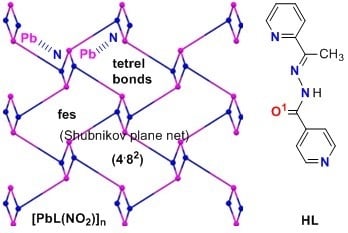
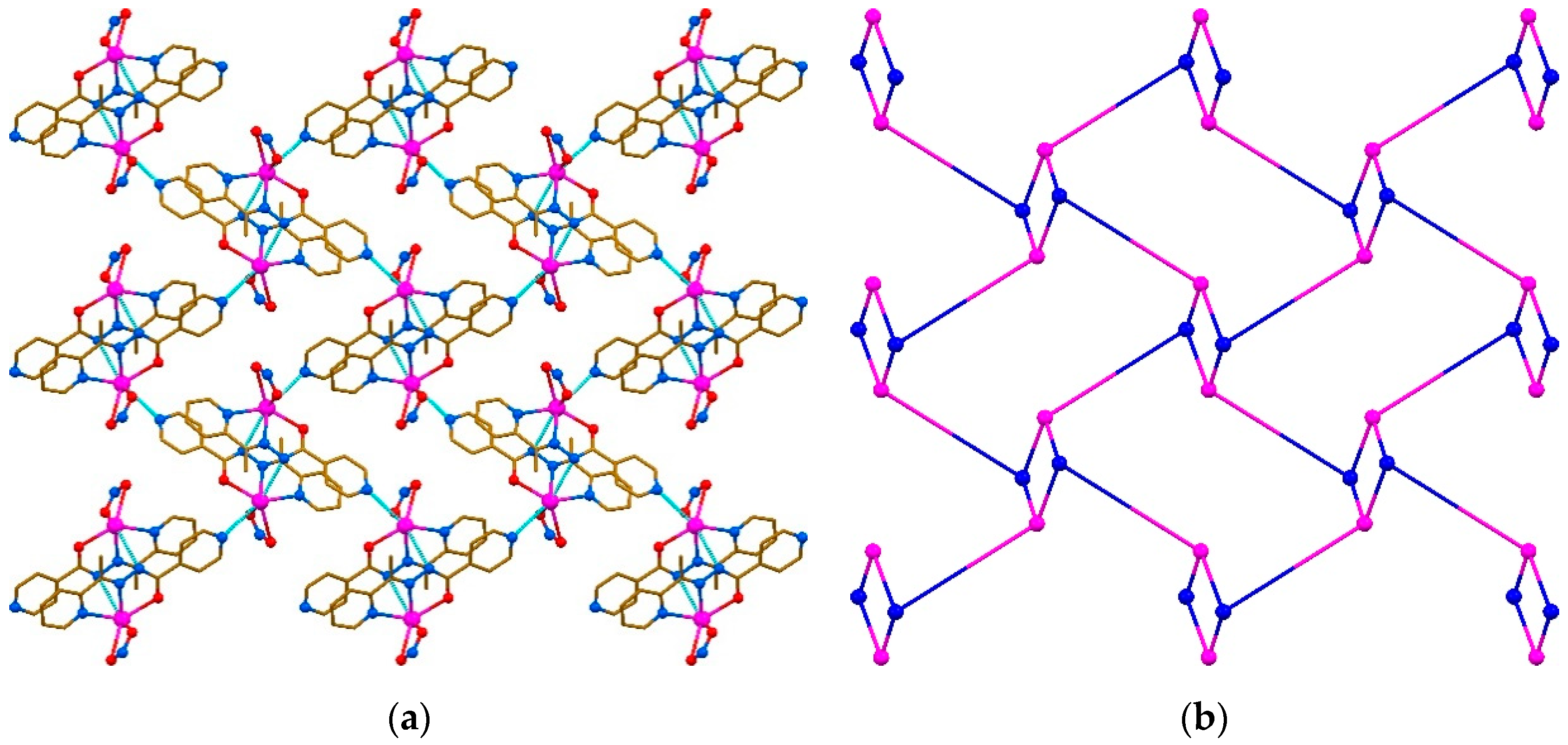
Tetrel Bonding and Other Non-Covalent Interactions Assisted Supramolecular Aggregation in a New Pb(II) Complex of an Isonicotinohydrazide
Abstract
1. Introduction
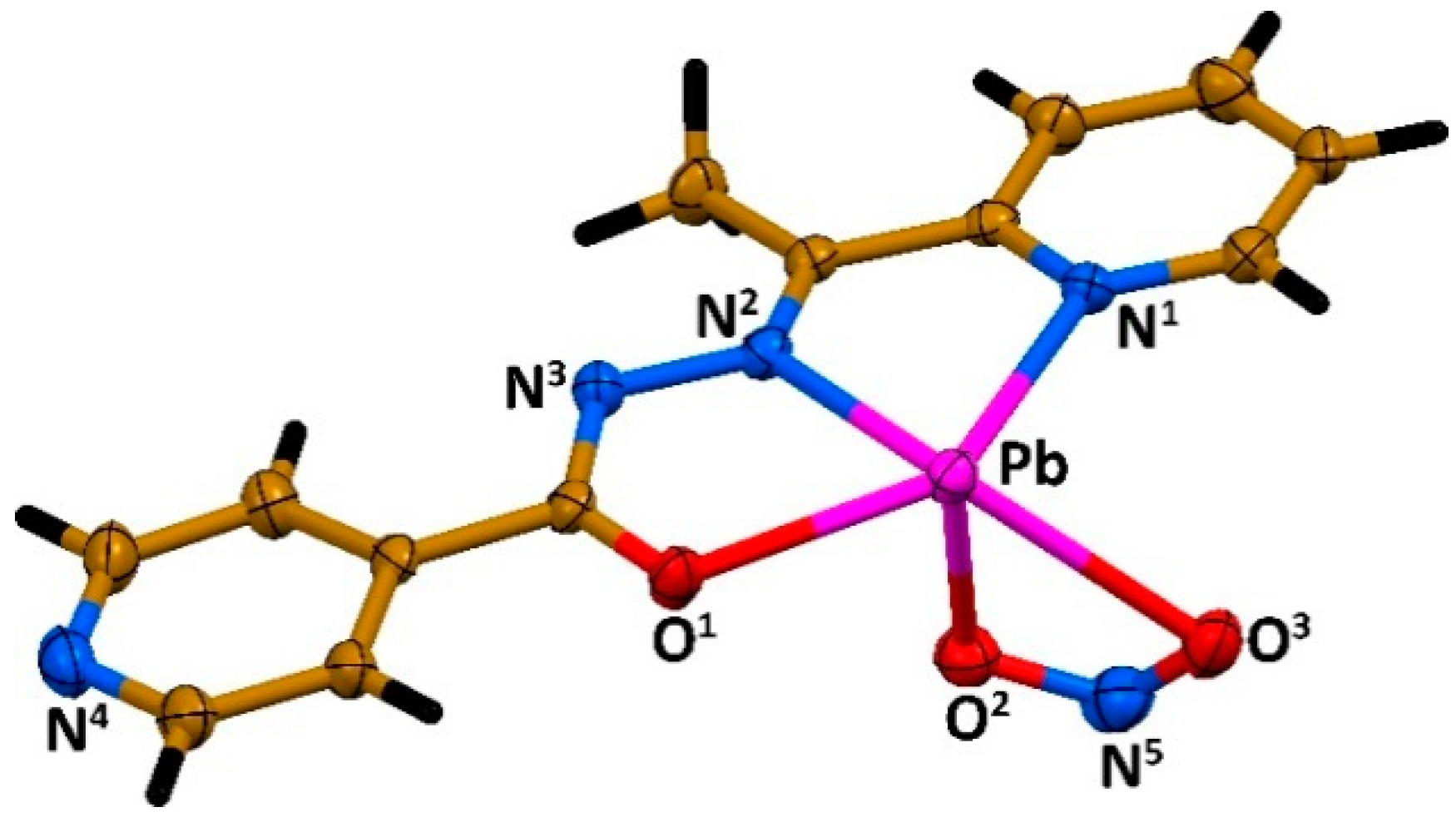
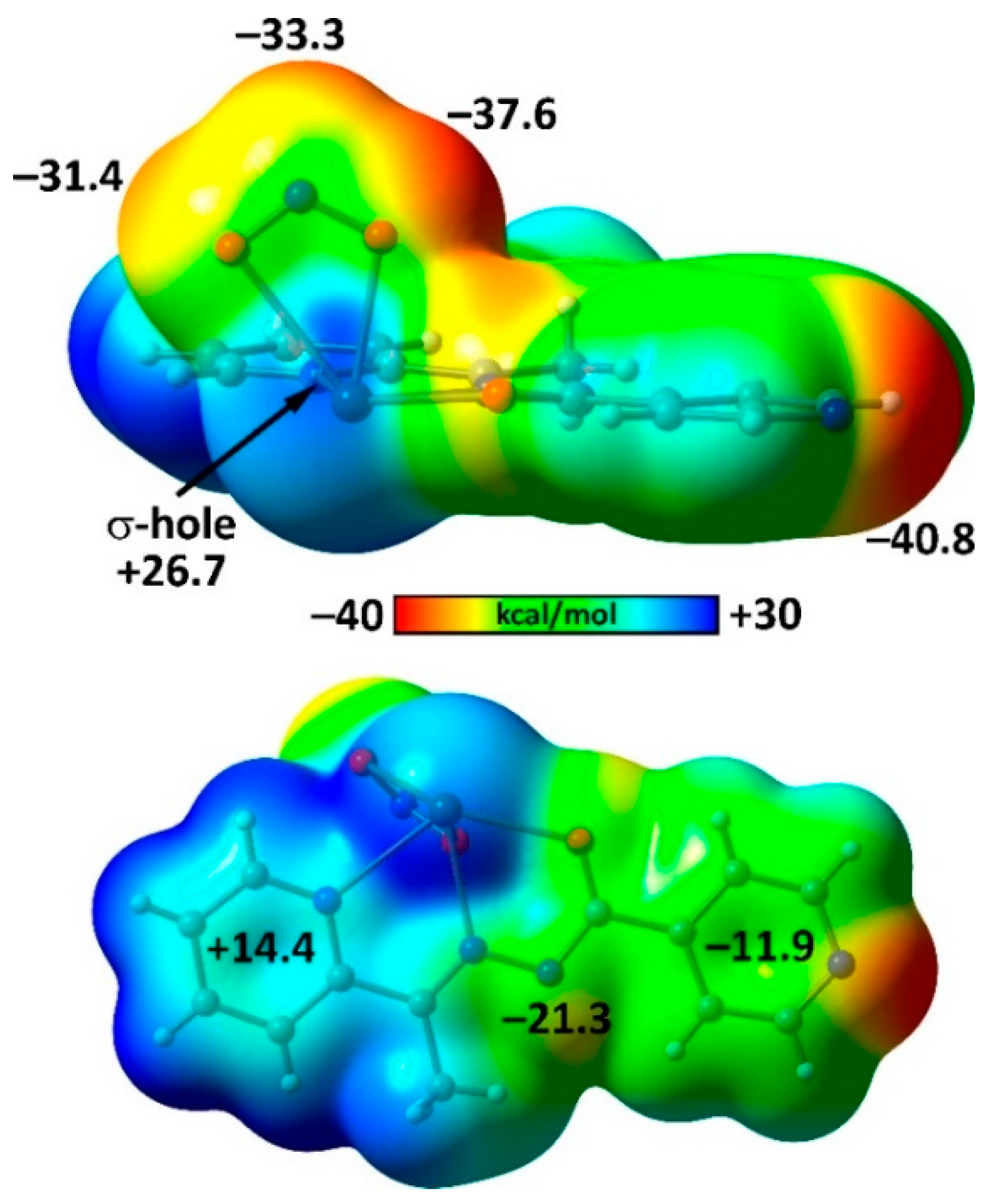
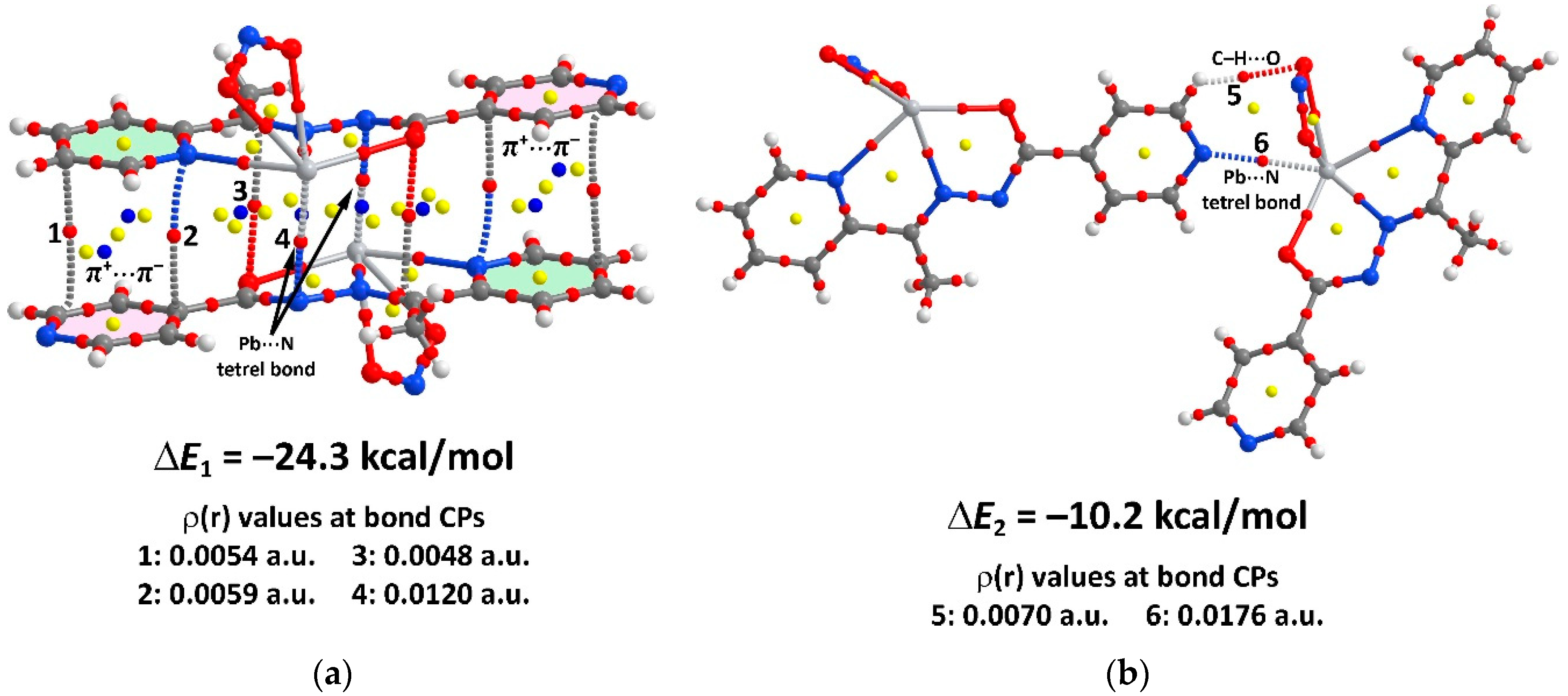
2. Results and Discussion
3. Materials and Methods
3.1. Reagents
3.2. Instrumentation
3.3. Synthesis of [PbL(NO2)]n
3.4. Single-Crystal X-Ray Diffraction of [PbL(NO2)]n
3.5. DFT Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Van der Waals, J.D. Over de Continuiteit van den Gas-en Vloiestoftoestand. Ph.D. Thesis, University of Leiden, Leiden, The Netherlands, 1873. [Google Scholar]
- Watson, J.D.; Crick, F.H.C. Molecular Structure of Nucleic Acids: A Structure for Deoxyribose Nucleic Acid. Nature 1953, 171, 737–738. [Google Scholar] [CrossRef] [PubMed]
- Scheiner, S. New ideas from old concepts: The hydrogen bond. Biochemist 2019, 41, 6–9. [Google Scholar] [CrossRef]
- Hobza, P.; Zahradník, R. Intermolecular Interactions between Medium-Sized Systems. Nonempirical and Empirical Calculations of Interaction Energies: Successes and Failures. Chem. Rev. 1988, 88, 871–897. [Google Scholar] [CrossRef]
- Müller-Dethlefs, K.; Hobza, P. Noncovalent Interactions: A Challenge for Experiment and Theory. Chem. Rev. 2000, 100, 143–168. [Google Scholar] [CrossRef]
- Janiak, C. A critical account on π–π stacking in metal complexes with aromatic nitrogen-containing ligands. J. Chem. Soc. Dalton Trans. 2000, 3885–3896. [Google Scholar] [CrossRef]
- Ran, J.; Wong, M.W. Saturated Hydrocarbon−Benzene Complexes: Theoretical Study of Cooperative CH/π Interactions. J. Phys. Chem. A 2006, 110, 9702–9709. [Google Scholar] [CrossRef]
- Hobza, P.; Zahradník, R.; Müller-Dethlefs, K. The World of Non-Covalent Interactions: 2006. Collect. Czech. Chem. Commun. 2006, 71, 443–531. [Google Scholar] [CrossRef]
- Riley, K.E.; Pitoňak, M.; Jurećka, P.; Hobza, P. Stabilization and Structure Calculations for Noncovalent Interactions in Extended Molecular Systems Based on Wave Function and Density Functional Theories. Chem. Rev. 2010, 110, 5023–5063. [Google Scholar] [CrossRef]
