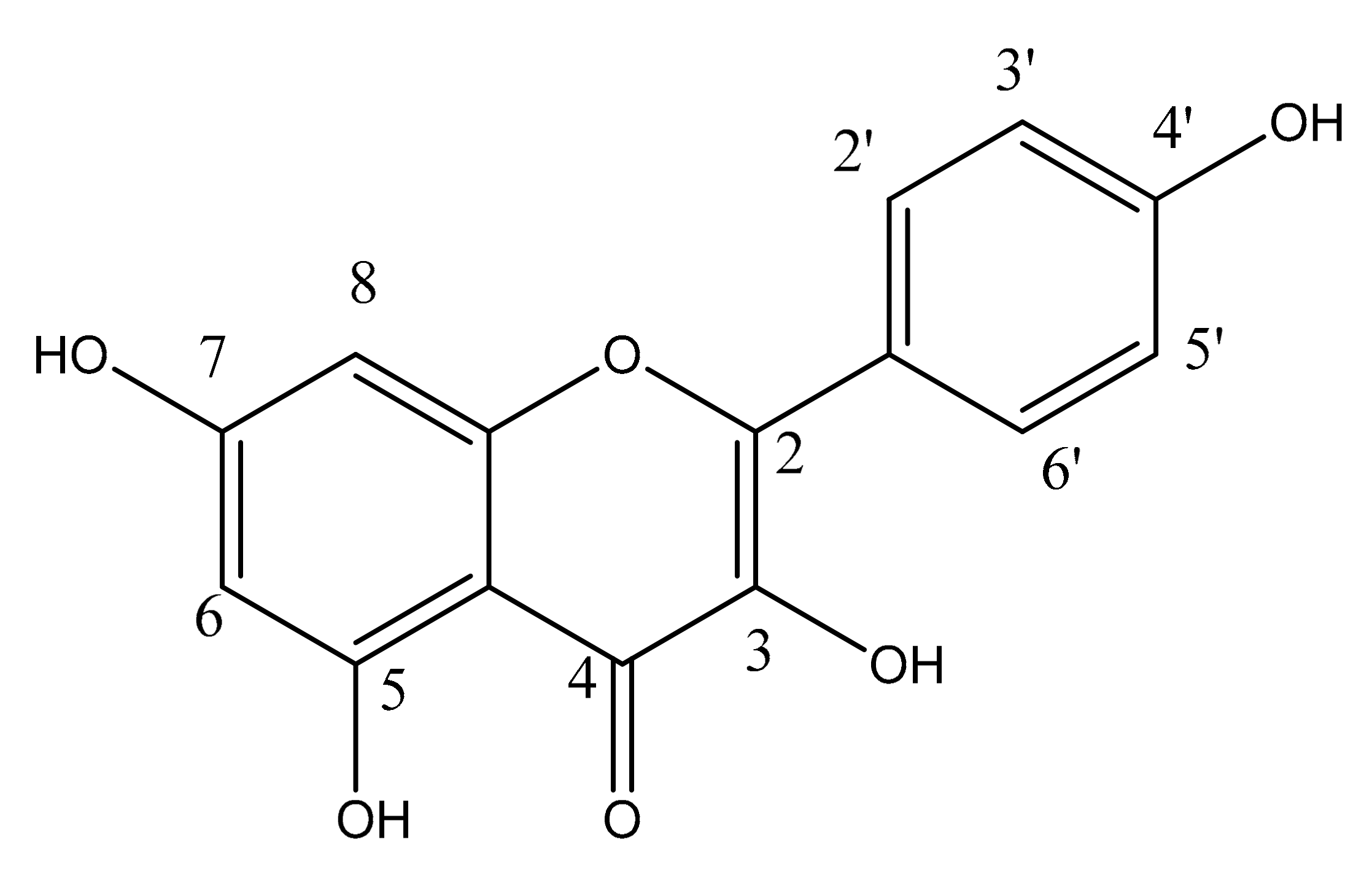
2. Chemistry of Kaempferol
3. Natural Sources of Kaempferol
4. Isolation of Kaempferol from Natural Sources
5. Biosynthesis of Kaempferol
6. Bioavailability, Oral Absorptionand Metabolism of Kaempferol
7. Anti-Inflammatory Effect of Kaempferol
7.1. In Vitro Studies
In Vivo Studies
7.2. Clinical Studies
8. Toxicity Profile of Kaempferol
9. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CYP 1A1 | cytochrome 1A1 |
| NK cells | Natural killer cells |
| COX | Cyclo-oxygenase |
| NOS | Nitric oxide synthase |
| LOX | Lipo oxygenase |
| TNF-α | Tumor necrotic factor alpha |
| LPS | Lipo polysaccharides |
| ROS | Reactive Oxygen Species |
| RNS | Reactive Nitrogen Species |
| PLA | Phospholipase A |
| Nrf-2 | Nuclear factor (erythroid-derive 2) -like-2 |
| NF-ĸB | Nuclear factor-kappa B |
| AGE | Advance glycation endproducts |
| TSLP | Thymic stromal lymphopoietin |
| IL | Interleukin |
| MAO | Mono amine oxidase enzyme |
| MMPs | Mettaloproteases |
| AP-1 | Activator protein |
References
- Panche, A.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, 5. [Google Scholar] [CrossRef]
- Burak, M.; Imen, Y. Flavonoids and their antioxidant properties. TürkiyeKlin Tip Bild. 1999, 19, 296–304. [Google Scholar]
- Metodiewa, D.; Kochman, A.; Karolczak, S. Evidence for antiradical and antioxidant properties of four biologically active N, N-Diethylaminoethyl ethers of flavaone oximes: A comparison with natural polyphenolic flavonoid rutin action. IUBMB Life 1997, 41, 1067–1075. [Google Scholar] [CrossRef] [PubMed]
- Havsteen, B. The biochemistry and medical significance of the flavonoids. Pharmacol. Ther. 2002, 96, 67–202. [Google Scholar] [CrossRef]
- Samanta, A.; Das, G.; Das, S.K. Roles of flavonoids in plants. Carbon 2011, 100, 12–35. [Google Scholar]
- García-Mediavilla, M.V.; Crespo, I.; Collado, P.S.; Esteller, A.; Sánchez-Campos, S.; Tuñón, M.J.; González-Gallego, J. The anti-inflammatory flavones quercetin and kaempferol cause inhibition of inducible nitric oxide synthase, cyclooxygenase-2 and reactive C-protein, and down-regulation of the nuclear factor kappaB pathway in Chang Liver cells. Eur. J. Pharmacol. 2007, 557, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Kwon, K.H.; Murakami, A.; Tanaka, T.; Ohigashi, H. Dietary rutin, but not its aglycone quercetin, ameliorates dextran sulfate sodium-induced experimental colitis in mice: Attenuation of pro-inflammatory gene expression. Biochem. Pharmacol. 2005, 69, 395–406. [Google Scholar] [CrossRef]
