Milk Authentication: Stable Isotope Composition of Hydrogen and Oxygen in Milks and Their Constituents
Abstract
1. Introduction
2. Results and Discussion
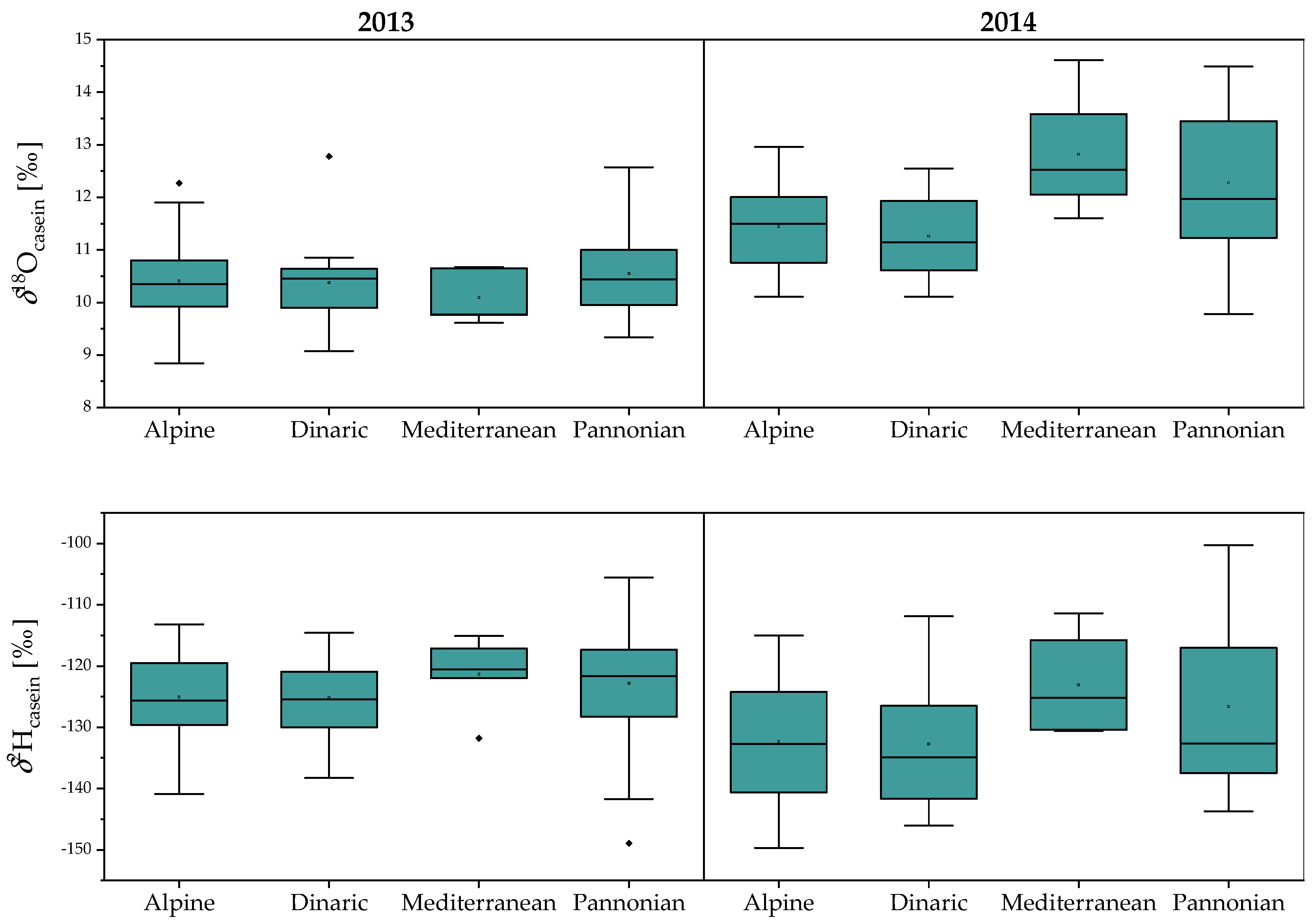
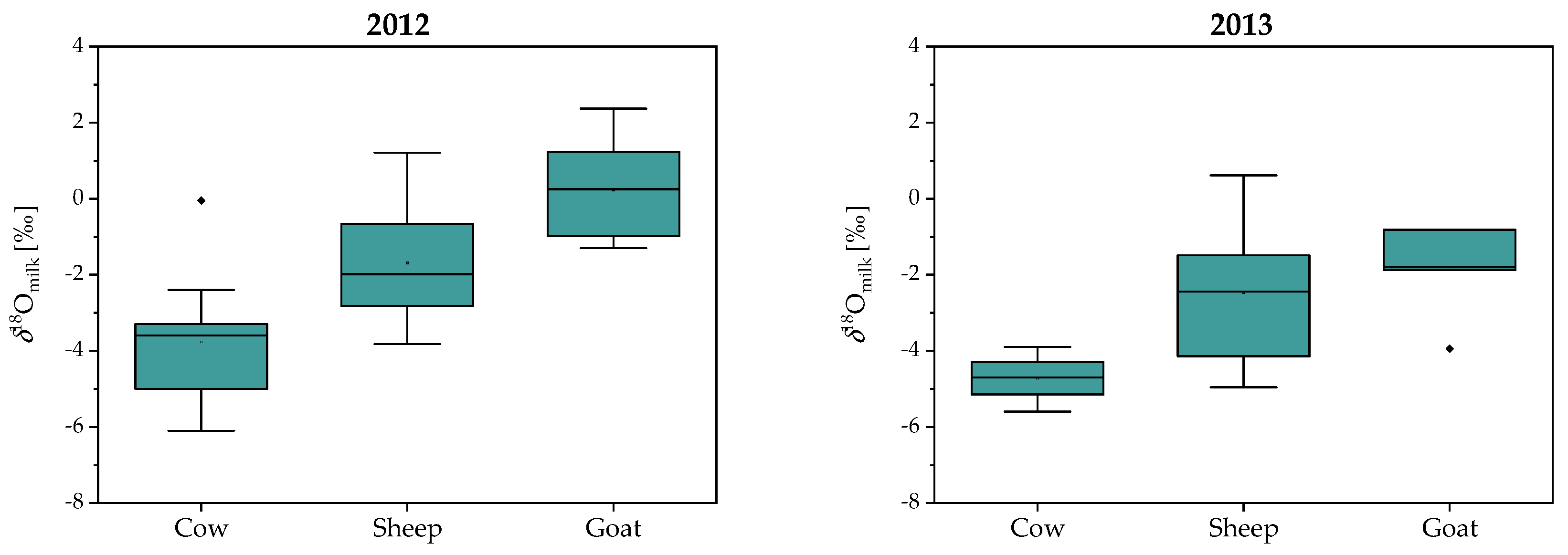
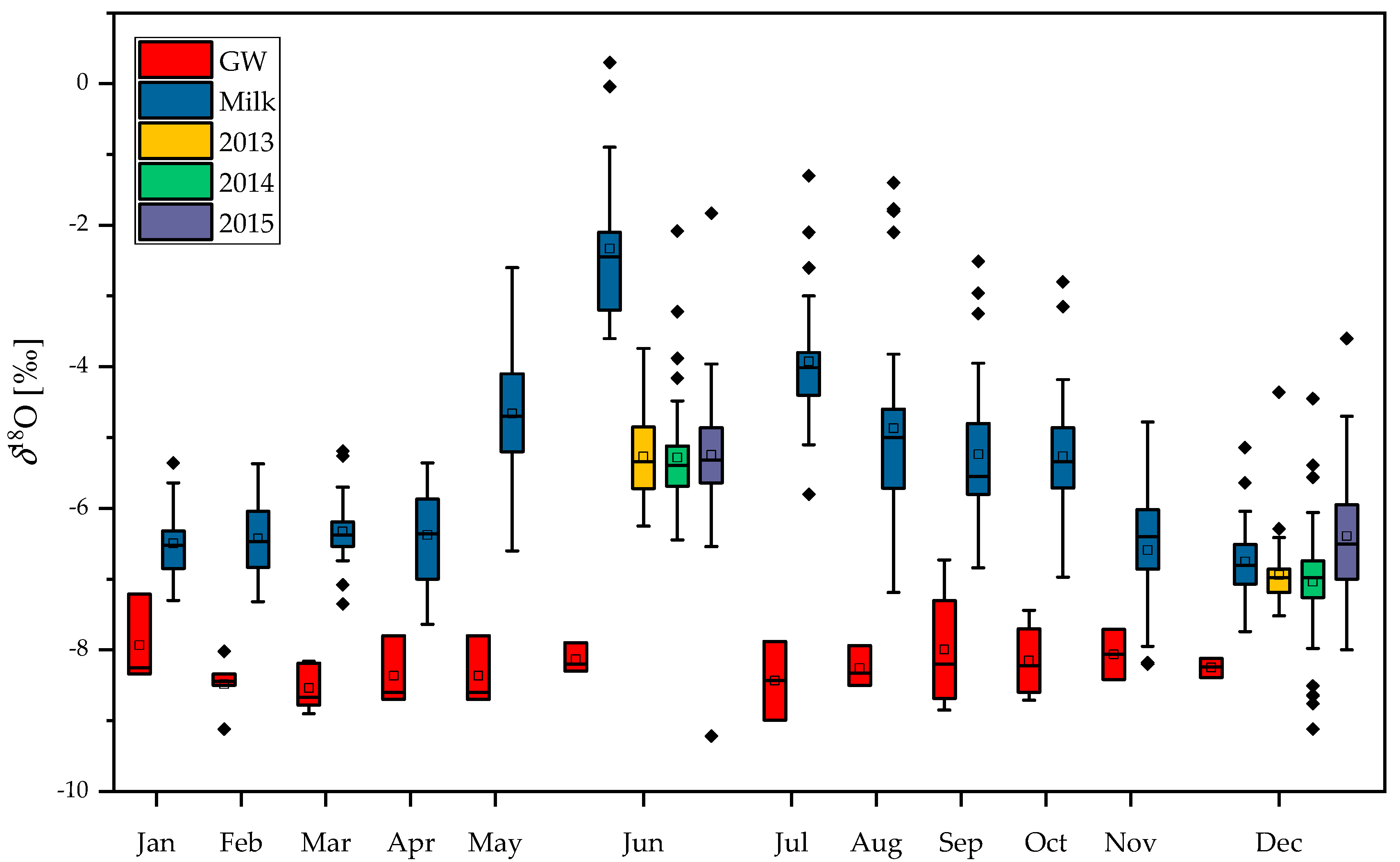
2.1. Stable Isotope Composition of Milk and Casein: Year, Season, Region and Species Variability
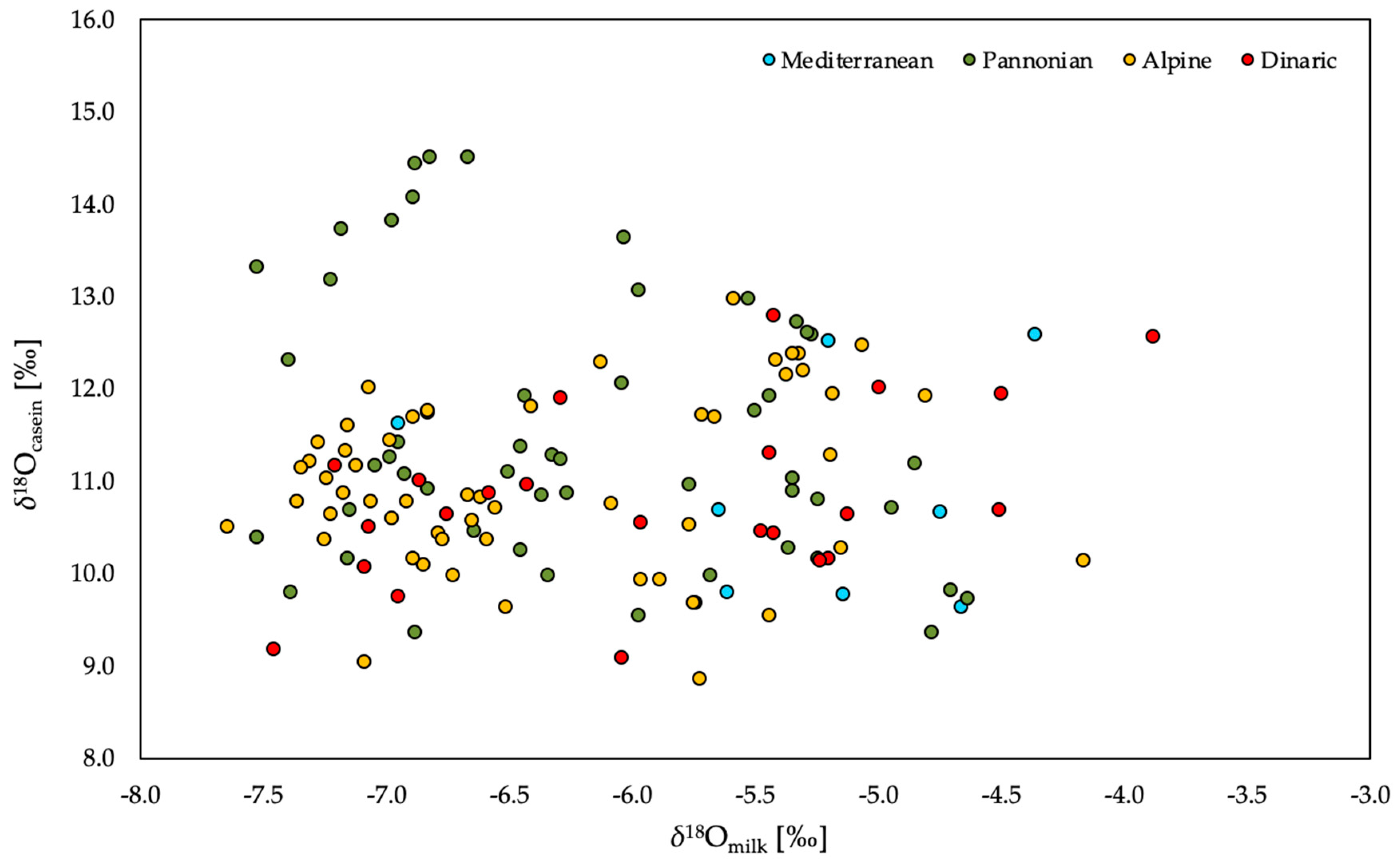
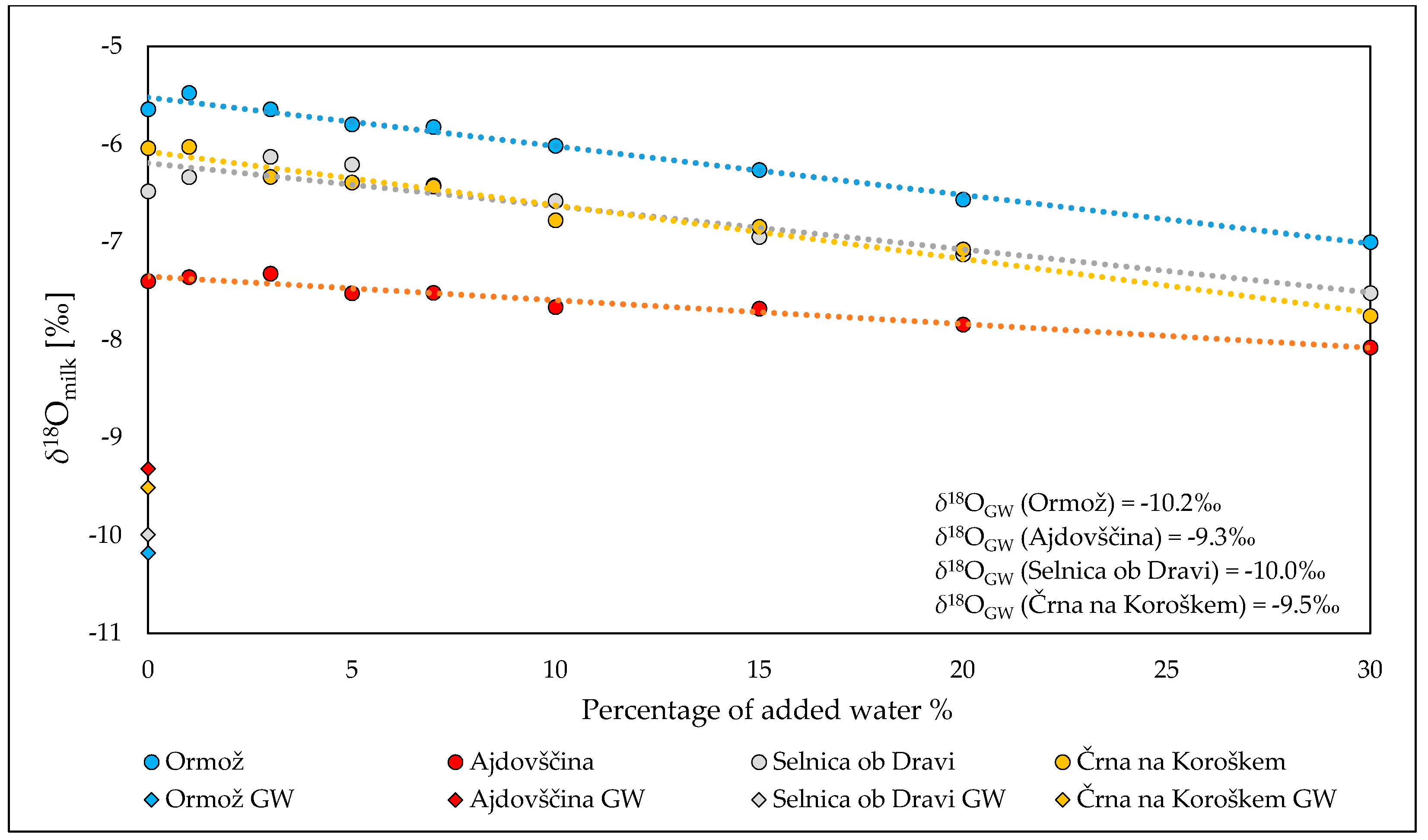
2.2. Stable Isotope Composition of Oxygen in Milk and Groundwater: Detection of Dilution with Water
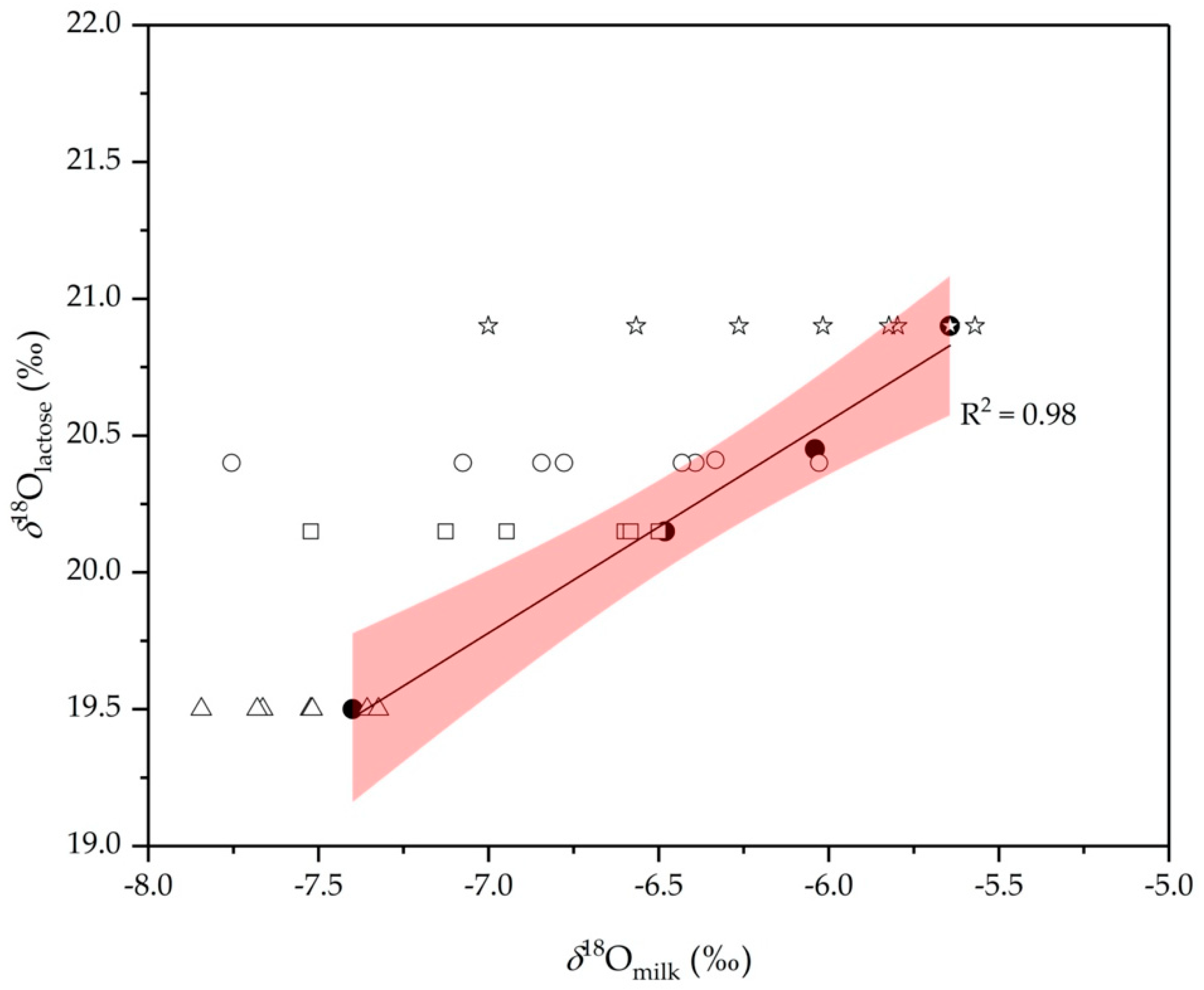
2.3. Lactose as an Internal Standard
3. Materials and Methods
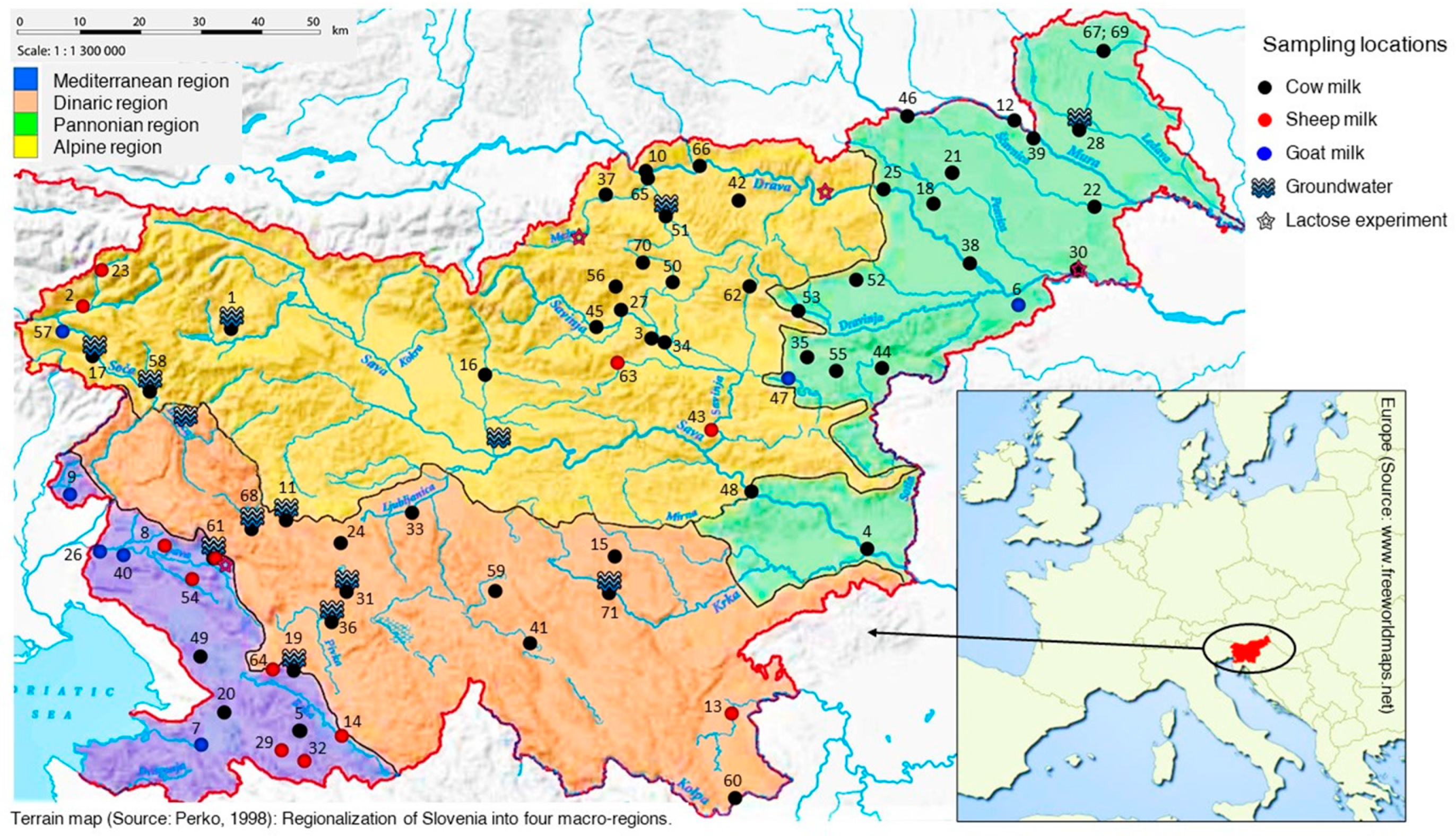
3.1. Sampling and Sample Preparation
3.2. Isolation of Casein
3.3. Water Addition Experiment
3.4. Isolation of Lactose
3.5. Determination of Stable Hydrogen and Oxygen Isotope Ratios
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kamal, M.; Karoui, R. Analytical methods coupled with chemometric tools for determining the authenticity and detecting the adulteration of dairy products: A review. Trends. Food Sci. Technol. 2015, 46, 27–48. [Google Scholar] [CrossRef]
- Handford, C.E.; Campbell, K.; Elliott, C.T. Impacts of Milk Fraud on Food Safety and Nutrition with Special Emphasis on Developing Countries: Health impacts of milk fraud. Com. Rev. Food Sci. Food Saf. 2016, 15, 130–142. [Google Scholar] [CrossRef]