- Kim, K.S.; Karthikeyan, S.; Singh, N.J. How Different Are Aromatic π Interactions from Aliphatic π Interactions and Non-π Stacking Interactions? J. Chem. Theory Comput. 2011, 7, 3471–3477. [Google Scholar] [CrossRef]
- Salonen, L.M.; Ellermann, M.; Diederich, F. Aromatic Rings in Chemical and Biological Recognition: Energetics and Structures. Angew. Chem. Int. Ed. 2011, 50, 4808–4842. [Google Scholar] [CrossRef]
- Riley, K.E.; Hobza, P. On the Importance and Origin of Aromatic Interactions in Chemistry and Biodisciplines. Acc. Chem. Res. 2013, 46, 927–936. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, S.E. Understanding Substituent Effects in Noncovalent Interactions Involving Aromatic Rings. Acc. Chem. Res. 2013, 46, 1029–1038. [Google Scholar] [CrossRef] [PubMed]
- Mahadevi, A.S.; Sastry, G.N. Cooperativity in Noncovalent Interactions. Chem. Rev. 2016, 116, 2775–2825. [Google Scholar] [CrossRef] [PubMed]
- Řezáč, J.; Hobza, P. Benchmark Calculations of Interaction Energies in Noncovalent Complexes and Their Applications. Chem. Rev. 2016, 116, 5038–5071. [Google Scholar] [CrossRef]
- Biedermann, F.; Schneider, H.-J. Experimental Binding Energies in Supramolecular Complexes. Chem. Rev. 2016, 116, 5216–5300. [Google Scholar] [CrossRef]
- Bauzá, A.; Mooibroek, T.J.; Frontera, A. Tetrel Bonding Interactions. Chem. Rec. 2016, 16, 473–487. [Google Scholar] [CrossRef]
- Thakuria, R.; Nath, N.K.; Saha, B.K. The Nature and Applications of π–π Interactions: A Perspective. Cryst. Growth Des. 2019, 19, 523–528. [Google Scholar] [CrossRef]
- Bauzá, A.; Seth, S.K.; Frontera, A. Tetrel bonding interactions at work: Impact on tin and lead coordination compounds. Coord. Chem. Rev. 2019, 384, 107–125. [Google Scholar] [CrossRef]
- Silvi, B.; Alikhani, E.; Ratajczak, H. Towards an unified chemical model of secondary bonding. J. Mol. Model. 2020, 26, 62. [Google Scholar] [CrossRef]
- Scheiner, S.; Michalczyk, M.; Zierkiewicz, W. Coordination of anions by noncovalently bonded σ-hole ligands. Coord. Chem. Rev. 2020, 405, 213136. [Google Scholar] [CrossRef]