- Nair, M.P.N.; Mahajan, S.; Reynolds, J.L.; Aalinkeel, R.; Nair, H.; Schwartz, S.A.; Kandaswami, C. The Flavonoid Quercetin Inhibits Proinflammatory Cytokine (Tumor Necrosis Factor Alpha) Gene Expression in Normal Peripheral Blood Mononuclear Cells via Modulation of the NF-κβ System. Clin. Vaccine Immunol. 2006, 13, 319–328. [Google Scholar] [CrossRef]
- Raso, G.M.; Meli, R.; Di Carlo, G.; Pacilio, M.; Di Carlo, R. Inhibition of inducible nitric oxide synthase and cyclooxygenase-2 expression by flavonoids in macrophage J774A.1. Life Sci. 2001, 68, 921–931. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef]
- Iwashina, T. Flavonoid properties of five families newly incorporated into the order Caryophyllales. Bull. Natl. Mus. Nat. Sci. 2013, 39, 25–51. [Google Scholar]
- Häkkinen, S.H.; Kärenlampi, S.O.; Heinonen, I.M.; Mykkänen, H.M.; Törrönen, A.R. Content of the flavonols quercetin, myricetin, and kaempferol in 25 edible berries. J. Agric. Food Chem. 1999, 47, 2274–2279. [Google Scholar] [CrossRef] [PubMed]
- Fieschi, M.; Codignola, A.; Mosca, A.L. Mutagenic flavonol aglycones in infusions and in fresh and pickled vegetables. J. Food Sci. 1989, 54, 1492–1495. [Google Scholar] [CrossRef]
- Miean, K.H.; Mohamed, S.J. Flavonoid (myricetin, quercetin, kaempferol, luteolin, and apigenin) content of edible tropical plants. Agric. Food Chem. 2001, 49, 3106–3112. [Google Scholar] [CrossRef] [PubMed]
- Hertog, M.G.; Hollman, P.C.; Katan, M.B. Content of potentially anti-carcinogenic flavonoids of 28 vegetables and 9 fruits commonly consumed in The Netherlands. J. Agric. Food Chem. 1992, 40, 2379–2383. [Google Scholar] [CrossRef]
- Herrmann, K. Flavonols and flavones in food plants: A review. Int. J. Food Sci. Technol. 1976, 11, 433–448. [Google Scholar] [CrossRef]
- Orhan, I.; Küpeli, E.; Terzioglu, S.; Yesilada, E. Bioassay-guided isolation of kaempferol-3-O-β-d-galactoside with anti-inflammatory and antinociceptive activity from the aerial part of Calluna vulgaris L. J. Ethnopharmacol. 2007, 114, 32–37. [Google Scholar] [CrossRef]
- Yang, J.-H.; Kondratyuk, T.P.; Marler, L.E.; Qiu, X.; Choi, Y.; Cao, H.; Yu, R.; Sturdy, M.; Pegan, S.; Liu, Y. Isolation and evaluation of kaempferol glycosides from the fern Neocheiropteris palmatopedata. Phytochemistry 2010, 71, 641–647. [Google Scholar] [CrossRef]
- Liang, Y.; Wei, L.; Zhu, Z.; Pan, Y.; Wang, H.; Liu, P. Isolation and purification of kaempferol-3, 7-O-α-L-dirhamnopyranoside from Siraitia grosvenori leaves by high-speed counter-current chromatograph and its free radical scavenging activity. Sep. Sci. Technol. 2011, 46, 1528–1533. [Google Scholar] [CrossRef]