- De La Fuente, M.A.; Juárez, M. Authenticity Assessment of Dairy Products. Crit. Rev. Food Sci. Nut. 2005, 45, 563–585. [Google Scholar] [CrossRef] [PubMed]
- Azad, T.; Ahmed, S. Common milk adulteration and their detection techniques. Food Contam. 2016, 3, 2. [Google Scholar] [CrossRef]
- Santos, P.M.; Pereira-Filho, E.R.; Rodriguez-Saona, L.E. Rapid detection and quantification of milk adulteration using infrared microspectroscopy and chemometrics analysis. Food Chem. 2013, 138, 19–24. [Google Scholar] [CrossRef]
- Musara, C.; Pote, W. Application of osmometry in quality analysis of milk. J. Food Sci. Technol. 2014, 51, 606–610. [Google Scholar] [CrossRef]
- Vujadinović, D.; Beribaka, M.; Vukić, M.; Marjanović-Balaban, Ž. Comparison of methods for determining the falsification of milk. J. Hyg. Engin. Des. 2017, 18, 19–24. [Google Scholar]
- Qu, J.-H. Applications of near-infrared spectroscopy in food safety evaluation and control: A review of recent research advances. Crit. Rev. Food Sci. Nutr. 2015, 55, 1939–1954. [Google Scholar] [CrossRef]
- Kasemsumran, S.; Thanapase, W.; Kiatsoonthon, A. Feasibility of Near-Infrared Spectroscopy to detect and to quantify adulterants in cow milk. Anal. Sci. 2007, 23, 907–910. [Google Scholar] [CrossRef]
- Büning-Pfaue, H. Analysis of water in food by near infrared spectroscopy. Food Chem. 2003, 82, 107–115. [Google Scholar] [CrossRef]