- Alkorta, I.; Elguero, J.; Frontera, A. Not Only Hydrogen Bonds: Other Noncovalent Interactions. Crystals 2020, 10, 180. [Google Scholar] [CrossRef]
- Gomilla, R.M.; Frontera, A. Charge assisted halogen and pnictogen bonds: Insights from the Cambridge Structural Database and DFT calculations. CrystEngComm 2020. [Google Scholar] [CrossRef]
- Zhao, Y.H.; Xu, H.B.; Fu, Y.M.; Shao, K.Z.; Yang, S.Y.; Su, Z.M.; Hao, X.R.; Zhu, D.X.; Wang, E.B. A Series of Lead(II)-Organic Frameworks Based on Pyridyl Carboxylate Acid N-Oxide Derivatives: Syntheses, Structures, and Luminescent Properties. Cryst. Growth Des. 2008, 8, 3566–3576. [Google Scholar] [CrossRef]
- Wang, X.L.; Chen, Y.Q.; Gao, Q.; Lin, H.Y.; Liu, G.C.; Zhang, J.X.; Tian, A.X. Coordination Behavior of 5,6-Substituted 1,10-Phenanthroline Derivatives and Structural Diversities by Coligands in the Construction of Lead(II) Complexes. Cryst. Growth Des. 2010, 10, 2174–2184. [Google Scholar] [CrossRef]
- Wibowo, A.C.; Vaughn, S.A.; Smith, M.D.; zur Loye, H.C. Novel Bismuth and Lead Coordination Polymers Synthesized with Pyridine-2,5-Dicarboxylates: Two Single Component “White” Light Emitting Phosphors. Inorg. Chem. 2010, 49, 11001–11008. [Google Scholar] [CrossRef]
- He, J.; Zeller, M.; Hunter, A.D.; Xu, Z. White Light Emission and Second Harmonic Generation from Secondary Group Participation (SGP) in a Coordination Network. J. Am. Chem. Soc. 2012, 134, 1553–1559. [Google Scholar] [CrossRef]
- Imran, M.; Mix, A.; Neumann, B.; Stammler, H.G.; Monkowius, U.; Gründlinger, P.; Mitzel, N.W. Hemi- and holo-directed lead(II) complexes in a soft ligand environment. Dalton Trans. 2015, 44, 924–937. [Google Scholar] [CrossRef]
- Mirdya, S.; Roy, S.; Chatterjee, S.; Bauzá, A.; Frontera, A.; Chattopadhyay, S. Importance of π-Interactions Involving Chelate Rings in Addition to the Tetrel Bonds in Crystal Engineering: A Combined Experimental and Theoretical Study on a Series of Hemi- and Holodirected Nickel(II)/Lead(II) Complexes. Cryst. Growth Des. 2019, 19, 5869–5881. [Google Scholar] [CrossRef]
- Mirdya, S.; Banerjee, S.; Chattopadhyay, S. An insight into the non-covalent Pb⋯S and S⋯S interactions in the solid-state structure of a hemidirected lead(II) complex. Cryst. Eng. Comm. 2020, 22, 237–247. [Google Scholar] [CrossRef]