- Winkel, B.S. Flavonoid Biosynthesis. A Colorful Model for Genetics, Biochemistry, Cell Biology, and Biotechnology. Plant Physiol. 2001, 126, 485–493. [Google Scholar] [CrossRef]
- Winkel, B.S. Biosynthesis of flavonoids and effects of stress. Curr. Opin. Plant Biol. 2002, 5, 218–223. [Google Scholar] [CrossRef]
- Calderón-Montaño, J.M.; Morón, E.B.; Pérez-Guerrero, C.; Lopez-Lazaro, M. A review on the dietary flavonoid kaempferol. Mini Rev. Med. Chem. 2011, 11, 298–344. [Google Scholar] [CrossRef] [PubMed]
- Gee, J.M.; Johnson, I.T. Polyphenolic compounds: Interactions with the gut and implications for human health. Curr. Med. Chem. 2001, 8, 1245–1255. [Google Scholar] [CrossRef] [PubMed]
- Lehtonen, H.-M.; Lehtinen, O.; Suomela, J.-P.; Viitanen, M.; Kallio, H. Flavonol Glycosides of Sea Buckthorn (Hippophaerhamnoides ssp. sinensis) and Lingonberry (Vaccinium vitis-idaea) Are Bioavailable in Humans and Monoglucuronidated for Excretion. J. Agric. Food Chem. 2010, 58, 620–627. [Google Scholar] [CrossRef]
- Crespy, V.; Morand, C.; Besson, C.; Cotelle, N.; Vezin, H.; Demigné, C.; Rémésy, C. The splanchnic metabolism of flavonoids highly differed according to the nature of the compound. Am. J. Physiol. Liver Physiol. 2003, 284, G980–G988. [Google Scholar] [CrossRef]
- Bokkenheuser, V.D.; Shackleton, C.H.; Winter, J. Hydrolysis of dietary flavonoid glycosides by strains of intestinal Bacteroides from humans. Biochem. J. 1987, 248, 953–956. [Google Scholar] [CrossRef]
- Schneider, H.; Blaut, M. Anaerobic degradation of flavonoids by Eubacteriumramulus. Arch. Microbiol. 2000, 173, 71–75. [Google Scholar] [CrossRef]
- Dupont, M.S.; Day, A.J.; Bennett, R.N.; Mellon, F.; Kroon, P. Absorption of kaempferol from endive, a source of kaempferol-3-glucuronide, in humans. Eur. J. Clin. Nutr. 2004, 58, 947–954. [Google Scholar] [CrossRef]
- De Vries, J.H.; Hollman, P.C.; Meyboom, S.; Buysman, M.N.; Zock, P.L.; Van Staveren, W.A.; Katan, M.B. Plasma concentrations and urinary excretion of the antioxidant flavonols quercetin and kaempferol as biomarkers for dietary intake. Am. J. Clin. Nutr. 1998, 68, 60–65. [Google Scholar] [CrossRef]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef]
- Folkerts, G.; Kloek, J.; Muijsers, R.B.; Nijkamp, F.P. Reactive nitrogen and oxygen species in airway inflammation. Eur. J. Pharmacol. 2001, 429, 251–262. [Google Scholar] [CrossRef]
- Mourits, M.P.; Prummel, M.F.; Wiersinga, W.M.; Koornneef, L. Clinical activity score as a guide in the management of patients with Graves’ ophthalmopathy. Clin. Endocrinol. 1997, 47, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Natella, F.; Nardini, M.; Virgili, F.; Scaccini, C. Role of dietary polyphenols in the platelet aggregation network—A review of the in vitro studies. Curr. Top. Nutraceutical Res. 2006, 4, 1–22. [Google Scholar]