- Pedretti, N.; Bertrand, D.; Semenou, M.; Robert, P.; Giangiacomo, R. Application of an experimental design to the detection of foreign substances in milk. J. Near Infra Spectrosc. 1993, 1, 174–184. [Google Scholar] [CrossRef]
- Castell-Palou, A.; Rosselló, C.; Femenia, A.; Simal, S. Simultaneous quantification of fat and water content in cheese by TD-NMR. Food Bioprocess. Technol. 2012, 6, 2685–2694. [Google Scholar] [CrossRef]
- Santos, P.M.; Pereira-Filho, E.R.; Colnago, L.A. Detection and quantification of milk adulteration using time domain nuclear magnetic resonance (TD-NMR). Microchem. J. 2016, 124, 15–19. [Google Scholar] [CrossRef]
- Danezis, G.P.; Tsagkaris, A.S.; Camin, F.; Brusic, V.; Georgiou, C.A. Food authentication: Techniques, trends & emerging approaches. TrAC—Trends Anal. Chem. 2016, 85, 123–132. [Google Scholar] [CrossRef]
- Dordevic, N.; Camin, F.; Marianella, R.M.; Postma, G.J.; Buydens, L.M.C.; Wehrens, R. Detecting the addition of sugar and water to wine: Detecting the addition of sugar and water to wine. Aust. J. Grape Wine Res. 2013, 19, 324–330. [Google Scholar] [CrossRef]
- Calderone, G.C.; Guillou, C. Analysis of isotopic ratios for the detection of illegal watering of beverages. Food Chem. 2008, 106, 1399–1405. [Google Scholar] [CrossRef]
- Meier-Augenstein, W.; Kemp, H.F.; Hardie, S.M.L. Detection of counterfeit scotch whisky by 2H and 18O stable isotope analysis. Food Chem. 2012, 133, 1070–1074. [Google Scholar] [CrossRef]