- Tan, Y.-X.; Meng, F.-Y.; Wu, M.-C.; Zeng, M.-H. Two Pb(II) dicarboxylates constructed by rigid terephthalate or flexible D(+)-camphorate with different 3D motif based on cooperative effect of steric hindrance of ligand and lone pair electrons. J. Mol. Struct. 2009, 928, 176–181. [Google Scholar] [CrossRef]
- Servati, G.M.; Stilinović, V.; Bauzá, A.; Frontera, A.; McArdle, P.; Van Derveer, D.; Ng, S.W.; Mahmoudi, G. Design of Lead(II) Metal-Organic Frameworks Based on Covalent and Tetrel Bonding. Chem. Eur. J. 2015, 21, 17951–17958. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, G.; Bauzá, A.; Frontera, A.; Garczarek, P.; Stilinović, V.; Kirillov, A.M.; Kennedy, A.; Ruiz-Pérez, C. Metal–organic and supramolecular lead(II) networks assembled from isomeric nicotinoylhydrazone blocks: The effects of ligand geometry and counter-ion on topology and supramolecular assembly. CrystEngComm 2016, 18, 5375–5385. [Google Scholar] [CrossRef]
- Mahmoudi, G.; Bauzá, A.; Frontera, A. Concurrent agostic and tetrel bonding interactions in lead(II) complexes with an isonicotinohydrazide based ligand and several anions. Dalton Trans. 2016, 45, 4965–4969. [Google Scholar] [CrossRef]
- Mahmoudi, G.; Bauza, A.; Amini, M.; Molins, E.; Mague, J.T.; Frontera, A. On the importance of tetrel bonding interactions in lead(II) complexes with (iso)nicotinohydrazide based ligands and several anions. Dalton Trans. 2016, 45, 10708–10716. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, G.; Dey, L.; Chowdhury, H.; Bauzá, A.; Ghosh, B.K.; Kirillov, A.M.; Seth, S.K.; Gurbanov, A.V.; Frontera, A. Synthesis and crystal structures of three new lead(II) isonicotinoylhydrazone derivatives: Anion controlled nuclearity and dimensionality. Inorg. Chim. Acta 2017, 461, 192–205. [Google Scholar] [CrossRef]
- Mahmoudi, G.; Safin, D.A.; Mitoraj, M.P.; Amini, M.; Kubicki, M.; Doert, T.; Locherere, F.; Fleck, M. Anion-driven tetrel bond-induced engineering of lead(II) architectures with N′-(1-(2-pyridyl)ethylidene)nicotinohydrazide: Experimental and theoretical findings. Inorg. Chem. Front. 2017, 4, 171–182. [Google Scholar] [CrossRef]