- Rathee, P.; Chaudhary, H.; Rathee, S.; Rathee, D.; Kumar, V.; Kohli, K. Mechanism of action of flavonoids as anti-inflammatory agents: A review. Inflamm. Allergy Drug Targets 2009, 8, 229–235. [Google Scholar] [CrossRef]
- Funk, C.D. Prostaglandins and Leukotrienes: Advances in Eicosanoid Biology. Science 2001, 294, 1871–1875. [Google Scholar] [CrossRef] [PubMed]
- Guzik, T.J.; Korbut, R.; Adamek-Guzik, T. Nitric oxide and superoxide in inflammation and immune regulation. J. Physiol. Pharmacol. 2003, 54, 469–487. [Google Scholar]
- Moens, U.; Kostenko, S.; Sveinbjörnsson, B. The Role of Mitogen-Activated Protein Kinase-Activated Protein Kinases (MAPKAPKs) in Inflammation. Genes 2013, 4, 101–133. [Google Scholar] [CrossRef]
- Zhang, R.; Ai, X.; Duan, Y.; Xue, M.; He, W.; Wang, C.; Xu, T.; Xu, M.; Liu, B.; Li, C. Kaempferol ameliorates H9N2 swine influenza virus-induced acute lung injury by inactivation of TLR4/MyD88-mediated NF-κB and MAPK signaling pathways. Biomed. Pharmacother. 2017, 89, 660–672. [Google Scholar] [CrossRef]
- Tang, X.-L.; Liu, J.; Dong, W.; Li, P.; Li, L.; Hou, J.-C.; Zheng, Y.-Q.; Lin, C.-R.; Ren, J.-G. Protective Effect of Kaempferol on LPS plus ATP-Induced Inflammatory Response in Cardiac Fibroblasts. Inflammation 2015, 38, 94–101. [Google Scholar] [CrossRef]
- Saw, C.L.L.; Guo, Y.; Yang, A.Y.; Paredes-Gonzalez, X.; Ramirez, C.; Pung, D.; Kong, A.-N.T. The berry constituents quercetin, kaempferol, and pterostilbene synergistically attenuate reactive oxygen species: Involvement of the Nrf2-ARE signaling pathway. Food Chem. Toxicol. 2014, 72, 303–311. [Google Scholar] [CrossRef]
- Nam, S.-Y.; Jeong, H.-J.; Kim, H.-M. Kaempferol impedes IL-32-induced monocyte-macrophage differentiation. Chem. Biol. Interactions 2017, 274, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Ricciotti, E.; FitzGerald, G.A. Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 986–1000. [Google Scholar] [CrossRef] [PubMed]
- Vane, J.R.; Mitchell, J.A.; Appleton, I.; Tomlinson, A.; Bishop-Bailey, D.; Croxtall, J.; Willoughby, D.A. Inducible isoforms of cyclooxygenase and nitric-oxide synthase in inflammation. Proc. Natl. Acad. Sci. USA 1994, 91, 2046–2050. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.; Mohammed, L.A. The role of leukotrienes in the pathophysiology of inflammatory disorders: Is there a case for revisiting leukotrienes as therapeutic targets? Inflammopharmacology 2006, 14, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Kim, G.H. Evaluation of Antioxidant and Inhibitory Activities for Different Subclasses Flavonoids on Enzymes for Rheumatoid Arthritis. J. Food Sci. 2010, 75, H212–H217. [Google Scholar] [CrossRef] [PubMed]