- Dansgaard, W. Stable isotopes in precipitation. Tellus 1964, 16, 436–468. [Google Scholar] [CrossRef]
- Gat, J.R. Oxygen and hydrogen isotopes in the hydrological cycle. Ann. Rev. Earth Plan. Sci. 1996, 24, 225–262. [Google Scholar] [CrossRef]
- Clark, I.D.; Fritz, P. Environmental Isotopes in Hydrogeology; CRC Press: London, UK, 1997. [Google Scholar]
- Craig, H. Isotopic Variations in Meteoric Waters. Science 1961, 133, 1702–1703. [Google Scholar] [CrossRef]
- Hobson, K.A.; Koehler, G. On the use of stable oxygen isotope (δ(18)O) measurements for tracking avian movements in North America. Ecol. Evol. 2015, 5, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Barbour, M.M. Stable oxygen isotope composition in plant tissue: A review. Funct. Plant. Biol. 2007, 34, 83–94. [Google Scholar] [CrossRef]
- Schmidt, H.-L.; Werner, R.A.; Roûmann, A. 18O Pattern and biosynthesis of natural plant products. Phytochem. 2001, 58, 9–32. [Google Scholar] [CrossRef]
- Krivachy (Tanz), N.; Rossmann, A.; Schmidt, H.L. Potentials and caveats with oxygen and sulfur stable isotope analyses in authenticity and origin checks of food and food commodities. Food Cont. 2015, 48, 143–150. [Google Scholar] [CrossRef]
- Camin, F.; Perini, M.; Colombari, G.; Bontempo, L.; Versini, G. Influence of dietary composition on the carbon, nitrogen, oxygen and hydrogen stable isotope ratios of milk. Rapid Commun. Mass Spectrom. 2008, 22, 1690–1696. [Google Scholar] [CrossRef] [PubMed]