- Mahmoudi, G.; Gurbanov, A.V.; Hemida, S.R.; Corballo, R.; Amini, M.; Bacchi, A.; Mitoraj, M.P.; Sagan, F.; Kukulka, M.; Safin, D.A. Ligand-Driven Coordination Sphere-Induced Engineering of Hybride Materials Constructed from PbCl2 and Bis-Pyridyl Organic Linkers for Single-Component Light-Emitting Phosphors. Inorg. Chem. 2017, 56, 9698–9709. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, G.; Zangrando, E.; Mitoraj, M.P.; Gurbanov, A.V.; Zubkov, F.I.; Moosavifar, M.; Konyaeva, I.A.; Kirillov, A.M.; Safin, D.A. Extended lead(II) architectures engineered via tetrel bonding interactions. New J. Chem. 2018, 42, 4959–4971. [Google Scholar] [CrossRef]
- Seth, S.K.; Bauzá, A.; Mahmoudi, G.; Stilinović, V.; López-Torres, E.; Zaragoza, G.; Keramidas, A.D.; Frontera, A. On the importance of Pb⋯X (X = O, N, S, Br) tetrel bonding interactions in a series of tetra- and hexa-coordinated Pb(II) compounds. CrystEngComm 2018, 20, 5033–5044. [Google Scholar] [CrossRef]
- Afkhami, F.A.; Mahmoudi, G.; Qu, F.; Gupta, A.; Köse, M.; Zangrando, E.; Zubkov, F.I.; Alkorta, I.; Safin, D.A. Supramolecular lead(II) architectures engineered by tetrel bonds. CrystEngComm 2020, 22, 2389–2396. [Google Scholar] [CrossRef]
- Afkhami, F.A.; Mahmoudi, G.; Qu, F.; Gupta, A.; Zangrando, E.; Frontera, A.; Safin, D.A. Supramolecular architecture constructed from the hemidirected lead(II) complex with N’-(4-hydroxybenzylidene)isonicotinohydrazide. Inorg. Chim. Acta 2020, 502, 119350. [Google Scholar] [CrossRef]
- Addison, A.W.; Nageswara, R.T.; Reedijk, J.; Van Rijn, J.; Verschoor, G.J. Synthesis, structure, and spectroscopic properties of copper(II) compounds containing nitrogen–sulphur donor ligands; the crystal and molecular structure of aqua[1,7-bis(N-methylbenzimidazol-2′-yl)-2,6-dithiaheptane]copper(II) perchlorate. J. Chem. Soc. Dalton Trans. 1984, 1349–1356. [Google Scholar] [CrossRef]
- Stroz, K. Plane groups—From basic to advanced crystallographic concepts. Z. Kristallogr. 2003, 218, 642–649. [Google Scholar]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld surface analysis. CrystEngComm 2009, 11, 19–32. [Google Scholar] [CrossRef]