- Davis, K.L.; Martin, E.; Turko, I.V.; Murad, F. Novel effects of nitric oxide. Annu. Rev. Pharmacol. Toxicol. 2001, 41, 203–236. [Google Scholar] [CrossRef] [PubMed]
- Cleeter, M.; Cooper, J.; Darley-Usmar, V.; Moncada, S.; Schapira, A. Reversible inhibition of cytochrome c oxidase, the terminal enzyme of the mitochondrial respiratory chain, by nitric oxide: Implications for neurodegenerative diseases. FEBS Lett. 1994, 345, 50–54. [Google Scholar] [CrossRef]
- Tracey, K.J.; Beutler, B.; Lowry, S.F.; Merryweather, J.; Wolpe, S.; Milsark, I.W.; Hariri, R.J.; Fahey, T.J.; Zentella, A.; Albert, J.D.; et al. Shock and tissue injury induced by recombinant human cachectin. Science 1986, 234, 470–474. [Google Scholar] [CrossRef]
- Rho, H.S.; Ghimeray, A.K.; Yoo, D.S.; Ahn, S.M.; Kwon, S.S.; Lee, K.H.; Cho, D.H.; Cho, J.Y. Kaempferol and Kaempferol Rhamnosides with Depigmenting and Anti-Inflammatory Properties. Molecules 2011, 16, 3338–3344. [Google Scholar] [CrossRef]
- Abo-Salem, O.M. Kaempferol Attenuates the Development of Diabetic Neuropathic Pain in Mice: Possible Anti-Inflammatory and Anti-Oxidant Mechanisms. Maced. J. Med. Sci. 2014, 2, 424–430. [Google Scholar] [CrossRef]
- Ďuračková, Z. Some current insights into oxidative stress. Physiol. Res. 2010, 59, 59. [Google Scholar]
- Gutteridge, J.M. Biological origin of free radicals, and mechanisms of antioxidant protection. Chem. Interact. 1994, 91, 133–140. [Google Scholar] [CrossRef]
- Fantone, J.C.; Ward, P.A. Role of oxygen-derived free radicals and metabolites in leukocyte-dependent inflammatory reactions. Am. J. Pathol. 1982, 107, 395–418. [Google Scholar] [PubMed]
- Erben-Russ, M.; Bors, W.; Saran, M. Reactions of Linoleic Acid Peroxyl Radicals with Phenolic Antioxidants: A Pulse Radiolysis Study. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1987, 52, 393–412. [Google Scholar] [CrossRef]
- Hyun, S.K.; Jung, H.A.; Chung, H.Y.; Choi, J.S. In vitro peroxynitrite scavenging activity of 6-hydroxykynurenic acid and other flavonoids from Gingko biloba yellow leaves. Arch. Pharmacal Res. 2006, 29, 1074–1079. [Google Scholar] [CrossRef]
- Lin, C.-W.; Chen, P.-N.; Chen, M.-K.; Yang, W.-E.; Tang, C.-H.; Yang, S.-F.; Hsieh, Y.-S. Kaempferol reduces matrix metalloproteinase-2 expression by down-regulating ERK1/2 and the activator protein-1 signaling pathways in oral cancer cells. PLoS ONE 2013, 8, e80883. [Google Scholar] [CrossRef]