- Abeni, F.; Petrera, F.; Capelletti, M.; Dal Prà, A.; Bontempo, L.; Tonon, A. Hydrogen and oxygen stable isotope fractionation in body fluid compartments of dairy cattle according to season, farm, breed, and reproductive stage. PLoS ONE 2015, 10, e0127391. [Google Scholar] [CrossRef] [PubMed]
- Kohn, M.J. Predicting animal δ18O: Accounting for diet and physiological adaptation. Geochim. Cosmochim. Acta 1996, 60, n4811–n4829. [Google Scholar] [CrossRef]
- Kohn, M.J.; Schoeninger, M.J.; Valley, J.W. Herbivore tooth oxygen isotope compositions: Effects of diet and physiology. Geochim. Cosmochim. Acta 1996, 60, 3889–3896. [Google Scholar] [CrossRef]
- Bryant, D.; Froelich, P.N. A model of oxygen isotope fractionation in body water of large mammals. Geochim. Cosmochim. Acta 1995, 59, 4523–4537. [Google Scholar] [CrossRef]
- Podlesak, D.W.; Torregrossa, A.-M.; Ehleringer, J.R.; Dearing, M.D.; Passey, B.H.; Cerling, T.E. Turnover of oxygen and hydrogen isotopes in the body water, CO2, hair, and enamel of a small mammal. Geochim. Cosmochim. Acta 2008, 72, 19–35. [Google Scholar] [CrossRef]
- Kornexl, B.E.; Werner, T.; Roßmann, A.; Schmidt, H.-L. Measurement of stable isotope abundances in milk and milk ingredients—A possible tool for origin assignment and quality control. Z. Für Lebensm. Und Forsch. A 1997, 205, 19–24. [Google Scholar] [CrossRef]