- Spackman, M.A.; McKinnon, J.J. Fingerprinting intermolecular interactions in molecular crystals. CrystEngComm 2002, 4, 378–392. [Google Scholar] [CrossRef]
- Wolff, S.K.; Grimwood, D.J.; McKinnon, J.J.; Turner, M.J.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer 3.1; University of Western Australia: Crawley, WA, Australia, 2012. [Google Scholar]
- Jelsch, C.; Ejsmont, K.; Huder, L. The enrichment ratio of atomic contacts in crystals, an indicator derived from the Hirshfeld surface analysis. IUCrJ 2014, 1, 119–128. [Google Scholar] [CrossRef]
- Safin, D.A.; Mitoraj, M.P.; Robeyns, K.; Filinchuk, Y.; Vande Velde, C.M.L. Luminescent mononuclear mixed ligand complexes of copper(I) with 5-phenyl-2,2′-bipyridine and triphenylphosphine. Dalton Trans. 2015, 44, 16824–16832. [Google Scholar] [CrossRef]
- Babashkina, M.G.; Robeyns, K.; Filinchuk, Y.; Safin, D.A. Detailed studies of the interaction of 3-chloroaniline with O,O′-diphenylphosphorylisothiocyanate. New J. Chem. 2016, 40, 1230–1236. [Google Scholar] [CrossRef]
- Safin, D.A.; Vande Velde, C.M.L.; Babashkina, M.G.; Robeyns, K.; Filinchuk, Y. Mononuclear heteroleptic complexes of copper(I) with 5-phenyl-2,2′-bipyridine and triphenylphosphine: Crystal structures, Hirshfeld surface analysis and luminescence properties. New J. Chem. 2016, 40, 6156–6163. [Google Scholar] [CrossRef]
- Safin, D.A.; Robeyns, K.; Babashkina, M.G.; Filinchuk, Y.; Rotaru, A.; Jureschi, C.; Mitoraj, M.P.; Hooper, J.; Brela, M.; Garcia, Y. Polymorphism driven optical properties of an anil dye. CrystEngComm 2016, 18, 7249–7259. [Google Scholar] [CrossRef]
- Safin, D.A.; Robeyns, K.; Garcia, Y. 1,2,4-Triazole-based molecular switches: Crystal structures, Hirshfeld surface analysis and optical properties. CrystEngComm 2016, 18, 7284–7296. [Google Scholar] [CrossRef]
- Safin, D.A.; Babashkina, M.G.; Mitoraj, M.P.; Kubisiak, P.; Robeyns, K.; Bolte, M.; Garcia, Y. An intermolecular pyrene excimer in the pyrene-labeled N-thiophosphorylated thiourea and its nickel(II) complex. Inorg. Chem. Front. 2016, 3, 1419–1431. [Google Scholar] [CrossRef]
- APEX3, SAINT; v6.28A; Bruker AXS Inc.: Madison, WI, USA, 2016.