- Sharma, D.; Gondaliya, P.; Tiwari, V.; Kalia, K. Kaempferol attenuates diabetic nephropathy by inhibiting RhoA/Rho-kinase mediated inflammatory signalling. Biomed. Pharmacother. 2019, 109, 1610–1619. [Google Scholar] [CrossRef]
- Kong, L.; Luo, C.; Li, X.; Zhou, Y.; He, H. The anti-inflammatory effect of kaempferol on early atherosclerosis in high cholesterol fed rabbits. Lipids Health Dis. 2013, 12, 115. [Google Scholar] [CrossRef]
- Kim, J.M.; Lee, E.K.; Kim, D.H.; Yu, B.P.; Chung, H.Y. Kaempferol modulates pro-inflammatory NF-κB activation by suppressing advanced glycation endproducts-induced NADPH oxidase. Age 2010, 32, 197–208. [Google Scholar] [CrossRef]
- Sekiguchi, A.; Motegi, S.-I.; Fujiwara, C.; Yamazaki, S.; Inoue, Y.; Uchiyama, A.; Akai, R.; Iwawaki, T.; Ishikawa, O. Inhibitory effect of kaempferol on skin fibrosis in systemic sclerosis by the suppression of oxidative stress. J. Dermatol. Sci. 2019, 96, 8–17. [Google Scholar] [CrossRef]
- Estakhri, F.; Panjehshahin, M.R.; Tanideh, N.; Gheisari, R.; Mahmoodzadeh, A.; Azarpira, N.; Gholijani, N. The effect of kaempferol and apigenin on allogenic synovial membrane-derived stem cells therapy in knee osteoarthritic male rats. Knee 2020, 27, 817–832. [Google Scholar] [CrossRef] [PubMed]
- Hosseinpour-Niazi, S.; Mirmiran, P.; Fallah-Ghohroudi, A.; Azizi, F. Non-soya legume-based therapeutic lifestyle change diet reduces inflammatory status in diabetic patients: A randomised cross-over clinical trial. Br. J. Nutr. 2015, 114, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Navarro, S.L.; Schwarz, Y.; Song, X.; Wang, C.-Y.; Chen, C.; Trudo, S.P.; Kristal, A.R.; Kratz, M.; Eaton, D.L.; Lampe, J.W. Cruciferous Vegetables Have Variable Effects on Biomarkers of Systemic Inflammation in a Randomized Controlled Trial in Healthy Young Adults. J. Nutr. 2014, 144, 1850–1857. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-del-Río, I.; Villar, C.J.; Lombó, F. Therapeutic uses of kaempferol: Anticancer and antiinflammatory activity. In Biosynthesis, Food Sources and Therapeutic Uses; Nova Science Publishers: Hauppauge, NY, USA, 2016; Volume 71. [Google Scholar]
- Chen, A.Y.; Chen, Y.C. A review of the dietary flavonoid, kaempferol on human health and cancer chemoprevention. Food Chem. 2013, 138, 2099–2107. [Google Scholar] [CrossRef]
- Francis, A.R.; Shetty, T.K.; Bhattacharya, R.K. Modulating effect of plant flavonoids on the mutagenicity of N-methyl-N′-nitro-N-nitrosoguanidine. Carcinogenesis 1989, 10, 1953–1955. [Google Scholar] [CrossRef]
- Francis, A.; Shetty, T.; Bhattacharya, R. Modifying role of dietary factors on the mutagenicity of aflatoxin B1: In vitro effect of plant flavonoids. Mutat. Res. Genet. Toxicol. 1989, 222, 393–401. [Google Scholar] [CrossRef]