- Chesson, L.A.; Valenzuela, L.O.; O’Grady, S.P.; Cerling, T.E.; Ehleringer, J.R. Hydrogen and oxygen stable isotope ratios of milk in the United States. J. Agric. Food Chem. 2010, 58, 2358–2363. [Google Scholar] [CrossRef] [PubMed]
- Crittenden, R.G.; Andrew, A.S.; LeFournour, M.; Young, M.D.; Middleton, H.; Stockmann, R. Determining the geographic origin of milk in Australasia using multi-element stable isotope ratio analysis. Int. Dairy J. 2007, 17, 421–428. [Google Scholar] [CrossRef]
- Vander Zanden, H.B.; Soto, D.X.; Bowen, G.J.; Hobson, K.A. Expanding the isotopic toolbox: Applications of hydrogen and oxygen stable isotope ratios to food web studies. Front. Ecol. Evol. 2016, 4, 20. [Google Scholar] [CrossRef]
- Magdas, D.A.; Cristea, G.; Cordea, D.V.; Bot, A.; Puscas, R.; Radu, S.; Mirel, V.; Mihaiu, M. Measurements of Stable Isotope Ratios in Milk Samples from a Farm Placed in the Mountains of Transylvania. In AIP Conference Proceedings, Proceedings of the Isotopes and Molecules (PIM 2013), Cluj Napoca, Romania, 25–27 September 2013; American Institute of Physics: University Park, MD, USA, 2013. [Google Scholar]
- Chung, I.-M.; Kim, J.K.; Yang, Y.J.; An, Y.J.; Kim, S.Y.; Kwon, C.; Kim, S.H. A case study for geographical indication of organic milk in Korea using stable isotope ratios-based chemometric analysis. Food Cont. 2020, 107, 106755. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, Q.; Guo, X.; Tang, C.; Yu, X.; Zhan, T.; Qin, Y.; Zhang, J. Application of isotopic and elemental fingerprints in identifying the geographical origin of goat milk in China. Food Chem. 2019, 277, 448–454. [Google Scholar] [CrossRef]
- Garbaras, A.; Skipitytė, R.; Šapolaitė, J.; Ežerinskis, Ž.; Remeikis, V. Seasonal variation in stable isotope ratios of cow milk in Vilnius region, Lithuania. Animals 2019, 9, 69. [Google Scholar] [CrossRef]
- Ehtesham, E.; Hayman, A.; Van Hale, R.; Frew, R. Influence of feed and water on the stable isotopic composition of dairy milk. Int. Dairy J. 2015, 47, 37–45. [Google Scholar] [CrossRef]
- Mezga, K.; Urbanc, J.; Cerar, S. The isotope altitude effect reflected in groundwater: A case study from Slovenia. Isot. Env. Health Stud. 2014, 50, 33–51. [Google Scholar] [CrossRef]
- Lin, G.P.; Rau, Y.H.; Chen, Y.F.; Chou, C.C.; Fu, W.G. Measurements of δD and δ18O stable isotope ratios in milk.”. J. Food Sci. 2003, 68, 2192–2195. [Google Scholar] [CrossRef]
- Golc-Teger, S. “Sadržaj laktoze kao indikator dodate vode u mleku. Mljekarstvo 1985, 37, 195–204. [Google Scholar]
- SMT4-CT98-2236 (EU project): The Development and Validation of Methods to Determine the Origin of Milk, Butter and Cheese; Central Science Laboratory; Final Report; December 2001; Available online: https://www.bats.ch/trace/?file5=coreforms/organisation.php&record_number=1 (accessed on 31 December 2001).