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Cryst. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision A.03; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Adamo, C.; Barone, V. Toward reliable density functional methods without adjustable parameters: The PBE0 model. J. Chem. Phys. 1999, 110, 6158–6169. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A dispersion correction for density functionals, Hartree-Fock and semi-empirical quantum chemical methods DFT-D3. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Weigend, F. Accurate Coulomb-fitting basis sets for H to Rn. Phys. Chem. Chem. Phys. 2006, 8, 1057–1065. [Google Scholar] [CrossRef]
- Bader, R.F.W. A quantum theory of molecular structure and its applications. Chem. Rev. 1991, 91, 893–928. [Google Scholar] [CrossRef]
- Keith, T.A. AIMAll; Version 19.10.12; TK Gristmill Software: Overland Park, KS, USA, 2019. [Google Scholar]
Sample Availability: Samples of the compound [PbL(NO2)]n are available from the authors. |



| [PbL(NO2)]n (This Work) | [PbL’(NO2)]n [37] | |
|---|---|---|
| Bond lengths (Å) | ||
| Pb–N1 (L/L’) | 2.583(4) (covalent) | 2.535(5) (covalent) |
| Pb–N2 (L/L’) | 2.462(4) (covalent) | 2.452(4) (covalent) |
| Pb∙∙∙N3 (L/L’) | 3.235(4) (tetrel) | 3.258(4) (tetrel) |
| Pb∙∙∙N4 (L/L’) | 3.039(5) (tetrel) | 2.752(5) (covalent) |
| Pb–O1 (L/L’) | 2.387(3) (covalent) | 2.384(5) (covalent) |
| Pb–O2 (NO2) | 2.387(4) (covalent) | 2.547(4) (covalent), 3.299(4) (tetrel) |
| Pb–O3 (NO2) | 2.768(4) (covalent) | 2.904(5) (covalent) |
| Bond angles (°) | ||
| O1–Pb–O2 | 82.35(13) | 79.90(14) |
| O1–Pb–O3 | 124.07(13) | 114.84(15) |
| O1–Pb–N1 | 128.82(12) | 130.02(14) |
| O1–Pb–N2 | 65.50(12) | 65.51(14) |
| O1–Pb–N4 | – | 91.30(14) |
| O2–Pb–O3 | 47.63(14) | 44.26(14) |
| O2–Pb–N1 | 78.05(13) | 81.12(14) |
| O2–Pb–N2 | 75.04(13) | 70.61(14) |
| O2–Pb–N4 | – | 148.15(13) |
| O3–Pb–N1 | 70.93(12) | 79.40(16) |
| O3–Pb–N2 | 112.30(13) | 109.41(14) |
| O3–Pb–N4 | – | 153.72(15) |
| N1–Pb–N2 | 63.90(13) | 64.62(14) |
| N1–Pb–N4 | – | 81.49(14) |
| N2–Pb–N4 | – | 77.86(13) |
| Torsion angles (°) | ||
| 2-Py⋯4-/3-Py | 3.5(3) | 3.0(3) |
| Cg(I) | Cg(J) | d[Cg(I)⋯Cg(J)] | α | β | γ | Slippage | Symmetry Transformation |
|---|---|---|---|---|---|---|---|
| 2-Py | 4-Py | 3.544(3) | 3.5(3) | 19.7 | 18.1 | 1.194 | 2 − x, 2 −y, 1 − z |
| 4-Py | 2-Py | 3.544(3) | 3.5(3) | 18.1 | 19.7 | 1.100 | 2 − x, 2 − y, 1 − z |
| C–H(I) | d[C–H(I)] | Cg(J) | d[H(I)⋯Cg(J)] | d[C⋯Cg(J)] | ∠ [CH(I)⋯Cg(J)] | Symmetry Transformation |
|---|---|---|---|---|---|---|
| C7–H7B | 0.98 | 4-Py | 2.81 | 3.657(6) | 145 | 1 – x, 2 – y, 1 – z |
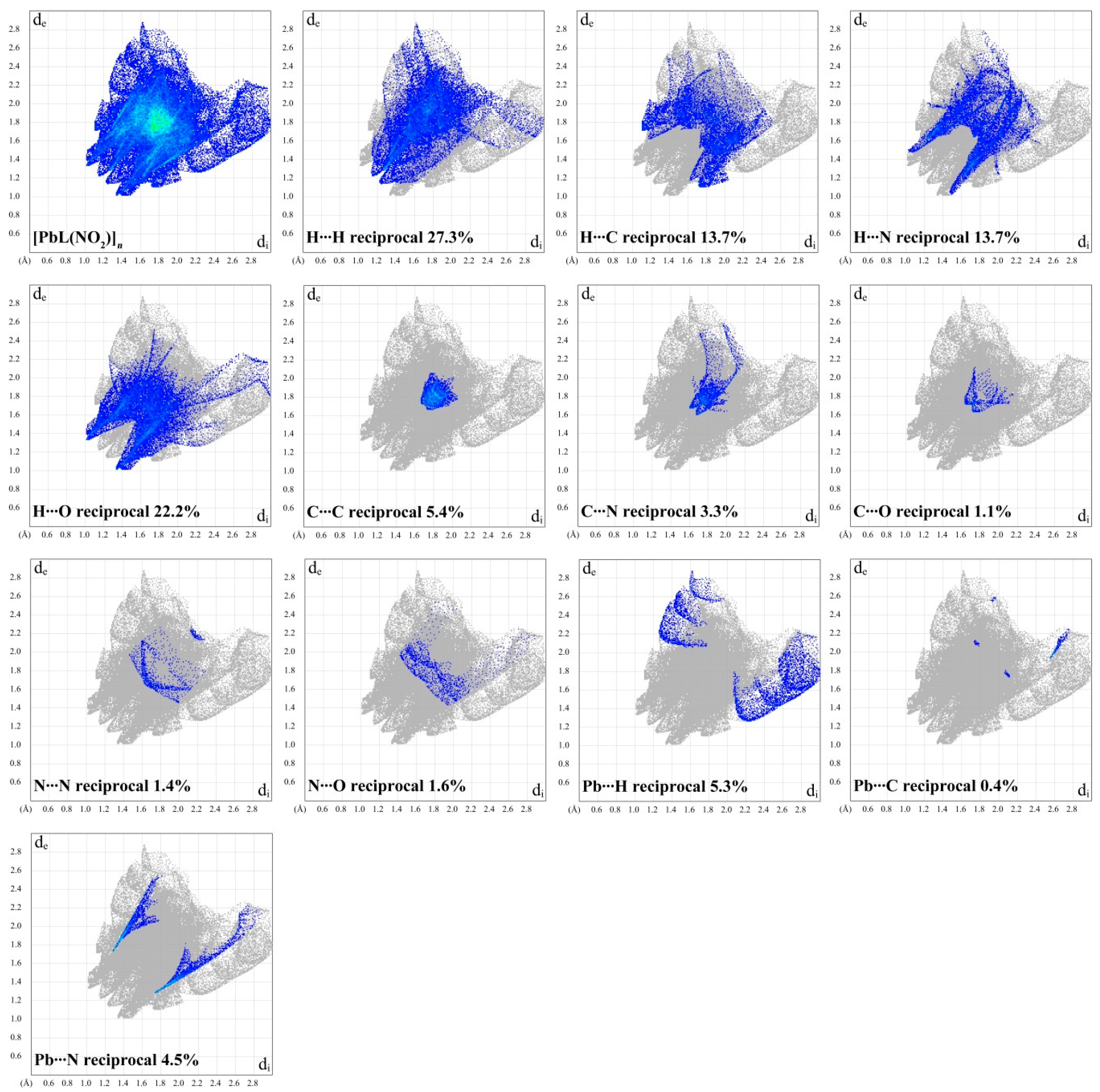
| H | C | N | O | Pb | |