- MacGregor, J.T.; Jurd, L. Mutagenicity of plant flavonoids: Structural requirements for mutagenic activity in Salmonella typhimurium. Mutat. Res. Environ. Mutagenesis Relat. Subj. 1978, 54, 297–309. [Google Scholar] [CrossRef]
- Niering, P.; Michels, G.; Wätjen, W.; Ohler, S.; Steffan, B.; Chovolou, Y.; Kampkötter, A.; Proksch, P.; Kahl, R. Protective and detrimental effects of kaempferol in rat H4IIE cells: Implication of oxidative stress and apoptosis. Toxicol. Appl. Pharmacol. 2005, 209, 114–122. [Google Scholar] [CrossRef]
- Mira, L.; Fernandez, M.T.; Santos, M.; Rocha, R.; Florêncio, M.H.; Jennings, K.R. Interactions of flavonoids with iron and copper ions: A mechanism for their antioxidant activity. Free. Radic. Res. 2002, 36, 1199–1208. [Google Scholar] [CrossRef]
- Pietta, P.-G. Flavonoids as Antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef]
- Sahu, S.C.; Gray, G.C. Pro-oxidant activity of flavonoids: Effects on glutathione and glutathione S-transferase in isolated rat liver nuclei. Cancer Lett. 1996, 104, 193–196. [Google Scholar] [CrossRef]
- Galati, G.; Sabzevari, O.; Wilson, J.X.; O’Brien, P.J. Prooxidant activity and cellular effects of the phenoxyl radicals of dietary flavonoids and other polyphenolics. Toxicology 2002, 177, 91–104. [Google Scholar] [CrossRef]
- Silva, I.D.; Rodrigues, A.; Gaspar, J.; Mala, R.; Laires, A.; Rueff, J. Mutagenicity of kaempferol in V79 cells: The role of cytochromes P450. Teratog. Carcinog. Mutagenesis 1996, 16, 229–241. [Google Scholar] [CrossRef]
- Silva, I.D.; Rodrigues, A.; Gaspar, J.; Maia, R.; Laires, A.; Rueff, J. Involvement of rat cytochrome 1A1 in the biotransformation of kaempferol to quercetin: Relevance to the genotoxicity of kaempferol. Mutagenesis 1997, 12, 383–390. [Google Scholar] [CrossRef]
- Takanashi, H.; Aiso, S.; Hirono, I.; Matsushima, T.; Sugimura, T. Carcinogenicity test of quercetin and kaempferol in rats by oral administration. J. Food Saf. 1983, 5, 55–60. [Google Scholar] [CrossRef]
- Hu, Y.; Cheng, Z.; Heller, L.I.; Krasnoff, S.B.; Glahn, R.P.; Welch, R.M. Kaempferol in Red and Pinto Bean Seed (Phaseolus vulgaris L.) Coats Inhibits Iron Bioavailability Using an in Vitro Digestion/Human Caco-2 Cell Model. J. Agric. Food Chem. 2006, 54, 9254–9261. [Google Scholar] [CrossRef]
- Lemos, C.; Peters, G.J.; Jansen, G.; Martel, F.; Calhau, C. Modulation of folate uptake in cultured human colon adenocarcinoma Caco-2 cells by dietary compounds. Eur. J. Nutr. 2007, 46, 329–336. [Google Scholar] [CrossRef]