- Brand, W.A.; Coplen, T.B.; Vogl, J.; Rosner, M.; Prohaska, T. Assessment of international reference materials for isotope-ratio analysis (IUPAC technical report). Pure Appl. Chem. 2014, 86, 425–467. [Google Scholar] [CrossRef]
Sample Availability: not available. |
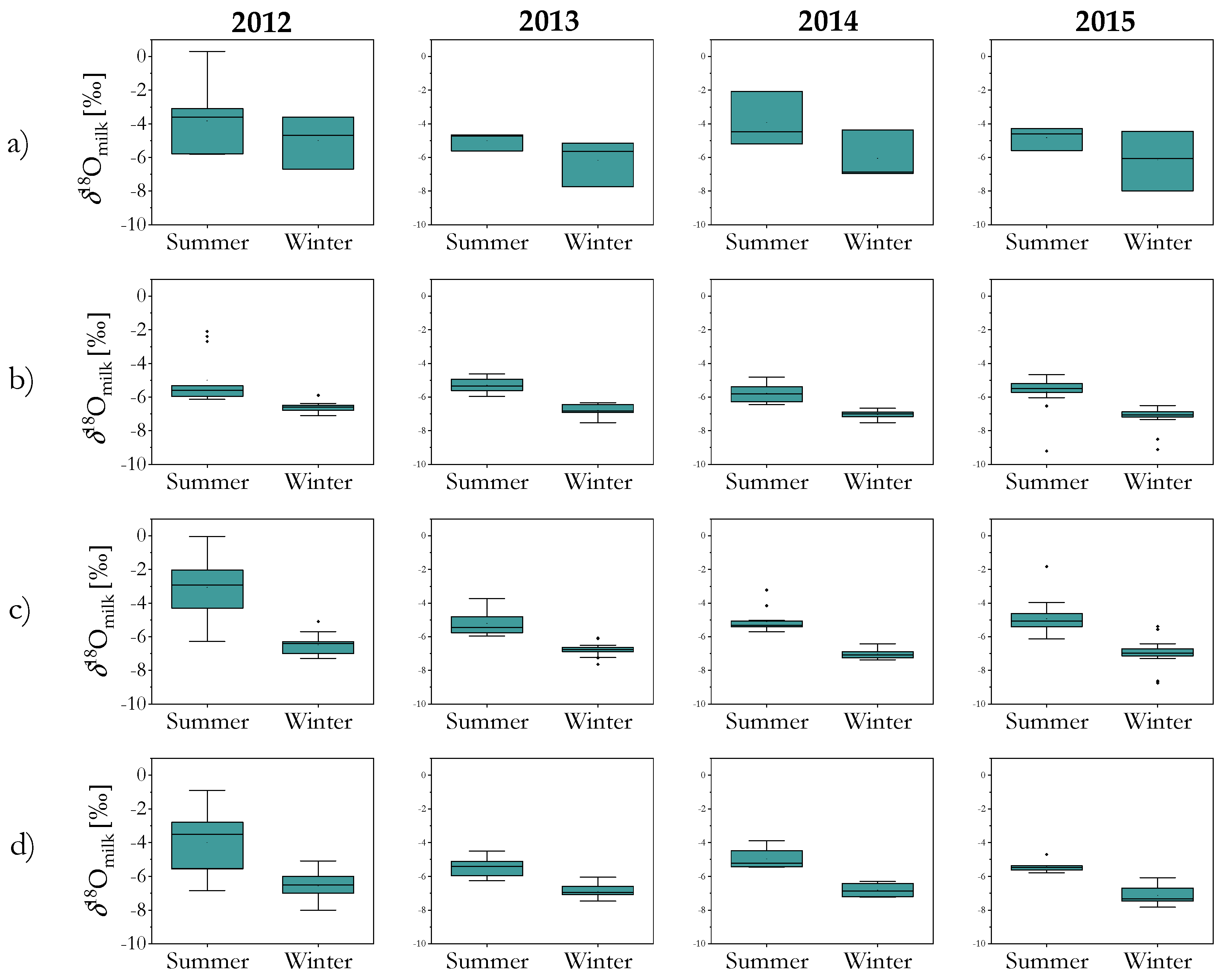
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamzić Gregorčič, S.; Potočnik, D.; Camin, F.; Ogrinc, N. Milk Authentication: Stable Isotope Composition of Hydrogen and Oxygen in Milks and Their Constituents. Molecules 2020, 25, 4000. https://doi.org/10.3390/molecules25174000
Hamzić Gregorčič S, Potočnik D, Camin F, Ogrinc N. Milk Authentication: Stable Isotope Composition of Hydrogen and Oxygen in Milks and Their Constituents. Molecules. 2020; 25(17):4000. https://doi.org/10.3390/molecules25174000
Chicago/Turabian StyleHamzić Gregorčič, Staša, Doris Potočnik, Federica Camin, and Nives Ogrinc. 2020. "Milk Authentication: Stable Isotope Composition of Hydrogen and Oxygen in Milks and Their Constituents" Molecules 25, no. 17: 4000. https://doi.org/10.3390/molecules25174000
APA StyleHamzić Gregorčič, S., Potočnik, D., Camin, F., & Ogrinc, N. (2020). Milk Authentication: Stable Isotope Composition of Hydrogen and Oxygen in Milks and Their Constituents. Molecules, 25(17), 4000. https://doi.org/10.3390/molecules25174000