|---|---|---|---|---|---|
| Contacts (C, %) | |||||
| H | 27.3 | – | – | – | – |
| C | 13.7 | 5.4 | – | – | – |
| N | 13.7 | 3.3 | 1.4 | – | – |
| O | 22.2 | 1.1 | 1.6 | 0.0 | – |
| Pb | 5.3 | 0.4 | 4.5 | 0.0 | 0.0 |
| Surface (S, %) | |||||
| 54.8 | 14.7 | 13.0 | 12.5 | 5.1 | |
| Random contacts (R, %) | |||||
| H | 30.0 | – | – | – | – |
| C | 16.1 | 2.2 | – | – | – |
| N | 14.2 | 3.8 | 1.7 | – | – |
| O | 13.7 | 3.7 | 3.3 | 1.6 | – |
| Pb | 5.6 | 1.5 | 1.3 | 1.3 | 0.3 |
| Enrichment (E) b | |||||
| H | 0.91 | – | – | – | – |
| C | 0.85 | 2.45 | – | – | – |
| N | 0.96 | 0.87 | 0.82 | – | – |
| O | 1.62 | 0.30 | 0.48 | 0.00 | – |
| Pb | 0.95 | 0.27 | 3.46 | 0.00 | – |
| Parameter | [PbL(NO2)]n |
|---|---|
| Chemical formula | C13H11N5O3Pb |
| Formula weight | 492.46 |
| Crystal system | monoclinic |
| Space group | P21/n |
| a (Å) | 8.1660(11) |
| b (Å) | 13.2850(17) |
| c (Å) | 13.7842(18) |
| β (°) | 92.860(4) |
| V (Å3) | 1493.5(3) |
| Z | 4 |
| Dcalc (g/cm3) | 2.190 |
| μ(Mo-Kα) (mm−1) | 11.316 |
| F(000) | 920 |
| θ range (°) | 2.13–27.32 |
| Reflections collected | 18486 |
| No. of unique data | 3318 |
| Rint | 0.0505 |
| Observed data [I > 2σ(I)] | 2666 |
| Parameters refined | 200 |
| Goodness of fit (F2) | 1.033 |
| R1 [I > 2σ(I)] 1 | 0.0288 |
| wR2 [I > 2σ(I)] 2 | 0.0625 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahmoudi, G.; Abedi, M.; Lawrence, S.E.; Zangrando, E.; Babashkina, M.G.; Klein, A.; Frontera, A.; Safin, D.A. Tetrel Bonding and Other Non-Covalent Interactions Assisted Supramolecular Aggregation in a New Pb(II) Complex of an Isonicotinohydrazide. Molecules 2020, 25, 4056. https://doi.org/10.3390/molecules25184056
Mahmoudi G, Abedi M, Lawrence SE, Zangrando E, Babashkina MG, Klein A, Frontera A, Safin DA. Tetrel Bonding and Other Non-Covalent Interactions Assisted Supramolecular Aggregation in a New Pb(II) Complex of an Isonicotinohydrazide. Molecules. 2020; 25(18):4056. https://doi.org/10.3390/molecules25184056
Chicago/Turabian StyleMahmoudi, Ghodrat, Marjan Abedi, Simon E. Lawrence, Ennio Zangrando, Maria G. Babashkina, Axel Klein, Antonio Frontera, and Damir A. Safin. 2020. "Tetrel Bonding and Other Non-Covalent Interactions Assisted Supramolecular Aggregation in a New Pb(II) Complex of an Isonicotinohydrazide" Molecules 25, no. 18: 4056. https://doi.org/10.3390/molecules25184056
APA StyleMahmoudi, G., Abedi, M., Lawrence, S. E., Zangrando, E., Babashkina, M. G., Klein, A., Frontera, A., & Safin, D. A. (2020). Tetrel Bonding and Other Non-Covalent Interactions Assisted Supramolecular Aggregation in a New Pb(II) Complex of an Isonicotinohydrazide. Molecules, 25(18), 4056. https://doi.org/10.3390/molecules25184056