- Li, C.; Li, X.; Choi, J.-S. Enhanced bioavailability of etoposide after oral or intravenous administration of etoposide with kaempferol in rats. Arch. Pharmacal Res. 2009, 32, 133–138. [Google Scholar] [CrossRef]

| Food/Plant Beverages | Quantity | References | |
| Kaempferol | Strawberry | 5–8 mg/kg | [12] |
| Gooseberry yellow | 16 mg/kg | [12] | |
| Gooseberry red | 19 mg/kg | [12] | |
| Onion leaves | 832 mg/kg | [13] | |
| Black tea | 118 mg/kg | [14] | |
| Green chili | 39 mg/kg | [14] | |
| Papaya shoots | 453 mg/kg | [14] | |
| Brinjal | 80 mg/kg | [14] | |
| Pumpkin | 371 mg/kg | [14] | |
| Carrot | 140 mg/kg | [14] | |
| White radish | 38 mg/kg | [14] | |
| Beans | 14 mg/kg | [15] | |
| Broccoli | 72 mg/kg | [15] | |
| Broccoli | 30 mg/kg | [16] | |
| Cauliflower | 270 mg/kg | [16] |
| Mechanism of Action | References | |
| Anti-Inflammatory Effect | Inhibits the NF-κB binding activity of DNA and myeloid differentiation factor 88 | [38] |
| Suppresses the release of IL-6, IL-1β, IL-18 and TNF-α. | [39] | |
| Increases mRNA and protein expression of Nrf2-regulated genes | [40] | |
| Inhibits the toll-like receptor 4 (TLR4) | [41] |
| Clinical Trials | Anti-Inflammatory Response | References |
|---|---|---|
| Type-2 diabetic patients with inflammation were treated with kaempferol-rich diet | Decreased the levels of inflammatory biomarkers (C-reactive protein (CRP), IL-6 and TNF-α) | [62] |
| Cruciferous diet (kaempferol-rich diet) was administered to patients | Recovered the inflammatory biomarkers like IL-6 and IL-8 | [63] |
| Male smokers with inflammation were treated with kaempferol-rich diet (broccoli) for 10 days. | Reduced the TNF-α and IL-6 levels (inflammatory biomarkers) | [64] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alam, W.; Khan, H.; Shah, M.A.; Cauli, O.; Saso, L. Kaempferol as a Dietary Anti-Inflammatory Agent: Current Therapeutic Standing. Molecules 2020, 25, 4073. https://doi.org/10.3390/molecules25184073
Alam W, Khan H, Shah MA, Cauli O, Saso L. Kaempferol as a Dietary Anti-Inflammatory Agent: Current Therapeutic Standing. Molecules. 2020; 25(18):4073. https://doi.org/10.3390/molecules25184073
Chicago/Turabian StyleAlam, Waqas, Haroon Khan, Muhammad Ajmal Shah, Omar Cauli, and Luciano Saso. 2020. "Kaempferol as a Dietary Anti-Inflammatory Agent: Current Therapeutic Standing" Molecules 25, no. 18: 4073. https://doi.org/10.3390/molecules25184073
APA StyleAlam, W., Khan, H., Shah, M. A., Cauli, O., & Saso, L. (2020). Kaempferol as a Dietary Anti-Inflammatory Agent: Current Therapeutic Standing. Molecules, 25(18), 4073. https://doi.org/10.3390/molecules25184073










